Abstract
We studied two rhesus monkeys before and after surgical ablation of the nodulus and uvula (Nod/Uv) of the cerebellum. Three-axis eye movements were recorded with the magnetic-field scleral search coil system during a variety of vestibular and ocular motor tasks. Here we describe the effects of the Nod/Uv lesions on dynamic (head translation) and static (head tilt) otolith-mediated vestibulo-ocular reflexes. The main findings were: 1) eye velocity during sinusoidal vertical translation (1.5 Hz) was reduced by 59% in the dark and 36% in the light; 2) eye velocity during steps of horizontal translation was reduced, but only in the dark and more so during the sustained (constant-velocity) than the initial (acceleration) part of the response, and 3) there was a torsional nystagmus that depended on the position of roll head tilt, but static ocular counterroll was unchanged. These results suggest new roles for the Nod/Uv in the processing of otolith signals. This is likely important not only for facilitating gaze during linear head motion, but also for maintaining postural stability and one’s orientation relative to gravity. The lesions appeared to have a greater effect on responses to vertical motion, particularly in the light (in contrast responses to interaural translation in the light were nearly normal), suggesting a particular importance of the Nod/Uv in processing signals arising from the sacculi.
INTRODUCTION
The nodulus and the uvula (Nod/UV) of the inferior cerebellar vermis belong to the phylogenetically old vestibulocerebellum. They are densely interconnected with the vestibular nuclei and other brainstem areas involved in eye movements (reviewed in Voogd, Barmack 2005), and they receive direct afferent input from the vestibular nerve. Prior studies have shown that the Nod/Uv plays an important role in the several key aspects of vestibular reflexes, in particular the ability to relate one’s own motion and orientation to the gravitational upright. An example of this is the alignment of the eye-velocity axis with the gravito-inertial vector during sustained off-vertical-axis rotation; this function depends on both otolith inputs and an intact velocity-storage mechanism and is abolished by lesions of the Nod/Uv (Angelaki, Hess 1995, Wearne, Raphan & Cohen 1996). In fact, it has been suggested that the role of velocity storage in the rotational VOR may be less important than its function in the control of body orientation and posture (Green, Angelaki 2003).
The sense of orientation depends critically on the proper combination of otolith inputs and semicircular canal signals. Thus, it is expected that the Nod/Uv would play an important role in the processing of otolith information. Although prior studies have shown that diffuse cerebellar diseases can impair the tVOR, the specific role of individual cerebellar areas, including the Nod/Uv, is not well known. In this study, we investigated in two rhesus monkeys the effect of Nod/Uv lesions on the otolith-ocular reflexes, in response to static head tilts (counterroll) and to linear head motion (translation). Here we will focus attention on the role of the Nod/Uv in the control of static and dynamic otolith reflexes. These reflexes both serve vision, by reducing gaze errors induced by head movement, and orientation sense. In the same series of experiments, we also found that the Nod/Uv are critical for normal vertical pursuit but not large-field ocular following (Walker et al. 2008).
METHODS
Two juvenile rhesus monkeys were studied under a protocol that was approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University. Eye movements were recorded using the magnetic field scleral search coil system with three magnetic fields and dual coils in each eye. The eye movement calibration method has been previously described (Tian, Zee & Walker 2007). Lesions of the Nod/Uv were performed by neurosurgical aspiration with direct visualization under inhalation anesthesia, using full sterile technique and postoperative analgesia. Lesion extent was confirmed with MRI and post-mortem histology.
Motion stimuli
Different systems were used to test horizontal and vertical tVORs. Horizontal translation was performed on a belt-driven linear sled (Acutronic, Zürich, Switzerland). The stimulus consisted of abrupt transient motion (0.4 g to a plateau speed of 40 cm/s), either in the dark or with fixation of a laser target that was back-projected onto a tangent screen in front of the monkey (27 or 70 cm viewing distance). Chair position and velocity were monitored by a linear sensor and fed back to the host computer.
