Abstract
We have carried out conformational energy calculations on alanine-based copolymers with the sequence Ac-AAAAAXAAAA-NH2 in water, where X stands for lysine or glutamine, to identify the underlying source of stability of alanine-based polypeptides containing charged or highly soluble polar residues in the absence of charge–charge interactions. The results indicate that ionizable or neutral polar residues introduced into the sequence to make them soluble sequester the water away from the CO and NH groups of the backbone, thereby enabling them to form internal hydrogen bonds. This solvation effect dictates the conformational preference and, hence, modifies the conformational propensity of alanine residues. Even though we carried out simulations for specific amino acid sequences, our results provide an understanding of some of the basic principles that govern the process of folding of these short sequences independently of the kind of residues introduced to make them soluble. In addition, we have investigated through simulations the effect of the bulk dielectric constant on the conformational preferences of these peptides. Extensive conformational Monte Carlo searches on terminally blocked 10-mer and 16-mer homopolymers of alanine in the absence of salt were carried out assuming values for the dielectric constant of the solvent ɛ of 80, 40, and 2. Our simulations show a clear tendency of these oligopeptides to augment the α-helix content as the bulk dielectric constant of the solvent is lowered. This behavior is due mainly to a loss of exposure of the CO and NH groups to the aqueous solvent. Experimental evidence indicates that the helical propensity of the amino acids in water shows a dramatic increase on addition of certain alcohols, such us trifluoroethanol. Our results provide a possible explanation of the mechanism by which alcohol/water mixtures affect the free energy of helical alanine oligopeptides relative to nonhelical ones.
The α-helical conformation of short homopolypeptides containing only alanines is unstable in water at room temperature (1). This behavior is consistent with the value of the Zimm-Bragg parameters (2) s and σ determined from experiments on random copolymers (3). If charged (4) or neutral polar (5) residues are introduced into a chain of 13 alanine residues to solubilize it in water, the helix becomes stable, more so when charged residues rather than neutral polar residues are incorporated. This behavior has been attributed to desolvation of the backbone NH and CO groups of the alanines by the preferentially hydrated charged or polar groups (6) rather than to a high intrinsic tendency of alanines to form an α-helix. By being deprived of a high degree of hydration, the backbone NH and CO groups can form the α-helical hydrogen bonds.
Previous experiments (4, 5) and calculations (6, 7) treated polyalanine chains containing several charged or neutral polar guest groups, e.g., three lysines or glutamines in the copolymers AAAAKAAAAKAAAAKA and AAQAAAAQAAAAQAAY, respectively, in which the helix-stabilizing effect might be influenced by interactions between the guest residues. In fact, the helix content increases with the number of guest residues (8). Therefore, to investigate the helix-forming properties of polyalanine in the absence of such interactions between the guest residues, we have carried out calculations on copolymers containing nine alanines and only one guest residue, viz., Ac-AAAAAXAAAA-NH2, in water, where X designates lysine or glutamine, and on homopolymers of alanine.
As a related problem, in which desolvation of the backbone NH and CO groups might be the source of the α-helical stability of polyalanine, we have examined the effect of varying dielectric constant on the stability of ALA10 and ALA16 α-helical homopolymers. By lowering the dielectric constant, the tendency to hydrate the backbone NH and CO group is lowered; this lowering might be the reason why alcohols such as trifluorethanol (TFE) increase α-helical stability.
Methodology
The details of the computational methodology are presented in a previous paper (6). The theoretical values of the helix content were computed in two ways: from computed Boltzmann-averaged successive spin–spin vicinal coupling constants (θcoupling) and from the computed Boltzmann-averaged number of residues having dihedral angles in the helical conformation (θdihedral) (6). When treating the effect of dielectric environment on helix stability of ALA10 and ALA16, the electrostatic free energy component of the total free energy was computed with the fast Multigrid Boundary Element method (9), with dielectric constants of 80, 40, and 2, corresponding to the values for pure water, ≈70% TFE in water (10), and a medium with the same dielectric constant as the solute to avoid solvent polarization effects, respectively. The accessible surface areas of the backbone polar atoms NH and CO were computed by using the MSEED algorithm (11).
