Abstract
BACKGROUND
The development of valid methods for repeatedly measuring red blood cell (RBC) volume (RCV) in the same individual would be useful in furthering understanding of the physiology and pathophysiology of the pregnant woman, fetus, and infant under a variety of conditions.
STUDY DESIGN AND METHODS
Small volumes (5 to 100 mL) of either sheep or human blood were used to test the hypothesis that there is no significant difference in RCV and blood volume determined in vitro using as many as five populations of RBCs labeled at distinct biotin densities. By varying the mass of biotinylating reagent, the density of biotin on the surface of RBCs was incrementally increased to produce discrete populations as assessed by flow cytometric enumeration. Calculation of RCV for each biotin-labeled RBC population was based on the dilution principle.
RESULTS
All biotin densities, except the most densely labeled, where variance was the greatest, accurately quantitated the in vitro blood volume to within 10 percent of the correct value. There was no bias of either overestimation or underestimation in the determination of the blood volume using either sheep or human RBCs.
CONCLUSION
These in vitro results provide evidence that the multidensity biotin labeling method is sufficiently accurate to utilize in vivo for repeated determination of circulating RCV and blood volume.
Advances in neonatal medicine include therapies that have resulted in improved survival and outcome of extremely low birth weight (<1 kg) and other critically ill infants. While substantial evidence exists supporting the use of surfactant, improved ventilator support, inhaled nitric oxide, and extracorporeal membrane oxygenation, other therapies such as erythropoietin and use of autologous placental blood transfusion have weaker supporting bases of evidence and are being actively investigated.
Reliable methods permitting repeated measurements of red blood cell (RBC) volume (RCV) and survival in the pregnant woman, fetus, and infant would be useful in furthering understanding of the physiology and pathophysiology of a variety of perinatal conditions and their responses to treatment. One such method is the biotinylation of RBCs.1 When used in combination with flow cytometry, the biotin labeling method requires only microliter volumes while achieving sufficient sensitivity to be used in 500-g neonates.2–4 Additional advantages of the biotin labeling RBC method over other RBC labeling methods include the following: 1) it does not expose the subject to radiation; 2) it has been successfully applied in numerous mammalian species;2,5–11 3) the required reagents are inexpensive and readily available;2,4 4) the equipment required is available in clinical laboratories of most academic medical centers;2,4 and 5) technician time required is modest.
Because ovine fetal and newborn cardiovascular, pulmonary, and hematologic development resembles human physiology, sheep are commonly used to model preclinical studies. In particular, ovine erythropoiesis in the fetus, neonate, and adult is similar to the human.12 Accordingly, regulation of erythropoiesis and RBC kinetics during the perinatal period in the ovine model are areas of active investigation13–15 that could be advanced by methods permitting multiple, repeated measurement of RCV and survival.
In this study, our objective was to investigate whether multiple discrete densities of biotin-labeled sheep and human RBCs can be simultaneously applied to independently and accurately quantitate RCV in vitro. Our hypotheses were that RCV determined using each of the different densities of biotinylated RBC would not be significantly different from one another and that the RCV values would be accurate within 5 percent under conditions that simulate anticipated in vivo situations.
MATERIALS AND METHODS
Animal and human study subjects
Studies using sheep RBCs were approved by the institutional animal care and use committees at both Arkansas and Iowa. Studies using human RBCs were approved by the institutional review boards of the University of Arkansas for Medical Sciences and the University of Iowa and were carried out according to the principles of the Declaration of Helsinki. Human blood was obtained from healthy adult volunteers. Informed consent was obtained from all subjects.
For sheep studies, freshly obtained venous blood was shipped overnight from Iowa City. Human blood was obtained from healthy adult volunteers. Informed consent was obtained from all human subjects.
Biotinylation of RBCs
The biotin labeling method for measurement of RCV in sheep and humans has been previously described.2,16 Briefly, blood was centrifuged at 1000 RCF for 10 minutes and the plasma layer was aspirated and saved for use in reconstituting RBCs after biotin labeling. The RBCs were washed three times with 5 vol of pH 7.4 buffer (“wash buffer”) containing 11.1 mmol per L glucose, 20 mmol per L sodium bicarbonate, 2.3 mmol per L NaHPO4, 1.14 mmol per L Na2PO4, and 154 mmol per L NaCl (356 mOsm/L). Washed RBCs were resuspended in sufficient wash buffer to yield a 25 percent suspension of RBCs. Up to five aliquots of RBCs were biotinylated with increasing amounts of sulfo-NHS-biotin reagent (Pierce Chemical Co., Rockford, IL). To prevent hydrolysis of the reagent before addition to the RBCs, this biotinylation reagent was prepared in wash buffer adjusted to pH 5.0 with HCl immediately before use. The concentration of biotinylation reagent per milliliter of RBCs was chosen empirically to produce discrete individual peaks in the flow histogram generated by plotting event number versus the log of the fluorescent intensity.
