Abstract
Background
In vitro and animal studies demonstrate that myeloperoxidase catalytically consumes nitric oxide as a substrate, limiting its bioavailability and function. We therefore hypothesized that circulating levels of myeloperoxidase would predict risk of endothelial dysfunction in human subjects.
Methods and Results
Serum myeloperoxidase was measured by enzyme-linked immunoassay, and brachial artery flow–mediated dilation and nitroglycerin-mediated dilation were determined by ultrasound in a hospital-based population of 298 subjects participating in an ongoing study of the clinical correlates of endothelial dysfunction (age, 51±16; 61% men, 51% with cardiovascular disease). A strong inverse relation between brachial artery flow–mediated dilation and increasing quartile of serum myeloperoxidase level was observed (11.0±6.0%, 9.4±5.3%, 8.6±5.8%, and 6.4±4.5% for quartiles 1 through 4, respectively; P<0.001 for trend). Using the median as a cut point to define endothelial dysfunction, increasing quartile of myeloperoxidase predicted endothelial dysfunction after adjustment for classic cardiovascular disease risk factors, C-reactive protein levels, prevalence of cardiovascular disease, and ongoing treatment with cardiovascular medications (OR, 6.4; 95% CI, 2.6 to 16; P=0.001 for highest versus lowest quartile).
Conclusions
Serum myeloperoxidase levels serve as a strong and independent predictor of endothelial dysfunction in human subjects. Myeloperoxidase-mediated endothelial dysfunction may be an important mechanistic link between oxidation, inflammation, and cardiovascular disease.
Keywords: atherosclerosis, free radicals, inflammation, peroxidase, nitric oxide
Recent studies have emphasized the importance of myeloperoxidase for cardiovascular disease.1–4 Myeloperoxidase levels are higher in patients with coronary artery disease1 and predict future cardiovascular events after risk factors and C-reactive protein are controlled for.2, 3 Myeloperoxidase-derived oxidants, including hypochlorous acid, play a physiological role in innate host defenses. However, the enzyme and products of myeloperoxidase-catalyzed oxidation reactions (tyrosylation, nitration, halogenation) have been identified in human atherosclerotic lesions, further suggesting that myeloperoxidase may play a pathophysiological role in atherogenesis.5–8
Another potentially important consequence of myeloperoxidase activity is consumption of nitric oxide and induction of endothelial dysfunction. Kinetic studies first showed that myeloperoxidase can serve as a catalytic sink for nitric oxide.9 Consistent with such an effect, myeloperoxidase impairs nitric oxide–dependent vasodilation in isolated arterial and tracheal rings and decreases nitric oxide bioavailability in cultured cells.10–12 Myeloperoxidase is rapidly taken up by endothelial cells by a transcytotic process and accumulates within the subendothelial space, positioning it anatomically to interfere with the effects of nitric oxide in the vessel wall.12,13
The purpose of the present study was to seek evidence that myeloperoxidase impairs the biological activity of endothelium-derived nitric oxide in human subjects. We hypothesized that serum myeloperoxidase levels would correlate inversely with nitric oxide–dependent flow-mediated dilation of the brachial artery.14 Given the important antiatherogenic effects of endothelium-derived nitric oxide,15 this finding would provide insight into the causes of endothelial dysfunction and additional evidence that myeloperoxidase may play a pathogenic role in cardiovascular disease.
Methods
Research Subjects
Sequential patients receiving care from the Cardiology Service of Boston Medical Center and volunteers responding to advertisements were eligible for study as part of an ongoing investigation of the clinical determinants of endothelial function. Subjects with unstable coronary disease, blood pressure >180/110 mm Hg on medications, or clinical evidence of congestive heart failure were excluded. All participants gave written informed consent, and the Institutional Review boards of Boston Medical Center and the Cleveland Clinic Foundation approved all study protocols.