Results and Discussion
Computations were carried out for the four different polypeptide sequences with acetyl (CH3CO—) and amino(—NH2) end groups described in Table 1. Two of the sequences are 10- and 16-residue homopolymers of alanine. For each sequence, runs were carried out at three different values of the dielectric constant, namely ɛ = 80, 40, and 2. For each run, more than 40,000 conformations (see Table 1) were generated and energy minimized, and the total free energy was then computed by the procedure described in previous publications (6, 12, 13). The same procedures were used for the copolymers Ac-AAAAAKAAAA-NH2 (9A1K) and Ac-AAAAAQAAAA-NH2 (9A1Q), with pKa0 for lysine taken as 10.5 (14). The resulting Boltzmann-averaged helix contents are displayed in Table 1. Table 2 shows the Boltzmann-averaged values of the total accessible surface area of the CO and NH groups at different dielectric constants for all of the runs. The conformation, and hence the accessible surface area, depends on the dielectric constant. It is worth noting that the accessible surface area of the NH groups in the 16-residue homopolymer of alanine is not a monotonic function of the dielectric constant, as observed for the CO groups. This behavior for the lowest-energy conformation (6) of the 16-residue homopolymer of alanine at ɛ = 80, which resembles a torus, arises because it maximizes the solvent-exposed accessible surface area of the backbone CO groups while burying many NH groups.
Table 1.
Summary of computations at 25°C
| Peptide sequence | No. of generated conformations* | No. of accepted conformations | Lowest energy, Kcal/mol | Helical fraction θcoupling, % | Helical fraction θdihedral, % | Dielectric constant ɛ |
|---|---|---|---|---|---|---|
| ALA16 | 43,752 | 1,345 | −255.8 | 7 (6†) | 12 (6†) | 80 |
| ALA16 | 42,256 | 1,222 | −215.7 | 59 | 68 | 40 |
| ALA16 | 49,151 | 1,416 | −79.8 | 90 | 100 | 2 |
| ALA10 | 50,824 | 1,645 | −181.6 | 0 (0‡) | 10 (0‡) | 80 |
| ALA10 | 34,105 | 1,626 | −154.0 | 54 | 71 | 40 |
| ALA10 | 45,357 | 1,739 | −49.5 | 90 | 100 | 2 |
| 9A1K§ | 38,482 | 1,649 | −176.5 | 77 | 76 | 80 |
| 9A1Q | 39,381 | 1,689 | −252.5 | 31 | 50 | 80 |
These values correspond to the number of generated conformations for the runs using the procedure described in refs 11 and 12.
Theoretical estimates obtained by using the experimental value of the Zimm-Bragg parameters (2), as derived from Host-Guest experiments (3), i.e., σ = 8 × 10−4 and s = 1.06 at 25°C.
Experimental value obtained by using the tri-block copolymer technique for the thermally induced helix-coil transition for copolymers of the type (d,l-lysine)m-(l-alanine)n-(d,l-lysine)m, with n = 10 for the alanyl residues in the central block (1).
Values in this row were computed at pH 6, with a value of 10.5 adopted for pKa0 for the lysine residue (14).
Table 2.
Boltzmann-averaged values of the accessible surface area of backbone polar atoms at different dielectric constants
| Peptide sequence | ɛ* | NH†, Å2 | CO†, Å2 |
|---|---|---|---|
| ALA10 | 80 | 16.7 | 244.8 |
| ALA10 | 40 | 10.9 | 143.9 |
| ALA10 | 2 | 4.3 | 118.5 |
| 9A1K | 80 | 10.6 | 106.6 |
| 9A1Q | 80 | 4.8 | 144.2 |
| ALA16 | 80 | 13.8 | 326.1 |
| ALA16 | 40 | 22.7 | 236.8 |
| ALA16 | 2 | 4.3 | 130.0 |
Value of the dielectric constant used during the simulation.
Boltzmann-averaged over all accepted conformations for the solvent accessible surface area (11) of the peptide amide and carbonyl group.
Helix Content of 9A1K at pH 6.
