Abstract
Exposure to sounds during early development causes enlarged cortical representations of those sounds, leading to the commonly held view that the size of stimulus representations increases with stimulus exposure. However, representing stimuli based solely on their prevalence may be inefficient, because many frequent environmental sounds are behaviorally irrelevant. Here, we show that cortical plasticity depends not only on exposure time but also on the temporal modulation rate of the stimulus set. We examined cortical plasticity induced by early exposure to 7 kHz tone pips repeated at a slow (2 Hz), fast (15 Hz), or ethological (6 Hz) rate. Certain rat calls are modulated near 6 Hz. We found that spectral representation of 7 kHz increased only in the ethological-rate-reared animals, whereas improved entrainment of cortical neurons was seen in animals reared in the slow- and fast-rate condition. This temporal rate dependence of spectral plasticity may serve as a filtering mechanism to selectively enlarge representations of species-specific vocalizations. Furthermore, our results indicate that spectral and temporal plasticity can be separately engaged depending on the statistical properties of the input stimuli.
Introduction
Cortical sensory representations can be reorganized during early development and in adulthood (Diamond and Weinberger, 1986; Edeline et al., 1993; Recanzone et al., 1993; Bakin et al., 1996; Irvine and Rajan, 1996; Kilgard and Merzenich, 1998a; Bao et al., 2001; Zhang et al., 2001; Beitel et al., 2003; Polley et al., 2004, 2006; Blake et al., 2006; Noreña et al., 2006; de Villers-Sidani et al., 2008; Zhou et al., 2008). Such cortical plasticity processes are believed to enlarge representations of behaviorally important stimuli, thereby optimizing the processing capacity for these stimuli. Consistent with such a view, behaviorally important sounds, such as species-specific vocalizations, are preferentially represented in the auditory cortex of many species (Rauschecker et al., 1995; Wang et al., 1995; Ohlemiller et al., 1996; Tian et al., 2001; Wang and Kadia, 2001). Although cortical plasticity in adult animals is induced by behaviorally important sensory stimuli associated with activity in the neuromodulatory systems, plasticity in developing animals can be induced by passive sensory exposure (Zhang et al., 2001; Chang and Merzenich, 2003; de Villers-Sidani et al., 2007; de Villers-Sidani et al., 2008; Zhou and Merzenich, 2008). A potential problem with exposure-induced plasticity is that both behaviorally important and irrelevant stimuli are present in the sensory environment. In some environments, behaviorally irrelevant stimuli may even dominate the sensory input. Thus, representation of stimuli based solely on frequency of occurrence or acoustic power could be highly inefficient.
Natural animal vocalizations are often repeated in bouts (Liu et al., 2003; Schnupp et al., 2006). The temporal repetition rate of vocalizations within these bouts is an important feature that may distinguish animal vocalizations from other environmental sounds. For instance, mouse vocalization calls are typically produced 5–10 times per second, whereas insect chirps may be repeated at much higher rates (Liu et al., 2003; Schnupp et al., 2006). A plausible mechanism that could allow for selective representations of species-specific calls is temporal filtering of the sensory input so that only sounds modulated near an ethologically relevant modulation rates induce experience-dependent plasticity.
In the present study, we investigated how cortical plasticity depends on temporal repetition rate. We characterized rat calls and showed that they are typically repeated at 3–10 Hz. We then exposed rat pups to brief tones repeated at 2, 6, or 15 pips per second and subsequently examined spectral frequency and temporal rate representations. Our results indicate that exposure to tones repeated at an ethologically relevant rate, but not a slower or faster rate, enlarged cortical representations of the exposure frequency. Although spectral representation was not changed for animals reared in the faster or slower rate, temporal rate representation was improved.
Materials and Methods
Recording and analysis of animal vocalization.
All procedures used in this study were approved by the University of California Berkeley Animal Care and Use Committee. To record rat pup isolation calls, individual rat pups were placed on a platform located in an anechoic chamber where the ambient temperature is maintained at 21.5°C. A 1/4 inch Bruel and Kjaer (B&K) model 4135 microphone was connected to a B&K 2669 preamplifier and B&K 2690 conditioning amplifier, and the output signal was digitized with a 16-bit analog-to-digital converter (National Instrument) at 200 kHz. Adult encounter calls were recorded after an adult female rat was introduced to the home cage of a single adult male. Five postnatal 11 (P11), six P15 rat pups, and two adult pairs were used.