Study Protocol
Subjects discontinued all medications for 24 hours before study, fasted overnight, and refrained from smoking for 12 hours (if applicable). A fasting blood sample was obtained, and heart rate and blood pressure were measured with an automatic physiological recorder (Johnson & Johnson, Inc). A medical history and record review were performed to define coronary risk factors, including diabetes mellitus (fasting blood glucose >125 mg/dL or hypoglycemic medications), hypertension (blood pressure >140/90 mm Hg or antihypertensive medications in the absence of known cardiac disease), family history of premature coronary heart disease (first-degree relative with coronary heart disease before 60 years of age by subject report), hypercholesterolemia (fasting LDL cholesterol >160 mg/dL or lipid-lowering medications), and cigarette smoking (any smoking within 1 year of study). The Framingham Risk Score was calculated for each subject as defined.16 Cardiovascular disease was defined clinically as the presence of coronary artery disease, peripheral arterial disease, or cerebral vascular disease.
Endothelium-dependent brachial artery flow–mediated dilation was determined as previously described.17 Briefly, 2D images were obtained at baseline and 55 to 65 seconds after induction of reactive hyperemia by 5-minute cuff occlusion of the upper arm. After a 10-minute period to reestablish baseline conditions, endothelium-independent dilation of the brachial artery was assessed from images obtained before and 4 minutes after sublingual nitroglycerin (0.4 mg). The nitroglycerin portion was omitted if systolic blood pressure was <100 mm Hg or if the patient declined because of prior migraine headaches or adverse reaction to nitroglycerin. Images were digitized online, and arterial diameters were measured with customized software (Medical Imaging Applications, Inc) by individuals blinded to clinical and laboratory status of the subjects. Flow-mediated dilation was expressed as the absolute change in diameter in millimeters and as the percent increase in diameter from baseline.
Biochemical Analyses
Serum lipoprotein profile (total cholesterol, triglycerides, and HDL cholesterol) was determined by automated analyzer (Hitachi Instruments). LDL cholesterol was estimated by the Friedewald formula unless triglyceride levels were >300 mg/dL, in which case direct LDL cholesterol determinations were performed. C-reactive protein was determined with a high-sensitivity nephelometer method (Dade Behring). Myeloperoxidase was measured by high-sensitivity sandwich ELISA (PrognostiX, Inc). Each plate included a standard curve with isolated human myeloperoxidase (extinction coefficient, 178 000 mol·L−1·cm−1; RZ >0.85) and replicates of controls (known high- and low-myeloperoxidase samples) were used on every plate to correct for interplate variability. Personnel blinded to clinical outcomes and patient identifiers performed all laboratory studies within a reference laboratory at the Cleveland Clinic Foundation.
Statistical Analysis
Patient characteristics are presented as either mean±SD or median and interquartile range for continuous measures and as number (percentage) for categorical measures. For continuous variables, the univariate and multivariable correlates of vasodilator function were determined with linear regression analysis (SPSS 10.1). For categorical variables, the median vasodilator function value was described by group and compared by use of the Wilcoxon rank-sum test. Adjustments were made for traditional risk factors (age, gender, diabetes, hypertension, hypercholesterolemia, family history, smoking) or Framingham Risk Score, prevalent cardiovascular disease, and cardiovascular medications known to influence endothelial function (ACE inhibitors, aspirin, and statins).15,18
To evaluate myeloperoxidase as a predictor of endothelial dysfunction expressed in a dichotomous manner, subjects were categorized as having endothelial dysfunction or not using the sex-specific median value of flow-mediated dilation (0.31 mm for men, 0.35 mm for women). Differences between groups were evaluated with the Mann-Whitney U test or the χ2 test, for continuous and categorical variables, respectively. Levels of myeloperoxidase and C-reactive protein were divided into quartiles because neither variable was normally distributed. Risk for endothelial dysfunction according to quartile of myeloperoxidase or C-reactive protein was evaluated by use of logistic regression (SAS 8.0).
Results
Subject Characteristics
The clinical characteristics of the enrolled subjects are displayed in Table 1. Approximately half had cardiovascular disease. As expected in a hospital-based population, there was a high prevalence of risk factors and use of cardiovascular medications.
TABLE 1.