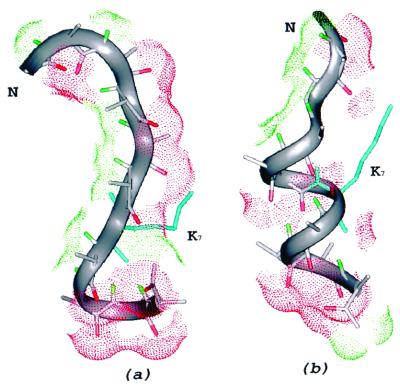
To test the effect of charged residues on the conformational preference of alanine, we carried out a run for the copolymer 9A1K, i.e., a 10-mer in which ALA-6 is replaced by LYS. This sequence involves no interactions of lysine with other ionizable groups within the chain or with the end groups, but preserves the influence of an ionizable group on the solvation preference of the backbone CO and NH groups. The simulation at pH 6 led to a Boltzmann-averaged helix content, θcoupling, of 77% (θdihedral = 76%), which is consistent with previous theoretical calculations (6) showing how lysine contributes to modify the apparent intrinsic helix-forming tendency of the amino acids by forcing the CO and NH groups to be shielded from the solvent. The results of this run (summarized in Table 1) show that, even in the absence of charge–charge interactions, a lysine residue introduced into the sequence to enhance solubility is able to modify the solvation preference of the CO and HN groups and alter the conformational preference of the all-alanine sequence so as to favor the α-helical conformation. Fig. 1 shows the initial and lowest-energy conformations found during the electrostatically driven Monte Carlo run for 9A1K.
Figure 1.
(a) Conformation used as the starting point of the simulation for the 9A1K peptide at pH 6. The initial values of the φ and ψ dihedral angles of the backbone correspond to those of the lowest-energy conformation of ALA10, with ɛ = 80 shown in Fig. 2c; after replacement of ALA-6 of Fig. 2c by LYS, the energy was minimized to produce the conformation of a. (b) The lowest-energy conformation found for the 9A1K peptide. The backbone of the polypeptide chain is traced with a gray ribbon, the single lysine residue is shown in blue, and the solvent-accessible surfaces of the oxygen (red) and polar hydrogens (green) are displayed using dots.
The Boltzmann-averaged helix contents obtained for the sequences ALA10 and 9A1K at ɛ = 80 in our simulations follow the trend of the CD experimental results obtained at high-salt concentration by Kallenbach and coworkers (N. R. Kallenbach, personal communication) for the general sequence Ac-AOOAAAAAAXAAAAAAOOAGGY-NH2 (with X representing either alanine or lysine, G = glycine, Y = tyrosine, and O = ornithine). Their experimental results show a larger helix content after substitution of the central alanine for lysine.
The CD data for Ac-AOOAAAAAAKAAAAAAOOAGGY-NH2 at low [0.01 M potassium fluoride (KF)] and high salt (1.0 M KF) concentrations collected by these researchers (N. R. Kallenbach, personal communication) show a significant change in the helix content, i.e., a doubling of the helix content at high salt concentration. Fluoride ions are known to have a strong solvent-ordering effect, as is apparent from its position in the Hofmeister series. This effect of the F− ions cannot be the only explanation for the experimental observations of Kallenbach et al. (personal communication) because the helix content changes by less than 30% when the central lysine residue is replaced by alanine. This behavior can be attributed mainly to: (i) electrostatic interactions between lysine and the ornithines or (ii) the effect of salt on the interactions between the nonpolar groups of lysine (i.e., the nonpolar part of the side chain of this residue) and the methyl groups of alanines (15). Sequences in which lysine residues are close to each other, such as the 16-residue alanine-based peptides containing six lysines (6K) (4), show similar variations of the helix content as the one reported by Kallenbach and coworkers (N. R. Kallenbach, personal communication) for the same range of salt concentrations. The dependence of the helix content on salt concentration, as found for 6K, indicates that there is a strong electrostatic effect at low salt concentration that is mostly screened out at high salt concentration. On the other hand, in sequences designed to avoid electrostatic interactions at low or high salt concentrations, such as the peptides 3K(I)[(Ac-AAAAKAAAAKAAAAKA-NH2 (one-letter code)] and 3K(II) (Ac-AKAAAAKAAAAKAAAA-NH2) of Marqusee et al. (4), the lysines are sufficiently far apart so that a very small variation of helix content (≈15%) is observed when the salt concentration is varied between 0.001 M and 1.0 M NaCl. The experimental evidence from Marqusee et al., with the 3K(I) and 3K(II) sequences, clearly rules out an effect of salt (on both the interactions between nonpolar groups and on charge–charge interactions) as the main source of stability in alanine-based copolypeptides. Consequently, the dependence of the helix content on salt concentration in the experiments of Kallenbach and coworkers (N. R. Kallenbach, personal communication) indicates that a strong electrostatic effect occurs at low salt concentration. Hence, the low helix content found by Spek et al. (16) at low salt concentration, when the central alanine was replaced by lysine, is attributable to repulsive electrostatic interactions between the central lysine and ornithines; these interactions are screened out at high-salt concentration (N. R. Kallenbach, personal communication), showing that addition of a lysine residue into a row of alanine residues augments the helix content, in good qualitative agreement with our simulations.