Visual examination of all recorded rat calls indicated that all pup calls were in the frequency range from 25 to 50 kHz, and all adult encounter calls were in the frequency range from 25 to 70 kHz (Brudzynski et al., 1999; Brudzynski and Pniak, 2002; Liu et al., 2003). Thus, we bandpass filtered all calls to obtain signals in the ranges of 25–50 kHz for pup calls or 25–75 kHz for adult calls, for further automatic identification of the calls based on their amplitude envelopes. The start of a call was defined as an upward crossing of a threshold of six SDs above the mean amplitude of a no-call period, and the end occurred when the amplitude envelope was below the threshold for at least 40 ms. Calls <5 ms (or 10 ms) long were automatically excluded for the pups (or adults). Call-onset asynchrony (COA) was defined as the time between the start of two consecutive calls.
Acoustic rearing of young rat pups.
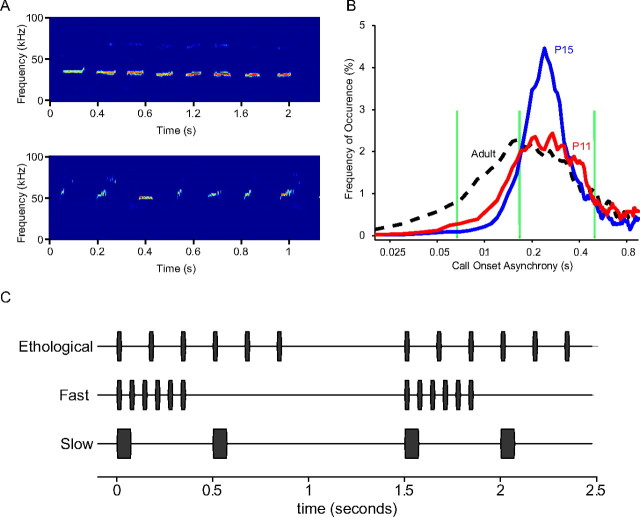
Four groups (ethological, slow, fast, and mixed) of Sprague Dawley rat pups were placed with their mothers in anechoic sound-attenuation chambers from P8 to P30. This time range covers the critical period for spectral plasticity in auditory cortex (AI) and has been used previously (Zhang et al., 2001; de Villers-Sidani et al., 2007; Han et al., 2007). The ethological, fast, and slow rat pups experienced tone pips (7.071 kHz, 60 dB SPL, 25 ms) presented at one of three repetition rates 24 h a day. The ethological and fast groups heard trains of six tone pips presented at the rate of 6 and 15 Hz, respectively, with one train every 1.5 s (see Fig. 1D). A pair of tone pips were played to the slow group every 1.5 s, with 0.5 s onset asynchrony between the tone pips. To ensure that the slow group receives the same amount of acoustic energy as the other groups, the duration of the tone pips was set at 75 ms (see Fig. 1D). The mix litter was exposed to trains of 15 kHz tone pips (60 dB SPL, 25 ms) presented at the ethological rate (6 Hz) and trains of 5 kHz tone pips (60 dB SPL, 25 ms) presented at the fast rate (15 Hz). The respective trains were presented once every 3 s and interleaved so they never overlapped (see Fig. 3A). After sound exposure, rats were moved to a regular animal room environment until they were mapped (typically 4–20 d after the end of rearing). A control litter was reared in a regular animal room environment.
Figure 1.
Characterization of rat calls. A, Example spectrograms of a bout of rat pup isolation calls (top) and adult encounter calls (bottom). B, Distributions of COA within a bout. Green vertical lines indicate the COAs of the three experimental conditions: fast (15 Hz, COA = 0.067 s, far left), ethological (6 Hz, COA = 0.167 s, middle), and slow (2 Hz, CAO = 0.5 s, far right). C, A schematic of the stimuli used in the ethological, fast, and slow experimental-rearing conditions.
Figure 3.
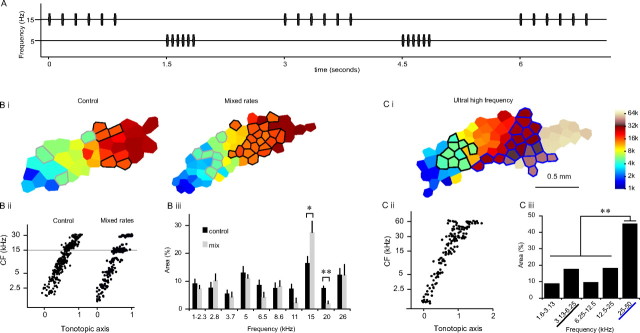
Over-representation of sounds repeated at the ethological rates. A, A schematic of “mix-rate” rearing stimuli. A train of 15 kHz tone consisted of six tone pips presented at the ethological rate (6 Hz), and a train of 5 kHz tone consisted of six tone pips presented at the fast rate (15 Hz). Trains of the two repetition rates were interleaved such that one train was heard every 1.5 s. B, CF map reorganization resulted from the mixed-rate rearing. Bi, Example maps of control and mixed-rate animals. Control animal is the same as seen in Figure 2A. Area represented 5 kHz ±0.2 octaves are outlined in gray, whereas area representing 15 kHz ±0.2 octaves are outlined in black. Bii, Distributions of CFs along the tonotopic axis. Biii, Sizes of cortical areas representing different frequency bands. There was a significant increase in representation at 15 kHz and a significant decrease at 20 kHz. Error bars indicate SEM. *p < 0.05, **p < 0.001. C, Cortical representation of ultrasonic frequencies. Ci, An example CF map from a control animal mapped up to 74 kHz. Areas representing 25–50 kHz are outlined in blue, whereas areas representing 3.13–6.25 kHz are outlined in black. Cii, Distribution of CFs along the tonotopic axis. Ciii, Sizes of cortical areas representing one-octave frequency bands. The representation of the 25–50 kHz band was significantly larger than those of the other bands.