The incubation of biotinylation reagent with the washed RBCs was conducted at pH 7.4 and was terminated after 30 minutes (empirically determined to be the optimal length of reaction time)by the addition of a fivefold volume of wash buffer. The biotinylated RBCs were washed four times in wash buffer and resuspended in autologous plasma at a hematocrit (Hct) level of 25 percent.
Complexing of biotin-labeled RBCs
For each of the samples from the standard curve, the reference mixture, and the individual densities of biotinylated RBCs, 1-to 2-µL volumes were aliquoted in triplicate, washed to remove plasma, and resuspended with 12.5 µg of fluorescein-labeled avidin (FITC-AV). After being incubated for 10 minutes at room temperature, 1 mL of wash buffer containing 164 µmol per L biotin was added to the RBC suspension to prevent cross-linking of biotinylated RBCs by tetrameric biotin-binding sites on FITC-AV. Unbound FITC-AV was removed by sedimenting the RBCs and washing four times as described previously.2 The first two washes contained approximately 164 µmol per L biotin; the third and fourth washes contained biotin and 2 percent bovine serum albumin (BSA) to reduce nonspecific aggregation of RBCs (which manifest as “shoulders” on the high-fluorescence aspects of histogram peaks).
Enumeration of each population of biotin-labeled RBCs using flow cytometry
After being complexed with FITC-AV, washed RBCs were resuspended in 0.5 mL of wash buffer with 164 µmol per L biotin and 2 percent BSA previously filtered through a 0.45-µmfilter and enumerated with at least 200,000 events by a flow cytometer (FACSCalibur, BD Biosciences, San Jose, CA). A flow rate of the cytometer and dilution factor for the RBC suspension were chosen to produce 2000 to 5000 RBC counting events per second to ensure sufficient single event counting essential for accurate RCV determination.
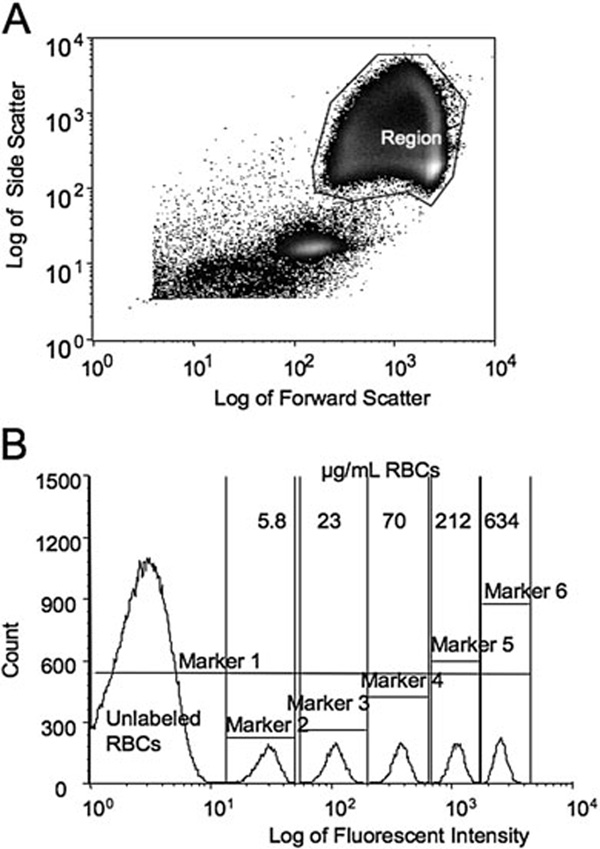
In the plot of log side scatter versus log forward scatter (“scatter” plot), the region containing the RBCs was manually selected to include the vast majority of all RBCs while excluding debris and photomultiplier noise (Fig. 1A). The histogram display of event number versus the log of the fluorescent intensity (Fig. 1B) was divided into discrete populations of biotinylated RBCs using the interval region “markers”; the total number of events, that is, RBCs, within each region was determined. To minimize variability from day to day due to machine performance, the leading edge of the endogenous fluorescence of the unlabeled RBCs was arbitrarily set to 10 on the fluorescent intensity histogram by adjusting the gain of the photomultiplier of the flow cytometer.
Fig. 1.
Flow cytometric results from a representative in vitro mixture of human unlabeled and biotinylated RBCs from one study subject (Human Study 2 from Table 1). (A) Enumeration of RBCs. Selected region includes unlabeled and labeled RBCs. (B) Histogram of unlabeled and labeled RBCs complexed with FITC-AV.Markers delineate the different densities of biotinylated RBCs and the total events enumerated for each population. Marker 1 includes all the RBCs; Marker 2 through 6 denote individual populations labeled at 5.8, 23, 70, 212, and 634 µg per mL RBCs, respectively.
