Abstract
We previously demonstrated that the α2B-adrenergic receptor mutant, in which the F(x)6IL motif in the membrane-proximal carboxyl terminus were mutated to alanines (α2B-ARm), is deficient in export from the endoplasmic reticulum (ER). In this report, we determined if α2B-ARm could modulate transport from the ER to the cell surface and signaling of its wild-type counterpart. Transient expression of α2B-ARm in HEK293T cells markedly inhibited cell-surface expression of wild-type α2B-AR, as measured by radioligand binding. Subcellular localization demonstrated that α2B-ARm trapped α2B-AR in the ER. The α2B-AR was shown to form homodimers and heterodimers with α2B-ARm as measured by co-immunoprecipitation of the receptors tagged with green fluorescent protein and hemagglutinin epitopes. In addition to α2B-AR, the transport of α2A-AR and α2C-AR to the cell surface was also inhibited by α2B-ARm. Furthermore, transient expression of α2B-ARm significantly reduced cell-surface expression of endogenous α2-AR in NG108-15 and HT29 cells. Consistent with its effect on α2-AR cell-surface expression, α2B-ARm attenuated α2A-AR- and α2B-AR-mediated ERK1/2 activation. These data demonstrated that the ER-retained mutant α2B-ARm conferred a dominant negative effect on the cell-surface expression of wild-type α2-AR, which is likely mediated through heterodimerization. These data indicate a crucial role of ER export in the regulation of cell-surface targeting and signaling of G protein-coupled receptors.
Keywords: G protein-coupled receptor, α2-adrenergic receptor, ER export-motif, Export trafficking, Dimerization, Signal transduction
1. Introduction
Cell-surface receptors coupled to heterotrimeric G proteins represent a superfamily of membrane proteins that respond to a vast array of sensory and chemical stimuli [1,2]. G protein-coupled receptors are synthesized in the endoplasmic reticulum (ER)2, transported to the Golgi apparatus for posttranslational modification (e.g. glycosylation), and then moved on to their functional destinations at the plasma membrane. Therefore, ER export represents the first step in intracellular trafficking of G protein-coupled receptors. There are several events associated with the ER that critically regulate G protein-coupled receptor export. First, similar to many other membrane proteins, receptors must be correctly folded in order to pass the ER quality control mechanism [3,4]. Second, export from the ER and further transport to the cell surface of some G protein-coupled receptors requires specific accessory proteins or chaperones [5–8]. Interaction of the receptors with chaperones may regulate correct folding/assembly of the receptors within the ER and facilitates receptor transport from the ER and on to the cell surface. Third, dimerization of the receptors (homo-and/or hetero-dimerization) in the ER may be required for ER export and further cell-surface targeting. Recently, Bouvier’s group has demonstrated that mutation of the putative dimerization motif GxxxGxxxL in the β2-adrenergic receptor (AR) prevents normal trafficking of the receptor to the plasma membrane [9]. Although dimerization has been demonstrated for a variety of G protein-coupled receptors [10–15], whether α2-AR is able to homodimerize and the role of dimerization in the trafficking of α2-AR remain unknown. Fourth, receptor exit from the ER may be directed by specific signals or motifs embedded in the receptors. Recent studies have identified two classes of ER-export signals in the cytoplasmic carboxyl termini of a variety of membrane proteins. The diacidic motif (DxE) was found in the cytoplasmic carboxyl termini of vesicular stomatitis viral glycoprotein, cystic fibrosis transmembrane conductance regulator and potassium channels [16–19]. The double phenylalanine motif (FF) is required for efficient ER-to-Golgi transport of the p24 family proteins and ER-Golgi intermediate compartment-53 [20]. These ER-export motifs mediate the interaction of transported proteins with the COP II vesicles, directing the export of proteins from the ER. In regards to G protein-coupled receptors, a triple phenylalanine sequence (FxxxFxxxF) seems to act as an ER-exit motif for the dopamine D1 receptor [21].
We demonstrated that a phenylalanine and double leucine spaced by 6 residues [F(x)6LL] in the membrane-proximal carboxyl termini of α2B-AR and angiotensin II type 1 receptor (AT1R) are required for exit from the ER [22]. Mutation of the F(x)6LL motif to alanines abolishes export of the receptors out of the ER and further transport to the cell surface. We report here that, similar to many other G protein-coupled receptors, α2B-AR apparently homodimerizes in the ER, and that the α2B-AR mutant deficient in ER export confers an inhibitory effect on the cell-surface targeting and function of its wild-type (WT) counterpart through heterodimerization in the ER. These data indicate a crucial role of ER export in the regulation of cell-surface targeting and signaling of G protein-coupled receptors.
2. Experimental procedures
2.1. Materials
Rat α2A-, α2B- and α2C-AR in vector pcDNA3 were kindly provided by Dr. Stephen M. Lanier (Department of Pharmacology and Experimental Therapeutics, Louisiana State University Health Sciences Center, New Orleans, LA) and rat AT1R in vector pCDM8 by Dr. Kenneth E. Bernstein (Department of Pathology, Emory University, Atlanta, GA). Antibodies against phospho-ERK1/2, green fluorescent protein (GFP), HA (conjugated with rhodamine) and calregulin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-ERK1/2 antibodies were from Cell Signaling Technology (Beverly, MA). Anti-HA mouse monoclonal antibody 12CA5 was from Roche Applied Science (Mannheim, Germany). Fluorescently labeled secondary antibodies (Alexa Fluor 488-labeled anti-rabbit)and 4,6-diamidino-2-phenylindole were obtained from Molecular Probes, Inc. (Eugene, OR). pDsRed2-ER, an ER marker, was from BD Biosciences (Palo Alto, CA). UK14304, rauwolscine, phentolamine and protein G immobilized on Sepharose 4B were obtained from Sigma (St. Louis, MO). [3H]-RX-821002 (specific activity = 41 Ci/mmol) was purchased from PerkinElmer Life Sciences (Boston, MA). Penicillin–streptomycin, L-glutamine and trypsin-EDTA were from Invitrogen. All other materials were obtained as described elsewhere [23–26].
2.2. Plasmid constructions
α2B-AR and AT1R tagged with green fluorescent protein (GFP) at their carboxyl termini (α2B-AR-GFP and AT1R-GFP) and α2B-AR tagged with HA epitope at its amino terminus (HA-α2B-AR) were generated as previously described [22,23]. The GFP and HA epitopes have been used to label G protein-coupled receptors, including α2B-AR and AT1R, resulting in receptors with similar characteristics to the WT receptors [22–24,27]. Receptor mutants, in which Phe436Ile443Leu444 in α2B-AR (α2B-ARm) and Phe309Ile315Leu316 in AT1R (AT1Rm) were mutated to alanines, were generated using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The sequence of each construct used in this study was verified by restriction mapping and nucleotide sequence analysis (Louisiana State University Health Sciences Center DNA Sequence Core).