Electrophysiological-recording procedure.
The primary AI of sound-reared and control rats were mapped at comparable ages from P34 to P52. Care was taken to ensure that animals in different groups were recorded at comparable ages (control, 47.9 ± 18.4 d; ethological, 39.8 ± 6.3; fast, 39.2 ± 6; slow, 37 ± 2.2). Rats were preanesthetized with buprenorphine (0.05 mg/kg, s.c.) a half hour before they were anesthetized with sodium pentobarbital (50 mg/kg, followed by 10–20 mg/kg supplements as needed). Atropine sulfate (0.1 mg/kg) and dexamethasone (1 mg/kg) were administered once every 6 h. The head was secured in a custom head-holder that left the ears unobstructed, and the cisterna magna was drained of CSF. The right auditory cortex was exposed through a craniotomy and duratomy and was kept under a layer of silicone oil to prevent desiccation. Sound stimuli were delivered to the left ear through a custom-made speaker that had been calibrated to have <3% harmonic distortion and flat output in the entire frequency range.
Cortical responses were recorded with tungsten microelectrodes (FHC). Recording sites were chosen to evenly and densely sample the primary auditory cortex while avoiding surface blood vessels and were marked on an amplified digital image of the cortex. Microelectrodes were lowered orthogonally into the cortex to a depth of 450–600 μm where responses to noise bursts could be found. Multiunit responses to 25 ms tone pips of 51 frequencies (1–32 kHz, 0.1 octave spacing, 5 ms cosine-squared ramps) and eight sound pressure levels (0–70 dB SPL, 10-dB steps) were recorded to reconstruct the frequency-intensity receptive field. In two control animals, 12 additional frequencies (32–74 kHz, 0.1 octave spacing) were included to quantify representations of high ultrasonic frequencies up to 74 kHz.
Responses to trains of tone pips and noise bursts were recorded in two additional rats per group using 4 × 4 silicon polytrodes, with ∼1 mΩ impedance (NeuroNexus Technologies; N2T). After finding AI by coarse mapping with tungsten microelectrodes, a polytrode was lowered into cortex. Six noise bursts or pure tone pips were presented in trains at six different presentation rates (3, 6, 9, 12, 15, 18 Hz). The noise bursts and tone pips were 25 ms long (with 5 ms cosine-squared ramps) and presented at a sound pressure level of 50 dB. Each carrier-rate combination was repeated 10 times and presented in a pseudo-randomized order. One train was presented once every 3 s.
Data analysis.
The characteristic frequency (CF) was defined as the frequency at which responses are evoked at threshold: the lowest sound pressure level that activate the neuron. The bandwidth at 30 dB above threshold (BW30) measures the width of the receptive field (in octaves). The CF, threshold, and BW30 for each penetration site were determined visually. AI was functionally defined by well tuned neurons and fine tonotopic gradient with increasing CFs going rostrodorsally. Penetrations that were not in AI were removed leaving a total of 1590 AI recording sites (389 from control, 368 from ethological, 343 from fast, 157 from slow, 194 from mix, and 139 from ultrasonic recordings). Cortical area representing a specific frequency was measured using voronoi tessellation (Matlab; Mathworks).
Repetition rate transfer functions (RRTFs), normalized responses as functions of presentation rates, were calculated as follows. First, only trials in which the response to the first noise burst (or pure tone) was greater than two SDs above mean spontaneous spike rate were included. The normalized response was calculated by taking the average response of the last 5 sound presentations (response being the number of spikes triggered 7–40 ms after onset of the noise/tone) and dividing it by the response to the first sound. A normalized response greater than one indicates that the unit responded better to subsequent sounds than to single noise pulse/tone pip. All reported statistics are two-tailed t tests unless indicated otherwise.