The vertical TVOR was tested on a manually driven, spring-assisted, sled that was mounted on tracks to constrain its motion. The chair faced a visual stimulus that was 122 cm away, and recordings were made in both light and complete darkness. Vertical head motion was measured using an accelerometer that was firmly attached to the animal chair, oriented with the vertical axis. To derive head velocity from recorded head acceleration, an optimization algorithm was first used to determine the offset (bias) of the acceleration signal due to gravity. This bias was subtracted from the accelerometer signal and was also used to calibrate the signal (offset = 1g = 980 cm/s2). The normalized acceleration signal was numerically integrated (cumtrapz, www.scipy.org) to yield linear head velocity in cm/s. Angular eye velocity was determined using standard rotation vector techniques.
Quick phases and saccades were detected and removed from the analysis. The quick-phase detection algorithm was based on acceleration and jerk thresholds (Wyatt 1998). The right-hand-rule convention is used for all data: positive positions and velocities are leftward, downward, and clockwise.
RESULTS
Vertical tVOR
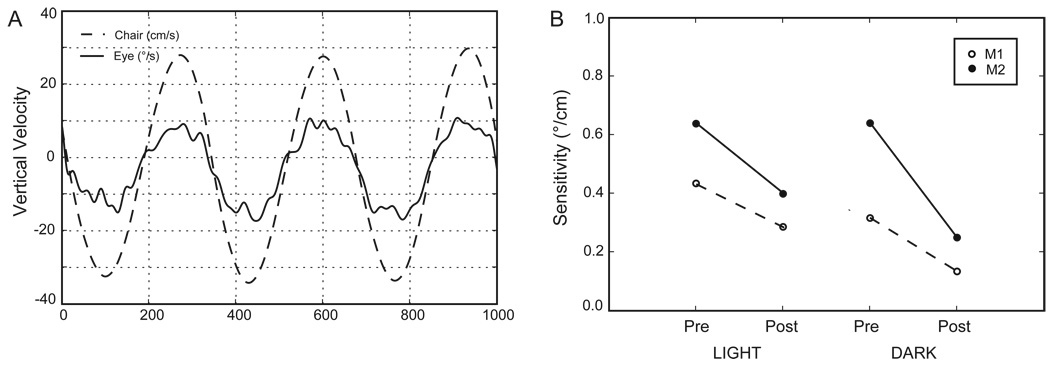
Smooth eye movements elicited by vertical translation (bob) were substantially reduced by the Nod/Uv lesions (Walker et al. 2008 (in press)). This decrease was greater for translation in the dark (59% decrease) but was also present during translation in the light (36% decrease) (Figure 1). Thus, although a visual stimulus was still able to enhance the response after the lesion, this enhancement was not enough to restore eye velocity to its pre-lesion values. Visual enhancement was seen during both upward (85% enhancement) and downward (108% enhancement) motion.
Figure 1.
Responses to vertical translation on a manually driven spring-assisted chair. The stimulus frequency was approximately 1.5 Hz. A. Example of a response during translation in complete darkness. Head velocity was calculated from the accelerometer signal as described in the Methods. Head velocity is inverted for easier comparison to eye velocity (vertical component of angular eye velocity). B. Response sensitivities (eye velocity / head velocity). These were calculated by robust linear regression (rlm in R / rpy, www.cran.r-project.org); the regression slope is the sensitivity.
