Abstract
Obstructive sleep apnea (OSA) patients have elevated tonic and phasic inspiratory activity in the genioglossus and other upper airway muscles during wakefulness; this protects their upper airway from collapse. In this group, sleep-related decrements of upper airway motor tone result in sleep-related upper airway obstructions. We previously reported that in the rat, a species widely used to study the neural mechanisms of both sleep and breathing, lingual electromyographic activity (EMG) is minimal or absent during slow-wave sleep (SWS) and then gradually increases after the onset of rapid eye movement sleep (REMS) due to the appearance of large phasic bursts. Here, we investigated whether sleep-wake patterns and respiratory modulation of lingual EMG depend on the site of EMG recording within the tongue. In nine chronically instrumented rats, we recorded from 17 sites within the tongue and from the diaphragm across sleep-wake states. We quantified lingual EMG in successive 10 s intervals of continuous 2 h recordings (1–3 pm). We found that sleep-wake patterns of lingual EMG did not differ between the base and tip of the tongue, and that respiratory modulation was extremely rare regardless of the recording site. We also determined that the often rhythmic lingual bursts during REMS do not occur with respiratory rhythmicity. This pattern differs from that in OSA subjects who, unlike rats, have collapsible upper airway, exhibit prominent respiratory-modulation of upper airway motor tone during quiet wakefulness, retain considerable tonic and inspiratory phasic activity during SWS, and show nadirs of activity during REMS.
Keywords: atonia, genioglossus, hypoglossal motoneurons, obstructive sleep apnea, REM sleep, upper airway
1. Introduction
Obstructive sleep apnea (OSA) is a syndrome characterized by episodic nocturnal hypoventilations or apneas caused by recurrent narrowing of the upper airway in the oropharyngeal region. The episodes are precipitated by sleep-related decrements of upper airway muscle tone (Sauerland and Harper, 1976; Remmers et al., 1978). The primary cause of the disorder is anatomical, a narrow upper airway that, to stay patent, requires active contraction of upper airway dilator muscles. OSA subjects have a higher than normal persons tonic and phasic inspiratory upper airway muscle tone; this protects their airway from collapse at the time of negative inspiratory pressure (Suratt et al., 1988; Sériès et al., 1989; Mezzanotte et al., 1992; Hendricks et al., 1993). When awake, OSA patients can maintain adequate ventilation, indicating an effective adaptation to altered upper airway anatomy. However, the adaptation is not fully effective during sleep, which highlights an important role in the pathophysiology of the disorder of the central interaction between the neural mechanisms that control behavioral state and those that control upper airway muscles and breathing.
OSA subjects often have prominent and only partially compensated for by reflexes decrements of upper airway motor tone at the onset of slow-wave sleep (SWS) and then further decrements during rapid eye movement sleep (REMS) (Hendricks et al., 1993; Okabe et al., 1994; Mezzanotte et al., 1996; Schwartz et al., 1998; Katz and White, 2003; Wilkinson et al., 2008; reviewed by Kubin and Davies, 2002). This pattern accounts for more prolonged and more severe obstructive episodes during REMS than during SWS. They also typically exhibit some phasic inspiratory activation of upper airway motor tone superimposed on the tonic (expiratory) activity in both wakefulness and sleep (Wiegand et al., 1991; Carlson et al., 1995; Mezzanotte et al., 1996; Katz and White, 2003; 2004)
Animal models have been used to study the effects of sleep on the central neural control of the upper airway (Carley and Radulovacki, 2002). Few such models have naturally compromised upper airway anatomy (Hendricks et al., 1987; Ogasa et al., 2004; Brennick et al., 2009). Normal rodents have been extensively used, but they have fully patent upper airway and the reports differ considerably regarding the levels and patterns of their upper airway motor tone across the sleep-wake cycle (see Discussion). We previously reported that, in adult rats, lingual electromyographic activity (EMG) is minimal or absent during SWS and then gradually increases during REMS due to the appearance of large phasic bursts (Lu et al., 2005). However, in that study, lingual recording sites tended to be located in relatively distal parts of the tongue, which could account for low levels of tonic activity, absence of obvious respiratory modulation and high incidence of non-respiratory phasic bursts. Based on the hydrostat theory of tongue movements, one would predict that muscle fibers located throughout the tongue contribute to the control of tongue movements and position, whereas the concept of an antagonistic control of tongue protrudors and retractors would suggest that the tonic levels and respiratory modulation of lingual EMG have different patterns and/or magnitudes at the base and near the tip of the tongue (Kier and Smith, 1985; Sokoloff, 2004; see Discussion). Therefore, our present goal was to determine whether, in normal, adult rats, sleep-wake patterns of lingual EMG and its respiratory modulation vary with the location of the recording site within the tongue. A preliminary report has been published (Lu and Kubin, 2007).
2. Methods
Experiments were conducted on nine adult, male Sprague-Dawley rats obtained from Charles River Laboratories (Wilmington, MA). The animals were housed individually under a 12 h light (7:00–19:00)/12 h dark cycle and with standard rodent chow and water available ad lib. All surgical and animal handling procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania and followed the guidelines of the American Physiological Society for the care and use of animals in research.
2.1. Instrumentation procedures
Instrumentation was conducted following at least four days of adaptation to housing in our facility. The animals were given atropine (0.04 mg/kg, i.m.) prior to surgery. Under ketamine (60 mg/kg, i.m.) and xylazine (7.5 mg/kg, i.m.) anesthesia followed by isoflurane administered through a nose mask (0.5–0.8%), the animals were instrumented for recording of the cortical EEG and lingual and nuchal EMGs. The two wires that were implanted into the tongue (10-stranded, stainless steel, Teflon-coated except ~0.5 mm at the tip; catalog number A361; Cooner Wire, Chatsworth, CA) were inserted into the tongue on the opposite sides of the midline with the aim to place the tip of one wire in a distal part of the tongue and the tip of the other wire at the base of the tongue. The details of EEG, nuchal EMG and lingual EMG electrode implantation and recording procedures were described previously (Lu et al., 2005). In five animals, two additional EMG leads were implanted into the sternal diaphragm. All leads were tunneled subcutaneously and attached to a mini-socket (220-9 pin ABS Plug, Ginder Scientific, Ottawa) that was then attached to the skull with dental acrylic and all skin openings were tightly sutured. At the conclusion of the instrumentation surgery, the animals were given gentamicin (5 mg/kg, i.m), yohimbine (5.0 mg/kg, i.m.) and an analgesic (Butorphanol, 2 mg/kg, s.c.), and their recovery was periodically observed for at least two subsequent days.
2.2. Habituation and recording procedures
Starting on day 7–10 after instrumentation, each animal was placed in a ventilated, dimly illuminated and sound-attenuated recording chamber for 1–3 h/day on at least three separate days and attached to the recording apparatus to habituate it to the recording procedures and establish optimal recording conditions. The cortical EEG, lingual, nuchal and diaphragmatic EMGs were amplified using Grass amplifiers with a bandwidth of 0.3–100 Hz for the EEG and 30–1000 Hz for the EMGs. All signals were continuously monitored and digitally stored using a sampling rate of 100 Hz and 1000 Hz, respectively (Power-1401 converter and Spike-2 v.5 data acquisition hardware and software; Cambridge Electronic Design, Inc., Cambridge, England). Gains were set to obtain maximal amplification without saturation of the amplifiers or converters. The animal was left undisturbed during the recordings. The data for this report were obtained from the middle 2 h of a fourth or later recording session conducted between noon and 4 pm on day 15–50 after instrumentation (mean: 26 days).
The animals were sacrificed 2–30 days after the recording session that yielded the data for this report (mean: 11 days). For this, they were deeply anesthetized (Nembutal; 80 mg/kg, i.p.), decapitated, the head was fixed in 10% formalin, and the tongue carefully dissected to verify the location of the tips of the recording wires. The locations of the recording sites were then plotted onto a standard, parasgittal cross-section of the tongue produced in-house from serial, Neutral red-stained, sections of the tongue.
