Abstract
Objective
To determine whether the co-prescribing of two or more antipsychotics, a relatively frequent practice with little data to support its safety and efficacy, is associated with an increased prevalence of metabolic syndrome.
Methods
364 newly admitted adults treated with second-generation antipsychotics underwent assessments evaluating antipsychotic polytherapy, and of the presence of metabolic syndrome and triglycerides/high-density lipoprotein cholesterol ratio >3.5 (TG/HDL), a sensitive marker of insulin resistance. The correlates of antipsychotic polytherapy and associations with metabolic syndrome and TG/HDL were determined by univariate comparisons and multiple logistic regression analyses.
Results
Antipsychotic polytherapy was present in 70 patients (19.2%) and was significantly more likely in patients with schizophrenia and those treated with clozapine, quetiapine or ziprasidone (p<0.0001). Compared with antipsychotic monotherapy, polytherapy was associated with elevated rates of metabolic syndrome (50.0% vs. 34.3%, p=0.015) and TG/HDL (50.7% vs. 35.0%, p=0.016). However, in logistic regression analyses, metabolic syndrome was significantly associated with higher body mass index (BMI), older age, a diagnosis of bipolar disorder or schizophrenia, and cotreatment with a first-generation antipsychotic (r2: 0.25, p<0.0001). The TG/HDL marker of insulin resistance was associated with higher BMI, male sex, Caucasian race and absence of aripiprazole treatment (r2: 0.14, p<0.0001). Antipsychotic polypharmacy dropped out of both multivariate models.
Conclusions
Compared with patients receiving antipsychotic monotherapy, patients on antipsychotic polytherapy have higher rates of metabolic syndrome and lipid markers of insulin resistance. However, antipsychotic polytherapy is not independently associated with the prevalence of these abnormalities, which are related to known demographic, clinical and anthropometric risk factors.
Keywords: Antipsychotics, Polypharmacy, Correlates, Metabolic Syndrome, Insulin Resistance
The prescribing of more than one antipsychotic at the same time is becoming common in the treatment of patients with severe mental illnesses, despite authoritative treatment guidelines that advocate for antipsychotic monotherapy, recommending antipsychotic cotreatment as the last option after other strategies, including clozapine, have failed (1–3). Depending on treatment settings, populations, time of study and study methodology, prevalence rates of antipsychotic polytherapy for schizophrenia and other chronic psychotic disorders range between 3% and 71% (4–19). The increased cost of combining two atypical antipsychotics and the lack of substantial proof of the efficacy and safety of antipsychotic polytherapy has raised considerable reservations regarding this practice (20–22).
Among the four controlled studies in patients with clozapine-refractory schizophrenia, two trials demonstrated a moderate benefit of augmentation with sulpiride (23) and risperidone (24), while the other two trials did not find clozapine augmentation with risperidone to be superior compared to augmentation with placebo (25,26). However, since only less than 5% of patients are treated with clozapine in the US, these controlled trials are limited in addressing the efficacy of combining two antipsychotics in patients with schizophrenia and other severe mental disorders who are either not considered fully treatment refractory, or in those unwilling or unable to benefit from clozapine.
On the other hand, the safety of antipsychotic polytherapy has received scant attention in published work. In several studies, antipsychotic combination treatment has been associated with higher rates of extrapyramidal side effects, as well as increased use of anticholinergic agents (12,17,27) compared to antipsychotic monotherapy. In addition, sedation (25) and hyperprolactinemia (23,28) have been observed more often in patients treated with antipsychotic polytherapy. In addition, recent data suggest that antipsychotic polytherapy may be associated with greater metabolic risk. In a case-control study of patients treated with antipsychotics, including 181 cases with new antidiabetic prescriptions and 1,448 matched controls, an elevated risk for treatment-emergent diabetes mellitus was found in association with antipsychotic combination treatment (29). The association of antipsychotic treatment with weight gain, hyperglycemia, dyslipidemia, and the metabolic syndrome (30–34), as well as naturalistic reports linking cardiovascular mortality to antipsychotic polytherapy (35,36), suggest that combining antipsychotics, particularly second-generation antipsychotics, could be associated with an increased risk of metabolic syndrome, which is linked with coronary heart disease and diabetes (37,38). However, the antipsychotics used in polytherapy may produce different effects on the prevalence of metabolic syndrome (39,40), because clozapine and olanzapine are associated with greater weight increase and metabolic abnormalities than other SGAs, particularly ziprasidone and aripiprazole (41).
The relationship between antipsychotic polytherapy and metabolic syndrome has not been examined. In this cross-sectional study of an inpatient population treated with second-generation antipsychotics, we aimed to determine whether antipsychotic polytherapy is associated with an increased point prevalence of metabolic syndrome and lipid markers of insulin resistance. We hypothesized that the frequency of metabolic syndrome and insulin resistance would be elevated in patients receiving more than one antipsychotic for the treatment of a severe mental disorder.
Methods
Setting and Patient Population
In May 2004, the Pharmacy and Therapeutics Committee of the Zucker Hillside Hospital instructed all physicians to follow the recommendations of the 2004 Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes (41). The policy required that the following measures be obtained within 24 hours of admission, or as soon as clinically feasible: personal and family history of obesity, diabetes, dyslipidemia, hypertension or cardiovascular disease; weight and height; waist circumference at the level of the umbilicus; fasting plasma glucose; and fasting lipid profile. These data and demographic and clinical information were collected for this study from the hospital records after discharge. Psychiatric diagnoses were made by board certified or board eligible psychiatrists according to DSM-IV criteria. The Institutional Review Board of the North Shore–Long Island Jewish Health System approved the study.
Data were collected from the records of 458 psychiatric inpatients treated with second-generation antipsychotics at the time of presentation to the hospital’s intake unit. The patients were randomly selected from 1,420 consecutive admissions between August 1, 2004 and March 1, 2005. Ninety-four subjects were excluded from this study, leading to a total sample of 364 patients with complete data. Reasons for exclusion were: a) age younger than 20 years or older than 79 years (n=49) and b) missing data required to calculate prevalence rates for antipsychotic polytherapy and metabolic syndrome (n=45).