Clinical Characteristics
| Endothelial Dysfunction |
||||
|---|---|---|---|---|
| Characteristic | Mean Value | No | Yes | P |
| Age, y | 51±16 | 47±17 | 55±14 | <0.001 |
| Male gender, % (n) | 61 (182) | 61 (91) | 61 (91) | 1.00 |
| Diabetes mellitus, % (n) | 19 (55) | 9 (13) | 28 (42) | <0.001 |
| Hypertension, % (n) | 45 (134) | 34 (50) | 56 (84) | <0.001 |
| Hypercholesterolemia, % (n) | 39 (115) | 36 (53) | 42 (62) | 0.28 |
| Family history of CAD, % (n) | 27 (80) | 23 (34) | 31 (46) | 0.12 |
| Cigarette smoking, % (n) | 60 (180) | 60 (89) | 61 (91) | 0.81 |
| Cardiovascular disease, % (n) | 51 (153) | 41 (61) | 62 (92) | <0.001 |
| Body mass index, kg/m2 | 28.9±7.5 (182) | 28.0±7.0 | 30.1±8.1 | 0.07 |
| Total cholesterol, mg/dL | 192±44 | 193±48 | 191±40 | 0.58 |
| LDL cholesterol, mg/dL | 115±39 | 113±43 | 117±34 | 0.10 |
| HDL cholesterol, mg/dL | 51±16 | 53±16 | 48±15 | 0.02 |
| Triglycerides, mg/dL | 139±110 | 139±131 | 139±83 | 0.27 |
| ACE inhibitor therapy, % (n) | 23 (69) | 17 (26) | 29 (43) | 0.02 |
| Aspirin, % (n) | 38 (114) | 30 (45) | 46 (69) | 0.004 |
| Statin therapy, % (n) | 20 (59) | 16 (24) | 24 (35) | 0.11 |
| Framingham Risk Score | 4.5±7.1 | 2.3±7.2 | 6.6±6.3 | <0.001 |
| C-reactive protein, mg/L | 0.74±0.96 | 0.65±0.91 | 0.83±1.00 | 0.006 |
| Myeloperoxidase,pmol/L | 470±466 | 343±362 | 598±521 | <0.001 |
CAD indicates coronary artery disease. Data are mean±SD when appropriate. Endothelial dysfunction was defined using the median value of flow-mediated dilation as a cut point. Body mass index data were available for 182 subjects. n=298.
Correlates of Vasodilator Function
The unadjusted correlations of flow-mediated dilation (expressed in millimeters) were age (r=−0.25, P<0.001), HDL cholesterol (r=0.22, r<0.001), Framingham Risk Score (r=−0.29, P<0.001), serum C-reactive protein (r=−0.27, P<0.001), and serum myeloperoxidase (r=−0.31, P<0.001). There was a significant association of flow-mediated dilation with male gender (median, 0.31 versus 0.35 mm; P=0.02), diabetes mellitus (0.20 versus 0.35 mm; P<0.001), hypertension (0.27 versus 0.38 mm; P<0.001), family history (0.29 versus 0.34 mm; P=0.02), and prevalent cardiovascular disease (0.28 versus 0.39 mm; P<0.001). The correlations and associations of flow-mediated dilation expressed as percent change from baseline were similar, except that the association with gender was stronger (7.0% versus 10.5%; P<0.001). The unadjusted correlates of nitroglycerin-mediated dilation were similar to those for flow-mediated dilation, including C-reactive protein (r=−0.19, P=0.01) and serum myeloperoxidase (r=−0.20, P=0.006).
Flow-mediated dilation was unexpectedly worse in subjects taking cardiovascular medications, including aspirin (median, 0.29 versus 0.37 mm; P<0.001) and ACE inhibitors (0.28 versus 0.36 mm; P=0.003) but not statins (0.31 versus 0.35 mm; P=0.17). These findings likely reflect confounding by indication bias, whereby patients with a higher level of disease are more likely to be taking medications.18 There were similar univariate relations between cardiovascular medications and nitroglycerin-mediated dilation.