2.3. Signal processing, scoring of sleep-wake states and data analysis
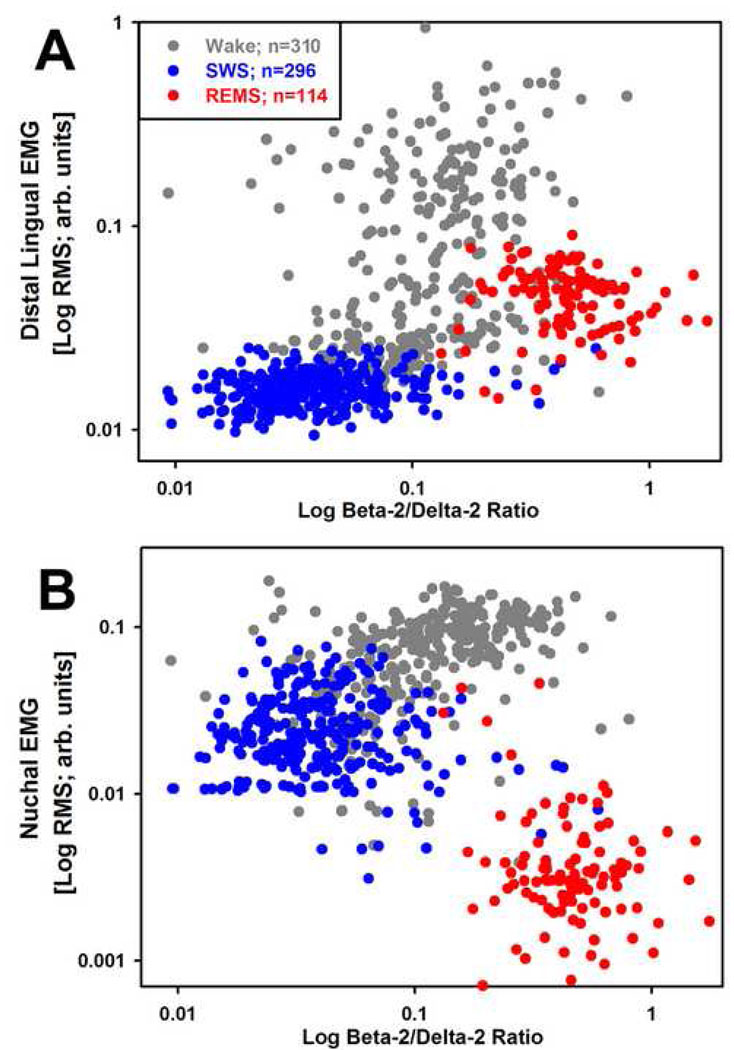
The nuchal and diaphragmatic EMGs were recorded differentially between the two wires implanted in the corresponding muscles, whereas lingual EMGs were recorded between each wire implanted into the tongue and a reference electrode on the parietal bone. To eliminate EEG signal from lingual EMG, the signal was high pass-filtered at 75 Hz and, for consistency, the same filtering was also applied to the nuchal and diaphragmatic EMGs. The EEG was band pass filtered at 0.75–40 Hz. Filtered signals were then displayed and behavioral states scored using commercial sleep-scoring software (Somnologica; Medcare, Buffalo, NY). Three behavioral states, wakefulness (W), SWS and REMS, were distinguished in successive 10 s epochs based on the appearance of the cortical EEG and nuchal EMG and the shape of EEG power spectrum simultaneously displayed for each scored interval. To reduce overestimation of EMG levels during SWS or REMS introduced by often large burst of activity associated with awakenings, the epochs in which awakenings occurred were scored as SWS or REMS only when these states occupied at least 75% of the duration of the epoch; all other epochs were scored according to the state that occupied more than 50% of their duration. After the initial scoring, root mean squares (RMSs) of the nuchal and lingual EMGs were calculated for each scoring interval and the values exported to a spreadsheet together with concurrently calculated cortical EEG powers in selected bands (delta-2: 0.75–2.0 Hz; delta-1: 2.0–4.5 Hz; theta: 5.5–8.0 Hz; alpha: 8.0–13.5 Hz; beta-1: 13.5–20 Hz and beta-2: 20–25 Hz). Two procedures were then used to verify the correctness of scoring. First, the hypnogram was plotted together with delta-1 and theta powers of the cortical EEG and RMS of nuchal EMG because these measures assume characteristic levels in each of the three distinguished behavioral states (cf. Fig. 2 in Lu et al., 2005). Then, logarithmic scatter plots of the RMSs of the nuchal and lingual EMGs vs. the ratio of EEG powers in the beta-2 to delta-2 bands were generated for all scoring intervals. Such plots exhibit distinct clustering in relation to behavioral states, with lingual EMG assuming the highest and most variable levels during W, lowest levels during SWS and intermediate levels during REMS (Fig. 1A), whereas nuchal EMG exhibits intermediate levels during SWS (Fig. 1B). If the plots contained isolated data points that significantly deviated from the clusters typical of a given behavioral state, the original records were reviewed and, if warranted by visual inspection, scoring was corrected (occasional data points located outside the main clusters represent scoring epochs associated with state transitions).
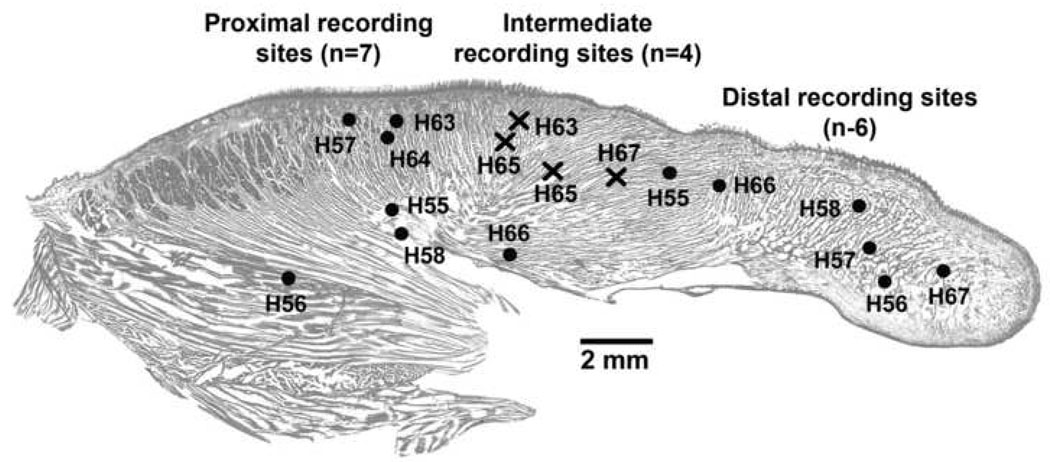
Figure 2.
Location within the tongue of the recording sites classified as proximal (near the tongue base or close to the pharyngeal lumen; n=7), distal (near the tip of the tongue; n=6), or intermediate (crosses; n=4). The sites were localized post-mortem and superimposed on a standard, Neutral red-stained cross-section of the tongue. Numbers preceded by “H” denote subjects.
Figure 1.
Typical clustering with behavioral states of the magnitudes of lingual (A) and nuchal (B) EMGs relative to the beta-2/delta 2 power ratio of the cortical EEG. Individual data points represent root mean squares (RMS) of EMG signals and the corresponding power ratios for each 10 s-long scoring interval in a 2 h-long recording session (720 points color-coded by behavioral state). Note that the levels of lingual EMG during REMS characteristically cluster on the right side (high levels of the beta-2/delta 2 power ratio) and at levels intermediate between those during SWS and wake (A), whereas nuchal EMG attains intermediate levels during SWS and nadirs during REMS. Logarithmic scales are used to accommodate the full dynamic range of both variables. The legend lists the total numbers of scoring intervals for each of the three behavioral states.
The data from each recording session comprised 720 sets of measurements derived from successive 10 s epochs covering the entire 2 h of each recording session. Since movement artefacts were absent during sleep and rare in W, no data points were excluded to eliminate any potential bias and obtain representative data across all animals and behavioral states. For quantification of lingual EMGs using RMS, we defined the recording baseline (signal level corresponding to electrical noise only) as the lowest value among the 720 analyzed intervals. This approach was based on the assumption (verified by visual inspection of the raw data) that at least one out of 720 scoring intervals in each session contained electrical noise and only residual, or no, EMG activity (cf., Lu et al., 2005). This lowest value was then subtracted from all 720 EMG levels obtained for each data set. Subsequently, the data from each recording session were sorted by behavioral state, the mean RMS levels of lingual and nuchal EMGs were calculated for all W epochs, and the mean activity in W was then used to normalize EMG levels in all scoring epochs.