Results
Repetition rate of rat vocalizations
The repetition rate of rat vocalizations were measured by recording pup isolation calls and adult encounter calls. A total of 1610, 1210, and 1063 calls were extracted for adults, P15s and P11s, respectively. A bout was defined as a series of successive calls with COAs <1 s. Only calls that were produced in bouts were included for further analysis, resulting in 1410 (88%), 724 (60%), and 819 (77%) calls over 295, 197, and 209 bouts. An analysis of pup isolation and adult encounter vocalizations revealed that calls within bouts are typically repeated at 3–10 Hz (Fig. 1). The repetition rate of adult calls is faster than that of the pup calls (Fig. 1A,B). The COA distribution of the P15 group showed a very clear peak (240 ms, ∼4.2 Hz), as the isolation calls from P15 pups were very stereotypical and repeated regularly. The repetitions were less regular for the P11 and the adult calls, resulting in the larger spreads. The COA distribution of the P11 group has a peak at 270 ms, corresponding to ∼3.7 Hz, whereas that of adults has a peak at 160 ms, corresponding to 6.3 Hz.
Effect of presentation rate on spectral plasticity
To investigate the impact of repetition rates on cortical plasticity, we exposed three groups of rat pups to trains of 7 kHz tone pips, with tone trains presented once every 1.5 s. Within each train, tone pips were repeated at a rate of 2 Hz for the slow group, 6 Hz for the ethological group, and 15 Hz for the fast group. The slow and fast rates are below and above the range of the ethological repetition rates of rat vocalizations (Fig. 1B). The duration of the tone pips was increased for the slow group so that the total acoustic energy of tone pips experienced by the animals was the same for all three groups (Fig. 1C).
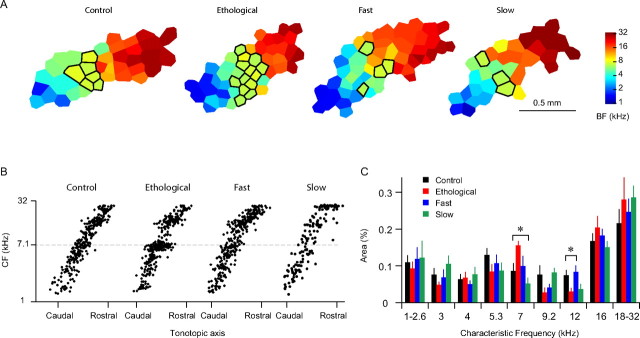
Previous studies have shown that exposure to a tone increases cortical representations of that tone (Zhang et al., 2001; de Villers-Sidani et al., 2007; Han et al., 2007). In this study, we mapped the auditory cortex of several animals for each group (control, n = 10; ethological, n = 6; fast, n = 6; slow, n = 4) and found enlarged representations of the exposure frequency in animals reared with the ethological rate but not with the slow or fast rate (Fig. 2). A four-conditions by nine-frequencies ANOVA showed no differences across condition (p = 0.95) and a significant interaction (p < 0.04). One-way ANOVAs across the frequency bins showed significant differences between conditions only in the 7 kHz (p < 0.037) and 12 kHz bins (p < 0.041). Animals reared in the ethological rate (6 Hz) showed a significant increase in the cortical area representing 7 kHz (±0.2 octaves) when compared with the naive control, slow and fast groups (p < 0.013, 0.041, 0.001, respectively, one-tailed t test). Animals reared with the ethological rate showed smaller representations of 12 kHz tone when compared with the control (p < 0.034) and fast (p < 0.018) groups (Fig. 2C).
Figure 2.
Influences of stimulus presentation rate on spectral plasticity. A, Representative cortical CF maps of the control, ethological, fast, and slow groups. The areas representing 7 kHz ±0.2 octaves were outlined in black. B, Distributions of CFs along the tonotopic axis. C, Sizes of cortical areas representing different frequency bands. Significant differences were seen for the 7 kHz and 12 kHz bands. Error bars indicate SEM. *p < 0.05.
An additional group of rat pups were exposed to two different carrier frequencies presented at two different rates: trains of 15 kHz tone pips were presented at the ethological rate (6 Hz) and trains of 5 kHz tone pips were presented at the fast rate (15 Hz). The respective trains were presented once every 3 s and were interleaved so that one train was played every 1.5 s (Fig. 3A). A comparison with the naive control animals showed an increase in representation of 15 kHz (p < 0.05) and a decrease in the representation of the neighboring 20 kHz (p < 0.001). No changes were observed around the representation of 5 kHz (p = 0.58) (Fig. 3B). These results confirm our finding that sounds that are repeated at an ethological rate are over-represented.
The average threshold, response latency, BW30, and recording depth are shown in Table 1. One-way ANOVAs comparing across the five groups showed no significant differences for threshold (p > 0.2), latency (p > 0.05), BW30 (p > 0.2), or recording depth (p > 0.5).
Table 1.