Helix Content of 9A1Q.
Experiments conducted by Scholtz et al. (5) on the 16-residue peptide Ac-(AAQAA)3-Y(NH2) indicate that helix formation in an alanine-based copolymer can be induced not only by incorporation of ionizable residues but also by highly soluble polar residues such as glutamine. An important distinction found in these experiments, however, is that the helix content is somewhat lower (35%) compared to the reported value (≈70%) for a similar alanine-based 16-residue peptide in which the glutamine residues were replaced by lysines, i.e., for the peptide 3K(I) studied by Marqusee et al.¶ (4).
A previous theoretical analysis (7) of the sequence Ac-(AAQAA)3-YNH2 showed good agreement with the available experimental evidence (5), indicating that the presence of a bulky polar glutamine residue influences the hydration of the backbone peptide groups. However, the mechanism by which this residue affects the solvation of the CO and NH groups was not addressed in that paper. To further our understanding of the effect of a highly soluble polar residue on the solvation preference of the NH and CO groups, we carried out a Monte Carlo search on the peptide 9A1Q. Analysis of this sequence enables us to compare the results with those from simulations of 9A1K, in which a charged residue is included in the central part of the sequence, and with those from the simulation of an all-alanine chain. From this comparison, we are able to identify the effect arising from the disturbance of the solvation shell of the backbone of the poly(L-alanine) chain when other residues are introduced to render the sequence soluble.
The computed Boltzmann-averaged helix content for 9A1Q is 31% (50%) (see Table 1), which is lower than the 77% (76%) obtained for the 9A1K peptide. Analysis of the results of the simulations for 9A1K and 9A1Q shows that their behavior follows qualitatively the experimentally observed tendency described by Scholtz et al. (5) in their comparison between 3K(I) and Ac-(AAQAA)3-Y(NH2), i.e., a reduction in the observed helical content.
In agreement with the results obtained for the peptide 9A1K and with previous simulations (6), we have also found that there is a tendency of the backbone CO and NH of 9A1Q to group in clusters when a highly soluble polar residue is present in the sequence. The lower helix content obtained in our simulations for 9A1Q compared with 9A1K, as well as the experimental evidence for 3K(I) and Ac-(AAQAA)3-Y(NH2), may be due to a weaker effect of the polar side chain of glutamine on the solvent compared with that of a charged lysine.
TFE Dependence of the Average Helix Content for the Blocked 10-mer and 16-mer Alanine Homopolymers.
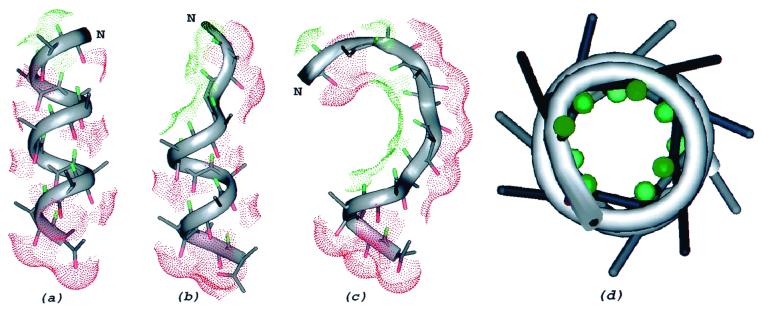
We modeled the dielectric effect of the solution surrounding the peptides by carrying out simulations at different dielectric constant, ɛ = 80, 40, and 2, for the terminally blocked 10-mer and 16-mer homopolymers of alanine. For Ac-AAAAAAAAAA-NH2 (ALA10) and Ac-AAAAAAAAAAAAAAAA-NH2 (ALA16), our results for a dielectric constant of ɛ = 80, i.e., in an aqueous solution, show Boltzmann-averaged helix contents near 0% (10%) for both sequences, in agreement with previous experimental results for a tri-block copolymer (1), for Host-Guest copolymers (3), and with recent experiments by Williams et al. (8). On the other hand, our calculations for ALA10 and ALA16 with a dielectric constant of ɛ = 40 show helix contents of 54% (71%) and 59% (68%), respectively, in agreement with experimental evidence from short template-nucleated helices in water containing TFE (17). As a further test, we carried out additional calculations for ALA10 and ALA16 at ɛ = 2 (the same value inside and outside the peptide cavity). For both sequences, our calculations show helix contents over 90%, providing evidence that lower dielectric media disfavor conformations with solvent-exposed CO and NH groups (17). Nonhelical conformations are energetically more favorable in water, with a higher ɛ, because the CO and NH groups tend to cluster in highly solvated forms (Fig. 2c). A distinctive aspect of these clusters is that the NH and CO groups tend to separate from each other, occupying, in some cases, different faces of the molecule and avoiding competition between solvation and internal NH⋅⋅⋅CO hydrogen