Horizontal Interaural tVOR
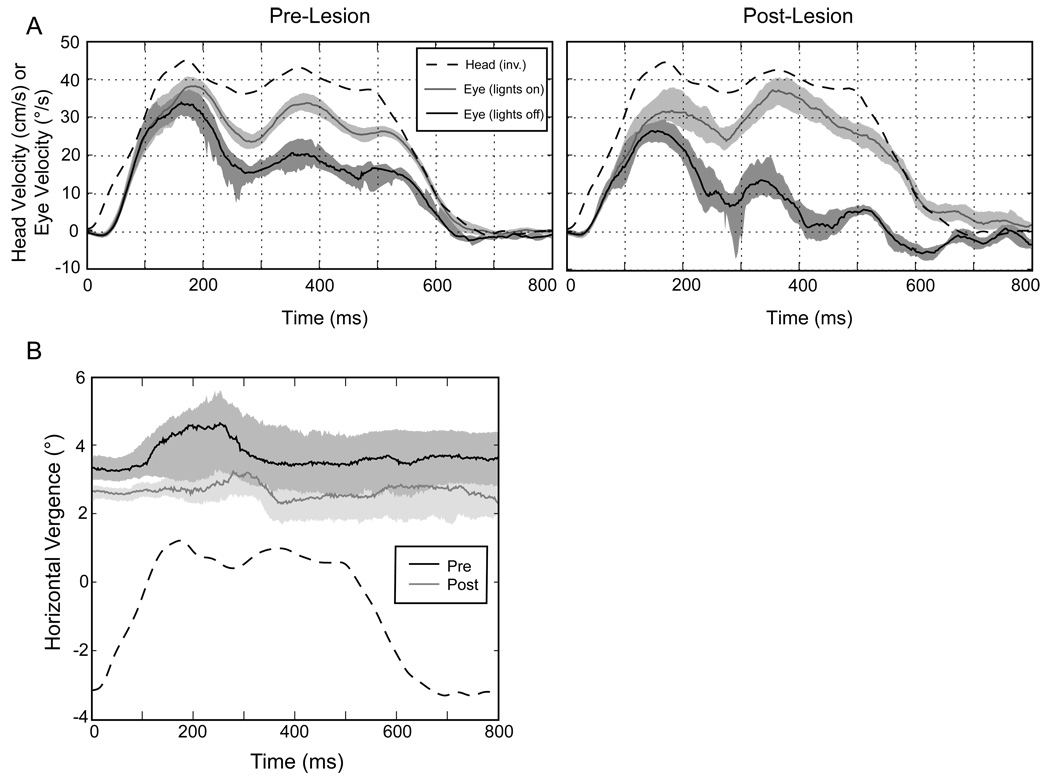
The interaural tVOR (IA tVOR) was also affected by the Nod/Uv lesions. Figure 2 shows eye velocity during a representative step of leftward motion, before and after the lesion, both in darkness and with lights on. As for the vertical tVOR, the post-lesion response was reduced. The use of abrupt steps of motion allowed us to examine the timing of this change, and we found that the earliest part of the response was less affected than the plateau portion. Elsewhere, we have shown that the response change is consistent with a deficit in the integration of head acceleration to maintain eye speed during constant-velocity head translation (Walker et al. 2007).
Figure 2.
A. Responses to rightward interaural translation in M1 (70 cm viewing distance). The left panel shows responses before the Nod/Uv lesion and the right panel responses after the lesion. Solid lines represent the medians of the full set of similar trials, and the corresponding shaded areas include the 25th to 75th percentiles of eye velocity. Quick phases and saccades have been excluded. The dashed line shows head velocity (in cm/s) inverted for comparison. Note that this is not the same as the ideal eye velocity, which depends on both head velocity and viewing distance. Each panel compares responses for trials in which the target remained on to those in which the target went out (i.e., translation in the dark). B. Horizontal vergence during translation in the dark after viewing a target at 70 cm (same data as in A). Convergence is positive. Again the solid lines show the median values and the shaded areas the two middle quartiles. The head velocity (dashed line) is included for reference only and is not scaled.
Although the Nod/Uv lesions impaired the processing of otolith signals, and hence the tVOR in the dark, they did not prevent visual tracking from compensating for this deficit. In fact, responses in the light had similar magnitudes before and after the lesions (Figure 2A). Thus, visual mechanisms seemed to play an even greater role for the IA tVOR post-lesion than for the vertical tVOR, although the difference in the stimuli (steps vs. sinusoidal translation) might account for at least some of this difference.
We also examined the binocularity of the IA tVOR. How well is vergence position maintained during prolonged translation, and is this dependent on an intact Nod/Uv? This is important, because a loss of sustained convergence, particularly in the dark, might indicate an impaired ability to maintain the percept of target distance, and hence the response amplitude. We did not find a general loss of horizontal vergence control after the Nod/Uv lesions. For the example of Figure 2B (M1, 70cm), there was only a minimal change (< 1∘) in the vergence angle. Most importantly, there was no loss of vergence during the course of the trials in the dark, either before or after the lesion. Thus, the decrease of the sustained interaural tVOR after the Nod/Uv lesions cannot be attributed to a inability to hold vergence in the dark.