In vitro simulation of in vivo determination of RCV (the reference mixture)
We chose various volumes of blood varying from as little as 5 mL to asmuch as 100 mL to simulate in vivo circulating blood volume of extremely low birth weight infants and small animals. Sufficient numbers of biotinylated RBCs of each biotin density were added to whole blood to produce an enrichment of 1 to 3 percent, which is approximately equal to those deemed appropriate in previous in vivo studies.4,16,17 Known volumes of different densities of biotinylated RBCs were added to produce equal enrichment of each. The number of RBCs added for each biotinylation density was quantitated by gravimetric determination of the mass of the individual densities and the mass of unlabeled blood to within 0.1 mg. Sample weights were converted to volumes using the specific gravity (SG) of blood for sheep with a normal Hct of 30 percent and for humans with a normal Hct of 40 percent of 1.04 and 1.05 g/mL, respectively. The total red blood cell count (RCC) per femtoliter of each volume was calculated as: Hct/mean cell volume (MCV; determined in fL/RBC from the whole blood sample or resuspended RBC sample at the time of measurement of Hct and use).
Preparation of the standard curve
A 5-point standard curve of biotinylated RBCs was prepared as previously described.2,3 Briefly, known volumes of biotinylated RBCs for each of the different densities were diluted into a known volume of unlabeled blood to produce a standard curve of known percentages of biotinylated RBCs that encompassed the estimated likely enrichment percentage of the reference sample. The weight of each component was recorded, and the dilution of each density-labeled population was calculated for each point on a standard calculated enrichment versus measured enrichment curve using the equations below according to the dilution principle18 as follows:
and
In the original development of the biotin method, slopes of the standard curves varied between 1.2 and 1.8.2–4 With the better regional gating and background subtraction used for this study, slopes are typically 1.0 ± 0.10, suggesting that omission of the standard curve and assumption of a slope of 1.0 would potentially introduce an error in volume determination caused by the deviation of the true slope of the standard curve from a slope of 1.0.
Calculation of RCV
The RCVs were calculated independently based on the dilution principle (i.e., the number of RBCs in each population of biotinylated RBCs as a percentage of the total number of RBCs counted).2,4,16,19 For each density i, RCVi was calculated as follows:
where RCVi is the RCV determined by biotin density i, %Bi is the percentage of biotinylated RBCs in the aliquot of the ith density (100%), RCCi is the RCCs in RBCs per fL for the aliquot of RBC of the ith density, Wi is the mass in g of the aliquot of biotinylated RBCs of the ith density, MCVu is the MCV in fL per cell for the reference mixture, SGi is the SG in g per mL in the aliquot added of the ith density, and %BUi is the percentage of biotinylated RBC of ith density in the reference mixture after addition of all the biotin densities. The true volume of the reference mixture was determined gravimetrically after the addition of all biotinylated RBCs.
Statistical analysis
Statistical analyses were performed with computer software (StatView, Abacus Concepts, Berkeley, CA). Significance of differences of mean of triplicate determination of RCVs among the different biotinylation densities was tested by one-way analysis of variance (ANOVA). Within each experimental set, differences between RCVs determined using each biotin density and the true gravimetric RCV were calculated to estimate method bias.
RESULTS
To achieve discrete separation with predictable spacing of multiple densities of biotinylated RBCs, we initially investigated the biotinylation characteristics of RBCs from humans and from sheep. Once these capabilities were achieved, in vitro simulations of multiple, simultaneous in vivo determination of multiple density biotinylated RBC measurements were performed to determine RCV.
Relationship of mass of biotinylating reagent to fluorescence intensity at multiple densities
During method development, RBC aggregates were observed when higher concentrations of biotinylation reagent produced increased biotin on the RBC surface. After the addition of FITC-AV, these aggregates were visible by eye as a granular appearance instead of the typical pellucid haze. The presence of doublets and clusters of RBCs was confirmed by light microscopy. RBCs with higher biotin densities had histogram peaks with “shoulders” of higher fluorescence. The addition of free biotin to the wash buffer used after incubation with FITC-AV prevented aggregation. We interpret this finding as prevention of cross-linking of biotin by tetrameric avidin bridge between two or more RBCs.