Laboratory tests
Fasting blood glucose levels were measured at bedside with Accu-Chek Inform, Model 2001201, Roche, Mannheim, Germany. This microreflectometric method has excellent overall correlation (r=0.974) with the standard laboratory glucose-oxidase method for serum glucose levels up to 400 mg/dl (42). The fasting lipid levels were measured spectrophotometrically at the Long Island Jewish Medical Center laboratory with the Chemistry Immunanalyzer, Model AU 2700, Olympus, Melville, New York.
Metabolic Syndrome and Lipid markers of Insulin Resistance
The metabolic syndrome was diagnosed in patients who fulfilled 3 or more of the following 5 criteria: waist circumference at the level of umbilicus greater than 88 cm in women and greater than 102 cm in men; fasting blood glucose level of 110 mg/dL or greater; serum triglyceride level of 150 mg/dL or greater; high-density lipoprotein cholesterol (HDL-C) less than 40 mg/dL in men and less than 50 mg/dl in women; and arterial blood pressure 130/85 or greater (43). Current treatment with antihypertensive medications fulfilled the metabolic syndrome criterion for high blood pressure. Treatment with lipid-lowering drugs fulfilled the metabolic syndrome criteria for low HDL and high triglycerides. A diagnosis of diabetes mellitus fulfilled the fasting hyperglycemia criterion. The insulin resistance was assessed indirectly with lipid markers using a triglyceride/HDL ratio > 3.5 (44).
Data Analyses
Analyses of variance (ANOVAs) and chi-square tests were used to compare demographic, treatment and clinical variables in patients treated with one or more than one antipsychotic. To test whether individual combinations were associated with a differential effect on the prevalence of metabolic syndrome and lipid markers of insulin resistance, we carried out three additional analyses, for which we grouped antipsychotics by class, i.e., second-generation/second-generation vs. second-generation/first-generation; combinations including the “low-risk drugs” aripiprazole and/or ziprasidone vs. other combinations; and combinations using the “high-risk drugs” clozapine and/or olanzapine vs. other combinations.
We conducted stepwise multiple regression analyses to examine correlates of antipsychotic polypharmacy and to examine the relevance of variables other than antipsychotic polytherapy for the presence of metabolic syndrome and TG/HDL > 3.5. For the multivariate analyses, we entered into the model antipsychotic polytherapy, sex, age, race as well as all variables that in univariate analyses were different between patients on antipsychotic monotherapy or polytherapy at a level of p≤0.1 (i.e., body mass index (BMI), diagnosis of schizophrenia, bipolar disorder and depressive disorder, treatment with olanzapine, quetiapine, risperidone, aripiprazole, ziprasidone, clozapine or a first-generation antipsychotic, and cotreatment with antidepressant or anticholinergic drugs). Given the strong correlation between BMI and waist circumference, the multiple regression for metabolic syndrome was also repeated without BMI in the model. Furthermore, since the value of TG/HDL ratio as a marker of insulin resistance in individuals of African descent is controversial (45,46), the multiple regression analyses for TG/HDL ratio >3.5 was also repeated for the subgroup of Caucasian patients. Data were analyzed using JMP 5.0.1, 1989–2003, SAS Institute Inc.
Results
Demographic and Clinical Features of Patients With and Without Antipsychotic Polypharmacy
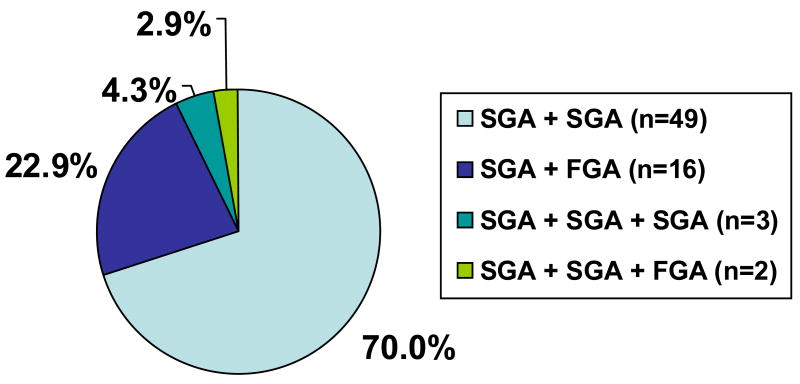
The mean age of the 364 patients included in this study was 42.9 years, 54.4% were male and 67.8% were White. Seventy-one patients (19.2%) were receiving antipsychotic polytherapy. Sixty-five patients (17.9%) were on two antipsychotics and five patients were treated with three antipsychotics concurrently. Combinations of two second-generation antipsychotics were most common (n=49, 70.0%), followed by combinations of a first- with a second-generation antipsychotic (n=16, 22.9%). Three patients (4.3%) were on combinations of three second-generation antipsychotics (n=3, 4.2%) and two patients (2.9%) were receiving two second-generation antipsychotics and one first-generation antipsychotic (Figure 1).
Figure 1.
Combination Patterns by Antipsychotic Class in 70 (19.2%) of 364 Patients on Antipsychotic Polypharmacy
Compared with the 294 patients on antipsychotic monotherapy, patients on antipsychotic polytherapy had a higher body mass index (31.0±8.4 vs. 28.1±6.3, p=0.0012), were more likely to be diagnosed with schizophrenia (77.1% vs. 40.8%, p<0.0001) and less likely to be diagnosed with a depressive disorder (7.1% vs. 24.1%, p=0.0017) or bipolar disorder (11.4% vs. 22.8%, p=0.035) (Table 1). Polytherapy patients were also more likely to receive a first-generation antipsychotic (27.1% vs. 0.0%, p<0.0001), clozapine (21.4% vs. 4.1%, p<0.0001), ziprasidone (18.6% vs. 5.8%, p=0.0005), quetiapine (42.9% vs. 26.5%, p= 0.0072) and risperidone (38.6% vs. 25.5%, p= 0.029) than patients receiving antipsychotic monotherapy. Except for a modestly higher rate of anticholinergic drug use (14.3% vs. 6.8%, p=0.041) and a lower rate of antihypertensive medications (7.1% vs. 16.7%, p=0.044), patients on antipsychotic polypharmacy did not differ from those on monotherapy regarding comedications.