2.3. Cell culture and transient transfection
HT-29 human colon cancer cells expressing endogenously α2A-AR and HEK293T human embryonic kidney cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum, 100 units/ml penicillin and 100 µg/ml streptomycin. DDT-MF2 hamster smooth muscle cells stably expressing α2A-AR [28] were cultured DMEM containing 2.5% bovine calf serum, 2.5% horse serum, 100 units/ml penicillin and 100 µg/ml streptomycin. NG108-15 neuroblastoma-glioma cells expressing endogenous α2B-AR subtype were cultured in DMEM containing 10% fetal bovine serum, 100 units/ml penicillin, 100 µg/ml streptomycin, 100 µM hypoxanthine, 0.4 µM aminopterin, and 16 µM thymidine. Transient transfection of the cells was carried out using Lipofect-AMINE 2000 reagent (Invitrogen) as described previously [22,23]. Based on the GFP fluorescence, 70–85% of the cells were transfected.
2.4. Radioligand binding
Radioligand binding of membrane preparation were carried out as described [29] with modifications [22]. HEK293 cells were cultured on 100-mm dishes and transiently transfected with 4 µg of α2-AR (α2A-, α2B- or α2C-AR) in pcDNA3 vector together with 6 µg of pEGFP-N1 vector or α2B-ARm-GFP. NG108-15, HT29 and DDT-MF2 cells were transfected with 10 µg of pEGFP-N1 vector or α2B-ARm-GFP. At 48 h after transfection, the cells were homogenized in 3 ml per plate of buffer containing 5 mM Tris–HCl, pH 7.4, 5 mM EGTA and 5 mM EDTA supplemented with Complete Mini protease inhibitor cocktail (Roche Applied Science, Mannheim, Germany). After centrifugation at 100,000 ×g for 30 min, the pellet was resuspended in 300 µl per plate of membrane buffer containing 50 mM Tris–HCl, pH 7.4, 0.6 mM EDTA and 5 mM MgCl2. The membrane suspension (25 µg from HEK293T and DDT-MF2 cells) was incubated with increasing concentrations of [3H]-RX-821002 (1.25–160 nM) in a total volume of 100 µl. The membrane fraction prepared from NG108-15 and HT29 cells (200 µg) was incubated with [3H]-RX-821002 at a concentration of 40 nM. Nonspecific binding was determined in the presence of the selective α2-AR antagonist rauwolscine or phentolamine (10 µM). Duplicate samples were incubated for 30 min at room temperature with constant shaking, and the reaction was terminated by vacuum filtration. After washing with 100 mM Tris–HCl, pH 7.4 (4 × 4 ml), the retained radioactivity was measured by liquid scintillation spectrometry in 8 ml of Ecoscint A scintillation solution (National Diagnostics, Inc., Atlanta, GA).
2.5. Fluorescence microscopy
HEK293T cells were grown on poly-L-lysine coated coverslips in 6-well dishes and transfected with 30 ng of pDsRed2-ER together with 30 ng of α2B-AR-GFP, 30 ng of α2B-ARm-GFP or 30 ng of α2B-AR-GFP plus 300 ng of HA-α2B-AR. At 36–48 h after transfection, the cells were fixed with 4% paraformaldehyde–4% sucrose mixture in PBS for 15 min. For direct fluorescence microscopy, the cells were stained with 4,6-diamidino-2-phenylindole for 5 min. For immunofluorescence microscopy, the fixed cells were permeabilized with PBS containing 0.2% Triton X-100 for 5 min, and blocked with 5% normal donkey serum for 1 h. The cells were then incubated with primary antibody for 1 h. After washing with PBS (3 × 5 min), the cells were incubated with Alexa Fluor 488-labeled secondary antibody (1 :2000 dilution) for 1 h at room temperature and stained with 4,6-diamidino-2-phenylindole for 5 min. The cover-slips were mounted, and fluorescence was detected with a Leica DMRA2 epifluorescent microscope. Images were deconvolved using SlideBook software and the nearest neighbors deconvolution algorithm (Intelligent Imaging Innovations, Denver, CO) as previously described [22,23].
2.6. Immunoprecipitation of receptors
HEK293T cells were cultured on 100-mm dishes and transfected with 4 µg of HA-or GFP-tagged receptor constructs individually or in combination for 48 h. The cells were washed twice with PBS and harvested. The cells were then lysed with 800 µl of lysis buffer containing 50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS and Complete Mini protease inhibitor cocktail. After gentle rotation for 1 h, samples were centrifuged for 15 min at 14,000 ×g and the supernatant was incubated with 50 µl of protein G Sepharose for 1 h at 4 °C to remove non-specific bound proteins. Samples were then incubated with 5 µg of anti-HA antibodies overnight at 4 °C with gentle rotation followed by an incubation with 50 µl of protein G sepharose beads for 5 h. Resin was collected by centrifugation and washed three times with 500 µl of lysis buffer. Immunoprecipitated receptors were eluted with 100 µl of 1 X SDS-PAGE loading buffer, separated by 8% SDS-PAGE and visualized by immunoblotting using anti-GFP antibodies.
2.7. Measurement of ERK1/2 activation
HEK293T cells were cultured on 100-mm dishes and transfected with 4 µg of α2-AR with or without 8 µg of α2B-ARm. At 12 h after transfection, the transfected cells were split into 6-well dishes. At 36 h after transfection, HEK293T cells were starved for at least 3 h and then stimulated with the α2-AR agonists UK14304 at concentrations from 0.01 to 10 µM for 5 min at 37 °C as described previously [22,23]. Stimulation was terminated by addition of 1 × SDS gel loading buffer. After solubilizing the cells, 20 µl of total cell lysates was separated by 10% SDS-PAGE and ERK1/2 activation was determined by Western blotting by measuring the levels of phosphorylation of ERK1/2 with phosphospecific ERK1/2 antibodies. The membranes were stripped and reprobed with anti-ERK1/2 antibodies to determine the total amount of ERK1/2 and to confirm equal loading of proteins.
2.8. Immunoblotting
Western blotting was carried out as described previously [22,23]. Samples were separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes. The signal was detected using ECL Plus (PerkinElmer Life Sciences) and a Fuji Film luminescent image analyzer (LAS-1000 Plus).
2.9. Statistical analysis
Differences were evaluated using Student’s t test, and P <0.05 was considered as statistically significant. Data are expressed as the mean±SE.