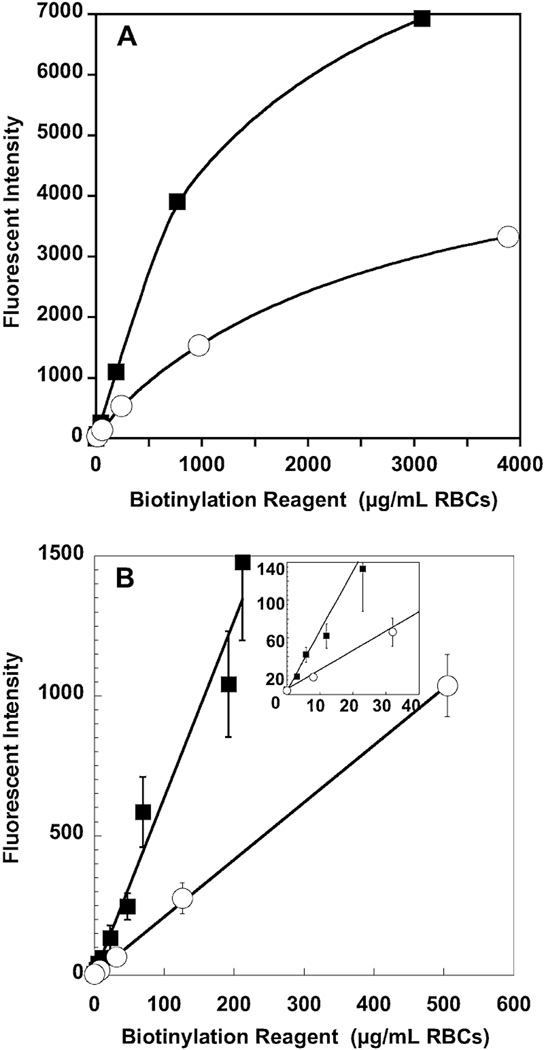
Biotinylation at different reagent masses per volume of RBCs resulted in distinct, discretely spaced populations of biotinylated RBCs (Fig. 1B). Representative plots for RBCs from an individual sheep and individual human subject (Fig. 2A) exhibit linear increase in fluorescent intensity per RBC with mass of biotinylation reagent per milliliter of RBCs until approximately 200 µg of biotinylation reagent per milliliter of RBCs; correlation coefficients for the two linear regressions (for values <500 and 200 for sheep and human, respectively) were 0.9999 and 0.9999, and the two intercepts were 4.2 and −1.4. For both sheep and human, fluorescent intensity per RBC for biotinylation reagent mass of more than 200 exhibit decreasing slopes, consistent with saturation of available biotinylation sites on the RBC membrane (Fig. 2A).
Fig. 2.
Effect of increasing concentrations of biotinylation reagent on fluorescent intensity of RBCs from human (■) and sheep (○). (A) Saturation of biotin binding sites. A study of RBCs from one human and one sheep shows evidence of saturation. (B) Relationship of biotinylation reagent in µg per mL RBCs to fluorescent intensity per RBC. There is a linear relationship between mass of sulfo-NHS-biotin and the fluorescent intensity of the biotinylated RBC for masses of 200 µg per mL or less for humans and 500 µg per mL or less for sheep. Data are depicted as mean ± 1SD.
The combined data for sheep (n = 5) and the combined data for humans (n = 7; Fig. 2B) demonstrate that sheep consistently exhibit lesser biotinylation density per RBC at any given amount of reagent; these data also illustrate the smaller variation between individuals of the same species. For any given individual, linearity with reagent mass was consistently observed (correlation coefficients >0.997 for all 12 RBC samples), but the slopes varied among the individuals. For sheep, the mean ± 1 standard deviation (SD) for the slope was 2.0 ± 0.2; for humans, the mean ± 1SD for the slope was 5.8 ± 1.1.
Determination of RCV by multiple densities
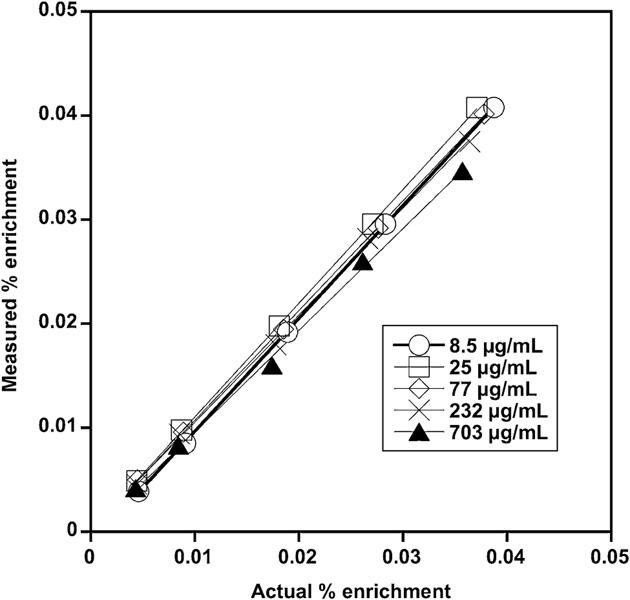
The standard curves for each RBC biotinylation density were similar, with each curve approximating the line of identity. The representative family of standard curves from Sheep Study 3 (Fig. 3) exhibited the characteristic linearity and agreement among densities observed for all sheep and humans studied. The method was accurate as the mean errors were −3 and 1 percent for the sheep and human studies, respectively, and precise as the standard deviation of the errors were 6 and 4 percent, respectively (Table 1).
Fig. 3.
Representative family of standard curves for an in vitro sheep study. The measured enrichment determined by flow cytometry versus the known enrichment determined gravimetrically yields slopes between 0.98 and 1.09 with correlation coefficients of 0.999 or more. A standard curve for each separate density of biotinylated RBCs was prepared using enrichments of biotinylated RBCs that encompassed the expected enrichment of that label in the total blood volume.