Table 1.
Demographic and Clinical Characteristics at Time of Psychiatric Admission
| Characteristic | Total (N=364) | Antipsychotic Monotherapy (N=294) | Antipsychotic Polypharmacy (N=70) | F/chi2 | P-value |
|---|---|---|---|---|---|
|
Demographics and Medical History | |||||
| Age (years±SD) | 42.9 ± 15.3 | 43.6 ±15.9 | 40.0 ± 12.1 | F: 3.03 | 0.08 |
| Sex (Male, N, %) | 198 (54.4) | 157 (53.4) | 41 (58.6) | χ2: 0.61 | 0.43 |
| Race (White, N, %)a | 244 (67.8) | 196 (67.3) | 48 (69.6) | χ2: 0.12 | 0.73 |
| Smoking (N, %) | 199 (54.7) | 158 (53.7) | 41 (58.6) | χ2: 0.54 | 0.47 |
| History/Presence of diabetes (N, %) | 50(13.7) | 43 (14.6) | 7 (10.0) | χ2: 1.02 | 0.31 |
| History of CAD (N,%) | 29 (8.0) | 26 (8.8) | 3 (4.3) | χ2: 1.60 | 0.21 |
| Body mass index (kg/m2±SD)b | 28.6 ±6.7 | 28.1 ±6.3 | 31.0 ±8.4 | F: 10.65 | 0.0012 |
| Primary Psychiatric Diagnosis | |||||
| Schizophrenia (N, %) | 174 (47.8) | 120 (40.8) | 54 (77.1) | χ2: 29.9 | <0.0001 |
| Bipolar Disorder (N, %) | 75 (20.6) | 67 (22.8) | 8 (11.4) | χ2: 4.46 | 0.035 |
| Depressive Disorder (N, %) | 76 (20.9) | 71 (24.1) | 5(7.1) | χ2: 9.99 | 0.0017 |
| Substance Use Disorder (N, %) | 17 (4.7) | 16 (5.4) | 1 (1.4) | χ2: 2.05 | 0.15 |
| Dementia (N, %) | 9 (2.5) | 8 (2.7) | 1 (1.4) | χ2: 0.39 | 0.52 |
| Other (N, %) | 13 (3.6) | 12(4.1) | 1 (1.4) | χ2: 1.16 | 0.28 |
| Antipsychotic Treatmentc | |||||
| Olanzapine (N, %) | 117 (32.1) | 88 (29.9) | 29 (41.4) | χ2: 3.43 | 0.064 |
| Quetiapine (N, %) | 108 (29.7) | 78 (26.5) | 30 (42.9) | χ2: 7.22 | 0.0072 |
| Risperidone (N, %) | 102 (28.0) | 75 (25.5) | 27 (38.6) | χ2: 4.78 | 0.029 |
| Aripiprazole (N, %) | 35 (9.6) | 24 (8.2) | 11 (15.7) | χ2: 3.71 | 0.054 |
| Ziprasidone (N, %) | 30 (8.2) | 17 (5.8) | 13 (18.6) | χ2: 12.23 | 0.0005 |
| Clozapine (N, %) | 27 (7.4) | 12 (4.1) | 15 (21.4) | χ2: 24.8 | <0.0001 |
| First-generation antipsychotic (N, %) | 19 (5.2) | 0 (0.0) | 19(27.1) | χ2: 84.2 | <0.0001 |
| Non-antipsychotic Treatmentc | |||||
| Anxiolytics/Hypnotics (N, %) | 188 (51.6) | 150 (51.0) | 38 (54.3) | χ2:0.24 | 0.62 |
| Antidepressants (N, %) | 168 (46.1) | 142 (48.3) | 26 (37.1) | χ2: 2.83 | 0.09 |
| Mood Stabilizers (N, %) | 132 (36.3) | 102 (34.7) | 30 (42.9) | χ2: 1.63 | 0.20 |
| Anticholinergics (N, %) | 30 (8.2) | 20 (6.8) | 10(14.3) | χ2: 4.19 | 0.041 |
| Lipid lowering drugs (N, %) | 49(13.5) | 39(13.3) | 10(14.3) | χ2: 0.051 | 0.82 |
| Hypoglycemic drugs (N, %) | 21 (5.8) | 17 (5.8) | 4 (5.7) | χ2: 0.003 | 0.98 |
| Antihypertensive drugs (N, %) | 54 (14.8) | 49 (16.7) | 5(7.1) | χ2: 4.06 | 0.044 |
FGA: First-generation Antipsychotic; SGA: Second-generation Antipsychotic
6 patients without ethnic/racial information;
one patient without BMI information;
total n> 364 due to polypharmacy
Metabolic Syndrome and Lipid Markers of Insulin Resistance
Compared to patients treated with one antipsychotic, those on two or three antipsychotics were more likely to fulfill criteria for the metabolic syndrome (50.0% vs. 34.4%, p=0.015) and to have a TG/HDL >3.5 (50.7% vs. 35.0%, p=0.016). Regarding individual metabolic syndrome criteria or continuous metabolic variables, patients on antipsychotic polytherapy were only more likely to fulfill the low HDL criterion (70.0% vs. 55.4%, p=0.026) and to have greater waist circumference (95.5±16.1 cm vs. 91.4±13.7 cm, p=0.028) compared with patients on antipsychotic monotherapy (Table 2).
Table 2.