3. Results
3.1. Effect of α2B-ARm on the cell-surface expression of α2B-AR
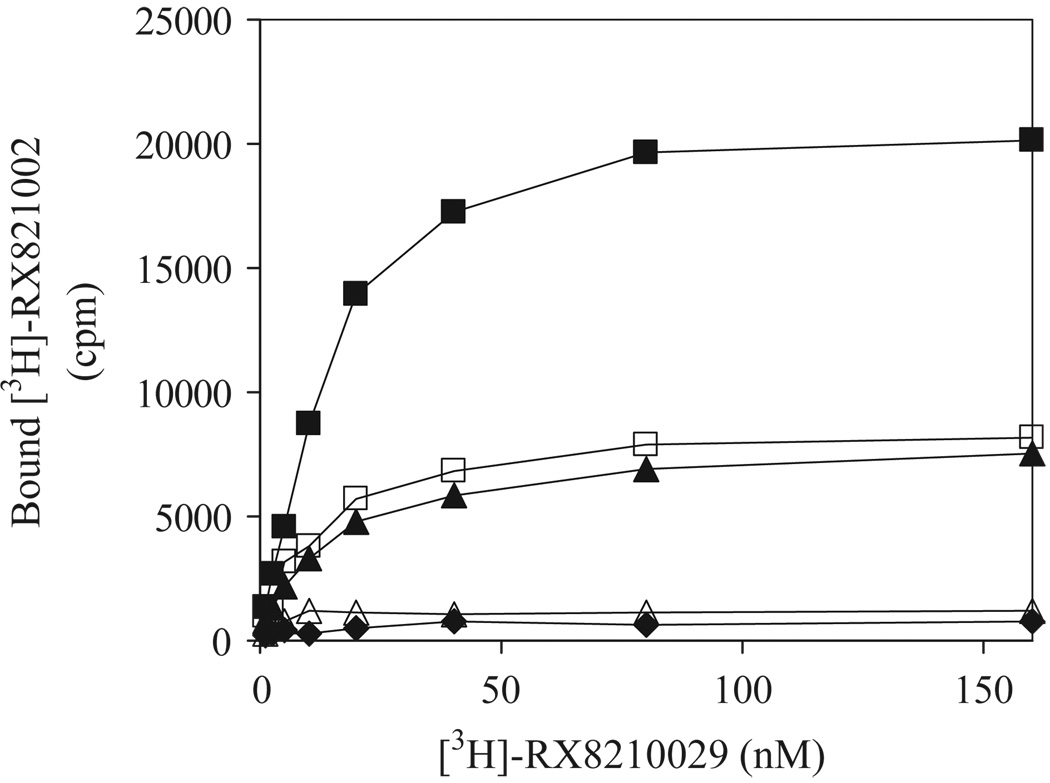
We have recently demonstrated that α2B-ARm, in which Phe436Ile443Leu444 in α2B-AR were mutated alanines is unable to export from the ER [22]. To determine whether α2B-ARm could function as a dominant negative mutant for the transport of its WT counterpart from the ER to the cell surface, we first determined the effect of α2B-ARm on the transport of α2B-AR to the cell surface by radioligand binding of membrane preparations and intact cells. The membrane fractions prepared from cells expressing α2B-AR bound the ligand RX-821002 in a dose-dependent manner (Fig. 1). Consistent with our previous data [22], membrane fractions prepared from cells expressing α2B-ARm-GFP or HA-α2B-ARm were unable to bind the ligand RX-821002. Ligand binding was significantly attenuated in membrane fractions prepared from cells expressing α2B-AR together with α2B-ARm-GFP or HA-α2B-ARm compared with that from cells expressing α2B-AR (Fig. 1). These data indicate that α2B-ARm conferred a dominant negative effect on the cell-surface targeting of its WT receptor.
Fig. 1.
Effect of transient expression of α2B-ARm on the transport of α2B-AR. HEK293T cells were transiently transfected with 4 µg of α2B-AR in pcDNA3 plus 6 µg of pEGFP-N1 vector (closed squares), 10 µg of α2B-ARm-GFP (diamonds), 10 µg of HA-α2B-ARm (open triangles), 4 µg of α2B-AR plus 6 µg of α2B-ARm-GFP (closed triangles) or 4 µg of α2B-AR plus 6 µg of HA-α2B-ARm (open squares). Membrane preparation (25 µg) was incubated with increasing concentrations of [3H]-RX-821002 (1.25–160 nM) for 30 min. Specific binding was determined in duplicate and nonspecific binding determined in the presence of 10 µM rauwolscine as described under “Experimental Procedures”. The data shown are representative of at least three separate experiments, each with similar results.
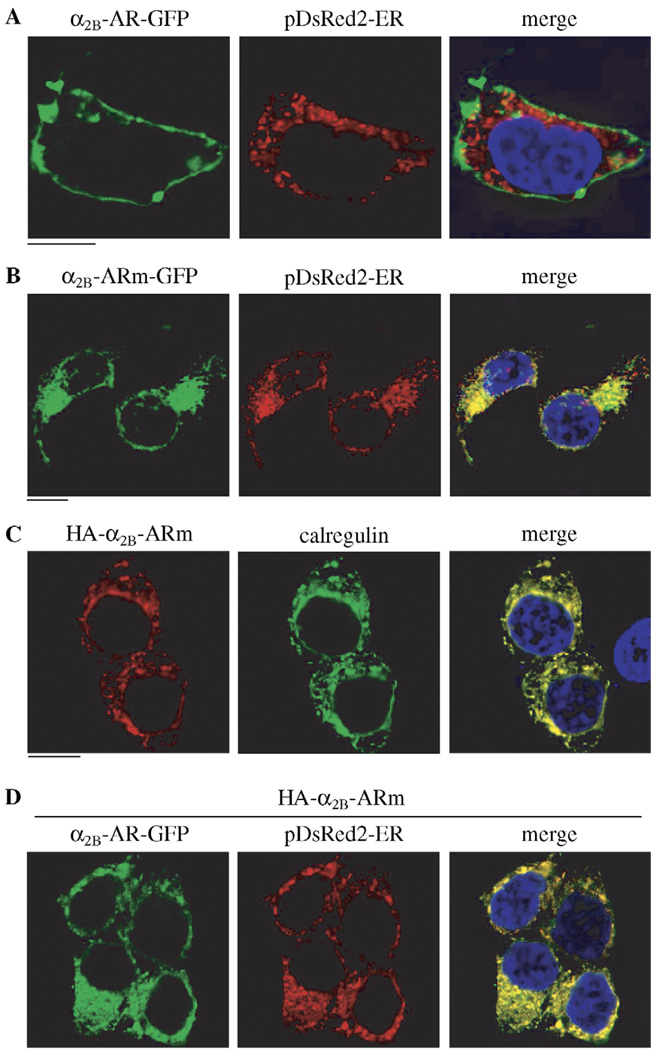
We then sought to determine the effect of α2B-ARm on the subcellular localization of α2B-AR by fluorescent microscopy. HEK293T cells were transiently transfected with GFP-or HA-tagged α2B-AR and/or α2B-ARm. The subcellular distribution of the GFP-tagged receptors at steady state was revealed by fluorescence microscopy detecting GFP signal and HA-tagged receptors were visualized by detecting immunofluorscent signal following staining with anti-HA antibodies. As anticipated, α2B-AR-GFP was mainly localized at the cell surface in the absence of mutant receptor (Fig. 2A). Cell-surface localization of α2B-AR-GFP was confirmed by co-localization with tetra-methylrhodamine-conjugated concanavalin A, a plasma membrane marker (not shown). α2B-ARm tagged with GFP or HA was unable to export from the ER as indicated by co-localization with the ER markers, pDsRed2-ER and calregulin (Fig. 2B and C). Consistent with its inhibitory effect on the cell-surface expression of α2B-AR as measured by radioligand binding, expression of HA-α2B-ARm induced an accumulation of α2B-AR-GFP in the perinuclear regions of the transfected cells. The accumulated receptors were extensively co-localized with the ER marker pDsRed2-ER (Fig. 2D). These data indicate that α2B-ARm is able to trap its WT counterpart in the ER, therefore, inhibiting the transport to the cell surface.
Fig. 2.