Responses to Static Roll Tilts
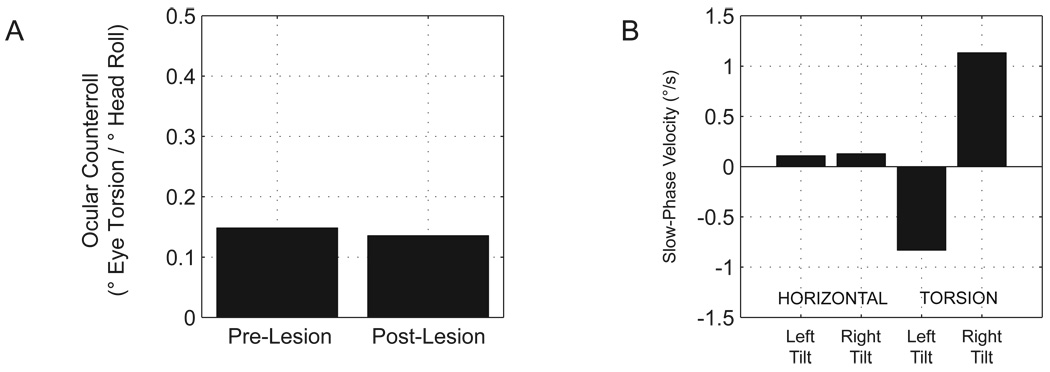
The otolith organs not only detect linear head motion but are also affected by the linear acceleration due to gravity, according to the orientation of each otolith organ relative to the gravity vector. Normally, the sensation of head tilt is less important for gaze stability (there is no ongoing head motion) than for maintaining balance and upright posture; nonetheless, the static otolith-ocular reflexes provide a measure of the integrity of the reflexes and of the brain’s ability to distinguish a head tilt from a translation that would produce a similar change in linear acceleration. Figure 3 shows the ocular torsion (ocular counterroll, OCR) elicited by roll head tilts in M2 before and after the Nod/Uv lesion (we focused attention on M2, because torsion in M1 may have been affected by cyclovertical muscle weakness related to a separate study of superior oblique paresis (Shan et al. 2007)). The lesion had little effect on OCR (15% pre vs. 14% post). Thus, even though Nod/Uv lesions have a large effect on the dynamic roll tVOR mediated by the semicircular canals (Angelaki, Hess 1994), they do not affect the otolith-mediated response to static roll. There was no head-tilt-dependent horizontal nystagmus (to suggest that tilt was misinterpreted as translation), although there was a small torsional nystagmus that consisted of clockwise slow-phases with the right ear down and counterclockwise slow phases with the left ear down, i.e., the direction of the slow phases was opposite to that of the change in the torsional position of the eyes that characterizes the OTR. The mechanism of the torsional nystagmus is uncertain. Unlike horizontal nystagmus, torsion is never a necessary response to translation, and thus it is unlikely that this is a tilt-translation misinterpretation.
Figure 3.
A. Ocular counterroll (OCR) before and after the Nod/Uv lesion (M2). OCR was calculated as the difference in the torsional offset of Listing’s plane when the head was tilted 15∘ to the left compared to 15∘ to the right, divided by the difference in roll head position (30∘). OCR was calculated separately for each eye, and the two values, which were similar, were averaged. B. Horizontal and torsional slow-phase velocity (SPV) in the dark with the head tilted 15∘ to the left or right, recorded after the Nod/Uv lesion. Each value is the median eye velocity, after exclusion of saccades, over the full range of spontaneous eye movements. There was no torsional nystagmus before the lesion (SPV 0.02 ∘/s with right tilt and −0.15∘/s with left tilt).