Following verification that the variables were normally distributed, paired or unpaired Student’s t-tests were used to compare EMG levels among behavioral states and recording sites. The variability of the means is characterized by the standard error (SE).
3. Results
3.1. Sleep-wake states
The average percentages of recording time that the nine rats spent in different sleep-wake states were: 36.2%±3.1 (SE) for W, 46.7%±2.5 for SWS, and 17.1%±1.6 for REMS, with the corresponding ranges being 30–56%, 34–55%, and 10–25%, respectively. This corresponded to the following total recording times in all nine rats: 391 min of W (2345 scoring epochs), 504 min of SWS (3026 epochs) and 185 min of REMS (1108 epochs).
3.2. Lingual EMG in different locations within the tongue across the sleep-wake cycle
Of the 18 lingual recording sites, 17 yielded satisfactory signal quality and were successfully localized within the tongue after the animals were sacrificed. Of those, six were classified as distal because they were found within the peripheral half of the tongue and seven as proximal because they were found at the pharyngeal level (Fig. 2). Of the six distal sites, four were located near the tip of the tongue and the remaining two slightly more centrally. Of the seven proximal sites, four were located close to the base of the tongue at locations where muscle fibers of the genioglossus assume the characteristic fan-like pattern and three were located close the pharyngeal surface of the tongue. The proximal sites located near the base and near the surface of the tongue could theoretically exhibit different changes with the sleep-wake cycle, but we found this not to be the case (Fig. 3.A). Therefore, in the absence of a compelling basis for distinguishing between the sites located close to the pharyngeal surface and at deeper locations, data from all seven proximal sites were combined. In addition to these 13 sites, four sites were found at locations that were neither clearly distal nor clearly proximal (crosses in Fig. 2). In an attempt to maximize the contrast between the activities recorded from different tongue compartments, the distal, proximal and intermediate sites were analyzed as three separate groups.
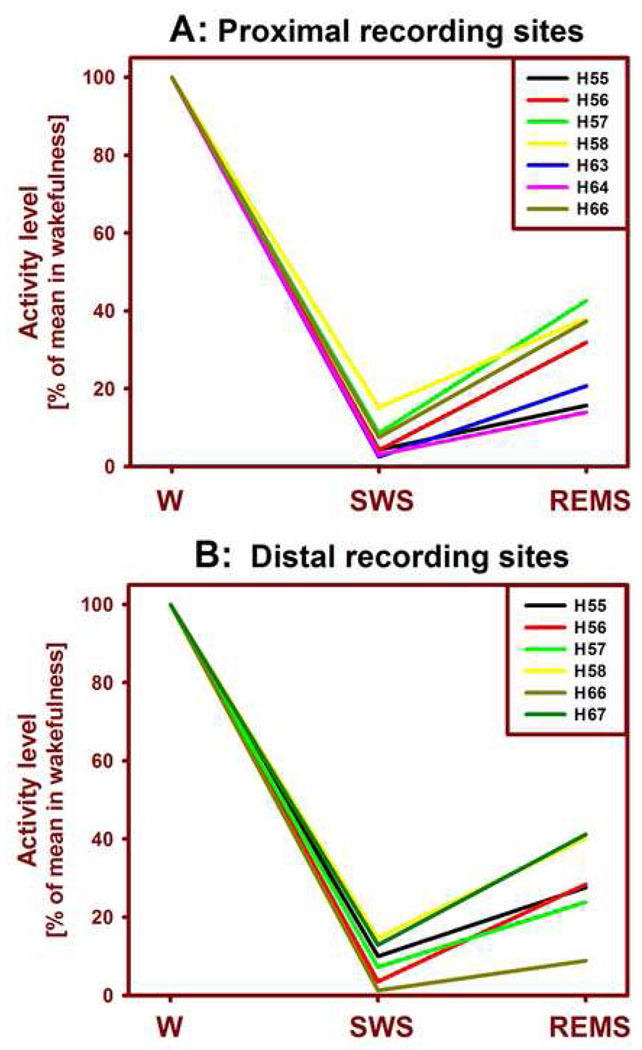
Figure 3.
Mean levels of lingual EMG across the three behavioral states in individual animals. The lingual EMG was lowest during SWS and then increased during REMS for both proximal (A) and distal (B) recording sites. Activity levels in SWS and REMS were normalized by their average levels during wakefulness (W). The levels of lingual EMG during SWS and REMS did not differ between the proximal and distal recording sites. Numbers preceded by “H” denote subjects (cf. Fig. 2).
The mean levels of lingual EMG had consistent patterns across the three behavioral states in all nine rats. At both proximal and distal locations, the mean lingual EMG normalized by its mean level in W was lowest during SWS and then significantly increased during REMS. The distribution of these means among individual rats is shown separately for the proximal and distal recording sites in Fig. 3. The mean proximal lingual EMG was 6.5%±1.7 (SE) during SWS and 28.6%±4.4 during REMS (relative to 100% in W). Both the decrease during SWS and the subsequent increase during REMS were statistically significant at p levels lower, or much lower, than 0.002; n=7, paired t-tests).
For the six distal recording sites, the mean lingual EMG levels were 8.4%±2.2 during SWS and 28.2%±4.8 during REMS, and the differences between the three states were as highly significant as for the proximal sites. The corresponding means obtained from the four intermediate recording sites were similar to those from the proximal and distal locations, 6.5%±1.7 during SWS and 30.2%±3.9 during REMS. The W-normalized levels of activity during either SWS or REMS did not differ among the three distinct regions of the tongue, proximal, intermediate and distal. In contrast to lingual EMG, the mean nuchal EMG was reduced to 34.0%±3.6 of its mean level in W during SWS and to 11.0%±2.3 during REMS (p< 0.0001 for the comparison between SWS and REMS; n=9).
3.3. Co-activation of lingual EMG at proximal and distal sites
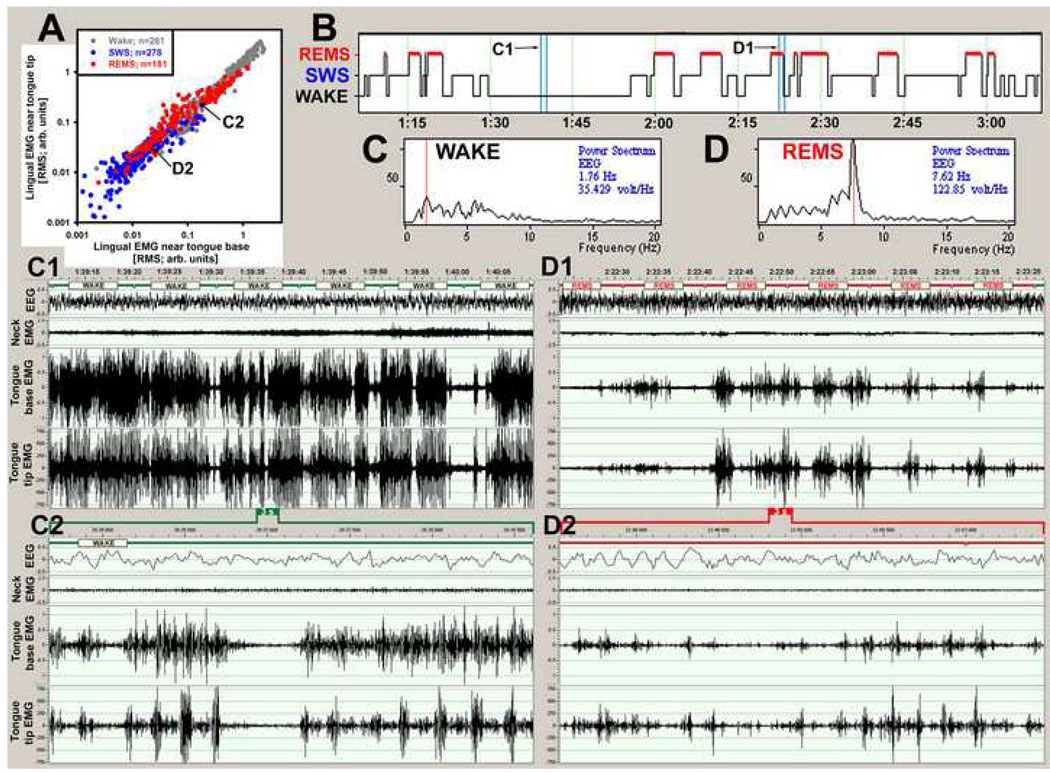
As expected, lingual EMGs exhibited prominent phasic activity during behaviors such as drinking and chewing. During grooming and scratching, lingual and nuchal EMGs often exhibited common rhythms. All these behaviors were associated with co-activation of lingual EMGs at both the proximal and distal locations within the tongue. Indeed, in all animals and across all behavioral states, there was a strong correlation between the mean lingual EMG levels calculated within successive scoring intervals (10 s) for each of the five simultaneously recorded proximal-distal EMG pairs. Figure 4A shows such a correlation for all 720 data points from an entire recording session with one lingual EMG signal recorded at a site near the base and the other near the tip of the tongue and panel B shows the hypnogram for this recording. Examples of raw lingual EMGs recorded during W (panels C1 and C2) and REMS (panels D1 and D2) reveal intricate patterns and complex phase relationships between the two lingual EMGs. However, when averaged over 10 s periods, lingual activities recorded from the two distinct tongue compartments are tightly correlated. In W, this high level of correlation reflects co-activations associated with distinct voluntary behaviors, and in REMS it shows a similarly strong correlation of activities associated with centrally generated activities of unknown purpose (Fig. 4A). It is of note that, on the time scale of less than 1 s, there are clear phase shifts and variable amplitude relationships between lingual EMG bursts simultaneously recorded from the two sites (panels C2 and D2 in Fig. 4). The frequency of these bursts is about 300 min−1, which is considerably faster than the respiratory rate in rats (80–130 min−1; cf. Fig. 5 and Fig 6). This example is typical of the co-activation, on the one hand, and intricate phasic bursting, on the other hand, obtained from all paired lingual EMG records analyzed in this study.