Effect of α2B-ARm on the subcellular distribution of α2B-AR. HEK293T cells cultured on coverslips were transiently transfected with α2B-AR-GFP and the ER marker pDsRed2-ER (A), α2B-ARm-GFP and pDsRed2-ER (B), HA-α2B-ARm (C) or α2B-AR-GFP, HA-α2B-ARm and pDsRed2-ER (D). GFP-tagged receptor subcellular distribution and co-localization with the ER marker pDsRed2-ER were revealed by direct fluorescence microscopy. HA-tagged receptor subcellular localization and co-localization with the ER marker calregulin were revealed following staining with anti-calregulin antibodies (1 :50 dilution). The data are representative images of three independent experiments. Green, GFP-tagged receptor (A, B and D) and calregulin (C); Red, the ER marker pDsRed2-ER (A, B and D) and HA-tagged receptor (C); Blue, DNA staining by 4,6-diamidino-2-phenylindole (nuclear); Yellow, co-localization of the receptor and pDsRed2-ER (A, B and D) or calregulin (C); Scale bars = 10 µm.
3.2. Homodimerization and heterodimerization of α2B-AR and α2B-ARm
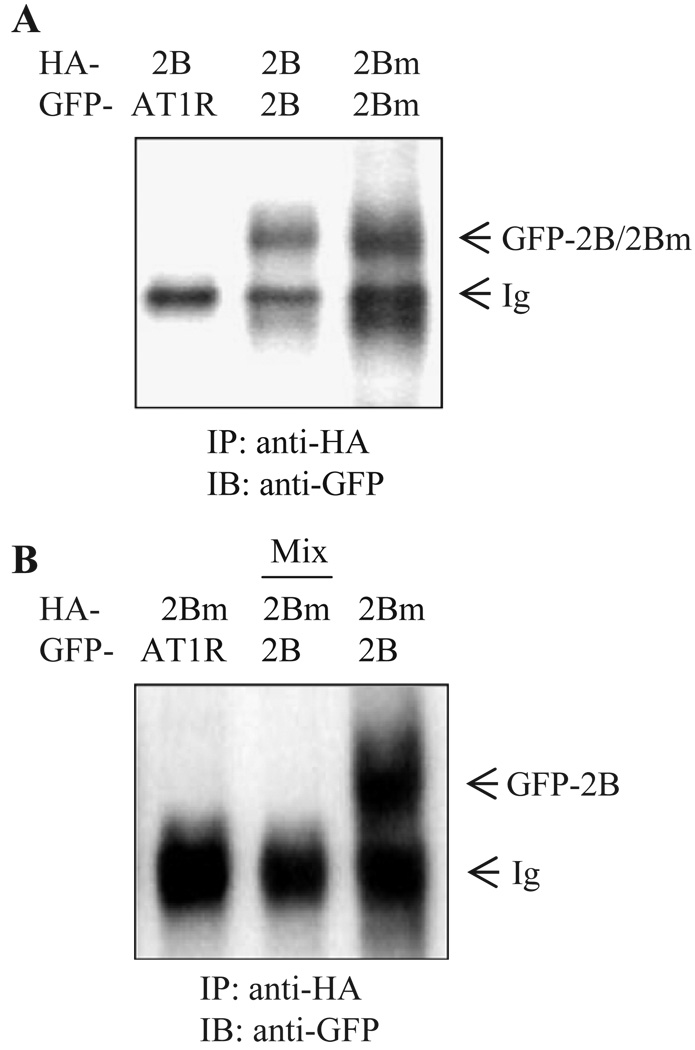
We hypothesized that the inhibition of transport to the cell surface of α2B-AR by its ER-retained mutant was mediated through heterodimerization of mutant and WT receptors, preventing the WT from ER export. To test this hypothesis, we first determined whether α2B-AR is able to form homodimers. α2B-AR-GFP was transiently expressed in HEK293T cells, and its expression was visualized by immunoblotting using anti-GFP antibodies. As anticipated, a α2B-AR-GFP monomer with apparent molecular weight of ~60 kDa was revealed. Another band with apparent molecular weight of ~120 kDa, twice that of the monomer, was also observed (not shown). Consistently, two bands, apparently monomer and dimer were detected in the immunoprecipitate using anti-GFP antibodies from cells expressing α2B-AR-GFP (not shown). To obtain direct evidence for α2B-AR homodimerization, we determined if α2B-AR tagged with two different epitopes could be co-immunoprecipitated. HEK293T cells were co-transfected with HA-α2B-AR and α2B-AR-GFP, and the receptors were then immunoprecipitated with anti-HA antibodies. The presence of α2B-AR-GFP in the anti-HA immunoprecipitate was then revealed by immunoblotting using anti-GFP antibodies. As shown in Fig. 3A, α2B-AR-GFP was detected in the anti-HA immunoprecipitate. Similarly, α2B-ARm-GFP was found in the anti-HA immunoprecipitate from the cells expressing HA-α2B-ARm. In contrast, AT1R-GFP was not found in the anti-HA immunoprecipitate when co-expressed with HA-α2B-AR. These data indicate that HA- and GFP-tagged α2B-AR or α2B-ARm were co-immunoprecipitated as a complex and suggest that α2B-AR as well as α2B-ARm underwent homodimerization, similar to many other G protein-coupled receptors [9–15].
Fig. 3.
Western blot analysis of homodimerization and heterodimerization of α2B-AR and α2B-ARm. A. Homodimerization of α2B-AR and α2B-ARm. HEK293T cells were transfected with HA-α2B-AR plus AT1R-GFP (left lane), HA-α2B-AR plus α2B-AR-GFP (middle lane), or HA-α2B-ARm plus α2B-ARm-GFP (right lane). The cells were solubilized and immunoprecipitated with anti-HA antibodies. The anti-HA immunoprecipitate was separated by SDS-PAGE and immunoprecipitated receptors were revealed by Western blotting with anti-GFP antibodies. B. Heterodimerization of α2B-AR and α2B-ARm. HEK293T cells were transfected with HA-α2B-ARm plus AT1R-GFP (left lane) and HA-α2B-ARm plus α2B-AR-GFP (right lane), solubilized and immunoprecipitated with anti-HA antibodies. HEK293 cells transfected with only HA-α2B-ARm or α2B-AR-GFP were mixed together for immunoprecipitation with anti-HA antibodies (middle lane). The immunoprecipitated receptors were separated by SDS-PAGE and visualized by Western blotting with anti-GFP antibodies. The data shown are representative of at least four independent experiments.
We then determined whether α2B-AR and α2B-ARm could form heterodimers. α2B-AR and α2B-ARm differentially tagged with epitopes HA and GFP were co-expressed in HEK293 cells and immunoprecipitated with anti-HA antibodies. As shown in Fig. 3B, α2B-AR-GFP was found in the anti-HA immunoprecipitate from cells transfected with HA-α2B-ARm and α2B-AR-GFP. These data indicate that α2B-AR and α2B-ARm form heterodimers.
To determine whether heterodimerization of α2B-AR and α2B-ARm was mediated through non-specific interaction (such as hydrophobic) of the receptors caused by solubilization, HEK293T cells were separately transfected with α2B-AR or α2B-ARm, mixed and immunoprecipitated with anti-HA antibodies. Anti-HA antibodies did not immunoprecipitate α2B-AR-GFP from the mixture (Fig. 3B, middle lane). These data indicate that α2B-AR and α2B-ARm did not heterodimerize when they were transfected separately into different cell populations. These data indicate that the interaction observed in cells co-expressing α2B-AR and α2B-ARm was not an artifact and that such an interaction required co-expression of the receptors in the same cells.