Table 1.
In vitro RCV determinations of sheep and human RBCs labeled at four or five biotin densities
| Species and Hct | Reference RCV (mL) | Biotinylating reagent (µg/mL of RBCs) | Measured RCV | Difference from reference RCV (%) | p Value* |
|---|---|---|---|---|---|
| Sheep, 26% | 1.3 | 18 | 1.3 | −1 | 0.36 |
| 36 | 1.2 | −8 | |||
| 72 | 1.2 | −8 | |||
| 144 | 1.3 | −5 | |||
| Mean ± SD | −5 ± 3 | ||||
| Sheep, 40% | 38 | 8.5 | 38 | 1 | 0.34 |
| 34 | 35 | −8 | |||
| 133 | 36 | −5 | |||
| 513 | 36 | −6 | |||
| 2150 | 35 | −7 | |||
| Mean ± SD | −5 ± 4 | ||||
| Sheep, 34% | 30 | 8.6 | 30 | −2 | 0.23 |
| 26 | 30 | 0 | |||
| 77 | 33 | 8 | |||
| 226 | 30 | −3 | |||
| 716 | 33 | 9 | |||
| Mean ± SD | 2 ± 6 | ||||
| Overall mean ± SD for sheep | −3 ± 6 | ||||
| Human, 41% | 36 | 3 | 36 | 0 | 0.95 |
| 7.5 | 36 | 0 | |||
| 19 | 37 | 3 | |||
| 47 | 37 | 2 | |||
| 117 | 36 | 1 | |||
| Mean ± SD | 1 ± 1 | ||||
| Human, 38% | 38 | 6 | 40 | 5 | 0.73 |
| 23 | 41 | 6 | |||
| 70 | 40 | 4 | |||
| 212 | 41 | 6 | |||
| 634 | 34 | −12 | |||
| Mean ± SD | 2 ± 8 | ||||
| Human, 45% | 45 | 6 | 46 | 3 | 0.26 |
| 23 | 45 | 2 | |||
| 71 | 46 | 3 | |||
| 213 | 43 | −3 | |||
| 638 | 44 | −1 | |||
| Mean ± SD | 1 ± 3 | ||||
| Overall mean ± SD for humans | 1 ± 4 |
ANOVA of RCV among the four or five biotinylation densities produced by the different reagent masses; RCV is mean of three replicates per density for each individual sheep or human.
To assess the accuracy and precision of RCV values determined simultaneously and independently using the four or five densities, in vitro simulations of in vivo RCV measurements were performed using blood from three additional sheep and three additional human subjects (Table 1). For both sheep and humans, the RCV measurements were both precise and accurate. The variation in RCV values among densities in any sheep was not significant and did not exhibit a trend with increasing biotinylation reagent. The mean bias in the estimate was 5 percent or less. Likewise, the variation in RCV values among densities in any human subject was not significant and did not exhibit a trend with increasing biotinylation reagent. The mean bias in the estimate was 2 percent or less.
DISCUSSION
Results of the present in vitro studies indicate that RCV can be simultaneously and independently quantitated using sheep or human RBCs labeled with biotin at multiple discrete densities. The corresponding blood volumes can be calculated from the Hct. The successful in vitro validation of this method demonstrated here is a necessary prerequisite before in vivo validation in an animal model and then in human subjects.
The ability to independently perform RCV measurements at multiple, discrete RBC biotin densities in individual subjects has several potential clinical applications. This capability is particularly useful for dynamic studies in which RCV is undergoing change in different pathologic and physiologic states. As such, studies of pregnant women and their offspring are particularly well suited for studies of multiple RCV determinations. The discrete biotin densities can be used to measure RCV and blood volume repeatedly over time in the same individual. In determining new RBC production, the ability to determine RCV at different times during the life span of RBCs would be particularly important in accurately quantifying absolute RBC production despite dilution of labeled RBCs as blood volume expands with somatic growth. Pregnant women and their fetuses and newborns are especially important populations in which such blood volume expansion occurs. This ability could also be important in characterizing RBC kinetics in the following situations: 1) clinical situations in which an intervening transfusion is required; 2) clinical situations in which RBC loss due to hemorrhage or iatrogenic removal occurs; 3) normal conditions in which physiologic changes in blood volume occur immediately after birth; and 4) pathologic conditions such as sepsis, which induce changes in RCV and blood volume. The fact that these studies have not been done previously is largely due to the inability of the previous methods to reliably, accurately, and safely perform multiple RCV measurements using sufficiently small blood volumes to not confound these measurements or render them impractical.