Figure 4.
Co-activation of lingual EMGs recorded from the base and tip of the tongue. (A) scatter plot of lingual EMG levels, color-coded by behavioral state, as recorded from the two locations within the tongue and quantified in 720 successive 10 s-long intervals of a 2 h-long recording. Activity levels are tightly correlated, indicating co-activation of the two EMGs (when averaged over 10 s intervals). A more detailed examination of the raw records reveals intricate patterns within each 10 s epoch, with distinct bursts of activity occurring asynchronously or sequentially at the two recording sites. Differences in the amplitude and phase relationship between the two recording sites can be seen within individual bursts in the ~55 s long raw records in C1 and D1 derived from the periods marked by the blue bars superimposed on the hypnogram shown in B. The segment in C1 is from a period of wakefulness (the corresponding EEG power spectrum is shown in C), and the segment in D1 is from a period of REMS (EEG power spectrum is D). The records in C2 and D2 show further expanded (3 s-long) portions of the records C1 and D1, respectively. These fast records reveal many differences in the timing and pattern of lingual activities recorded at the two sites. The arrows in A, labeled C2 and D2, point to the two data points in the scatter plot that correspond to the records in C2 and D2, respectively. The records were obtained from the two sites marked in Fig. 2 as rat H56.
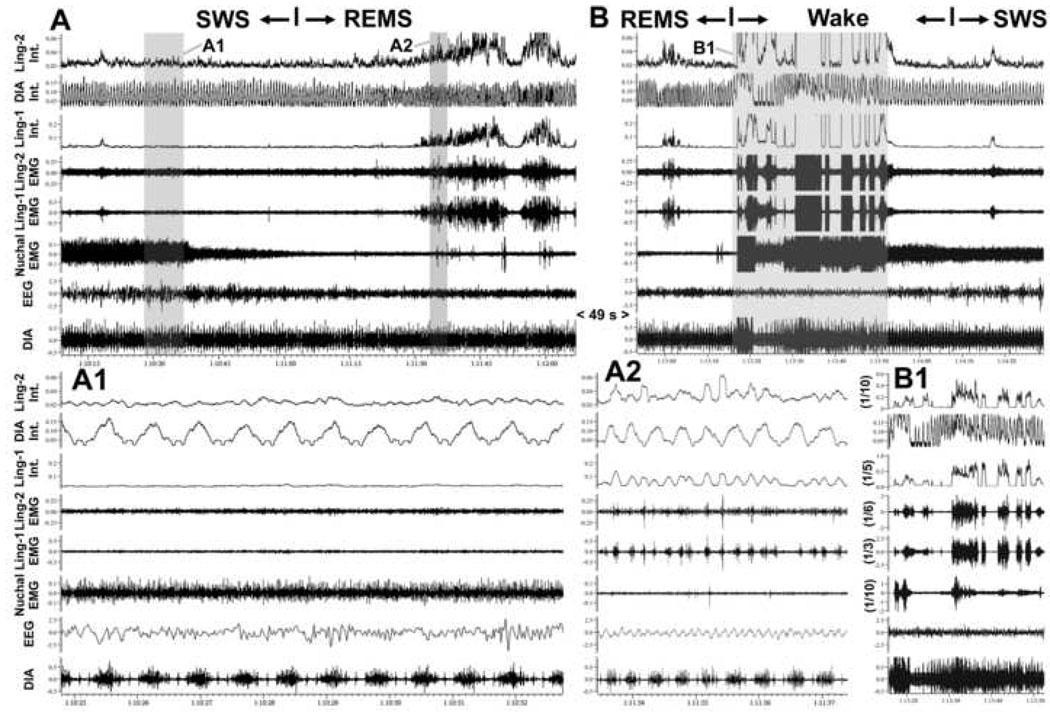
Figure 5.
Absence of respiratory modulation of lingual EMG during state transitions. A and B show two successive segments of EMG records from two intermediate sites in the tongue (Ling-1 and Ling-2; sites labeled in Fig. 2 as H65) and the diaphragm (DIA), as well as their integrated (Int.) versions, together with the cortical EEG and nuchal EMG taken from periods of state transitions. In the 113.5 s-long record shown in A, a transition from SWS to REMS was identified at the mark based on the atonia of nuchal EMG and appearance of theta rhythm in the EEG. In the 95.5 s-long record shown in B, awakening from REMS occurred and then the animal entered SWS. During the 49 s period separating A and B, REMS continued uninterrupted. The records in A1 (7.95 s) and A2 (3.95 s) show on an expanded time scale the shaded parts of the record in A. They correspond to the periods of SWS and REMS, respectively. A careful inspection of the raw and integrated diaphragmatic and lingual EMGs reveals no evidence of respiratory modulation of lingual EMG in A (Ling-1 is atonic and Ling-2 nearly atonic). In B, both lingual EMGs exhibit rhythmic, or semi-rhythmic bursts, but the phase and frequency of these rhythms is different from the respiratory rhythm in the diaphragm. B1 shows the 36.7 s-long, highlighted portion of the record in B using the same time scale as in B, but with the gains for lingual and nuchal EMGs reduced 3–10 times to show the full magnitude of activation present in these muscles around the time of awakening.
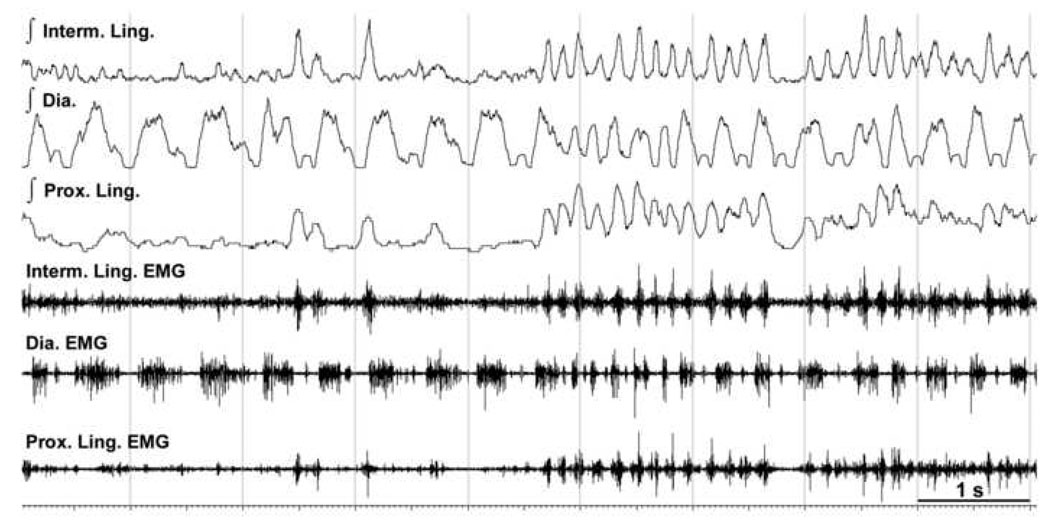
Figure 6.