3.3. Effect of α2B-ARm on the transport of α2A-AR and α2C-AR
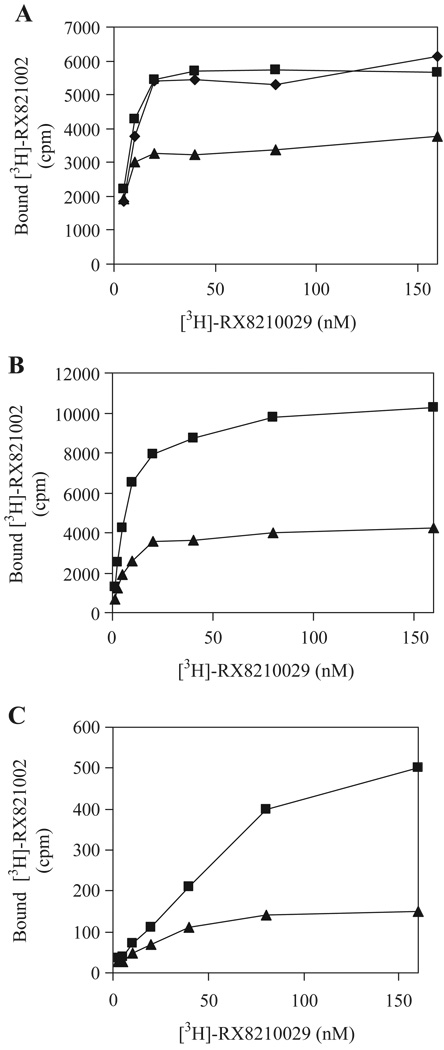
Our preceding data indicate that α2B-ARm functions as a dominant negative mutant for cell-surface targeting of its WT counterpart. We then investigated whether α2B-ARm could also regulate the transport of other α2-AR subtypes, α2A-AR and α2C-AR. We first determined the effect of α2B-ARm on the cell-surface expression of α2A-AR in DDT-MF2 cells stably expressing α2A-AR. Transient expression of α2B-ARm significantly reduced ligand binding of membrane preparations from DDT-MF2 compared with those from cells transfected with pEGFP-N1 vector (Fig. 4A). In contrast, transient expression of AT1Rm had no significant influence on the ligand binding of membrane fractions prepared from DDT-MF2 cells (Fig. 4A).
Fig. 4.
Effect of α2B-ARm on the transport of α2A-AR and α2C-AR. A. DDT-MF2 cells stably expressing α2A-AR were cultured on 100-mm dishes and transfected with 10 µg of pEGFP-N1 vector (squares), α2B-ARm-GFP (triangles) or AT1Rm-GFP (diamonds). B. HEK293T cells were co-transfected with 4 µg of α2A-AR in pcDNA3 and 6 µg of pEGFP-N1 vector (squares) or α2B-ARm-GFP (triangles). C. HEK293T cells were co-transfected with 4 µg of α2C-AR in pcDNA3 and 6 µg of pEGFP-N1 vector (squares) or α2B-ARm-GFP (triangles). Membrane preparation (25 µg) was incubated with increasing concentrations of [3H]-RX-821002 (1.25–160 nM) for 30 min. Specific binding was determined in duplicate and nonspecific binding determined in the presence of 10 µM rauwolscine as described under “Experimental Procedures”. The data shown are representative of three separate experiments.
We then determined the effect of α2B-ARm on the transport of α2A-AR and α2C-AR in HEK293T cells. HEK293T cells were co-transfected with α2A-AR or α2C-AR together with α2B-ARm-GFP and effect of α2B-ARm on the transport of α2A-AR and α2C-AR was evaluated by ligand binding of membrane preparations. The ligand binding was markedly reduced in membrane fractions from HEK293T cells co-transfected with α2B-ARm and α2A-AR or α2C-AR as compared with the cells transfected with α2A-AR (Fig. 4B) or α2C-AR alone (Fig. 4C). These data suggest that α2B-ARm may confer a similar inhibitory effect on the transport of the three α2-AR subtypes, α2A-AR, α2B-AR and α2C-AR.
3.4. Effect of α2B-ARm on the transport of endogenous α2-AR
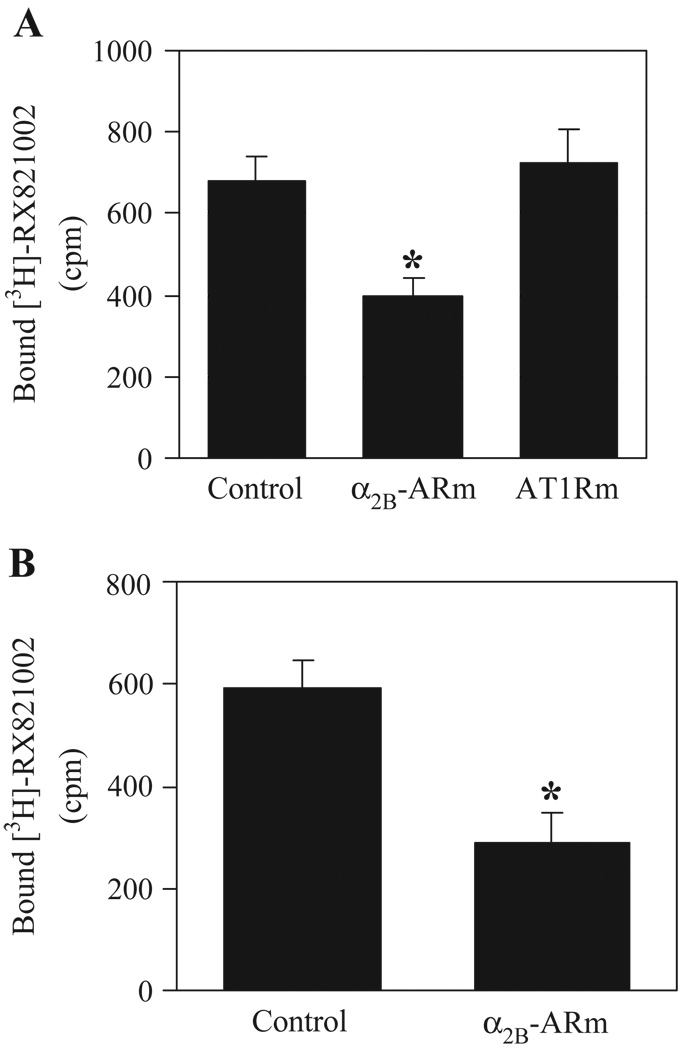
To determine if α2B-ARm could inhibit the transport of endogenous α2-AR, NG108-15 and HT29 cells expressing endogenous α2B-AR and α2A-AR, respectively, were transiently transfected with α2B-ARm-GFP. Ligand binding was significantly inhibited in membrane fractions prepared from NG108-15 (Fig. 5A) and HT29 cells (Fig. 5B) when transfected with α2B-ARm-GFP compared with that from cells transfected with pGEFP-N1 vector. Inhibition of α2B-AR transport by α2B-ARm was specific, as ligand binding was essentially the same in membrane fractions prepared from cells transfected with α2B-AR and pEGFP-N1 or AT1Rm-GFP (Fig. 5A).