Correlates of Serum Myeloperoxidase
The median serum myeloperoxidase level was 298.0 pmol/L, with an interquartile range of 154.1 to 638.1 pmol/L. Increasing myeloperoxidase level strongly correlated with prevalent cardiovascular disease (median, 548 versus 185 pmol/L; P<0.001) and with cardiovascular risk factors, including age (r=0.44, P<0.001), gender (median, 372 versus 238 pmol/L in men versus women; P<0.001), diabetes mellitus (573 versus 253 pmol/L; P<0.001), hypertension (433 versus 225 pmol/L; P<0.001), history of hypercholesterolemia (445 versus 248 pmol/L; P<0.001), HDL cholesterol (r=−0.34, P<0.001), serum triglycerides (r=0.26, P<0.001), smoking 364 versus 212 pmol/L; P<0.001), and Framingham Risk Score (r=0.41, r<0.001). Myeloperoxidase levels also correlated with levels of C-reactive protein (r=0.38, P<0.001) but not family history (339 versus 282 pmol/L; P=0.08). Myeloperoxidase levels were higher in individuals on cardiovascular medications, including statins (764 versus 254 pmol/L; P<0.001), ACE inhibitors (472 versus 259 pmol/L; P<0.001), and aspirin (420 versus 247 pmol/L; P<0.001), which likely reflects confounding by indication bias.
Relation Between Myeloperoxidase and Endothelial Dysfunction
As shown in Table 2, there were strong unadjusted inverse relations between increasing quartiles of myeloperoxidase and flow-mediated dilation and nitroglycerin-mediated dilation but not baseline brachial artery diameter. In multivariate regression models, myeloperoxidase remained a significant predictor of flow-mediated dilation after adjustment prevalent cardiovascular disease, cardiovascular medications, and the Framingham Risk Score (partial correlation coefficient, −0.14; P=0.01). When flow-mediated dilation was expressed as absolute change rather than the more commonly used percent change in diameter, the relation with myeloperoxidase was stronger (partial correlation coefficient, −0.19; P=0.001). There is growing evidence that expressing flow-mediated dilation as absolute change is preferable because is less influenced by baseline arterial diameter and is more reproducible.14,18 The inverse relation between myeloperoxidase levels and nitroglycerin-mediated dilation was lost after controlling for cardiovascular disease, cardiovascular medications, and the Framingham Risk Score (partial correlation coefficient, −0.03; P=0.71).
TABLE 2.
Brachial Artery Results by Quartile of Myeloperoxidase
| Myeloperoxidase Quartiles |
|||||
|---|---|---|---|---|---|
| 1 <154 pmol/L) |
2 (154–298 pmol/L) |
3 (298–638 pmol/L) |
4 (>638 pmol/L) |
P Trend | |
| Baseline diameter, mm | 4.01±0.83 | 4.11±0.86 | 4.12±0.82 | 3.97±0.68 | 0.80 |
| Flow-mediated dilation, % | 11.1±6.0 | 9.4±5.3 | 8.7±5.8 | 6.4±4.5 | <0.001 |
| Flow-mediated dilation, mm | 0.42±0.19 | 0.37±0.19 | 0.33±0.20 | 0.25±0.17 | <0.001 |
| Nitroglycerin-mediated dilation, % | 17.8±8.3 | 19.2±8.7 | 16.3±7.0 | 12.9±7.6 | 0.008 |
Nitroglycerin-mediated dilation data are available for 175 subjects.