CONCLUSION
Our study provides important new evidence for a critical role of the Nod/Uv in otolith-ocular reflexes. Not only is the Nod/Uv required for the modulation of the rotational VOR by otolith information, as others have shown, but here we show it is also important for the processing of otolith signals to drive the tVOR. Ablation of the Nod/Uv impairs the ocular response to vertical translation and reduces eye velocity during sustained horizontal translation along the interaural direction. Thus, processing of both utricular and saccular inputs is under the control of the Nod/Uv. Moreover, our data suggest that the function of the Nod/Uv in the horizontal and vertical tVOR may differ: Nod/Uv lesions reduced the interaural tVOR only in the dark, whereas the vertical tVOR was impaired substantially, even in the light.
ACKNOWLEDGEMENTS
This research was supported by NIH grant EY001849 (DSZ), the Albert Pennick Fund. and by the Arnold-Chiari Foundation. Dr. Walker was a Pollin Scholar. Adrian Lasker and Dale Roberts provided technical assistance. Measurement of the interaural tVOR was performed in the laboratory of Dr. Lloyd Minor. Histology was performed by Mitchell Smith, and slides were scanned by Camilo Pardo, and Dr. Carlos Pardo reviewed the slides. Dr. Peter Barker and Hugh Wall assisted with MRI scans.
REFERENCES
- Angelaki DE, Hess BJ. The cerebellar nodulus and ventral uvula control the torsional vestibulo-ocular reflex. J Neurophysiol. 1994;72(no 3):1443–1447. doi: 10.1152/jn.1994.72.3.1443. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Hess BJ. Inertial representation of angular motion in the vestibular system of rhesus monkeys. II. Otolith-controlled transformation that depends on an intact cerebellar nodulus. J Neurophysiol. 1995;73(no 5):1729–1751. doi: 10.1152/jn.1995.73.5.1729. [DOI] [PubMed] [Google Scholar]
- Green AM, Angelaki DE. Resolution of sensory ambiguities for gaze stabilization requires a second neural integrator. J Neurosci. 2003;23(no 28):9265–9275. doi: 10.1523/JNEUROSCI.23-28-09265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X, Tian J, Ying H, Quaia C, Optican LM, Walker MF, Tamargo RJ, Zee DS. Acute superior oblique palsy in monkeys: I. Changes in static eye alignment. Invest. Ophthalmol. Vis. Sci. 2007;48(no 6):2602–2611. doi: 10.1167/iovs.06-1316. [DOI] [PubMed] [Google Scholar]
- Tian J, Zee DS, Walker MF. Rotational and translational optokinetic nystagmus have different kinematics. Vision Res. 2007;vol. 47(no 7):1003–1010. doi: 10.1016/j.visres.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voogd J, Barmack NH. Oculomotor cerebellum. In: Buttner-Ennever JA, editor. Neuroanatomy of the Oculomotor System, Progress in Brain Research . vol. 151. Amsterdam: Elsevier; 2005. pp. 231–268. [Google Scholar]
- Walker MF, Tian J, Shan X, Tamargo RJ, Ying H, Zee DS. Effect of cerebellar lesions in monkey on gaze stability. In: Leigh RJ, Deveraux MW, editors. Advances in Understanding of Mechanisms and Treatment of Infantile Forms of Nystagmus. New York: Oxford University Press; (in press) [Google Scholar]
- Walker MF, Tian J, Shan X, Tamargo R, Zee DS. Program No. 861.3. Washington, DC: Society for Neuroscience; 2007. The cerebellar nodulus and uvula mediate integration of linear head acceleration. (abstract) [Google Scholar]
- Wearne S, Raphan T, Cohen B. Nodulo-uvular control of central vestibular dynamics determines spatial orientation of the angular vestibulo-ocular reflex. Ann. N. Y. Acad. Sci. 1996;781:364–384. doi: 10.1111/j.1749-6632.1996.tb15713.x. [DOI] [PubMed] [Google Scholar]
- Wyatt HJ. Detecting saccades with jerk. Vision Res. 1998;38(no 14):2147–2153. doi: 10.1016/s0042-6989(97)00410-0. [DOI] [PubMed] [Google Scholar]