Example of a brief occurrence of a seemingly common rhythm in lingual EMGs and diaphragm during REMS. During the second half of this 9 s-long record from the sites labeled in Fig. 2 as H63, the diaphragm and both the proximal (Prox.) and intermediate (Interm.) lingual EMGs have periods of rhythmic activity at rates that are considerably faster than typical respiratory rates during REMS (cf., integrated diaphragm EMG in the first half of the record). However, a close examination of these activities reveals variable phase relationships between the bursts in the diaphragm and lingual muscles. Such fast rhythms with variable phases of diaphragmatic and lingual activations frequently occur during REMS, suggesting that lingual and diaphragmatic EMGs are driven by different central pattern generators.
3.3. Respiratory modulation of lingual EMG
The analyses described in the preceding sections were based on the mean levels of lingual and nuchal EMGs across behavioral states calculated over successive 10 s periods of each recording. One limitation of this approach is that it does not capture phasic changes in EMG that occur on a 1-s or shorter time scale, such as distinct bursts of activity related to ingestive behaviors or breathing. To asses respiratory modulation of lingual EMG, we generated integrals of rectified EMG signals using a 100 ms time constant and examined all successive 30 s segments of our records for the presence of rhythmic activity temporally related to simultaneously recorded diaphragmatic activity. We operationally defined respiratory modulation as a rhythm in lingual EMG that persisted for at least four successive respiratory cycles and had a stable phase relationship relative to the simultaneously recorded diaphragmatic EMG. Data from four pairs of lingual recording sites from four rats were examined at high gain across the entire 2 h-long recording sessions. During a fraction of these records, observation of respiratory rhythm in the diaphragm during active W was obscured by postural influences and, consequently, respiratory modulation of lingual EMG could be missed. However, such cases represented less than 10% of the recording time. Figure 5 provides examples of raw and integrated EMGs recorded from the tongue and diaphragm during established states of W, SWS and REMS and during state transitions.
Despite our use of relatively liberal criteria for the presence of respiratory rhythm in lingual EMG, the instances of respiratory modulation were extremely rare and short-lasting. In three rats, we found a total of eight segments of records lasting from 4 to 8 respiratory cycles and two segments that were between 30 s and 2 min-long during which lingual EMG was respiratory-modulated. Out of these 10 segments, five occurred during REMS, two during SWS, two during quiet W, and one near a transition from W to SWS. Consistent with the common occurrence of co-activation of lingual EMG at different recording sites described in the preceding section, when respiratory modulation was present, it occurred at both recording sites. In the fourth rat, we found no respiratory-modulated lingual activity. The expanded record in panel A1 of Fig. 5 characteristically contains no respiratory modulation of lingual EMGs during SWS prior to transition into REMS or during the subsequent REMS period.
During REMS, we relatively often observed respiratory-like modulation, especially at times when the respiratory rhythm was particularly fast. However, with the few exceptions mentioned above, in all such cases, our period duration and/or phase relationship criteria for respiratory modulation were not met for at least four successive respiratory cycles. The expanded record in panel A2 of Fig. 5 and Fig. 6 show examples of semi-rhythmic lingual EMGs during REMS. Although lingual activity appears to be respiratory-modulated, especially at the time when the respiratory rhythm is transiently accelerated during the second half of the record in Fig. 6, the phase relationship between diaphragmatic and lingual activities is not constant during successive respiratory cycles. Thus, the total duration of the records with reliable respiratory modulation of lingual EMG that we identified in 8 h of continuous lingual records from four rats was less than 8 min.
4. Discussion
Our three main findings are that: (1) the mean level of lingual EMG changes with the sleep-wake cycle in a qualitatively and quantitatively similar manner in the proximal, intermediate and distal muscles of the tongue; (2) respiratory modulation of lingual EMG is extremely rare in adult rats in W and sleep; and (3) phasic activations of lingual EMG occur simultaneously throughout the tongue in a manner consistent with widespread co-activation of many different muscle fibers. These findings extend our previous study in which we quantified lingual EMG during established sleep and wake states and during state transitions in chronically instrumented, behaving rats, but did not investigate the site-dependence or respiratory modulation of lingual activity (Lu et al., 2005).
4.1. Lingual EMG in humans vs. rodents
Rats exhibit no propensity for sleep-related airway obstructions. This may explain why their lingual EMG is activated mainly in association with ingestive behaviors or grooming, whereas the tonic and inspiratory-related activity is sparse or absent. This is different from human subjects who often have both tonic and respiratory-modulated activity in the muscles of the tongue even when they do not present with clinically significant increases of upper airway resistance during sleep. An important anatomical reason for this difference may be that the hyoid bone to which several upper airway muscles (geniohyoid and hyoglossus) are attached is mobile in humans but firmly connected to the trachea in rodents. This mobility of the hyoid bone undoubtedly contributes to increased vulnerability of the human pharyngeal region to narrowing and collapse. Obesity also is an important factor affecting upper airway motor tone and airway collapsibility in humans (Schwartz et al., 1991; O'Donnell et al., 2000). Obese rodents, like obese humans, have less negative upper airway critical pressures than their lean controls (Ogasa et al., 2004; O'Donnell et al., 2000) and obese mice exhibit tonic upper airway activation under anesthesia (Liu et al., 2008; Brennick et al., 2009). However, the available evidence suggests that the levels and patterns of lingual EMG across sleep-wake states are similar in obese and lean rats (Sood et al., 2007). This would indicate that the upper airway of rodents is resistant to collapse under most natural conditions.
Subjects with OSA have significantly higher pharyngeal muscle activity during W than those with fully patent upper airway (Suratt et al., 1988; Sériès et al., 1989; Mezzanotte et al., 1992; Hendricks et al., 1993; Henke, 1998). A fraction of this increased upper airway tone is retained during SWS, and nadirs usually occur during REMS (e.g., Remmers et al., 1978; Suratt et al., 1988; Hendricks et al., 1993; Okabe et al., 1994; Schwartz et al., 1998). In contrast, this and our previous study (Lu et al., 2005) show that lingual EMG of rats becomes atonic, or nearly atonic, during SWS and then exhibits a gradual increase of phasic activity during REMS. Thus, it appears that the differences between sleep-wake patterns of lingual EMG in OSA subjects and healthy rodents can be explained on the grounds of anatomical differences. What is less clear is how common is the presence of tonic and respiratory-modulated activity in upper airway muscles of healthy humans and why some studies seem to suggest a relatively high level of tonic and respiratory-modulated lingual muscle activity during W and SWS in normal rats.
In healthy humans, upper airway motor tone is reported to increase, decrease or not change across sleep-wake states. For example, the genioglossus and geniohyoid, two major muscles innervated by the cranial nerve XII, may have increased (Basner et al., 1991; Tangel et al., 1992; Shea et al., 1999), decreased (Worsnop et al., 1998; Mezzanotte et al., 1996), minimally altered (Bailey et al., 2007a) or variable (Wilkinson et al., 2008) changes of activity during SWS when compared to quiet W. Then, during transitions from SWS into REMS, these activities are also reported to increase (Wiegand et al., 1990), decrease (Wiegand et al., 1991) or not change (Shea et al., 1999). However, it is of note that application of positive upper airway pressure did not reduce lingual activity recorded from the tongue in normal subjects during quiet W (Mezzanotte et al., 1996), suggesting that the recorded signal was not under central neural control. It is also of note that the peak level of lingual activity measured in normal humans during quiet W is of the order 5–10% of maximal activity, thus leaving little room for detecting sleep-related decrements (Mezzanotte et al., 1996; Stanchina et al., 2002).