Frequency of Metabolic Syndrome, Individual Metabolic Syndrome Criteria and Insulin Resistance in Patients Treated with Antipsychotic Monotherapy vs. Antipsychotic Polypharmacy
| Characteristic | Total (N=364) | Antipsychotic Monotherapy (N=294) | Antipsychotic Polypharmacy (N=70) | F/chi2 | P-value |
|---|---|---|---|---|---|
| Metabolic syndrome (N, %) | 136 (37.4) | 101 (34.3) | 35 (50.0) | χ2: 5.91 | 0.015 |
| # of metabolic syndrome criteria (±SD) | 1.97± 1.3 | 1.9 ± 1.4 | 2.2 ± 1.4 | F: 1.81 | 0.18 |
| Waist circumference >102 cm in males or >88 cm in females (N, %) | 122 (33.5) | 94 (32.0) | 28 (40.0) | χ2: 1.63 | 0.20 |
| Triglycerides ≥150 mg/dL (N, %)a | 151 (41.6) | 117(39.8) | 34 (49.3) | χ2: 2.07 | 0.15 |
| Fasting hyperglycemia ≥110 mg/dL (N, %)b | 42 (11.6) | 35 (11.9) | 7(10.1) | χ2: 0.18 | 0.67 |
| Hypertension 130/85 mm Hg (N, %) | 173 (47.5) | 139 (47.3) | 34 (48.6) | χ2: 0.038 | 0.85 |
| HDL <40 mg/dl in males or <50 mg/dl in females (N, %) | 212 (58.2) | 163 (55.4) | 49 (70.0) | χ2: 4.93 | 0.026 |
| Triglyceride/HDL-cholesterol >3.5 (N, %)a | 138 (38.0) | 103 (35.0) | 35 (50.7) | χ2: 5.84 | 0.016 |
| Continuous Metabolic Variables | |||||
| Waist circumference (cm±SD) | 92.2 ± 14.2 | 91.4 ± 13.7 | 95.5 ± 16.1 | F: 4.84 | 0.028 |
| Fasting glucose (mg/dL±SD)b | 93.1 ±22.5 | 92.6 ± 22.0 | 95.0 ± 24.6 | F: 0.65 | 0.42 |
| Systolic blood pressure (mm Hg±SD) | 124.0 ± 14.7 | 123.8 ± 14.3 | 124.9 ± 16.6 | F: 0.32 | 0.75 |
| Diastolic blood pressure (mm Hg±SD) | 79.0 ±9.1 | 78.8 ± 9.0 | 79.9 ± 9.7 | F: 0.91 | 0.34 |
| HDL (mg/dl ±SD) | 44.8 ± 12.1 | 45.2 ± 12.3 | 42.9 ± 10.9 | F: 2.09 | 0.15 |
| Triglycerides (mg/dL±SD)a | 136.2 ±81.8 | 134.2 ±81.3 | 144.6 ± 83.8 | F: 0.91 | 0.34 |
| Total cholesterol (mg/dL±SD)a | 171.4 ±40.1 | 172.5 ± 39.5 | 167.0 ±42.3 | F: 01.06 | 0.30 |
| LDL-cholesterol (mg/dL±SD)a | 115.5 ±37.2 | 115.7 ±36.6 | 114.9 ±39.5 | F: 0.03 | 0.87 |
| Triglyceride/HDL-cholesterol ratio (±SD)a | 3.5 ±2.8 | 3.4 ±2.8 | 3.7 ±2.5 | F: 0.70 | 0.4 |
: Based on 363 patients with available information;
: based on 362 patients with available information
Analyses of the polytherapy combinations grouped by drug class and weight gain potential indicated no significant differences in the prevalence of the metabolic syndrome in patients receiving second-generation/second generation vs. first generation/second-generation drugs (44.2% vs. 66.7%, p=0.10), “low-risk” combinations including ziprasidone and/or aripiprazole vs. other combinations (43.5% vs. 53.2%, p=0.44), and “high-risk” combinations including clozapine and/or olanzapine vs. other combinations (50% vs. 50%, p=1.0). Similarly, the prevalence of TG/HDL > 3.5 in these three comparisons were 47.1% vs. 61.1% (p=0.30); 45.4% vs. 53.2% (p=0.55); and 50% vs. 51.7% (p=0.82), respectively.
Multiple Regression Analysis of Variables Associated with Antipsychotic Polypharmacy, Metabolic Syndrome and Lipid Markers of Insulin Resistance
In multivariate analyses of demographic and clinical variables excluding antipsychotic medications, antipsychotic polytherapy was associated with a diagnosis of schizophrenia (p<0.0001) and higher body mass index (p=0.018) [r2: 0.10, p<0.0001].
In multivariate analyses of variables not used to define the metabolic syndrome, presence of the metabolic syndrome was associated with higher body mass index (p<0.0001), older age (p<0.0001), a diagnosis of bipolar disorder (p=0.0003) or schizophrenia (p=0.0041), and treatment with a first-generation antipsychotic (p=0.037) [r2: 0.25, p<0.0001], but not with antipsychotic polytherapy. Repeating these analyses without BMI in the model, the same remaining four variables emerged as significant correlates of metabolic syndrome, i.e., older age (p<0.0001), a diagnosis of schizophrenia (p=0.0010), treatment with a first-generation antipsychotic (p=0.0027) and a diagnosis of bipolar disorder (p=0.0032) and [r2: 0.09, p<0.0001]. Clarifying the association between first-generation antipsychotic cotreatment and metabolic syndrome, additional analyses indicated that patients cotreated with first-generation antipsychotics had significantly greater waist circumference (111.5±17.3 vs. 91.7±13.9, p=0.0035), a metabolic syndrome criterion that was not part of the multiple regression model.
The lipid marker of insulin resistance, measured with a categorical cut-off of TG/HDL ratio >3.5, was associated with higher body mass index (p<0.0001), male sex (p<0.0001), Caucasian race (p=0.028) and absence of aripiprazole treatment (p=0.040) [r2: 0.14, p<0.0001], but not with antipsychotic polytherapy. Repeating these analyses in the Caucasian subgroup, the same remaining three variables emerged as significant correlates of TG/HDL ratio >3.5, i.e., higher body mass index (p<0.0001), male sex (p<0.0001), Caucasian race (p=0.028) and absence of aripiprazole treatment (p=0.017) [r2: 0.15, p<0.0001].
Discussion
In this cross-sectional study of adults treated with at least one second-generation antipsychotic at the time of admission to a psychiatric hospital, one in five patients was treated with two or more antipsychotics. A combination of two second-generation antipsychotics was by far the most common polytherapy pattern. Clinical correlates of antipsychotic polytherapy were a diagnosis of schizophrenia and greater BMI. In univariate analyses, patients receiving antipsychotic polytherapy had significantly higher rates of metabolic syndrome and lipid markers of insulin resistance. Within the polytherapy group and taking into account the limited power of these analyses, the prevalence of metabolic syndrome and lipid markers of insulin resistance was not influenced by the drug class (i.e., second-generation/second-generation vs. second-generation/first-generation) or by metabolically high-risk (clozapine and olanzapine) or low-risk (aripiprazole and ziprasidone) SGAs. In multivariate logistic regression analyses, metabolic syndrome and lipid marker-derived insulin resistance were associated with demographic and clinical factors known to increase the risk for metabolic abnormalities, some of which that can be adversely affected by antipsychotic treatment, while antipsychotic polytherapy was not independently correlated with these abnormalities.