The studies presented here offer an enhancement of capabilities of RBCs biotinylated at a single density. RBCs biotinylated at a single density can be used to measure RCV and blood volume more than once in the same individual.16 This is accomplished by infusing additional RBCs biotinylated at the same single density but requires a correction for residual RBCs from the previous study based on a baseline blood sample taken just before the second study.16 Our group has previously validated and published an equation correcting for the residual biotinylated RBCs remaining in the circulation after all prior volume studies. However, multiple RCV determinations using RBCs biotinylated at a single density have practical limitations. When using the long-lived biotin label, accumulating residual biotin-labeled RBCs from previous studies are inevitable. Theoretical simulations indicated that at least doubling of the volume of labeled RBCs infused with each successive RCV measurement is needed to generate a signal to noise ratio (i.e., increase in blood concentration of the biotin label above baseline) adequate to yield less than 5 percent error. These theoretical conclusions were confirmed empirically, and these results indicated that multiple sequential measurements could be made in vivo using the biotin labeling method.16 The current validation of the five-density approach potentially multiplies the number of feasible RCV measurements by fivefold.
Safe, accurate, and reliable methods for measuring RCV and RBC survival in the pregnant woman, fetus, and infant are needed to further our understanding of the physiology and pathophysiology of a variety of perinatal conditions and their responses to treatment. While the greatest number of published studies of RCV and RBC survival in infants have used 51Cr, the most recent of these studies were published before 1970.20 Ethical concerns about radiation effects in infants, children, and pregnant women have discouraged these studies and motivated the development of alternative methods that avoid exposure to radioactivity.
In addition to safety concerns, all radioactive RBC labeling methods used to determine RCV are susceptible to artifacts arising from either dilution (“squeezing”) or concentration (“sludging”) while obtaining the venous or capillary blood samples.21,22 The common methods include labeling with 51Cr, 99mTc, and 14C-cyanate (sheep). Because it depends on the enumeration of labeled RBCs rather than blood concentration of label, the flow cytometric method for quantifying biotinylated RBCs is not susceptible to either dilution or concentration artifacts. Results of enumeration of biotin-labeled RBCs are expressed as a percentage of total RBCs counted.
As a result of ethical and methodologic problems, several nonradioactive methods have been developed for measuring RCV and RBC survival. These include methods based on nonradioactive stable isotope labels23 and flow cytometric methods based on dilution of hemoglobin (Hb)F by donor HbA24 or detection of minor antigens.25 Only the first of these is suitable for studies using autologous RBCs while the second is only suitable for studies in which allogeneic adult donor blood is administered to fetuses or newborns. The RCV methods that have been published using stable isotopes followed by neutron activation26 or X-ray fluorescence27 are labor-intensive and require highly specialized, expensive equipment26,27 and are not sufficiently sensitive for the small volumes of blood available from neonates27 or both. As a result, these stable isotope methods have not gained widespread use.
Human RBCs exhibit a greater biotinylation density per RBC than sheep RBCs when RBCs are biotinylated with the same mass of reagent per milliliter of RBCs. This difference is predictable from the observation that the mean corpuscular volume of individual human RBCs is approximately three times larger that sheep RBCs, 90 fL versus 30 fL, respectively. If, as an approximation, RBCs were spheres (which they are not), the difference in volume between species would dictate that the surface area of human RBCs would be more than twice that of sheep RBCs. Accordingly, the greater number of biotin moieties and greater fluorescence per RBC are likely attributable to the greater number of biotinylatable moieties (mainly lysine residues) exposed on the outer membrane of human RBCs, rather than to differences in the protein composition of the RBC membrane. The good agreement among the individuals in a species for biotinylation density per RBC at any given biotinylation reagent mass indicates that the fluorescence intensity at a given biotin density is relatively reproducible despite analytic and individual variation.
There are limitations in the interpretation of the results of this study. Although the RCV determinations did not differ significantly among biotin densities, there was a small, but significant overall tendency of the method to underestimate the blood volume in two of the three sheep experiments and in one of the human experiments. The source of the error is not clear. This remains to be assessed empirically and is under active investigation in our laboratories.
Another potential limitation is that the performance of the method in vitro may not be predictive of the method in vivo. For example, the formation of antibodies to biotinylated RBCs could affect repeated measurements of RCV in vivo. Indeed, in a previous study of 20 subjects who received biotinylated RBCs,28 15 percent of study subjects developed antibodies to biotinylated RBCs. However, there was no apparent effect of these antibodies on either measurement of RCV or even long-term survival of biotinylated RBCs,2,28,29 of course, because the emergence of antibodies develops over weeks or months and therefore did not affect initial measurements. This issue remains of concern and will be a focus of our ongoing in vivo human studies.
In summary, the present data provide evidence that RCV can be accurately quantitated in vitro in sheep and human blood using populations of RBCs labeled with different densities of biotin and using flow cytometric quantitation. Because biotin labeling of RBCs offers the promise of a nonradioactive method that is practical, safe, accurate, and more robust than other currently available methods, the next step will be to determine RCV in vivo using RBCs labeled at the biotin densities used here, first in sheep and then in humans. Based on the utility of the ovine model as a surrogate for studies of fetal and newborn infants, the biotin method is likely to be useful both in addressing biologic questions in this model and in establishing safe and effective procedures that can be applied in human studies.