Response properties of AI neurons
| Threshold (dB) | Latency (ms) | BW30 (oct) | Depth (μm) | |
|---|---|---|---|---|
| Control | 37.34 (2.63) | 17.93 (0.62) | 1.40 (0.073) | 550.8 (10.9) |
| Ethological | 32.42 (3.65) | 17.44 (0.46) | 1.31 (0.064) | 563.8 (13.1) |
| Fast | 35.29 (1.33) | 17.30 (0.41) | 1.33 (0.093) | 555.9 (15.4) |
| Slow | 26.2 (2.54) | 20.28 (1.07) | 1.13 (0.040) | 540.0 (5.8) |
| Mix | 32.88 (2.23) | 18.34 (0.37) | 1.22 (0.017) | 536.1 (3.2) |
SEM is indicated in parentheses. oct, Octave.
Over-representation of rat vocalization frequencies
The above results suggest that the representation of the frequency range of ultrasonic rat vocalizations should also be enlarged, because rat calls are mostly repeated at ethological rates. The ultrasonic (up to 74 kHz) region of the AI was mapped in two naive virgin female rats. Both animals showed large representation of ultrasonic frequencies (Fig. 4C). To facilitate statistical analysis, penetrations were separated into five one-octave-sized frequency bins (lowest bin covering 1.56–3.13 kHz and the highest bin covering 25–50 kHz, frequencies >50 kHz were not included, leaving 106 penetrations). A χ2 test showed that the representation of frequencies from 25 to 50 kHz was significantly greater than those of the other frequency ranges (p < 0.001) (Fig. 4Ciii).
Figure 4.
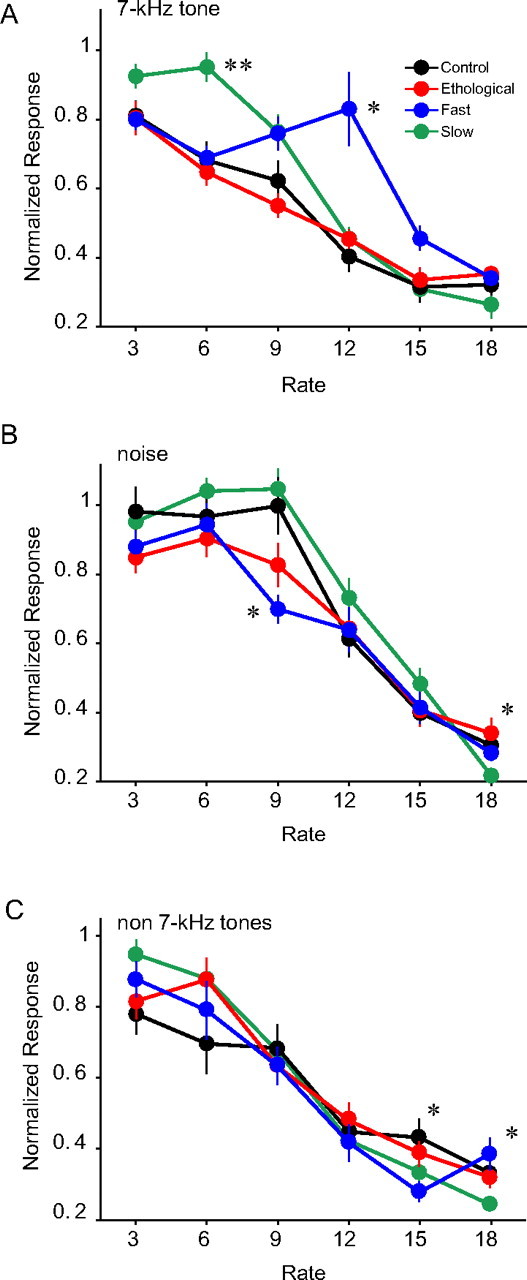
Effects of tonal exposure on cortical temporal response properties. A–C, Repetition rate transfer functions characterized with trains of 7 kHz tone pips (A), noise bursts (B), and non-7 kHz tone pips (C). Error bars indicate SEM. *p < 0.05, **p < 0.001, all for comparison to the control.
Temporal response plasticity
Plasticity in temporal response properties was tested in cortical neurons by measuring their responses to trains of tone pips presented at various rates. Only units that responded reliably to 7 kHz pure tones (see Materials and Methods) were included for the analysis (control, n = 26; ethological, n = 28; fast, n = 37; slow, n = 42). The 7 kHz-tone-derived RRTF of the control and ethological groups overlapped (Fig. 4A). A group by rate ANOVA showed significant main effects for group (p < 0.001) and rate (p < 0.0001) and a significant interaction (p < 0.00001). Post hoc t tests revealed that the normalized response at the 12 Hz repetition rate was significantly larger for the fast group when compared with all the other groups (p < 0.05). In addition, the slow group had greater normalized responses at the repetition rate of 6 Hz when compared with all the other groups (p < 0.001). Although neurons in the fast group showed slightly better following responses than the control groups at the repetition rate of 15 Hz, the rearing rate, the difference was not significant (p > 0.2). The slow group also showed slightly enhanced following responses at 3 Hz, but it was not different from that of the ethological group (p > 0.05).