Despite differences in settings, populations and definitions, our finding that almost 20% of patients were on two or more antipsychotics is in line with other recent studies in which antipsychotic polypharmacy ranged from 13% to 34% in patients with mixed psychiatric diagnoses (4–7,13,14). Consistent with previous studies, we found that antipsychotic polytherapy was most prevalent in schizophrenia (6,12,14) and that quetiapine was significantly more likely to be part of antipsychotic polytherapy (7,8,16), most likely due to the little added risk of extrapyramidal symptoms with this agent. In addition, clozapine and ziprasidone were also significantly associated with antipsychotic polytherapy. These practices are supported by the fact that clozapine non-responders are arguably the most appropriate candidates for antipsychotic polytherapy and that ziprasidone has been associated with little weight gain or metabolic burden in chronically treated patients (47,48), which makes ziprasidone a rational choice as an augmenting antipsychotic with the aim to not add to the metabolic burden of other antipsychotics. Moreover, first-generation antipsychotics and risperidone were also associated with antipsychotic polypharmacy, likely in an attempt to increase therapeutic efficacy (4, 16). Consistent with previous findings (49), olanzapine treatment was not significantly associated with antipsychotic polytherapy, while the number of patients on aripiprazole was insufficient to make conclusive statements. However, the fact that antipsychotic polytherapy consisted of a multitude of different combinations underscores the lack of prevailing data and of mechanistic theories that could guide rational antipsychotic cotreatment. Although in univariate analyses of our sample, patients on more than one antipsychotic were more likely to receive anticholinergic medications, this effect was lost in multiple regression analyses, most likely due to the fact that 95% of patients were on second-generation antipsychotics.
In univariate analyses, we observed the association between antipsychotic polytherapy and poor metabolic status that had previously been suggested (26,29). Body mass index and waist circumference values were significantly higher, and HDL-cholesterol levels were more frequently in the abnormally low category in patients on antipsychotic polytherapy. In addition, metabolic syndrome and lipid-marker derived insulin resistance were significantly more common in patients receiving two or more antipsychotics. However, in multivariate logistic regression analyses that were conducted to assess whether this association was independent of other potentially relevant factors, known risk factors for metabolic syndrome (i.e., body mass index, age, bipolar disorder and schizophrenia), and for insulin resistance (i.e., body mass index, sex, and race) emerged as significant contributing factors to poor metabolic health. This finding supports the postulated interaction between underlying risk factors and antipsychotic treatment (50, 51), rather than an independent effect of antipsychotic polypharmacy on poor metabolic outcome. The same interaction is likely to explain the weak association between first-generation antipsychotic cotreatment and metabolic syndrome, as suggested by the significantly greater waist circumference in the group receiving antipsychotic combinations that included a first-generation drug. Our findings suggest that patients receiving antipsychotic polytherapy represent a subgroup that is more obese and inactive and, thus, is more prone to metabolic risks than patients prescribed antipsychotic monotherapy.
In this context, it is important to note that even in a recent study that reported significant elevations in fasting glucose in the patients randomized to risperidone augmentation of clozapine, no differences between risperidone and placebo augmentation on any of the other metabolically relevant outcome variables were observed after eight weeks of treatment (26). Changes in body weight, body mass index, waist circumference and total cholesterol, LDL-cholesterol, HDL-cholesterol and triglycerides were similar among the monotherapy and polytherapy groups. This may be explained by a ceiling effect for these changes, at least after prolonged treatment with clozapine, and supports our finding of a lack of an independent association between antipsychotic polypharmacy and metabolic syndrome. Furthermore, in addition to potential differences in patient and other treatment variables between patients on antipsychotic polytherapy and those on monotherapy, the previously reported finding of increased prescriptions for antidiabetic drugs in patients on two or more antipsychotics (29) is sensitive to a possible measurement bias. This is suggested by a chart review study of 660 inpatients treated with at least one antipsychotic, where less than half of the patients were tested for diabetes, but testing occurred 2.6 times more often in patients on antipsychotic polypharmacy (52). Nevertheless, our findings do not exclude the possibility that combinations of certain antipsychotic drugs could worsen or improve the risk of increased BMI and abdominal adiposity found to be more prevalent in our polytherapy sample. The potential that individual combinations could improve metabolic risk is suggested by the signal that aripiprazole treatment, which has been reported to be associated with less weight gain and metabolic complications than most other second-generation antipsychotics (53,54), was associated with a lower prevalence of insulin resistance in our multiple regression analyses. This is also consistent with data from a recent 6-week, open-label study where aripiprazole augmentation of clozapine treatment at unaltered dose levels was associated with a significant decrease in weight, body mass index, fasting total serum cholesterol and triglycerides, without worsening of psychopathology scores (40).
The results of our study need to be interpreted within the limitations of its cross-sectional design, moderate sample size, lack of power to examine individual antipsychotic combinations, lack of sophisticated assessments of insulin resistance, and the potentially confounding presence of multiple diagnoses and comedications. In addition, it is possible that some patients were in the process of antipsychotic cross-titration at the time of admission and would not have been on antipsychotic polypharmacy if followed over a longer period of time (19). This could have attenuated a potential effect of sustained antipsychotic cotreatment. However, as the assessments were done at the time of admission, the bias of inpatient studies is reduced, which are more likely to capture patients during active treatment changes. Moreover, the rate of antipsychotic polytherapy was not excessive compared to other outpatient studies, suggesting that it is unlikely we included a substantial number of patients who were only on short-term antipsychotic cotreatment. Furthermore, even if some patients were on short-term antipsychotic polytherapy, this would not affect the determination of acute additive metabolic effects of combined antipsychotic treatments.