Fig. 5.
Effect of α2B-ARm on the transport of endogenous α2-AR. NG108-15 (A) or HT29 cells (B) were transiently transfected with 10 µg of pEGFP-N1 vector (control), AT1Rm-GFP or α2B-ARm-GFP. Membrane preparations (200 µg) were incubated with [3H]-RX-821002 at a concentration of 40 nM for 30 min. Specific binding was determined in triplicate and nonspecific binding determined in the presence of 10 µM phentolamine (A) or rauwolscine (B) as described under “Experimental Procedures”. The data are presented as the mean ± SE of three experiments. *P <0.05 versus control.
3.5. Effect of α2B-ARm on ERK1/2 activation
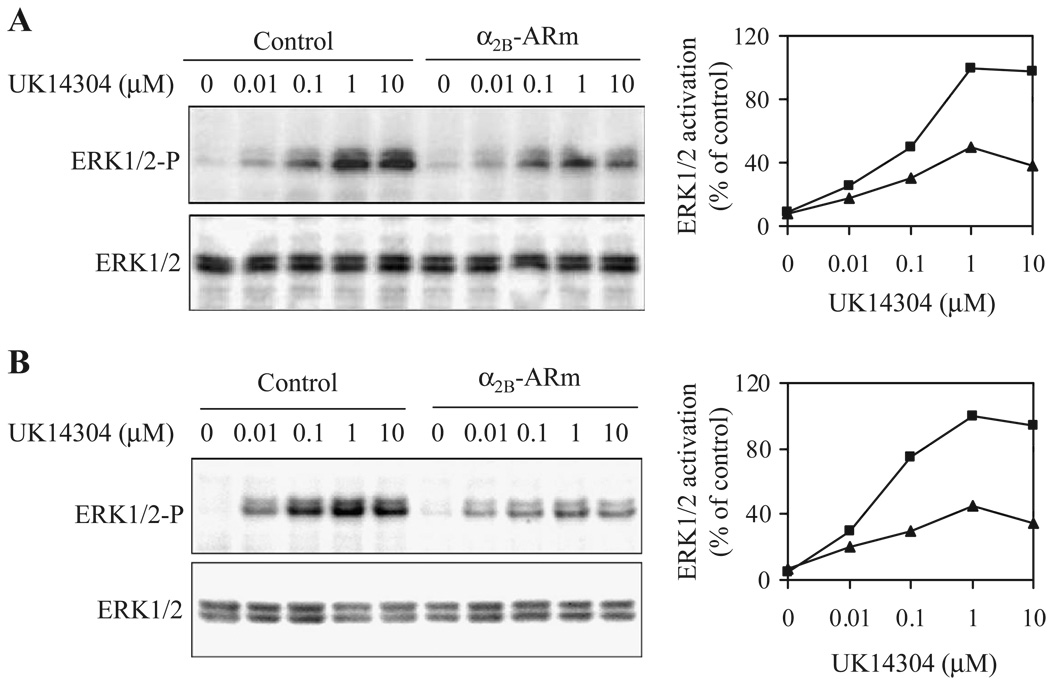
To determine whether α2B-ARm was able to modulate signaling of α2-AR, we determined the effect of α2B-ARm on the activation of ERK1/2. HEK293T cells were transfected with α2A-AR or α2B-AR with or without co-transfection of α2B-ARm and stimulated with the α2-AR agonist UK14304. Activation of ERK1/2 in response to stimulation with UK14304 was significantly attenuated in cells co-transfected with α2B-AR or α2A-AR and α2B-ARm compared with those from cells transfected with α2B-AR (Fig. 6A) or α2A-AR alone (Fig. 6B), consistent with the α2B-ARm- induced reduction in the cell-surface expression of both α2-AR subtypes. These data suggest that α2B-ARm may function as a dominant negative mutant for signal transduction via different α2-AR subtypes.
Fig. 6.
Effect of α2-ARm on ERK1/2 activation by α2A-AR and α2B-AR. HEK293 cells transfected with 4 µg of α2B-AR (A), or α2A-AR (B) together with 8 µg of pEGFP-N1 vector (control, squares) or α2B-ARm-GFP (triangles) were stimulated with increasing concentrations of UK14304 (0.01 to 10 µM) for 5 min. ERK1/2 activation was determined by Western blot analysis using phospho-specific ERK1/2 antibodies. Left panel-representative blots of ERK1/2 activation (upper panel) and total ERK1/2 expression (lower panel); Right panel-quantitative data expressed as percent of the ERK1/2 activation obtained in cells transfected with individual receptors and stimulated with 1 µM UK14304. Similar results were obtained in four separate experiments.
4. Discussion
The molecular mechanisms underlying the transport of G protein-coupled receptors from the ER to the cell surface and its role in regulation of receptor signaling remain poorly understood. To address these issues, we previously determined the role of Rab1, a Ras-like GTPase that regulates vesicle-mediated protein transport in the early secretory pathway, specifically from the ER to the Golgi, in the export trafficking of α2B-AR, β2-AR and AT1R [23,24]. We have demonstrated that, whereas the transport from the ER to the cell surface of AT1R and β2-AR is dependent on Rab1, α2B-AR export is independent of Rab1, indicating that different G protein-coupled receptors may use distinct pathways for their transport from the ER to the cell surface [23]. We then identified a motif consisting of a phenylalanine and double leucine spaced by 6 residues [F(x)6LL] in the membrane-proximal carboxyl termini of α2B-AR and AT1R, which is essential for the exit of the receptors from the ER [22]. The receptor mutants, α2B-ARm and AT1Rm in which the F(x)6LL motif were mutated to alanines, were unable to transport to the cell surface and were retained in the ER. In this report, we sought to determine if α2B-ARm could regulate the cell-surface targeting and signaling of its WT counterpart.
We first determined the effect of α2B-ARm on the transport of its WT receptor to the cell surface. Our data demonstrated that transient expression of α2B-ARm negatively regulated the cell-surface expression of α2B-AR in HEK293T cells as measured by radioligand binding. Consistently, subcellular localization analysis indicated that α2B-AR was trapped in the ER by its mutant. Furthermore, α2B-ARm expression also significantly attenuated the transport of endogenous α2B-AR to the cell surface in NG108-15 cells. These data strongly indicate that ER-retained α2B-ARm conferred a dominant negative effect on the cell-surface targeting of its WT counterpart. In contrast, AT1Rm had no significant effect on the cell-surface expression of α2B-AR, suggesting that inhibition of cell-surface targeting of α2B-AR by its mutant is not caused by an accumulation of the mutant receptors in the ER, which may interfere with WT receptor transport along the secretory pathway. These data are consistent with other reports demonstrating that the intracellularly retained mutants of G protein-coupled receptors negatively regulate the cell-surface expression of their WT counterparts [30–33], therefore functioning as dominant negative mutants.