Of the 133 units analyzed above, 116 were responsive to noise bursts (control, n = 24; slow, n = 25; fast, n = 31; slow, n = 36). A one-way ANOVA revealed significant differences between groups at the repetition rates of 9 Hz (p = 0.0002) and 18 Hz (p < 0.01) (Fig. 4B). Post hoc t tests showed that the fast group did not entrain to noise burst as well as the control or slow groups at the repetition rate of 9 Hz (p < 0.002 for both) and that the slow group did not entrain at 18 Hz as well as the other groups (p < 0.02, for all). However, we did not observe enhanced responses to noise bursts repeated at 12 Hz in the fast group or at 6 Hz in the slow group (Fig. 4B).
We also examined cortical responses to repeated tones of various carrier frequencies (4.5, 5.6, 8.9, 11.2, 14.1, or 17.8 kHz) in the control, ethological, fast, and slow groups (n = 21, 23, 32, and 40, respectively). The carrier frequencies were chosen to reliably activate the units, and only units used in the 7 kHz analysis were included. One-way ANOVAs across the different repetition rates showed significant differences only for the 15 and 18 Hz (p < 0.01, for both) (Fig. 4C). At the repetition rate of 15 Hz, the fast group did not entrain to tone pips as well as the control or ethological group (p < 0.02, for both); while in at the repetition rate of 18 Hz, the slow group showed lower normalized responses compared with all other groups (p < 0.04, for all). Thus, the enhanced responses to sounds repeated at 12 Hz in the fast group and enhanced response to sounds repeated at 6 Hz in the slow group were specific to the 7 kHz carrier frequency of the exposure tone.
Discussion
In the present study, we tested the hypothesis that temporal repetition rates influence how sound experiences shape cortical representations of the sound. All animals in the fast, slow, and ethological groups of animals were exposed to a 7 kHz tone of the same total acoustic energy, and yet the sensory experiences had completely different effects: a 40% increase in 7 kHz representations for the ethological group but not for the fast or the slow group. Furthermore, exposing developing animals to two tones (5 and 15 kHz) presented at two different rates (fast and ethological, respectively) lead to the over-representation of only the tone presented at the ethological rate. These results indicate that temporal repetition rates of sensory stimuli have a strong impact on experience-dependent plasticity. Earlier studies of sound exposure-induced cortical plasticity mostly used repetition rates similar to our ethological rate, and robust increases in representations of the exposed stimulus were observed (Zhang et al., 2001; Han et al., 2007). In contrast, reduced representations were observed for stimuli that are constantly present in the environment without temporal modulation (de Villers-Sidani et al., 2008; Zhou et al., 2008). Here, we show that exposure to tone pips that are repeated at 2 or 15 Hz does not result in greater representations of the tone. Such a temporal filtering mechanism would enlarge representations of stimuli that are repeated/modulated near the ethological rates of species-specific vocalization but not other potentially irrelevant stimuli that are modulated at other rates.
Repetition rates of the rat calls have considerable variability. However, the majority of calls were repeated at rates from 3 to 10 Hz (Fig. 1). Less than 1% of the calls had repetition rate higher than 15 Hz, whereas 26% had repetition rate <2 Hz. Thus, a large number of rat calls could pass through the presumptive temporal filter and shape cortical acoustic representations. The COAs that were >0.5 s (< 2 Hz) were mostly between two calls in different bouts. The enlarged representation of the frequency range of the rat vocalizations is consistent with the notion of a temporal filter for selective representation of sounds repeated at ethological rates. However, it could also be attributable to other experience-independent mechanisms.
The neural mechanisms of the temporal filter in cortical plasticity are unknown. It is well known that cortical neurons respond differently to sound repeated at different rates. For instance, cortical neurons in anesthetized rats do not respond well to sounds repeated >10 times per second (Kilgard and Merzenich, 1998b), whereas auditory thalamic neurons are capable to respond at much faster rates (Wehr and Zador, 2005). In the awake preparation, multiunit clusters have been shown on average to synchronize to clicks repeated at 72 Hz, but normalized responses still show a decrease beyond 10 Hz (Anderson et al., 2006). Such temporal response properties may contribute to the lack of spectral plasticity in the fast group. However, such cortical temporal response properties cannot account for the lack of spectral plasticity in the slow group, because cortical neurons respond well to slow-rate sounds.