Some degree of inspiratory modulation is commonly reported for the genioglossus, as well as other pharyngeal muscles, during quiet W, SWS and REMS in healthy humans (e.g., Basner et al., 1991; Wiegand et al., 1991; Mezzanotte et al., 1996; Henke, 1998; Saboisky et al., 2006; Bailey et al., 2007b; Wilkinson et al., 2008). However, in normal children in whom recordings were obtained with non-invasive electrodes, no respiratory modulation of genioglossal EMG was present during quiet W or SWS (Katz and White, 2004). Since most studies in normal humans are conducted with adult subjects and with the use of acutely implanted sharp electrodes, it is possible that some persons classified as clinically healthy (apnea-hypopnea index less than 5 or 10 per hour) are borderline OSA patients and that this and the discomfort caused by application of nasal mask and insertion of EMG electrodes contribute to increased incidence of respiratory modulation. In support of this, data from chronically instrumented cats show that lingual EMG exhibits inspiratory modulation and enhanced tonic activity on days 2–3 after instrumentation, but not after longer periods and following additional habituation (Neuzeret et al., 2009). Thus, the effects of an acute experimental setting need to be considered because there appears to be no reason why the tongue of a person with fully patent upper airway would need to maintain continuous active contraction or continuously generate respiratory movements.
4.2. Variable findings concerning sleep-wake patterns of lingual EMG in rats
In the rat, a species commonly used to study the central control of the upper airway, descriptions of lingual muscle activity levels and patterns across sleep-wake states are contradictory. In one early study, the proportion of XII motoneurons with tonic activity during quiet W was extremely low and phasic activity occurred in association with ingestive behaviors, rather than breathing (Travers and Jackson, 1992). However, another early study reported extremely strong inspiratory modulation with little tonic activity during SWS (Megirian et al., 1985), and several other studies found considerable levels of inspiratory modulation superimposed on tonic activity levels of comparable magnitude during both quiet W and SWS (Morrison et al., 2003b; Chan et al., 2006). In contrast, other studies appear to have encountered little or no inspiratory modulation of lingual EMG and nearly inactive tongue muscles during SWS (Jelev et al., 2001; Horner et al., 2002). One difficulty with the interpretation of these results is that not a single study to date provided information about the percentage of the recording time during which respiratory modulation was present in each behavioral state. Another problem is that it is often difficult to determine whether what is referred to as “inspiratory component” represents the genuine inspiratory-expiratory difference or the total level of activity measured during inspiration. Short post-surgery periods with little or no habituation may also contribute to enhanced respiratory modulation of lingual EMG through the discomfort factor considered in our discussion of EMG data from humans. In this regard, it is of note that our recordings were obtained at least 15 days after instrumentation and following at least three habituation sessions. Under these conditions, and with the entire records carefully reviewed for the presence of respiratory modulation with a stable phase relationship with diaphragmatic activity, we found inspiratory modulation of lingual EMG in less than 2% of our records.
Most rat studies report significant decreases of lingual EMG on transition from quiet W to SWS (e.g., (Jelev et al., 2001; Morrison et al., 2003b; Lu et al., 2005); but see Sood et al., 2007). In contrast, the transition from SWS to REMS have been reported to occur with an increase (Megirian et al., 1978), decrease (Megirian et al., 1985; Morrison et al., 2003b; Morrison et al., 2003a; Chan et al., 2006) or no change (Lu et al., 2005; Sood et al., 2007) of lingual EMG. This discrepancy is likely due to different methods of lingual EMG quantification during REMS that either include or exclude the large phasic bursts that occur with increasing amplitude and frequency towards the end of REMS episodes (Lu et al., 2005) (see also Fig. 5A). If the analysis includes only the last ~30 s of the SWS and the first ~30 s of the ensuing REMS episode, we and others observed no change because, under normal conditions, lingual muscles become atonic during SWS and no further decrease can be measured (Megirian et al., 1985; Morrison et al., 2003b; Lu et al., 2005). On the other hand, quantification across the entire period of REMS yields a mean level of lingual EMG that is significantly higher than during SWS and may be as high as 30–60% of the mean level of activity in W (Fig. 3; cf. also Morrison et al., 2003b; Lu et al., 2005).
4.3. Co-activation and lack of site-dependence of lingual activity across sleep-wake states
Throughout the tongue, extrinsic muscle fibers are intermixed with intrinsic fibers (Sokoloff, 2000). Therefore, it is impossible to distinguish between the intrinsic and extrinsic origin of lingual EMG when one uses a multi-unit recording approach. Nevertheless, recordings from the tongue usually aim at the base of the tongue where the fibers of the genioglossus, a major extrinsic tongue protrudor, are located. The implicit rationale for this is that lingual activity recorded at the base of the tongue, and thus at least in part from the genioglossus muscle, may be particularly revealing about the tongue’s airway-protecting function. This rationale is consistent with the known tongue-protruding action of the genioglossus when tested by means of electrical stimulation, but it is not supported by either the muscular hydrostat theory of the tongue functions (Kier and Smith, 1985; see Sokoloff, 2004 for a review) or the data showing that protrudor, retractor and intrinsic muscle fibers are extensively co-activated during centrally generated tongue contractions (Fuller et al., 1998; Bailey and Fregosi, 2004; Bailey et al., 2006).
Our present data lend further support to the hydrostat concept by showing that the state-dependent levels and patterns of lingual activity do not show a detectable with our methods site dependence. Our results from the sites near the base and near the tip of the tongue yielded similar magnitudes of activity changes in SWS and REMS relative to W. We also observed that, when tonic activity was present or phasic bursts occurred, they appeared in both proximal and distal locations. Moreover, the magnitude of activity was highly correlated between the proximal and distal sites and across all behavioral states (averaged over 10 s intervals; our approach did not allow us for a detailed analysis of intricate sequences of lingual bursts on the time scale of 1 s or less). Notably, the inspiratory modulation of lingual EMG that we found to be extremely rare, when present, also occurred both at the base and near the tip of the tongue. Such a widespread co-activation may be somewhat unique to the tongue because its muscle fibers have only one or no bony attachment. Consequently, any movement of the tongue needs to involve many muscle fibers in different locations in order to achieve the appropriate shape and position of the entire organ.
It is of note that the widespread co-activation and tight correlation of the magnitudes of activity in lingual muscle fibers in different locations within the tongue occurred not only during voluntary behaviors during W but also during phasic bursts in REMS. The role of these bursts is not known. During periods of REMS with strong central activation, medullary respiratory neurons have variable burst durations and variable phase relationships relative to the diaphragm (Orem et al., 2000). Thus, the semi-rhythmic lingual activity during REMS in rats could be driven by an increased but disorganized activity of central respiratory neurons. However, our present finding that respiratory modulation of lingual EMG is extremely rare regardless of the sleep-wake state makes this possibility less likely. As an alternative, we recently proposed that the phasic and often semi-rhythmic bursts that appear in lingual muscles during REMS may represent a dream-driven activation of suckling-like movements (Kubin and Volgin, 2008). This would be consistent with the broader idea that many organismal controls activated during REMS revert to the mechanisms typical of fetal or early postnatal period (Zepelin et al., 2005), and also with the practical observation that phasic activities of rat tongue are strongly driven by central generators of ingestive behaviors.
4.4. Sleep-wake patterns of upper airway activity under conditions of increased basal tone
The activity of XII motoneurons that innervate the tongue is generally low under quiet baseline conditions but it can be experimentally augmented by increased chemical respiratory drive (e.g., Parisi et al., 1987; Hwang et al., 1983; Horner et al., 2002), vagotomy (see Kubin and Davies, 1995; Fregosi and Fuller, 1997 for reviews), or stimulants applied directly onto XII motoneurons (e.g., Kubin et al., 1992; Kubin et al., 1993; Fenik et al., 1999; Jelev et al., 2001; Morrison et al., 2003b; Neuzeret et al., 2009). Under such conditions, one can observe both SWS and REMS-related decrements of upper airway activity that are difficult to detect under baseline conditions when there is only residual or no lingual muscle activity (Kimura et al., 1990; Kubin et al., 1993; Kubin et al., 1996; Jelev et al., 2001; Horner et al., 2002; Morrison et al., 2003b; Fenik et al., 2004). The depressant effect of SWS on lingual EMG also has a larger magnitude in healthy humans when the baseline level of activity is increased by resistive loads (Pillar et al., 2000). Thus, studies in which the baseline level of lingual EMG is experimentally enhanced are of both basic and clinical interest because they allow one to study the effects of sleep-wake states on lingual EMG that may approximate those in OSA patients.