bonds. Table 1 shows a clear tendency toward higher helix content for diminishing values of the dielectric constant, independent of the degree of polymerization. Moreover, from Table 2, a direct dependence of the (Boltzmann-averaged) exposed surface area of the CO and NH groups on the dielectric constant of the solvent is observed. There is a clear correlation between helix content and exposed surface area of the CO and NH groups that follows the same tendency described in previous work (6). The lowest-energy conformations found in the set of simulations with dielectric constant ɛ = 40 and 80, displayed in Fig. 2 b and c, respectively, are arranged with the CO and NH groups forming separated clusters. On the other hand, runs with ɛ = 2 led to lowest-energy conformations that were almost entirely α-helical (see Fig. 2a), in which all of the NH groups are buried (Fig. 2d).
Figure 2.
View of the lowest-energy conformations of ALA10 found in the set of simulations at different dielectric constants: (a) ɛ = 2, (b) ɛ = 40, and (c) ɛ = 80. All of the atoms have been colored in gray, except for the carbonyl O (red) and the amino HN (green). The backbone of the chain is traced with a gray ribbon, and the solvent-accessible surfaces of the oxygen and polar hydrogens are displayed using dots. Only a fraction of the backbone HN groups, not involved in intramolecular hydrogen bonds, remain exposed to the solvent when the dielectric constant ɛ is 40. (d) Top view of the lowest-energy conformation encountered in the runs for ɛ = 2, showing that all of the polar hydrogen (green) atoms are buried in a helix.
It is well known that TFE acts to enhance helicity. Despite the widespread use of TFE, the mechanism by which it induces helix formation remains unclear. From these simulations, we infer that it is possible that the TFE added to water modifies mainly the solvation environment in such a manner as to prevent CO and NH from being exposed to solvent, i.e., reducing backbone exposure (18). The effect of TFE on the stability of the α-helix formed by the ribonuclease S-peptide (residues 1–19 of ribonuclease A) was studied by Nelson and Kallenbach (19) using circular dichroism techniques. According to these authors, the most striking observation from their experiments is that the helix-stabilizing interactions afforded by the charged groups in aqueous solution are not altered by the addition of TFE. In other words, they suggest that the dielectric properties of the TFE/water mixtures do not appear to have a significant effect on charge–charge interactions that stabilize the peptide conformation in these experiments.
However, recent experiments (20) presented new evidence showing that the concentration of TFE seems to influence charge–charge interactions. In fact, the sequence Ac-AAQAAAAQAAGY-NH2 in 40% TFE exhibits a helix content of 81%, whereas a similar sequence with lysine instead of glutamine residues (Ac-AAKAAAAKAAGY-NH2) gives a helix content of 68%. On the other hand, it is known from earlier experiments in pure water (4, 5) that the helix preference of 16-residue alanine-based peptides is higher for lysine than glutamine (see Helix Content of 9A1Q). These contradictory results lead us to infer that the bulk dielectric constant may affect the charge–charge interactions in quite a complex manner. It is worth noting that the effect of TFE is also present in sequences without ionizable residues (e.g., Ac-AAQAAAAQAAGY-NH2), indicating that one possible effect of TFE is to influence the preferential reduction of backbone solvation.
From these experimental results it seems that changes in the bulk dielectric constant may affect both solvation of the peptide NH and CO groups, and the charge–charge interactions. In this work, we have identified these effects, and showed that, in the absence of net charges in the sequence, changes in the bulk dielectric constant (such as in TFE/water solutions at different concentrations) will shift the equilibrium toward the α-helical conformation. As already noted, charges in the sequences also affect the conformational equilibrium. Thus, it is possible that, for a given sequence, changes in the bulk dielectric constant will influence both charge–charge interactions [as also suggested by Avbelj (21)] and solvent exposure of the CO and NH groups.