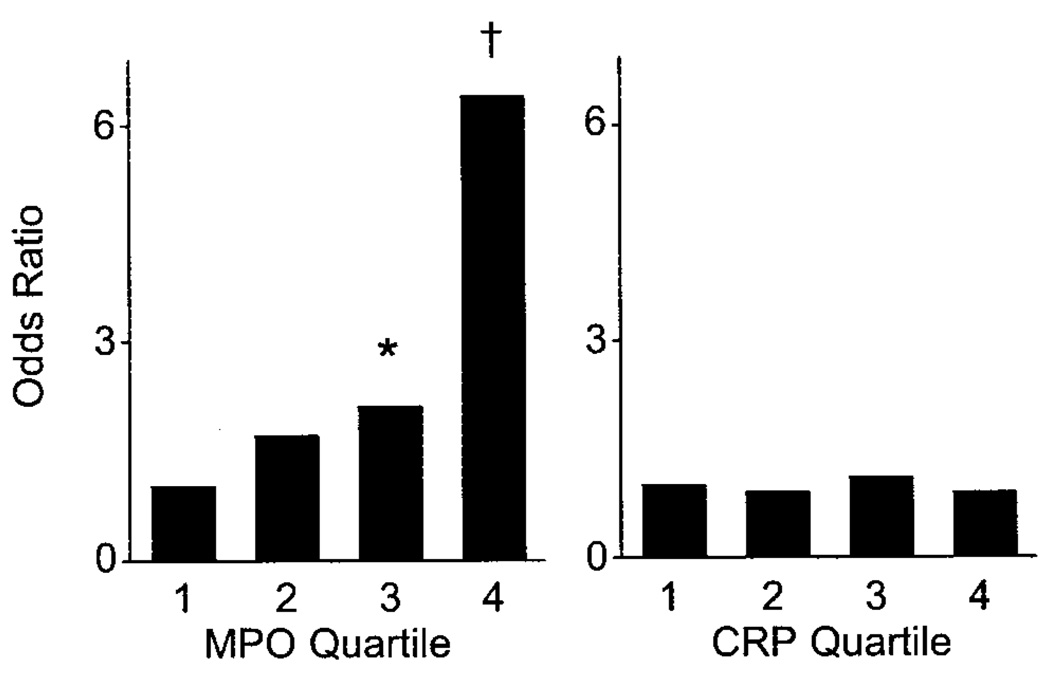
To gain further insight into the relative strength of the relation between myeloperoxidase and endothelial dysfunction, we divided subjects into 2 groups using the sex-specific median value of flow-mediated dilation as a cut point. A comparison of the clinical characteristics for patients with and without endothelial dysfunction is presented in Table 1. As expected, cardiovascular risk factors and prevalent cardiovascular disease were associated with endothelial dysfunction, and cardiovascular medication use was more common in individuals with endothelial dysfunction. As shown in Table 3, the ORs for endothelial dysfunction increased with increasing quartile of myeloperoxidase and remained significant after multivariable adjustment, including adjustment for C-reactive protein (the Figure).
TABLE 3.
Risk for Endothelial Dysfunction
| Myeloperoxidase Quartiles |
|||||
|---|---|---|---|---|---|
| 1 (<154 pmol/L) |
2 (154–298 pmol/L) |
3 (298–638 pmol/L) |
4 (>638 pmol/L) |
P Trend | |
| Unadjusted MPO | 1 | 1.7 (0.9–3.4) | 2.2 (1.1–4.3)* | 6.5 (3.2–13)† | <0.001 |
| MPO+Framingham Risk Score | 1 | 1.6 (0.8–3.2) | 1.9 (0.9–3.9) | 4.1 (1.9–8.9)† | <0.001 |
| MPO+Framingham Risk Score+CVD | 1 | 1.8 (0.9–3.6) | 2.2 (1.1–4.4)* | 5.6 (2.4–13)† | <0.001 |
| MPO+Framingham Risk Score+CVD+cardiovascular medications | 1 | 1.7 (0.9–3.6) | 2.1 (1.04–4.4)* | 6.4 (2.7–15)† | <0.001 |
| C-Reactive Protein Quartiles |
|||||
| 1 (<0.13 mg/L) |
2 (0.13–0.37 mg/L) |
3 (0.37–0.99 mg/L) |
4 (>0.99 mg/L) |
P Trend | |
| Unadjusted CRP | 1 | 1.1 (0.6–2.1) | 2.6 (1.3–5.0)† | 2.0 (1.1–3.9)* | 0.01 |
| CRP+Framingham Risk Score | 1 | 0.9 (0.4–1.7) | 1.4 (0.7–2.9) | 1.1 (0.5–2.3) | 0.52 |
| CRP+Framingham Risk Score+CVD | 1 | 0.9 (0.4–1.7) | 1.4 (0.7–3.0) | 1.1 (0.5–2.4) | 0.51 |
| CRP+Framingham Risk Score+CVD+cardiovascular | 1 | 0.8 (0.4–1.7) | 1.4 (0.7–2.9) | 1.1 (0.5–2.4) | 0.54 |
MPO indicates myeloperoxidase; CVD, cardiovascular disease; and CRP, C-reactive protein. Cardiovascular medications include statins, ACE inhibitors, or aspirin therapy. Data are ORs and 95% CIs for each quartile vs lowest quartile as predictors of endothelial dysfunction defined as a flow-mediated dilation less than the median.