Although exposure to fast-rate tone pips did not enlarge representations of the tone, it did improve entrainment of responses to quickly repeating tone pips in neurons of the fast group. Similarly, exposure to slow-rate tone pips enhanced cortical responses to slowly repeating tone pips. In previous studies, temporal plasticity was induced either with noise bursts (Kilgard et al., 2001; Chang and Merzenich, 2003; Bao et al., 2004; de Villers-Sidani et al., 2008; Zhou and Merzenich, 2008) or tone pips of several carrier frequencies (Kilgard et al., 2001). Tone pips of a single frequency were previously found ineffective in inducing temporal plasticity (Kilgard et al., 2001). We show here that temporal plasticity can be induced with a single-frequency tone and can be specific to the tonal stimulus. The discrepancy between the earlier and the present results may be related to differences in the experimental methods. The earlier study used stimulation of Nucleus Basalis to induce plasticity in adult animals, whereas we simply exposed young animals to the sound stimuli. Furthermore, we restricted our analysis only to neurons that responded reliably to the exposure tone, which might be necessary to reveal frequency-specific plasticity effects. These results are consistent with earlier findings of enhanced entrainment of cortical responses in mother rats to trains that are spectrally and temporally similar to pup calls (Liu et al., 2006). Our results suggest that both spectral and temporal information of specific stimuli can be represented in the same population of neurons. They also indicate that spectral and temporal plasticity can be separately engaged depending on the characteristics of input stimuli, such as the temporal modulation rate.
It has long been hypothesized that efficient representations of sensory stimuli depends on the stimulus statistics (Barlow, 1961; Lewicki, 2002; Singh and Theunissen, 2003) and that the learning and plasticity processes that shape sensory representations must be sensitive to the statistics of the sensory input (Kilgard et al., 2001; Maye et al., 2002; Toro and Trobalón, 2005). Our results support this hypothesis by showing that cortical circuits are sensitive to temporal rates and may use this feature to selectively represent sounds that are likely to be behaviorally relevant.
Footnotes
This work was supported by National Institutes of Health Grants DC007883 and DC009259 and by the American Tinnitus Association. H.K. is supported by the National Science Foundation Graduate Research Fellowship Program.
References
- Anderson SE, Kilgard MP, Sloan AM, Rennaker RL. Response to broadband repetitive stimuli in auditory cortex of the unanesthetized rat. Hear Res. 2006;213:107–117. doi: 10.1016/j.heares.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Bakin JS, South DA, Weinberger NM. Induction of receptive field plasticity in the auditory cortex of the guinea pig during instrumental avoidance conditioning. Behav Neurosci. 1996;110:905–913. doi: 10.1037//0735-7044.110.5.905. [DOI] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Bao S, Chang EF, Woods J, Merzenich MM. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat Neurosci. 2004;7:974–981. doi: 10.1038/nn1293. [DOI] [PubMed] [Google Scholar]
- Barlow HB. Possible principle underlying the transformation of sensory messages. In: Rosenblith W, editor. Sensory communication. Cambridge, MA: MIT; 1961. pp. 217–234. [Google Scholar]
- Beitel RE, Schreiner CE, Cheung SW, Wang X, Merzenich MM. Reward-dependent plasticity in the primary auditory cortex of adult monkeys trained to discriminate temporally modulated signals. Proc Natl Acad Sci U S A. 2003;100:11070–11075. doi: 10.1073/pnas.1334187100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DT, Heiser MA, Caywood M, Merzenich MM. Experience-dependent adult cortical plasticity requires cognitive association between sensation and reward. Neuron. 2006;52:371–381. doi: 10.1016/j.neuron.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudzynski SM, Pniak A. Social contacts and production of 50-kHz short ultrasonic calls in adult rats. J Comp Psychol. 2002;116:73–82. doi: 10.1037/0735-7036.116.1.73. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Kehoe P, Callahan M. Sonographic structure of isolation-induced ultrasonic calls of rat pups. Dev Psychobiol. 1999;34:195–204. doi: 10.1002/(sici)1098-2302(199904)34:3<195::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300:498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci. 2007;27:180–189. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villers-Sidani E, Simpson KL, Lu YF, Lin RC, Merzenich MM. Manipulating critical period closure across different sectors of the primary auditory cortex. Nat Neurosci. 2008;11:957–965. doi: 10.1038/nn.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Weinberger NM. Classical conditioning rapidly induces specific changes in frequency receptive fields of single neurons in secondary and ventral ectosylvian auditory cortical fields. Brain Res. 1986;372:357–360. doi: 10.1016/0006-8993(86)91144-3. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Pham P, Weinberger NM. Rapid development of learning-induced receptive field plasticity in the auditory cortex. Behav Neurosci. 1993;107:539–551. doi: 10.1037//0735-7044.107.4.539. [DOI] [PubMed] [Google Scholar]