4.5. Conclusions
Our data demonstrate common patterns of lingual activity across sleep-wake states near the base and near the tip of the tongue. These patterns may be similar to those in healthy humans with fully patent upper airway, but methodological differences in both human and animal studies likely contribute to variability of the results reported to date. Therefore, both a thorough analysis of the experimental records and detailed description of data acquisition and analysis are needed to help understand the source(s) of differences in the results and establish the extent of applicability of animal experiments to results from normal human subjects and patients with OSA.
Acknowledgments
The study was supported by grants HL-071097 and HL-092962 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailey EF, Fregosi RF. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J. Appl. Physiol. 2004;96:440–449. doi: 10.1152/japplphysiol.00733.2003. [DOI] [PubMed] [Google Scholar]
- Bailey EF, Fridel KW, Rice AD. Sleep/wake firing patterns of human genioglossus motor units. J. Neurophysiol. 2007a;98:3284–3291. doi: 10.1152/jn.00865.2007. [DOI] [PubMed] [Google Scholar]
- Bailey EF, Huang YH, Fregosi RF. Anatomic consequences of intrinsic tongue muscle activation. J. Appl. Physiol. 2006;101:1377–1385. doi: 10.1152/japplphysiol.00379.2006. [DOI] [PubMed] [Google Scholar]
- Bailey EF, Rice AD, Fuglevand AJ. Firing patterns of human genioglossus motor units during voluntary tongue movement. J. Neurophysiol. 2007b;97:933–936. doi: 10.1152/jn.00737.2006. [DOI] [PubMed] [Google Scholar]
- Basner RC, Ringler J, Schwartzstein RM, Weinberger SE, Weiss JW. Phasic electromyographic activity of the genioglossus increases in normals during slow-wave sleep. Respir. Physiol. 1991;83:189–200. doi: 10.1016/0034-5687(91)90028-h. [DOI] [PubMed] [Google Scholar]
- Brennick MJ, Pack AI, Ko K, Kim E, Pickup S, Maislin G, Schwab RJ. Altered upper airway and soft tissue structures in the New Zealand obese mouse. Am. J. Respir. Crit. Care Med. 2009;179:158–169. doi: 10.1164/rccm.200809-1435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carley DW, Radulovacki M, editors. Sleep-Related Breathing Disorders: Experimental Models and Therapeutic Potential. New York: Marcel Dekker; 2002. [Google Scholar]
- Carlson DM, Önal E, Carley DW, Lopata M, Basner RC. Palatal muscle electromyogram activity in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1995;152:1022–1027. doi: 10.1164/ajrccm.152.3.7663778. [DOI] [PubMed] [Google Scholar]
- Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am. J. Respir. Crit. Care Med. 2006;174:1264–1273. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- Fenik V, Davies RO, Kubin L. Adrenergic receptor subtypes mediating excitatory effects in hypoglossal motoneurons. Sleep. 1999;22 Suppl.:S37. [Google Scholar]
- Fenik V, Davies RO, Kubin L. Combined antagonism of aminergic excitatory and amino acid inhibitory receptors in the XII nucleus abolishes REM sleep-like depression of hypoglossal motoneuronal activity. Arch. Ital. Biol. 2004;142:237–249. [PubMed] [Google Scholar]
- Fregosi RF, Fuller DD. Respiratory-related control of extrinsic tongue muscle activity. Respir. Physiol. 1997;110:295–306. doi: 10.1016/s0034-5687(97)00095-9. [DOI] [PubMed] [Google Scholar]
- Fuller D, Mateika JH, Fregosi RF. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J. Physiol. 1998;507:265–276. doi: 10.1111/j.1469-7793.1998.265bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks JC, Kline LR, Kovalski RJ, O'Brien JA, Morrison AR, Pack AI. The English bulldog: a natural model of sleep-disordered breathing. J. Appl. Physiol. 1987;63:1344–1350. doi: 10.1152/jappl.1987.63.4.1344. [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Petrof BJ, Panckeri K, Pack AI. Upper airway dilating muscle hyperactivity during non-rapid eye movement sleep in English bulldogs. Am. Rev. Respir. Dis. 1993;148:185–194. doi: 10.1164/ajrccm/148.1.185. [DOI] [PubMed] [Google Scholar]
- Henke KG. Upper airway muscle activity and upper airway resistance in young adults during sleep. J. Appl. Physiol. 1998;84:486–491. doi: 10.1152/jappl.1998.84.2.486. [DOI] [PubMed] [Google Scholar]
- Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleep-wake state on the genioglossus vs. diaphragm muscle response to CO2 in rats. J. Appl. Physiol. 2002;92:878–887. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- Hwang J-C, Bartlett D, Jr, St.John WM. Characterization of respiratory-modulated activities of hypoglossal motoneurons. J. Appl. Physiol. 1983;55:793–798. doi: 10.1152/jappl.1983.55.3.793. [DOI] [PubMed] [Google Scholar]
- Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J. Physiol. 2001;532:467–481. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz ES, White DP. Genioglossus activity in children with obstructive sleep apnea during wakefulness and sleep onset. Am. J. Respir. Crit. Care Med. 2003;168:664–670. doi: 10.1164/rccm.200301-092OC. [DOI] [PubMed] [Google Scholar]
- Katz ES, White DP. Genioglossus activity during sleep in normal control subjects and children with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2004;170:553–560. doi: 10.1164/rccm.200403-262OC. [DOI] [PubMed] [Google Scholar]
- Kier WM, Smith KK. Tongues, tentacles and trunks: the biomechanics of movement in muscular hydrostats. Zool. J. Linn. Soc. 1985;83:307–324. [Google Scholar]
- Kimura H, Kubin L, Davies RO, Pack AI. Cholinergic stimulation of the pons depresses respiration in decerebrate cats. J. Appl. Physiol. 1990;69:2280–2289. doi: 10.1152/jappl.1990.69.6.2280. [DOI] [PubMed] [Google Scholar]
- Kubin L, Davies RO. Central pathways of pulmonary and airway vagal afferents. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. New York: Marcel Dekker; 1995. pp. 219–284. [Google Scholar]
- Kubin L, Davies RO. Mechanisms of airway hypotonia. In: Pack AI, editor. Sleep Apnea. Pathogenesis, Diagnosis, and Treatment. New York: Dekker; 2002. pp. 99–154. [Google Scholar]
- Kubin L, Kimura H, Tojima H, Davies RO, Pack AI. Suppression of hypoglossal motoneurons during the carbachol-induced atonia of REM sleep is not caused by fast synaptic inhibition. Brain Res. 1993;611:300–312. doi: 10.1016/0006-8993(93)90517-q. [DOI] [PubMed] [Google Scholar]
- Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci. Lett. 1992;139:243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- Kubin L, Tojima H, Reignier C, Pack AI, Davies RO. Interaction of serotonergic excitatory drive to hypoglossal motoneurons with carbachol-induced, REM sleep-like atonia. Sleep. 1996;19:187–195. [PubMed] [Google Scholar]
- Kubin L, Volgin DV. Developmental profiles of neurotransmitter receptors in respiratory motor nuclei. Respir. Physiol. Neurobiol. 2008;164:64–71. doi: 10.1016/j.resp.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Pichard L, Schneider H, Patil SP, Smith PL, Polotsky V, Schwartz AR. Neuromechanical control of the isolated upper airway of mice. J. Appl. Physiol. 2008;105:1237–1245. doi: 10.1152/japplphysiol.90461.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JW, Kubin L. Sleep-wake patterns of genioglossal EMG at the base and tip of the tongue in the rat. Sleep. 2007;30 Suppl.:A206. [Google Scholar]