Our study is the first attempt to examine the relationship between antipsychotic polytherapy and rates of metabolic syndrome as well as of a proxy measure for insulin resistance that is easy to obtain in clinical practice. The results confirm previous reports that patients with schizophrenia are most likely to receive antipsychotic polytherapy (6,12,14). However, in light of findings that patients with schizophrenia are also at particular risk for cardiovascular morbidity and mortality (55,56), the combined impact of more than one antipsychotic creates concern in clinical practice. Our data suggest that patients receiving antipsychotic polytherapy have poorer metabolic health, but that treatment with more than one antipsychotic is not a primary factor for this finding. Further research is needed to determine the metabolic effects of specific antipsychotic combinations, duration of treatment and individual dosages used in polytherapy. Moreover, the additive effect of antipsychotic coprescribing on other areas of health, such as cardiac conduction, neuromotor and reproductive system functioning, need to be evaluated. Until such data are available, antipsychotic polytherapy should most likely be reserved for the treatment of patients who cannot be treated with clozapine or have a clozapine-refractory psychotic or mood disorder.
Acknowledgments
The authors thank Bernadette M. Riordan, Zaimoon N. Hack and Leslie Randolph of the Zucker Hillside Hospital for assistance with primary data collection.
Grant Support: The Zucker Hillside Hospital Mental Advanced Center for Intervention and Services Research for the Study of Schizophrenia (MH074543-01) from the National Institute of Mental Health, Bethesda, Md.
Footnotes
Financial Disclosure: Dr. Correll has been a consultant to AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Intra-Cellular Therapeutics and has served on the speakers/advisory boards of AstraZeneca, Bristol-Myers Squibb, and Janssen. Dr. Kane has been a consultant to Janssen, Pfizer, Eli Lilly, and Bristol-Myers Squibb and has received honoraria from Abbott, Bristol-Myers Squibb, and Janssen.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 Suppl):1–56. [PubMed] [Google Scholar]
- 2.Miller AL, Hall CS, Buchanan RW, Buckley PF, Chiles JA, Conley RR, Crismon ML, Ereshefsky L, Essock SM, Finnerty M, Marder SR, Miller del D, McEvoy JP, Rush AJ, Saeed SA, Schooler NR, Shon SP, Stroup S, Tarin-Godoy B. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2003 update. J Clin Psychiatry. 2004 Apr;65(4):500–8. doi: 10.4088/jcp.v65n0408. [DOI] [PubMed] [Google Scholar]
- 3.Suppes T, Dennehy EB, Hirschfeld RM, Altshuler LL, Bowden CL, Calabrese JR, Crismon ML, Ketter TA, Sachs GS, Swann AC Texas Consensus Conference Panel on Medication Treatment of Bipolar Disorder. The Texas implementation of medication algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry. 2005 Jul;66(7):870–86. doi: 10.4088/jcp.v66n0710. [DOI] [PubMed] [Google Scholar]
- 4.Tapp A, Wood AE, Secrest L, Erdmann J, Cubberley L, Kilzieh N. Combination Antipsychotic Therapy in Clinical Practice. Psychiatric Services. 2003;54(1):55–59. doi: 10.1176/appi.ps.54.1.55. [DOI] [PubMed] [Google Scholar]
- 5.Keks NA, Altson K, Hope J, Krapivensky N, Culhane C, Tanaghow A, et al. Use of antipsychosis and adjunctive medications by an inner urban community psychiatric service. Aust N Z J Psychiatry. 1999;33(6):896–901. doi: 10.1046/j.1440-1614.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 6.Fourrier A, Gasquet I, Allicar MP, Bouhassira M, Lepine JP, Begaud B. Patterns of neuroleptic drug prescription: a national cross-sectional survey of a random sample of French psychiatrists. Br J Clin Pharmacol. 2000;49(1):80–6. doi: 10.1046/j.1365-2125.2000.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaffe AB, Levine J. Antipsychotic medication coprescribing in a large state hospital system. Pharmacoepidemiol Drug Saf. 2003 Jan-Feb;12(1):41–8. doi: 10.1002/pds.783. [DOI] [PubMed] [Google Scholar]
- 8.Ereshefsky L. Pharmacologic and pharmacokinetic considerations in choosing an antipsychotic. J Clin Psychiatry. 1999;60(Suppl 10):20–30.9. [PubMed] [Google Scholar]
- 9.Chee YK, Ungvari GS, Kin CH, et al. A survey of antipsychotic treatment for schizophrenia in Hong Kong. Chinese Medical Journal. 1997;110(792–796) [PubMed] [Google Scholar]
- 10.Brunot A, Lachaux B, Sontag H, Casadebaig F, Philippe A, Rouillon F, et al. [Pharmaco-epidemiological study on antipsychotic drug prescription in French Psychiatry: Patient characteristics, antipsychotic treatment, and care management for schizophrenia] Encephale. 2002;28(2):129–38. [PubMed] [Google Scholar]
- 11.Frangou S, Lewis M. Atypical antipsychotics in ordinary clinical practice: a pharmaco-epidemiologic survey in a south London service. Eur Psychiatry. 2000;15(3):220–6. doi: 10.1016/s0924-9338(00)00222-4. [DOI] [PubMed] [Google Scholar]
- 12.Procyshyn RM, Kennedy NB, Tse G, Thompson B. Antipsychotic polypharmacy: a survey of discharge prescriptions from a tertiary care psychiatric institution. Can J Psychiatry. 2001;46(4):334–9. doi: 10.1177/070674370104600404. [DOI] [PubMed] [Google Scholar]
- 13.Taylor D, Holmes R, Hilton T, Paton C. Evaluating and improving the quality of risperidone prescribing. Psych Bull. 1997;22:680–683. [Google Scholar]
- 14.Weissman EM. Antipsychotic prescribing practices in the Veterans Healthcare Administration--New York metropolitan region. Schizophr Bull. 2002;28(1):31–42. doi: 10.1093/oxfordjournals.schbul.a006924. [DOI] [PubMed] [Google Scholar]