P<0.05
P<0.01
Fully adjusted models for markers of inflammation and risk for endothelial dysfunction. Displayed are ORs for each quartile compared with first quartile of myeloperoxidase (MPO; left) or C-reactive protein (CRP; right) with endothelial dysfunction defined as flow-mediated dilation less than median. Models include Framingham Risk Score, prevalent cardiovascular disease, cardiovascular medications, and alternative marker of inflammation (C-reactive protein or myeloperoxidase). Myeloperoxidase predicts endothelial dysfunction (P=0.001 for trend), whereas C-reactive protein does not (P=0.87 for trend). *OR, 2.1 (95% CI, 1.02 to 4.5; P=0.04). †OR, 6.4; 95% CI, 2.6 to 16; P<0.001).
To compare the relative predictive value of myeloperoxidase with an alternative inflammatory marker, we examined the OR for endothelial dysfunction according to quartile of C-reactive protein. As shown in Table 3, unadjusted C-reactive protein predicted endothelial dysfunction, although the trend was nonlinear and the ORs were lower than those for myeloperoxidase. Interestingly, C-reactive protein did not remain a significant predictor of endothelial dysfunction after multivariable adjustment. The fully adjusted model, including adjustment for serum myeloperoxidase, is shown in the Figure.
Discussion
The present study demonstrates a strong relationship between serum myeloperoxidase levels and endothelial dysfunction, as monitored by nitric oxide–dependent flow-mediated dilation of the brachial artery. Patients in the highest quartile of myeloperoxidase were >6 times more likely to have endothelial dysfunction than patients in the lowest quartile. Myeloperoxidase did not relate to brachial artery diameter and was related only weakly to nitroglycerin-mediated dilation, suggesting a specific link with endothelial dysfunction rather than arterial geometry, the function of vascular smooth muscle, or the general dilator capacity of the brachial artery. The relation between myeloperoxidase and endothelial dysfunction remained significant after adjustment for prevalent cardiovascular disease, cardiac risk factors, and C-reactive protein levels. Moreover, myeloperoxidase predicted endothelial dysfunction more strongly than C-reactive protein, suggesting that myeloperoxidase is not simply a general marker of inflammation. Collectively, these findings are consistent with the hypothesis that myeloperoxidase directly impairs the biological activity of endothelium-derived nitric oxide.
Several years ago, Abu-Soud and Hazen9 first reported that myeloperoxidase has the remarkable catalytic activity of oxidatively consuming nitric oxide as a substrate under physiological conditions. Since that time, a growing number of kinetic,11 cell culture,12 organ chamber,11,12 histological,13 and animal model12 studies have provided supporting evidence that myeloperoxidase can function as a catalytic sink for nitric oxide, suggesting that this protein may play a role in the pathogenesis of endothelial dysfunction. The present results represent a direct translation of these prior experimental studies to the clinic, demonstrating a robust and independent link between myeloperoxidase levels and nitric oxide bioavailability and function, as monitored by endothelium-dependent flow-mediated dilation in subjects.
Although our findings are consistent with catalytic consumption of nitric oxide, a number of other potential mechanisms might contribute to the observed correlation between myeloperoxidase levels and endothelial dysfunction. Indeed, myeloperoxidase levels may serve merely as an excellent surrogate marker of a constellation of relevant inflammatory pathways that collectively participate in development of endothelial dysfunction. Myeloperoxidase and its reactive products, including hypochlorous acid, may have a number of additional adverse effects on endothelial function. For example, hypochlorous acid may react with l-arginine,10 reducing the availability of substrate for nitric oxide synthase and forming chlorinated arginine species that inhibit the enzyme.19 Myeloperoxidase and hypochlorous acid also reduce the availability of NADPH, an essential cofactor for nitric synthase.20 Myeloperoxidase has the capacity to nitrate proteins via production of reactive nitrogen species such as peroxynitrite and nitrogen dioxide.21 Reactive nitrogen species have been shown to oxidatively modify LDL and render it atherogenic22,23 and uncouple nitric oxide synthase.24 Furthermore, the contributions of alternative enzymatic sources of reduced oxygen species (superoxide and hydrogen peroxide) that serve as substrates for myeloperoxidase such as xanthine oxidase and vascular NADPH oxidases cannot be adequately assessed by the present study. Additional experimental studies are required to elucidate the relative importance of these mechanisms.