We hypothesized that inhibition of α2B-AR transport from the ER to the cell surface by its ER-retained mutant was mediated through heterodimerization between WT and mutant receptors in the ER. To test this hypothesis, we determined whether α2B-AR and α2B-ARm could form heterodimers by co-immunoprecipitation of receptors tagged with different epitopes (i.e. HA and GFP). When HA-α2B-ARm and α2B-AR-GFP were coexpressed in HEK293 cells, α2B-AR-GFP was found in the anti-HA immunoprecipitate, indicating that indeed α2B-AR and α2B-ARm form a stable complex. In contrast, two differentially tagged α2B-AR were not co-immunoprecipited when they were expressed in separate cell populations, suggesting that dimerization of α2B-AR and α2B-ARm requires their co-expression. Furthermore, co-expression of AT1R-GFP with HA-α2B-AR or HA-α2B-ARm did not result in co-immunoprecipitation with anti-HA antibodies. These data indicate that dimerization between WT and mutant α2B-AR is specific and suggest that α2B-AR does not heterodimerize with AT1R. Therefore, we conclude that the inhibition of WT α2B-AR transport from the ER to the cell surface by α2B-ARm is mediated through heterodimerization of α2B-ARm and α2B-AR. These data are consistent with inhibition of the transport to the cell surface of G protein-coupled receptors by heterodimerization of WT and intracellularly retained receptor mutants [30–33]. As the motif F(x)6LL is highly conserved among G protein-coupled receptors [22], the motif mutants, defective in ER export, may provide a useful tool for dissecting the role of dimerization in the trafficking of many other G protein-coupled receptors.
Why are the dimers of mutant and WT receptors unable to export from the ER? The F(x)6LL motif may be involved in correct folding and/or export from the ER of the receptors. It is possible that dimerization of α2B-AR may be required to form an ER export competent conformation. Disruption of such a conformation by mutation of the F(x)6LL motif in one of the receptors in the dimers may thus impair ER export. It is also possible that the dimers of mutant and WT receptors may retain proper conformation required for ER export, but lack the ER export signal [i.e. F(x)6LL motif]. Therefore, dimers are unable to export from the ER.
Dimerization has been well described for a variety of G protein-coupled receptors [9–15,34–41]. However, whether α2B-AR is capable of forming homodimers has not been reported. We have demonstrated that, similar to many other G protein-coupled receptors, α2B-AR is able to homodimerize by co-immunoprecipitation from cells expressing receptors tagged with different epitopes (i.e. HA-α2B-AR and α2B-AR-GFP). Whether α2B-AR could form homodimers in living cells is currently under investigation by using fluorescence resonance energy transfer techniques. In addition, we have demonstrated that, similar to WT α2B-AR, the mutant α2B-ARm is also capable of forming homodimers, suggesting that the motif F(x)6IL is not involved in the dimerization of α2B-AR.
Whereas some G protein-coupled receptor dimers are constitutively formed in the ER and then transported to the cell surface, other receptors are assembled as dimers at the cell surface in an agonist-dependent fashion [38,39]. The α2B-ARm, an ER-export deficient mutant, formed homodimers as well as heterodimers with α2B-AR and trapped α2B-AR in the ER. These data suggest that α2B-AR dimerization constitutively occurs as early as in the ER. Whether α2B-AR could dimerize at the plasma membrane and α2B-AR dimerization is regulated by agonist stimulation need further studies.
Expression of α2B-ARm inhibited cell-surface targeting of all three WT α2-AR subtypes, α2A-, α2B- and α2C-AR. Consistent with its effect on the cell surface receptor expression, α2B-ARm inhibited ERK1/2 activation by α2A-AR and α2B-AR, suggesting that α2B-ARm modulates not only α2-AR transport to the cell surface but also their signal transduction. Since we have demonstrated that inhibition of α2B-AR cell-surface expression is mediated through dimerization of mutant and WT receptors, it is possible that the inhibitory effect of α2B-ARm on the transport of α2A-AR and α2C-AR is also due to heterodimerization of α2B-ARm with α2A-AR or α2C-AR. This possibility is supported by a number of observations demonstrating that closely related members of G protein-coupled receptors may heterodimerize [11,14,37–39]. Furthermore, α2A-AR and α2C-AR indeed form heterodimers with β1-AR and M3-muscarinc receptor, respectively [40,41]. As homo-and hetero-dimerization may play a crucial role in the regulation of cell-surface targeting, signaling specificity/efficiency and ligand binding selectivity of individual receptors [34], the finding that α2B-AR exists in dimeric forms and possible heterodimerization between different α2-AR subtypes hints at the complexity of α2-AR and may open additional ways of understanding the function of this subfamily of G protein-coupled receptors.
The efficient trafficking of G protein-coupled receptors and the precise positioning of specific receptors at the cell membrane are critical aspects of integrated responses of the cell to hormones. Defective transport of the receptors from the ER to the cell surface is associated with the pathogenesis of a variety of human diseases [42,43]. The diseases may result from defective transport of the receptors out of the ER (resulting in receptor retention in the ER) and/or a defect in the machinery which transports receptors to their functional destination after ER exit. For example, numerous naturally occurring mutations in G protein-coupled receptors leading to ER retention have been implicated in inherited diseases such as retinitis pigmentosa, nephrogenic diabetes insipidus and male pseudohermaphroditism [42–44]. We have demonstrated that different G protein-coupled receptors may use distinct pathways to move from the ER to the cell surface [23] and that selective G protein-coupled receptors may carry a specific code for exit from the ER [22]. Therefore, selectivity of G protein-coupled receptor transport from the ER to the cell surface could be achieved at different regulatory sites within the transport process. Further elucidation of molecular mechanisms of export traffic of individual G protein-coupled receptors may provide a foundation for development of therapeutic strategies that influence specific components involved in the process of ER export of the receptors.
Acknowledgements
This work was supported in part by the National Institutes of Health Award “Mentoring in Cardiovascular Biology” 1P20RR018766 (Program Director: Stephen M. Lanier, Ph.D.-Louisiana State University Health Sciences Center) and by the Louisiana Board of Regents Grant LEQSF (2002-05)-RD-A-18 (to G. W.). Mathew T. Duvernay is a recipient of Louisiana Board Regents Graduate Fellowship. We acknowledge Stephen M. Lanier (Department of Pharmacology and Experimental Therapeutics) for review of this work and sharing reagents.
Footnotes
The abbreviations used are: ER, endoplasmic reticulum; AR, adrenergic receptor; AT1R, angiotensin II type 1 receptor; GFP, green fluorescent protein; HA, hemagglutinin; WT, wild type; ERK, extracellular signal-regulated kinase; PBS, phosphate-buffered saline.