- Han YK, Köver H, Insanally MN, Semerdjian JH, Bao S. Early experience impairs perceptual discrimination. Nat Neurosci. 2007;10:1191–1197. doi: 10.1038/nn1941. [DOI] [PubMed] [Google Scholar]
- Irvine DR, Rajan R. Injury- and use-related plasticity in the primary sensory cortex of adult mammals: possible relationship to perceptual learning. Clin Exp Pharmacol Physiol. 1996;23:939–947. doi: 10.1111/j.1440-1681.1996.tb01146.x. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998a;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nat Neurosci. 1998b;1:727–731. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Pandya PK, Vazquez J, Gehi A, Schreiner CE, Merzenich MM. Sensory input directs spatial and temporal plasticity in primary auditory cortex. J Neurophysiol. 2001;86:326–338. doi: 10.1152/jn.2001.86.1.326. [DOI] [PubMed] [Google Scholar]
- Lewicki MS. Efficient coding of natural sounds. Nat Neurosci. 2002;5:356–363. doi: 10.1038/nn831. [DOI] [PubMed] [Google Scholar]
- Liu RC, Miller KD, Merzenich MM, Schreiner CE. Acoustic variability and distinguishability among mouse ultrasound vocalizations. J Acoust Soc Am. 2003;114:3412–3422. doi: 10.1121/1.1623787. [DOI] [PubMed] [Google Scholar]
- Liu RC, Linden JF, Schreiner CE. Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. Eur J Neurosci. 2006;23:3087–3097. doi: 10.1111/j.1460-9568.2006.04840.x. [DOI] [PubMed] [Google Scholar]
- Maye J, Werker JF, Gerken L. Infant sensitivity to distributional information can affect phonetic discrimination. Cognition. 2002;82:B101–B111. doi: 10.1016/s0010-0277(01)00157-3. [DOI] [PubMed] [Google Scholar]
- Noreña AJ, Gourévitch B, Gourevich B, Aizawa N, Eggermont JJ. Spectrally enhanced acoustic environment disrupts frequency representation in cat auditory cortex. Nat Neurosci. 2006;9:932–939. doi: 10.1038/nn1720. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Kanwal JS, Suga N. Facilitative responses to species-specific calls in cortical FM-FM neurons of the mustached bat. Neuroreport. 1996;7:1749–1755. doi: 10.1097/00001756-199607290-00011. [DOI] [PubMed] [Google Scholar]
- Polley DB, Heiser MA, Blake DT, Schreiner CE, Merzenich MM. Associative learning shapes the neural code for stimulus magnitude in primary auditory cortex. Proc Natl Acad Sci U S A. 2004;101:16351–16356. doi: 10.1073/pnas.0407586101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Hauser M. Processing of complex sounds in the macaque nonprimary auditory cortex. Science. 1995;268:111–114. doi: 10.1126/science.7701330. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnupp JW, Hall TM, Kokelaar RF, Ahmed B. Plasticity of temporal pattern codes for vocalization stimuli in primary auditory cortex. J Neurosci. 2006;26:4785–4795. doi: 10.1523/JNEUROSCI.4330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NC, Theunissen FE. Modulation spectra of natural sounds and ethological theories of auditory processing. J Acoust Soc Am. 2003;114:3394–3411. doi: 10.1121/1.1624067. [DOI] [PubMed] [Google Scholar]
- Tian B, Reser D, Durham A, Kustov A, Rauschecker JP. Functional specialization in rhesus monkey auditory cortex. Science. 2001;292:290–293. doi: 10.1126/science.1058911. [DOI] [PubMed] [Google Scholar]
- Toro JM, Trobalón JB. Statistical computations over a speech stream in a rodent. Percept Psychophys. 2005;67:867–875. doi: 10.3758/bf03193539. [DOI] [PubMed] [Google Scholar]
- Wang X, Kadia SC. Differential representation of species-specific primate vocalizations in the auditory cortices of marmoset and cat. J Neurophysiol. 2001;86:2616–2620. doi: 10.1152/jn.2001.86.5.2616. [DOI] [PubMed] [Google Scholar]
- Wang X, Merzenich MM, Beitel R, Schreiner CE. Representation of a species-specific vocalization in the primary auditory cortex of the common marmoset: temporal and spectral characteristics. J Neurophysiol. 1995;74:2685–2706. doi: 10.1152/jn.1995.74.6.2685. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron. 2005;47:437–445. doi: 10.1016/j.neuron.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat Neurosci. 2001;4:1123–1130. doi: 10.1038/nn745. [DOI] [PubMed] [Google Scholar]
- Zhou X, Merzenich MM. Enduring effects of early structured noise exposure on temporal modulation in the primary auditory cortex. Proc Natl Acad Sci U S A. 2008;105:4423–4428. doi: 10.1073/pnas.0800009105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Nagarajan N, Mossop BJ, Merzenich MM. Influences of un-modulated acoustic inputs on functional maturation and critical-period plasticity of the primary auditory cortex. Neuroscience. 2008;154:390–396. doi: 10.1016/j.neuroscience.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]