- 15.Clark RE, Bartels SJ, Mellman TA, Peacock WJ. Recent trends in antipsychotic combination therapy of schizophrenia and schizoaffective disorder: implications for state mental health policy. Schizophr Bull. 2002;28(1):75–84. doi: 10.1093/oxfordjournals.schbul.a006928. [DOI] [PubMed] [Google Scholar]
- 16.Ganguly R, Kotzan JA, Miller LS, Kennedy K, Martin BC. Prevalence, trends, and factors associated with antipsychotic polypharmacy among Medicaid-eligible schizophrenia patients, 1998–2000. J Clin Psychiatry. 2004 Oct;65(10):1377–88. doi: 10.4088/jcp.v65n1013. [DOI] [PubMed] [Google Scholar]
- 17.Sim K, Su A, Fujii S, Yang SY, Chong MY, Ungvari GS, Si T, Chung EK, Tsang HY, Chan YH, Heckers S, Shinfuku N, Tan CH. Antipsychotic polypharmacy in patients with schizophrenia: a multicentre comparative study in East Asia. Br J Clin Pharmacol. 2004 Aug;58(2):178–83. doi: 10.1111/j.1365-2125.2004.02102.x. Erratum in: Br J Clin Pharmacol 2004 Nov;58(5):564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faries D, Ascher-Svanum H, Zhu B, Correll C, Kane J. Antipsychotic monotherapy and polypharmacy in the naturalistic treatment of schizophrenia with atypical antipsychotics. BMC Psychiatry. 2005 May 27;5(1):26. doi: 10.1186/1471-244X-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreyenbuhl J, Valenstein M, McCarthy JF, Ganoczy D, Blow FC. Long-term combination antipsychotic treatment in VA patients with schizophrenia. Schizophr Res. 2006 May;84(1):90–9. doi: 10.1016/j.schres.2006.02.023. Epub 2006 May 2. [DOI] [PubMed] [Google Scholar]
- 20.Weiden PJ, Casey DE. “Polypharmacy”: Combining antipsychotic medications in the treatment of schizophrenia. Jrnl Prac Psych and Behav Hlth. 1999;5:229–233. [Google Scholar]
- 21.Correll CU, Kane JM. Is there a rationale for antipsychotic polypharmacy in schizophrenia? In: Fleischhacker WW, Hummer M, editors. Schizophrene Stoerungen - State of the Art III. Innsbruck: Verlag Integrative Psychiatrie; 2004. pp. 95–112. [Google Scholar]
- 22.Stahl SM. Focus on antipsychotic polypharmacy: evidence-based prescribing or prescribing-based evidence? Int J Neuropsychopharmacol. 2004 Jun;7(2):113–6. doi: 10.1017/S1461145704004146. [DOI] [PubMed] [Google Scholar]
- 23.Shiloh R, Zemishlany Z, Aizenberg D, Radwan M, Schwartz B, Dorfman-Etrog P, et al. Sulpiride augmentation in people with schizophrenia partially responsive to clozapine. A double-blind, placebo-controlled study. Br J Psychiatry. 1997;171:569–73. doi: 10.1192/bjp.171.6.569. [DOI] [PubMed] [Google Scholar]
- 24.Josiassen RC, Joseph A, Kohegyi E, Stokes S, Dadvand M, Paing WW, Shaughnessy RA. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2005 Jan;162(1):130–6. doi: 10.1176/appi.ajp.162.1.130. [DOI] [PubMed] [Google Scholar]
- 25.Anil Yagcioglu AE, Kivircik Akdede BB, Turgut TI, Tumuklu M, Yazici MK, Alptekin K, et al. A double-blind controlled study of adjunctive treatment with risperidone in schizophrenic patients partially responsive to clozapine: efficacy and safety. J Clin Psychiatry. 2005;66(1):63–72. doi: 10.4088/jcp.v66n0109. [DOI] [PubMed] [Google Scholar]
- 26.Honer WG, Thornton AE, Chen EY, Chan RC, Wong JO, Bergmann A, et al. Clozapine alone versus clozapine and risperidone with refractory schizophrenia. N Engl J Med. 2006;354(5):472–82. doi: 10.1056/NEJMoa053222. [DOI] [PubMed] [Google Scholar]
- 27.Hida H, Faber M, Alberto-Gondouin MC, Jalaguier E. Analysis of prescriptions for psychotropic drugs in a psychiatric hospital. Therapie. 1997;52(6):573–8. [PubMed] [Google Scholar]
- 28.Montgomery J, Winterbottom E, Jessani M, Kohegyi E, Fulmer J, Seamonds B, Josiassen RC. Prevalence of hyperprolactinemia in schizophrenia: association with typical and atypical antipsychotic treatment. J Clin Psychiatry. 2004 Nov;65(11):1491–8. doi: 10.4088/jcp.v65n1108. [DOI] [PubMed] [Google Scholar]
- 29.Citrome L, Jaffe A, Levine J, Allingham B, Robinson J. Relationship between antipsychotic medication treatment and new cases of diabetes among psychiatric inpatients. Psychiatr Serv. 2004;55(9):1006–13. doi: 10.1176/appi.ps.55.9.1006. [DOI] [PubMed] [Google Scholar]
- 30.Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999 Nov;156(11):1686–96. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- 31.Wirshing DA, Boyd JA, Meng LR, Ballon JS, Marder SR, Wirshing WC. The effects of novel antipsychotics on glucose and lipid levels. J Clin Psychiatry. 2002 Oct;63(10):856–65. doi: 10.4088/jcp.v63n1002. [DOI] [PubMed] [Google Scholar]; 28 Cohn T, Prud’homme D, Streiner D, Kameh H, Remington G. Characterizing coronary heart disease risk in chronic schizophrenia: high prevalence of the metabolic syndrome. Can J Psychiatry. 2004 Nov;49(11):753–60. doi: 10.1177/070674370404901106. [DOI] [PubMed] [Google Scholar]
- 32.Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, Lamberti S, D’Agostino RB, Stroup TS, Davis S, Lieberman JA. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005 Dec 1;80(1):45–53. doi: 10.1016/j.schres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 33.McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, Meltzer HY, Hiao J, Scott Stroup T, Lieberman JA. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005 Dec 1;80(1):19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Correll CU, Frederickson AM, Kane JM, Manu P. Metabolic syndrome and the risk of coronary heart disease in 367 patients treated with second-generation antipsychotic drugs. J Clin Psychiatry. 2006 Apr;67(4):575–83. doi: 10.4088/jcp.v67n0408. [DOI] [PubMed] [Google Scholar]