References
- 1.Wess J. Pharmacol. Ther. 1998;80:231. doi: 10.1016/s0163-7258(98)00030-8. [DOI] [PubMed] [Google Scholar]
- 2.Rockman HA, Koch WJ, Lefkowitz RJ. Nature. 2002;415:206. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 3.Sitia R, Braakman I. Nature. 2003;426:891. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- 4.Petaja-Repo UE, Hogue M, Laperriere A, Bhalla S, Walker P, Bouvier M. J. Biol. Chem. 2001;276:4416. doi: 10.1074/jbc.M007151200. [DOI] [PubMed] [Google Scholar]
- 5.Colley NJ, Baker EK, Stamnes MA, Zuker CS. Cell. 1991;67:255. doi: 10.1016/0092-8674(91)90177-z. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira PA, Nakayama TA, Pak WL, Travis GH. Nature. 1996;383:637. doi: 10.1038/383637a0. [DOI] [PubMed] [Google Scholar]
- 7.Dwyer ND, Troemel ER, Sengupta P, Bargmann CI. Cell. 1998;93:455. doi: 10.1016/s0092-8674(00)81173-3. [DOI] [PubMed] [Google Scholar]
- 8.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. Nature. 1998;393:333. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 9.Salahpour A, Angers S, Mercier JF, Lagace M, Marullo S, Bouvier M. J. Biol. Chem. 2004;279:33390. doi: 10.1074/jbc.M403363200. [DOI] [PubMed] [Google Scholar]
- 10.Bouvier M. Nat. Rev., Neurosci. 2001;2:274. doi: 10.1038/35067575. [DOI] [PubMed] [Google Scholar]
- 11.Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C. Nature. 1998;396:674. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 12.Overton MC, Blumer KJ. Curr. Biol. 2000;10:341. doi: 10.1016/s0960-9822(00)00386-9. [DOI] [PubMed] [Google Scholar]
- 13.Overton MC, Blumer KJ. J. Biol. Chem. 2002;277:41463. doi: 10.1074/jbc.M205368200. [DOI] [PubMed] [Google Scholar]
- 14.Hague C, Uberti MA, Chen Z, Hall RA, Minneman KP. J. Biol. Chem. 2004;279:15541. doi: 10.1074/jbc.M314014200. [DOI] [PubMed] [Google Scholar]
- 15.Milligan G. Mol. Pharmacol. 2004;66:1. doi: 10.1124/mol.104.000497.. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura N, Balch WE. Science. 1997;277:556. doi: 10.1126/science.277.5325.556. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura N, Bannykh S, Slabough S, Matteson J, Altschuler Y, Hahn K, Balch WE. J. Biol. Chem. 1999;274:15937. doi: 10.1074/jbc.274.22.15937. [DOI] [PubMed] [Google Scholar]
- 18.Ma D, Zerangue N, Lin YF, Collins A, Yu M, Jan YN, Jan LY. Science. 2001;291:316. doi: 10.1126/science.291.5502.316. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Matteson J, An Y, Moyer B, Yoo JS, Bannykh S, Wilson IA, Riordan JR, Balch WE. J. Cell Biol. 2004;167:65. doi: 10.1083/jcb.200401035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nufer O, Kappeler F, Guldbrandsen S, Hauri HP. J. Cell Sci. 2003;116:4429. doi: 10.1242/jcs.00759. [DOI] [PubMed] [Google Scholar]
- 21.Bermak JC, Li M, Bullock C, Zhou QY. Nat. Cell Biol. 2001;3:492. doi: 10.1038/35074561. [DOI] [PubMed] [Google Scholar]
- 22.Duvernay MT, Zhou, Wu G. J. Biol. Chem. 2004;279:30741. doi: 10.1074/jbc.M313881200. [DOI] [PubMed] [Google Scholar]
- 23.Wu G, Zhao G, He Y. J. Biol. Chem. 2003;278:47062. doi: 10.1074/jbc.M305707200. [DOI] [PubMed] [Google Scholar]
- 24.Filipeanu CM, Zhou F, Claycomb WC, Wu G. J. Biol. Chem. 2004;279:41077. doi: 10.1074/jbc.M405988200. [DOI] [PubMed] [Google Scholar]
- 25.Wu G, Bogatkevich GS, Mukhin YV, Benovic JL, Hildebrandt JD, Lanier SM. J. Biol. Chem. 2002;275:9026. doi: 10.1074/jbc.275.12.9026. [DOI] [PubMed] [Google Scholar]
- 26.Wu G, Benovic JL, Hildebrandt JD, Lanier SM. J. Biol. Chem. 1998;273:7197. doi: 10.1074/jbc.273.13.7197. [DOI] [PubMed] [Google Scholar]
- 27.Hunyady L, Baukal AJ, Gaborik Z, Olivares-Reyes JA, Bor M, Szaszak M, Lodge R, Catt KJ, Balla T. J. Cell Biol. 2002;24:1211. doi: 10.1083/jcb.200111013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duzic E, Lanier SM. J. Biol. Chem. 1992;267:24045. [PubMed] [Google Scholar]
- 29.Hamamdzic D, Duzic E, Sherlock JD, Lanier SM. Am. J. Physiol. 1995;269:E162. doi: 10.1152/ajpendo.1995.269.1.E162. [DOI] [PubMed] [Google Scholar]
- 30.Kaykas A, Yang-Snyder J, Heroux M, Shah KV, Bouvier M, Moon RT. Nat. Cell Biol. 2004;6:52. doi: 10.1038/ncb1081. [DOI] [PubMed] [Google Scholar]
- 31.Benkirane M, Jin DY, Chun RF, Koup RA, Jeang KT. J. Biol. Chem. 1997;272:30603. doi: 10.1074/jbc.272.49.30603. [DOI] [PubMed] [Google Scholar]
- 32.Shioda T, Nakayama EE, Tanaka Y, Xin X, Liu H, Kawana-Tachikawa A, Kato A, Sakai Y, Nagai Y, Iwamoto A. J. Virol. 2001;75:3462. doi: 10.1128/JVI.75.7.3462-3468.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu X, Wess J. Biochemistry. 1998;37:15773. doi: 10.1021/bi981162z. [DOI] [PubMed] [Google Scholar]
- 34.Angers S, Salahpour A, Bouvier M. Annu. Rev. Pharmacol. Toxicol. 2002;42:409. doi: 10.1146/annurev.pharmtox.42.091701.082314. [DOI] [PubMed] [Google Scholar]
- 35.Angers S, Salahpour A, Joly E, Hilairet S, Chelsky D, Dennis M, Bouvier M. Proc. Natl. Acad. Sci. U. S. A. 2000;97:3684. doi: 10.1073/pnas.060590697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng FY, Wess J. J. Biol. Chem. 1999;274:19487. doi: 10.1074/jbc.274.27.19487. [DOI] [PubMed] [Google Scholar]
- 37.Jordan BA, Devi LA. Nature. 1999;399:697. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rocheville M, Lange DC, Kumar U, Sasi R, Patel RC, Patel YC. J. Biol. Chem. 2000;275:7862. doi: 10.1074/jbc.275.11.7862. [DOI] [PubMed] [Google Scholar]
- 39.Uberti MA, Hall RA, Minneman KP. Mol. Pharmacol. 2003;64:1379. doi: 10.1124/mol.64.6.1379. [DOI] [PubMed] [Google Scholar]
- 40.Maggio R, Vogel Z, Wess J. Proc. Natl. Acad. Sci. U. S. A. 1993;90:3103. doi: 10.1073/pnas.90.7.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J, He J, Castleberry AM, Balasubramanian S, Lau AG, Hall RA. J. Biol. Chem. 2003;278:10770. doi: 10.1074/jbc.M207968200. [DOI] [PubMed] [Google Scholar]
- 42.Olkkonen VM, Ikonen E, Engl N. J. Med. 2000;343:1095. doi: 10.1056/NEJM200010123431507. [DOI] [PubMed] [Google Scholar]
- 43.Aridor M, Hannan LA. Traffic. 2002;3:781. doi: 10.1034/j.1600-0854.2002.31103.x. [DOI] [PubMed] [Google Scholar]
- 44.Stojanovic A, Hwa J. Receptors Channels. 2002;8:33. [PubMed] [Google Scholar]