Conclusions
Aimed at understanding the effect of charge and highly soluble polar residues on the conformational preference of alanine-based polypeptides, we carried out simulations with a single substitution (a lysine or glutamine residue) in a central block of a 10-alanine chain. Our results show, in agreement with previous simulations (6), that the presence of a charged-soluble or highly soluble polar residue modifies the solvation preference of the CO and NH groups of the backbone. Analysis of the sequences with a single lysine or glutamine residue allowed us to identify the effect of altering the charge distribution on the side chain of the chosen residue and its influence on the conformational preference of the peptide. Our results show that a single lysine or glutamine residue influences the solvation preference of the backbone CO and NH groups in such a manner as to shift the conformational preference toward the helical conformation in agreement with experimental evidence (N. R. Kallenbach, personal communication). We have also shown that a single lysine residue has a larger effect on the solvation preference of the CO and NH groups than a single glutamine residue, in agreement with both experimental evidence (4, 5) that shows that alanine-based polypeptides containing lysine residues display a higher helix content than those containing glutamine, and with theoretical simulations of these copolypeptides (6, 7).
A recent theoretical study (22) that investigated the interaction between water and the helical peptide group shows that nonpolar side chains interfere with solvation of the peptide group and are a major factor affecting helix propensities. In a complementary manner, our work shows that highly soluble polar or ionizable side chains also affect solvation of the backbone in alanine-based oligopeptides, shifting the conformational preference toward helical conformations, and hence, affecting the apparent helical propensities of the residues in the sequence.
Modeling a TFE/water mixture, by adopting different values of the dielectric constant, allowed us to investigate one of the possible mechanisms of helix induction by TFE. A striking result, obtained for both the 10- and 16-mer of alanine, is that the shielding of the solvent-exposed CO groups is strongly correlated with the value of the dielectric constant assumed for the medium. These results indicate that TFE may act on the exposed CO and NH groups by diminishing their exposure to the solvent, i.e., shifting the conformational equilibrium toward more compact structures, such as the α-helical conformation, that shield the CO and NH groups from solvent while leading to hydrogen-bond formation between them. Such a behavior was proposed by Cammers-Goodwin et al. (17) after a series of experiments on conjugates Ac-Hel-Alan-OH, n = 1–6 at different TFE concentrations. Our treatment of TFE/water mixtures, by adopting different dielectric constants, follows physical evidence indicating that the dielectric constant of the mixture is a linear function of the TFE (10) concentration. However, our simulations are not intended to describe the dependence on the actual TFE concentration, but to mimic a key feature of TFE/water mixtures. Attempts to use a discrete molecular treatment of the solution [e.g., to account for additional factors such as the suggested clustering of alcohol molecules at intermediate TFE concentrations (10)] are extremely difficult and beyond the capability of a simplified representation of the solvent such us the one adopted in our simulations, i.e., a continuum representation of the solvent.
Our simulations show that alanine is not a strong helix-forming residue and that the helix-coil equilibrium of an all-alanine peptide can be shifted toward structures such as the α-helical conformation in two different ways: (i) by introducing charged or highly soluble polar residues into the sequence and (ii) by lowering the dielectric constant of the solvent. Both processes lead to the same effect, i.e., the hydration of the CO and NH groups of alanine-based polypeptides is affected by forcing the polar groups to interact more weakly with the solvent and strongly among themselves, leading to the formation of a net of internal hydrogen bonds.
Acknowledgments
This research was supported by grants from the National Institutes of Health (GM-14312 and 5 R03 TW00857-04), the National Institutes of Health National Center for Research Resources (P41RR-04293), and the National Science Foundation (MCB95-13167). Support was also received from the National Foundation for Cancer Research, the National Research Council of Argentina (CONICET), and Project No. P-328402 of the “Universidad Nacional de San Luis-Argentina.” This research was conducted by using the resources of the Cornell Theory Center, which receives funding from Cornell University, New York State, the National Center for Research Resources at the National Institutes of Health, and members of the Theory Center's Corporate Partnership Program.