ACKNOWLEDGMENTS
The authors acknowledge the advice of Robert S. Franco, PhD, Division of Hematology/Oncology, Department of Internal Medicine, University of Cincinnati College of Medicine, OH. The authors appreciate the technical assistance of Demet Nalbant, PhD, and Shan Zhu, MD. No author of this manuscript has any conflict of interest relevant to the subject matter of the article.
This work was supported by the National Heart, Lung, and Blood Institute via Grant NIH P01 HL046925 and the Thrasher Research Foundation Grant 02825-3.
ABBREVIATIONS
- FITC-AV
fluorescein-labeled avidin
- RCC
red blood cell count
- RCV
red blood cell volume
- SG
specific gravity (g/mL).
REFERENCES
- 1.Suzuki T, Dale GL. Biotinylated erythrocytes: in vivo survival and in vitro recovery. Blood. 1987;70:791–795. [PubMed] [Google Scholar]
- 2.Mock DM, Lankford GL, Widness JA, Burmeister LF, Kahn D, Strauss RG. Measurement of circulating red blood cell volume using biotin labeled red cells: validation against 51Cr labeled red cells. Transfusion. 1999;39:149–155. doi: 10.1046/j.1537-2995.1999.39299154728.x. [DOI] [PubMed] [Google Scholar]
- 3.Strauss RG, Mock DM, Johnson K, Mock NI, Cress G, Knosp L, Lobas L, Schmidt RL. Circulating RBC volume, measured with biotinylated RBCs, is superior to the Hct to document the hematologic effects of delayed versus immediate umbilical cord clamping in preterm neonates. Transfusion. 2003;43:1168–1172. doi: 10.1046/j.1537-2995.2003.00454.x. [DOI] [PubMed] [Google Scholar]
- 4.Mock DM, Lankford GL, Widness JA, Burmeister LF, Kahn D, Strauss RG. RBCs labeled at two biotin densities permit simultaneous and repeated measurements of circulating RBC volume. Transfusion. 2004;44:431–437. doi: 10.1111/j.1537-2995.2004.00654.x. [DOI] [PubMed] [Google Scholar]
- 5.Wardrop KJ, Tucker RL, Anderson EP. Use of an in vitro biotinylation technique for determination of posttransfusion viability of stored canine packed red blood cells. Am J Vet Res. 1998;59:397–400. [PubMed] [Google Scholar]
- 6.Hoffmann-Fezer G, Mysliwietz J, Mortlbauer W, Zeitler JJ, Eberle E, Honle U, Thierfelder S. Biotin labeling as an alternative nonradioactive approach to determination of red cell survival. Ann Hematol. 1993;67:81–87. doi: 10.1007/BF01788131. [DOI] [PubMed] [Google Scholar]
- 7.Rettig MP, Low PS, Gimm JA, Mohandas N, Wang J, Christian JA. Evaluation of biochemical changes during in vivo erythrocyte senescence in the dog. Blood. 1999;93:376–384. [PubMed] [Google Scholar]
- 8.Russo V, Barker-Gear R, Gates R, Franco R. Studies with biotinylated RBC: (1) use of flow cytometry to determine posttransfusion survival and (2) isolation using streptavidin conjugated magnetic beads. In: Magnani M, DeLoach JR, editors. The use of resealed erythrocytes as carriers and bioreactors. New York: Plenum Press; 1992. pp. 101–107. [DOI] [PubMed] [Google Scholar]
- 9.Valeri C, MacGregor H, Giorgio A, Srey R, Ragno G. Comparison of radioisotope methods and a nonradioisotope method to measure the RBC volume and RBC survival in the baboon. Transfusion. 2003;43:1366–1373. doi: 10.1046/j.1537-2995.2003.00528.x. [DOI] [PubMed] [Google Scholar]
- 10.Christian JA, Rebar AH, Boon GD, Low PS. Methodologic considerations for the use of canine in vivo aged biotinylated erythrocytes to study RBC senescence. Exp Hematol. 1996;24:82–88. [PubMed] [Google Scholar]
- 11.Manodori AB, Kuypers RA. Altered red cell turnover in diabetic mice. J Lab Clin Med. 2002;140:161–165. doi: 10.1067/mlc.2002.126504. [DOI] [PubMed] [Google Scholar]
- 12.Blunt MH, Huisman TH. The blood of sheep: composition and function. New York: Springer-Verlag; 1975. The hemoglobins of sheep. [Google Scholar]
- 13.Brace RA. Blood volume in the fetus and methods for its measurement. In: Nathanielsz PW, editor. Animal models in fetal medicine. Ithaca: Perinatology Press; 1984. pp. 19–36. [Google Scholar]
- 14.Widness JA, Lowe LS, Bell EF, Mock DM, Kistard JA, Bard H. Adaptive responses during anemia and its correction in lambs. J Appl Physiol. 2000;88:1397–1406. doi: 10.1152/jappl.2000.88.4.1397. [DOI] [PubMed] [Google Scholar]