No prior human study has examined the relation between myeloperoxidase and endothelium-dependent dilation, although several have investigated this issue with other systemic markers of inflammation and provided mixed results. For example, Fichtlscherer and colleagues25 reported that serum C-reactive protein correlates independently with impaired endothelium-dependent dilation in forearm microvessels. However, several larger-scale studies failed to find such a correlation.26,27 These latter findings are consistent with the present study, in which we observed an unadjusted relation between C-reactive protein and endothelial dysfunction that was lost after adjustment for risk factors and cardiovascular disease. Thus, the previously reported relation between C-reactive protein and endothelial dysfunction may reflect these confounding effects. In contrast, the strong independent relation with myeloperoxidase in the present study is consistent with the possibility that myeloperoxidase plays a mechanistic role in the pathogenesis of endothelial dysfunction.
The present study may have important clinical implications because it helps explain the now well-established relation between myeloperoxidase and cardiovascular disease.1–4 Loss of the biological activity of endothelium-derived nitric oxide promotes vasoconstriction, thrombosis, inflammation, and cellular proliferation in the vascular wall.15 Furthermore, loss of endothelium-derived nitric oxide often occurs in parallel with other changes in endothelial phenotype that further promote atherosclerosis.28 The present study is consistent with myeloperoxidase playing a direct pathogenic role in cardiovascular disease, in part, by impairing endothelial function.
Several limitations of the study warrant consideration. First, although the findings are suggestive, this cross-sectional study cannot prove a causal link between myeloperoxidase and endothelial dysfunction or definitive information about mechanism. It remains possible that the findings are attributable to unmeasured confounding factors. For example, myeloperoxidase also likely serves as a marker for general capacity for oxidative damage to the vasculature rather than functioning solely to directly consume nitric oxide. Second, it might be argued that the lack of an independent relation between myeloperoxidase and nitroglycerin-mediated dilation is not consistent with catalytic consumption of nitric oxide. However, it is increasingly recognized that there are important differences in the vasodilator responses to nitroglycerin and authentic nitric oxide.29 Furthermore, a more robust association might have been observed if submaximal doses of nitroglycerin had been used or a larger subset of subjects were monitored for their nitroglycerin responses. Finally, the study was performed in a relatively high-risk, hospital-based population. Further studies are warranted to assess the relationship between myeloperoxidase levels and measures of endothelial function in the general population.
In summary, we observed a strong, independent relation between serum myeloperoxidase level and endothelial dysfunction, as reflected by brachial artery flow–mediated dilation. These findings are consistent with experimental studies demonstrating that myeloperoxidase catalytically consumes nitric oxide in vitro and induces endothelial dysfunction in organ chamber and animal model studies. The present results provide further evidence for mechanistic links between myeloperoxidase, endothelial dysfunction, and cardiovascular disease. The results also suggest that further evaluation of myeloperoxidase inhibitors in cardiovascular disease is warranted.
Acknowledgments
This work was supported by NIH grants P01 HL-76491, HL-60886, HL-70621, HL-62526, HL-61878, and HL-55993. Dr Brennan is a recipient of an NIH NRSA fellowship (CA-93648). Dr Gokce is the recipient of a mentored patient-oriented research career transition award from the NIH (HL-04425). Support was also provided by the General Clinical Research Centers at Boston Medical Center (MO1RR00533) and the Cleveland Clinic Foundation (MO1RR018390).
Footnotes
Disclosure
Dr Hazen is named as a co-inventor on pending patents filed by the Cleveland Clinic Foundation that relate to the use of biomarkers for inflammatory and cardiovascular diseases.