Abbreviations
- TFE
trifluoroethanol
- 3K(I)
Ac-AAAAKAAAAKAAAAKA-NH2 (one-letter code)
- 3K(II)
Ac-AKAAAAKAAAAKAAAA-NH2
- 6K
Ac-AKAAKAKAAKAKAAKA-NH2
- ALA16
Ac-AAAAAAAAAAAAAAAA-NH2
- ALA10
Ac-AAAAAAAAAA-NH2
- 9A1K
Ac-AAAAAKAAAA-NH2
- 9A1Q
Ac-AAAAAQAAAA-NH2
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240455797.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240455797
These authors showed that the helix content of 16-residue peptides containing three lysines in the pattern KAAAAK is roughly independent of the position of the lysines in the sequence.
References
- 1.Ingwall R T, Scheraga H A, Lotan N, Berger A, Katchalski E. Biopolymers. 1968;6:331–368. doi: 10.1002/bip.1968.360060308. [DOI] [PubMed] [Google Scholar]
- 2.Zimm B H, Bragg J K. J Chem Phys. 1959;31:526–535. [Google Scholar]
- 3.Platzer K E B, Ananthanarayanan V S, Andreatta R H, Scheraga H A. Macromolecules. 1972;5:177–187. [Google Scholar]
- 4.Marqusee S, Robbins V H, Baldwin R L. Proc Natl Acad Sci USA. 1989;86:5286–5290. doi: 10.1073/pnas.86.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scholtz J M, York E J, Stewart J M, Baldwin R L. J Am Chem Soc. 1991;113:5102–5104. [Google Scholar]
- 6.Vila, J. A., Ripoll, D. R. & Scheraga, H. A. (2001) Biopolymers, in press. [DOI] [PubMed]
- 7.Vila J, Williams R L, Grant J A, Wojcik J, Scheraga H A. Proc Natl Acad Sci USA. 1992;89:7821–7825. doi: 10.1073/pnas.89.16.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams L, Kather K, Kemp D S. J Am Chem Soc. 1998;120:11033–11043. [Google Scholar]
- 9.Vorobjev Y N, Scheraga H A. J Comput Chem. 1997;18:569–583. [Google Scholar]
- 10.Hong D-P, Hoshino M, Kuboi R, Goto Y. J Am Chem Soc. 1999;121:8427–8433. [Google Scholar]
- 11.Perrot G, Cheng B, Gibson K D, Vila J, Palmer K A, Nayeem A, Maigret B, Scheraga H A. J Comput Chem. 1992;13:1–11. [Google Scholar]
- 12.Ripoll D R, Vorobjev Y N, Liwo A, Vila J A, Scheraga H A. J Mol Biol. 1996;264:770–783. doi: 10.1006/jmbi.1996.0676. [DOI] [PubMed] [Google Scholar]
- 13.Ripoll D R, Vila J A, Villegas M E, Scheraga H A. J Mol Biol. 1999;292:431–440. doi: 10.1006/jmbi.1999.3082. [DOI] [PubMed] [Google Scholar]
- 14.Perrin D D. Dissociation Constants of Organic Bases in Aqueous Solution. London: Butterworth; 1965. p. 390. [Google Scholar]
- 15.Némethy G, Steinberg I Z, Scheraga H A. Biopolymers. 1963;1:43–69. [Google Scholar]
- 16.Spek E J, Olson C A, Shi Z, Kallenbach N R. J Am Chem Soc. 1999;121:5571–5572. [Google Scholar]
- 17.Cammers-Goodwin A, Allen T J, Oslick S L, McClure K F, Lee J H, Kemp D S. J Am Chem Soc. 1996;118:3082–3090. [Google Scholar]
- 18.Kentsis A, Sosnick T R. Biochemistry. 1998;37:14613–14622. doi: 10.1021/bi981641y. [DOI] [PubMed] [Google Scholar]
- 19.Nelson J W, Kallenbach N R. Proteins Struct Funct Genet. 1986;1:211–217. doi: 10.1002/prot.340010303. [DOI] [PubMed] [Google Scholar]
- 20.Rohl C A, Chakrabartty A, Baldwin R L. Protein Sci. 1996;5:2623–2637. doi: 10.1002/pro.5560051225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avbelj F. J Mol Biol. 2000;300:1335–1359. doi: 10.1006/jmbi.2000.3901. [DOI] [PubMed] [Google Scholar]
- 22.Avbelj F, Luo P, Baldwin R L. Proc Natl Acad Sci USA. 2000;97:10786–10791. doi: 10.1073/pnas.200343197. [DOI] [PMC free article] [PubMed] [Google Scholar]