- 15.Moritz KM, Lim GB, Wintour EM. Developmental regulation of erythropoietin and erythropoiesis. Am J Physiol Regul Integr Comp Physiol. 1997;273:R1829–R1844. doi: 10.1152/ajpregu.1997.273.6.R1829. [DOI] [PubMed] [Google Scholar]
- 16.Mock DM, Mock NI, Lankford GL, Burmeister LF, Strauss RG, Widness JA. Red cell volume can be accurately determined in sheep using a nonradioactive biotin label. Pediatr Res. 2008;64:528–532. doi: 10.1203/PDR.0b013e318183f119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franco RS, Lohmann J, Silbertein EB, Mayfiled-Pratt G, Palascak M, Nemeth TA, Joiner CH, Weiner M, Rucknagel DL. Time-dependent changes in the density and hemoglobin F content of biotin-labeled sickle cells. J Clin Invest. 1998;101:2730–2740. doi: 10.1172/JCI2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Committee for Standardization in Hematology (ICSH) Panel on diagnostic applications of radioisotopes in haematology. Standard techniques for the measurement of red-cell and plasma volume. Br J Haematol. 1973;25:801–814. [PubMed] [Google Scholar]
- 19.Mock DM, Lankford GL, Burmeister LF, Strauss RG. Circulating red cell volume and red cell survival can be accurately determined in sheep using the [14C]cyanate label. Pediatr Res. 1997;41:916–921. doi: 10.1203/00006450-199706000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Brugnara C. The neonatal erythrocyte and its disorder. In: Nathan D, Ginsburg D, Orkin S, Look A, editors. Nathan and Oski’s hematology of infancy and childhood. Philadelphia (PA): W.B. Saunders Company; 2003. pp. 19–55. [Google Scholar]
- 21.Rivera LM, Rudolph N. Postnatal persistence of capillary-venous differences in hematocrit and hemoglobin values in low-birth-weight and term infants. Pediatrics. 1982;70:956–957. [PubMed] [Google Scholar]
- 22.Linderkamp O, Versmold HT, Strohhacker I, Messow-Zahn K, Riegel KP, Betke K. Capillary-venous hematocrit differences in newborn infants. I. Relationship to blood volume, peripheral blood flow, and acid base parameters. Eur J Pediatr. 1977;127:9–14. doi: 10.1007/BF00465560. [DOI] [PubMed] [Google Scholar]
- 23.Phillips HM, Holland BM, Abdel-Moiz A, Fayed S, Jones JG, Turner TL, Wardrop CJ, Cockburn F. Determination of red cell mass in assessment and management of anaemia in babies needing blood transfusion. Lancet. 1986;i:882–884. doi: 10.1016/s0140-6736(86)90988-8. [DOI] [PubMed] [Google Scholar]
- 24.Hudson IR, Cavill IA, Cooke A, Holland BM, Hoy TG, Trevett D, Turner TL, Wardrop CA. Biotin labeling of red cells in the measurement of red cell volume in preterm infants. Pediatr Res. 1990;28:199–202. doi: 10.1203/00006450-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Arndt PA, Kumpel BM. Blood doping in athletes—detection of allogeneic blood transfusions by flow cytofluorometry. Am J Hematol. 2008;83:657–667. doi: 10.1002/ajh.21196. [DOI] [PubMed] [Google Scholar]
- 26.Faxelius G, Raye J, Gutberlet R, Swanstrom S, Tsiantos A, Dolanski E, Dehan M, Dyer N, Lindstom D, Brill AB, Stahlman M. Red cell volume measurements and acute blood loss in high-risk newborn infants. J Pediatr. 1977;90:273–281. doi: 10.1016/s0022-3476(77)80650-1. [DOI] [PubMed] [Google Scholar]
- 27.Price DC, Swann SJ, Hung ST-C, Kaufman L, Huberty JP, Shohet SB. The measurement of circulating red cell volume using non-radioactive cesium and fluorescent excitation anaylsis. J Lab Clin Med. 1976;87:535–543. [PubMed] [Google Scholar]
- 28.Cordle DG, Strauss RG, Lankford GL, Mock DM. Antibodies provoked by the transfusion of biotin-labeled red cells. Transfusion. 1999;39:1065–1069. doi: 10.1046/j.1537-2995.1999.39101065.x. [DOI] [PubMed] [Google Scholar]
- 29.Mock DM, Lankford GL, Widness JA, Burmeister LF, Kahn D, Strauss RG. Measurement of red cell survival using biotin labeled red cells: validation against 51Cr labeled red cells. Transfusion. 1999;39:156–162. doi: 10.1046/j.1537-2995.1999.39299154729.x. [DOI] [PubMed] [Google Scholar]