References
- 1.Zhang R, Brennan ML, Fu X, et al. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286:2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 2.Brennan ML, Penn MS, Van Lente F, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 3.Baldus S, Heeschen C, Meinertz T, et al. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 4.Brennan ML, Hazen SL. Emerging role of myeloperoxidase and oxidant stress markers in cardiovascular risk assessment. Curr Opin Lipidol. 2003;14:353–359. doi: 10.1097/00041433-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker for myeloperoxidase-catalyzed halogenation, is present in human atherosclerotic aorta. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leeuwenburgh C, Hardy MM, Hazen SL, et al. Reactive nitrogen intermediates promote low density lipoprotein oxidation in human atherosclerotic intima. J Biol Chem. 1997;272:1433–1436. doi: 10.1074/jbc.272.3.1433. [DOI] [PubMed] [Google Scholar]
- 7.Sugiyama S, Okada Y, Sukhova GK, et al. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–891. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thukkani AK, McHowat J, Hsu FF, et al. Identification of α-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation. 2003;108:3128–3133. doi: 10.1161/01.CIR.0000104564.01539.6A. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Soud HM, Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem. 2000;275:37524–37532. doi: 10.1074/jbc.275.48.37524. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C, Patel R, Eiserich JP, et al. Endothelial dysfunction is induced by proinflammatory oxidant hypochlorous acid. Am J Physiol Heart Circ Physiol. 2001;281:H1469–H1475. doi: 10.1152/ajpheart.2001.281.4.H1469. [DOI] [PubMed] [Google Scholar]
- 11.Abu-Soud HM, Khassawneh MY, Sohn JT, et al. Peroxidases inhibit nitric oxide (NO) dependent bronchodilation: development of a model describing NO-peroxidase interactions. Biochemistry. 2001;40:11866–11875. doi: 10.1021/bi011206v. [DOI] [PubMed] [Google Scholar]
- 12.Eiserich JP, Baldus S, Brennan ML, et al. Myeloperoxidase: a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 13.Baldus S, Eiserich JP, Mani A, et al. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J Clin Invest. 2001;108:1759–1770. doi: 10.1172/JCI12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 15.Widlansky ME, Gokce N, Keaney JF, Jr, et al. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 16.Wilson PW, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 17.Vita JA. Nitric oxide–dependent vasodilation in human subjects. Methods Enzymol. 2002;359:186–200. doi: 10.1016/s0076-6879(02)59183-7. [DOI] [PubMed] [Google Scholar]
- 18.Benjamin EJ, Larson MG, Kupka MJ, et al. Clinical correlates and heritability of endothelial function in the community: the Framingham Heart Study. Circulation. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 19.Zhang C, Reiter C, Eiserich JP, et al. l-Arginine chlorination products inhibit endothelial nitric oxide production. J Biol Chem. 2001;276:27159–27165. doi: 10.1074/jbc.M100191200. [DOI] [PubMed] [Google Scholar]
- 20.Auchere F, Capeillere-Blandin C. NADPH as a co-substrate for studies of the chlorinating activity of myeloperoxidase. Biochem J. 1999;343(pt 3):603–613. [PMC free article] [PubMed] [Google Scholar]
- 21.Brennan ML, Wu W, Fu X, et al. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- 22.Podrez EA, Schmitt D, Hoff HF, et al. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest. 1999;103:1547–1560. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Podrez EA, Febbraio M, Sheibani N, et al. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocytegenerated reactive nitrogen species. J Clin Invest. 2000;105:1095–1108. doi: 10.1172/JCI8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fichtlscherer S, Rosenberger G, Walter DH, et al. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation. 2000;102:1000–1006. doi: 10.1161/01.cir.102.9.1000. [DOI] [PubMed] [Google Scholar]
- 26.Prasad A, Zhu J, Halcox JP, et al. Predisposition to atherosclerosis by infections: role of endothelial dysfunction. Circulation. 2002;106:184–190. doi: 10.1161/01.cir.0000021125.83697.21. [DOI] [PubMed] [Google Scholar]
- 27.Tan KC, Chow WS, Tam SC, et al. Atorvastatin lowers C-reactive protein and improves endothelium-dependent vasodilation in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2002;87:563–568. doi: 10.1210/jcem.87.2.8249. [DOI] [PubMed] [Google Scholar]
- 28.Vita JA, Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 29.Ignarro LJ. After 130 years, the molecular mechanism of action of nitroglycerin is revealed. Proc Natl Acad Sci U S A. 2002;99:7816–7817. doi: 10.1073/pnas.132271799. [DOI] [PMC free article] [PubMed] [Google Scholar]