- Lu JW, Mann GL, Ross RJ, Morrison AR, Kubin L. Differential effect of sleep-wake states on lingual and dorsal neck muscle activity in rats. Respir. Physiol. Neurobiol. 2005;147:191–203. doi: 10.1016/j.resp.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Megirian D, Cespuglio R, Jouvet M. Rhythmical activity of the rats's tongue in sleep and wakefulness. Electroencephalogr. Clin. Neurophysiol. 1978;44:8–13. doi: 10.1016/0013-4694(78)90100-1. [DOI] [PubMed] [Google Scholar]
- Megirian D, Hinrichsen CFL, Sherrey JH. Respiratory roles of genioglossus, sternothyroid, and sternohyoid muscles during sleep. Exp. Neurol. 1985;90:118–128. doi: 10.1016/0014-4886(85)90045-7. [DOI] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J. Clin. Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am. J. Respir. Crit. Care Med. 1996;153:1880–1887. doi: 10.1164/ajrccm.153.6.8665050. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu H, Park E, Liu X, Nolan P, Horner RL. Role of inhibitory amino acids in control of hypoglossal motor outflow to genioglossus muscle in naturally sleeping rats. J. Physiol. 2003a;552:975–991. doi: 10.1113/jphysiol.2003.052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu H, Park E, Nolan P, Horner RL. GABAA receptor antagonism at the hypoglossal motor nucleus increases genioglossus muscle activity in NREM but not REM sleep. J. Physiol. 2003b;548:569–583. doi: 10.1113/jphysiol.2002.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzeret P-C, Sakai K, Gormand F, Petitjean T, Buda C, Sastre J-P, Parrot S, Guidon G, Lin J-S. Application of histamine and serotonin to the hypoglossal nucleus increases genioglossus activity across the wake-sleep cycle. J. Sleep Res. 2009;18:113–121. doi: 10.1111/j.1365-2869.2008.00708.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell CP, Schwartz AR, Smith PL. Upper airway collapsibility: the importance of gender and adiposity. Am. J. Respir. Crit. Care Med. 2000;162:1606–1607. doi: 10.1164/ajrccm.162.5.ed11-00b. [DOI] [PubMed] [Google Scholar]
- Ogasa T, Ray AD, Michlin CP, Farkas GA, Grant BJ, Magalang UJ. Systemic administration of serotonin 2A/2C agonist improves upper airway stability in Zucker rats. Am. J. Respir. Crit. Care Med. 2004;170:804–810. doi: 10.1164/rccm.200312-1674OC. [DOI] [PubMed] [Google Scholar]
- Okabe S, Hida W, Kikuchi Y, Taguchi O, Takishima T, Shirato K. Upper airway muscle activity during REM and non-REM sleep of patients with obstructive apnea. Chest. 1994;106:767–773. doi: 10.1378/chest.106.3.767. [DOI] [PubMed] [Google Scholar]
- Orem J, Lovering AT, Dunin-Barkowski W, Vidruk EH. Endogenous excitatory drive in the respiratory system in rapid eye movement sleep in cats. J. Physiol. 2000;527:365–376. doi: 10.1111/j.1469-7793.2000.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi RA, Neubauer JA, Frank MM, Edelman NH, Santiago TV. Correlation between genioglossal and diaphragmatic responses to hypercapnia during sleep. Am. Rev. Respir. Dis. 1987;135:378–382. doi: 10.1164/arrd.1987.135.2.378. [DOI] [PubMed] [Google Scholar]
- Pillar G, Malhotra A, Fogel R, Beauregard J, Schnall R, White DP. Airway mechanics and ventilation in response to resistive loading during sleep: influence of gender. Am. J. Respir. Crit. Care Med. 2000;162:1627–1632. doi: 10.1164/ajrccm.162.5.2003131. [DOI] [PubMed] [Google Scholar]
- Remmers JE, DeGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J. Appl. Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Saboisky JP, Butler JE, Fogel RB, Taylor JL, Trinder JA, White DP, Gandevia SC. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J. Neurophysiol. 2006;95:2213–2221. doi: 10.1152/jn.00940.2005. [DOI] [PubMed] [Google Scholar]
- Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp. Neurol. 1976;51:160–170. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, Gold AR, Schubert N, Stryzak A, Wise RA, Permutt S, Smith PL. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am. Rev. Respir. Dis. 1991;144:t-8. doi: 10.1164/ajrccm/144.3_Pt_1.494. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, O'Donnell CP, Baron J, Schubert N, Alam D, Samadi SD, Smith PL. The hypotonic upper airway in obstructive sleep apnea. Role of structure and neuromuscular activity. Am. J. Resp. Crit. Care Med. 1998;157:1051–1057. doi: 10.1164/ajrccm.157.4.9706067. [DOI] [PubMed] [Google Scholar]
- Sériès F, Cormier Y, Desmeules M, La Forge J. Effects of respiratory drive on upper airways in sleep apnea patients and normal subjects. J. Appl. Physiol. 1989;67:973–979. doi: 10.1152/jappl.1989.67.3.973. [DOI] [PubMed] [Google Scholar]
- Shea SA, Edwards JK, White DP. Effect of wake-sleep transitions and rapid eye movement sleep on pharyngeal muscle response to negative pressure in humans. J. Physiol. 1999;520:897–908. doi: 10.1111/j.1469-7793.1999.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff AJ. Localization and contractile properties of intrinsic longitudinal motor units of the rat tongue. J. Neurophysiol. 2000;84:827–835. doi: 10.1152/jn.2000.84.2.827. [DOI] [PubMed] [Google Scholar]
- Sokoloff AJ. Activity of tongue muscles during respiration: it takes a village? J. Appl. Physiol. 2004;96:438–439. doi: 10.1152/japplphysiol.01079.2003. [DOI] [PubMed] [Google Scholar]
- Sood S, Liu X, Liu H, Horner RL. Genioglossus muscle activity and serotonergic modulation of hypoglossal motor output in obese Zucker rats. J. Appl. Physiol. 2007;102:2240–2250. doi: 10.1152/japplphysiol.01229.2006. [DOI] [PubMed] [Google Scholar]
- Stanchina ML, Malhotra A, Fogel RB, Ayas N, Edwards JK, Schory K, White DP. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am. J. Respir. Crit. Care Med. 2002;165:945–949. doi: 10.1164/ajrccm.165.7.2108076. [DOI] [PubMed] [Google Scholar]
- Suratt PM, McTier RF, Wilhoit SC. Upper airway muscle activation is augmented in patients with obstructive sleep apnea compared with that in normal subjects. Am. Rev. Respir. Dis. 1988;137:889–894. doi: 10.1164/ajrccm/137.4.889. [DOI] [PubMed] [Google Scholar]
- Tangel DJ, Mezzanotte WS, Sandberg EJ, White DP. Influences of NREM sleep on the activity of tonic vs. inspiratory phasic muscles in normal men. J. Appl. Physiol. 1992;73:1058–1066. doi: 10.1152/jappl.1992.73.3.1058. [DOI] [PubMed] [Google Scholar]
- Travers JB, Jackson LM. Hypoglossal neural activity during licking and swallowing in the awake rat. J. Neurophysiol. 1992;67:1171–1184. doi: 10.1152/jn.1992.67.5.1171. [DOI] [PubMed] [Google Scholar]
- Wiegand DA, Latz B, Zwillich CW, Wiegand L. Geniohyoid muscle activity in normal men during wakefulness and sleep. J. Appl. Physiol. 1990;69:1262–1269. doi: 10.1152/jappl.1990.69.4.1262. [DOI] [PubMed] [Google Scholar]
- Wiegand L, Zwillich CW, Wiegand D, White DP. Changes in upper airway muscle activation and ventilation during phasic REM sleep in normal men. J. Appl. Physiol. 1991;71:488–497. doi: 10.1152/jappl.1991.71.2.488. [DOI] [PubMed] [Google Scholar]
- Wilkinson V, Malhotra A, Nicholas CL, Worsnop C, Jordan AS, Butler JE, Saboisky JP, Gandevia SC, White DP, Trinder J. Discharge patterns of human genioglossus motor units during sleep onset. Sleep. 2008;31:525–533. doi: 10.1093/sleep/31.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J. Appl. Physiol. 1998;85:908–920. doi: 10.1152/jappl.1998.85.3.908. [DOI] [PubMed] [Google Scholar]
- Zepelin H, Siegel JM, Tobler I. Mammalian sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: Elsevier-Saunders; 2005. pp. 91–100. [Google Scholar]