- 35.Waddington JL, Youssef HA, Kinsella A. Mortality in schizophrenia. Antipsychotic polypharmacy and absence of adjunctive anticholinergics over the course of a 10-year prospective study. Br J Psychiatry. 1998;173:325–9. doi: 10.1192/bjp.173.4.325. [DOI] [PubMed] [Google Scholar]
- 36.Joukamaa M, Heliovaara M, Knekt P, Aromaa A, Raitasalo R, Lehtinen V. Schizophrenia, neuroleptic medication and mortality. Br J Psychiatry. 2006 Feb;188:122–7. doi: 10.1192/bjp.188.2.122. [DOI] [PubMed] [Google Scholar]
- 37.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 38.Grundy SM. Obesity, metabolic syndrome and cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2595–2600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- 39.Reinstein AU, Sirotovskara LA, Jones LE, Mohan S, Chasanov MA. Effects of clozapine-quetiapine combination therapy on weight and glycaemic control. Clinical Drug Investigation. 1999;19:99–104. [Google Scholar]
- 40.Henderson DC, Kunkel L, Nguyen DD, Borba CP, Daley TB, Louie PM, Freudenreich O, Cather C, Evins AE, Goff DC. An exploratory open-label trial of aripiprazole as an adjuvant to clozapine therapy in chronic schizophrenia. Acta Psychiatr Scand. 2006 Feb;113(2):142–7. doi: 10.1111/j.1600-0447.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 41.American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, et al. Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. J Clin Psychiatry. 2004;65:267–272. doi: 10.4088/jcp.v65n0219. [DOI] [PubMed] [Google Scholar]
- 42.Aziz S, Hsiang YH. Comparative study of home blood glucose monitoring devices: Visidex, Chemstrip bG, Glucometer, and Accu-Chek bG. Diabetes Care. 1983;6:529–532. doi: 10.2337/diacare.6.6.529. [DOI] [PubMed] [Google Scholar]
- 43.Expert Panel on Detection and Treatment of High Blood Cholesterol in Adults. Executive summary of The Third Report of the National Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol (Adult Treatment Part III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 44.McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, Simon J, Krauss RM. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005 Aug 1;96(3):399–404. doi: 10.1016/j.amjcard.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 45.Sumner AE, Finley KB, Genovese DJ, Criqui MH, Boston RC. Fasting triglyceride and the triglyceride-HDL cholesterol ratio are not markers of insulin resistance in African Americans. Arch Intern Med. 2005 Jun 27;165(12):1395–400. doi: 10.1001/archinte.165.12.1395. [DOI] [PubMed] [Google Scholar]
- 46.Bovet P, Faeh D, Gabriel A, Tappy L. The prediction of insulin resistance with serum triglyceride and high-density lipoprotein cholesterol levels in an East African population. Arch Intern Med. 2006 Jun 12;166(11):1236–7. doi: 10.1001/archinte.166.11.1236-b. [DOI] [PubMed] [Google Scholar]
- 47.Weiden PJ, Daniel DG, Simpson G, Romano SJ. Improvement in indices of health status in outpatients with schizophrenia switched to ziprasidone. J Clin Psychopharmacol. 2003 Dec;23(6):595–600. doi: 10.1097/01.jcp.0000095347.32154.08. [DOI] [PubMed] [Google Scholar]
- 48.Simpson GM, Glick ID, Weiden PJ, Romano SJ, Siu CO. Randomized, controlled, double-blind multicenter comparison of the efficacy and tolerability of ziprasidone and olanzapine in acutely ill inpatients with schizophrenia or schizoaffective disorder. Am J Psychiatry. 2004 Oct;161(10):1837–47. doi: 10.1176/ajp.161.10.1837. [DOI] [PubMed] [Google Scholar]
- 49.Faries D, Ascher-Svanum H, Zhu B, Correll C, Kane J. Antipsychotic monotherapy and polypharmacy in the naturalistic treatment of schizophrenia with atypical antipsychotics. BMC Psychiatry. 2005 May 27;5(1):26. doi: 10.1186/1471-244X-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Straker D, Correll CU, Kramer-Ginsberg E, Abdulhamid N, Koshy F, Rubens E, Saint-Vil R, Kane JM, Manu P. Cost-effective screening for the metabolic syndrome in patients treated with second-generation antipsychotic medications. Am J Psychiatry. 2005 Jun;162(6):1217–21. doi: 10.1176/appi.ajp.162.6.1217. [DOI] [PubMed] [Google Scholar]
- 51.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19( Suppl 1):1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- 52.Taylor D, Young C, Esop R, Paton C, Walwyn R. Testing for diabetes in hospitalised patients prescribed antipsychotic drugs. Br J Psychiatry. 2004 Aug;185:152–6. doi: 10.1192/bjp.185.2.152. [DOI] [PubMed] [Google Scholar]
- 53.Marder SR, McQuade RD, Stock E, Kaplita S, Marcus R, Safferman AZ, Saha A, Ali M, Iwamoto T. Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophr Res. 2003 Jun 1;61(2–3):123–36. doi: 10.1016/s0920-9964(03)00050-1. [DOI] [PubMed] [Google Scholar]
- 54.McQuade RD, Stock E, Marcus R, Jody D, Gharbia NA, Vanveggel S, Archibald D, Carson WH. A comparison of weight change during treatment with olanzapine or aripiprazole: results from a randomized, double-blind study. J Clin Psychiatry. 2004;65( Suppl 18):47–56. [PubMed] [Google Scholar]
- 55.Osby U, Correia N, Brandt L, Ekbom A, Sparen P. Mortality and causes of death in schizophrenia in Stockholm county, Sweden. Schizophr Res. 2000 Sep 29;45(1–2):21–8. doi: 10.1016/s0920-9964(99)00191-7. [DOI] [PubMed] [Google Scholar]
- 56.Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000 Sep;177:212–7. doi: 10.1192/bjp.177.3.212. [DOI] [PubMed] [Google Scholar]