Abstract
Common Variable Immunodeficiency Disorders are a mixed group of heterogeneous conditions linked by lack of immune globulin production and primary antibody failure. This variability results in difficulty in making coherent sense of either immunopathogenesis or the role of various genetic abnormalities reported in the literature. The recent attempt to collate the varied complications in these conditions and define particular clinical phenotypes, has improved our understanding of these diseases. Once refined and confirmed by studies in other series, these studies will facilitate improved accuracy of prognosis and better management of clinical complications; they may also provie a method of analysing outcomes as related to new immunopathologic and genetic findings.
Keywords: Common variable immune deficiency disorders, antibody failure, clinical phenotypes, genetic findings, management of patients
Introduction
Common Variable Immunodeficiency Disorders are a group of diseases in which failure to produce immune globulins and protective antibodies results in symptoms, usually but not always, of recurrent bacterial infections. The common feature is lack of antibodies to pathogens, though for many years it was only possible to demonstrate this by the surrogate measurement of levels of serum immunoglobulins, as assays for specific antibodies were previously unreliable or not available; now however assays for antibodies to panels of common vaccine or environmental antigens are used routinely.
Originally the name “variable” was used to describe a group of late-onset unclassified hypogammaglobulinemias in adults to distinguish these conditions from the more severe, inherited form of agammaglobulinaemia, X-linked agammaglobulinaemia (XLA) found in children (Fudenberg 1971). As more individual (often apparently single gene diseases) were discovered and named over time, the term “variable” was also used for those forms of primary antibody failure that presented in late childhood or in adults. These conditions came to be considered in the same way as the paediatric immune deficiencies, as likely to be single gene diseases due to a variety of mutations (as yet unknown) in non-redundant genes needed for antibody production. However, just as awareness of the multiplicity of genes involved has aided understanding of other late onset immunologically-based diseases (such as diabetes, inflammatory bowel disease or multiple sclerosis), it is equally likely that CVIDs are polygenic disorders in the out-bred human species
So why update our understanding of this group of conditions now? In the past several years improvements in the management of patients with a CVID, including replacement immune globulin and improved microbial therapies, have enabled patients to survive longer (Chapel 2008). This has increased awareness of non-infective clinical complications of these conditions and has enabled the definition of clinical and immunologic phenotypes that can be correlated with outcomes. It is particularly timely to provide the clinical context in which to interpret the newly emerging genetic markers and thus confirmation of these phenotypes is likely to be of use in the study of immunopathogenesis and genetic influences The different survival risks associated with the newly-defined clinical phenotypes may be helpful in guiding the use of riskier treatments that till now have been used sporadically to treat severe complications. The search for additional prognostic indicators continues. Finally we need improved definitions to ensure that only patients with recognisably similar immunological defects are grouped together, to avoid confusion in reporting complications and outcomes in patients with disparate types of CVIDs
Diagnosis
The need for an agreed definition was recognised internationally in the late 1990s; until this time there was confusion due to the variable definitions used in research papers, even though the need to exclude secondary causes of antibody failure was agreed universally. After much discussion, the original ESID and PAGID diagnostic criteria were published in 1999 (Conley 1999). Since then the criteria have been used variably; for example, the minimum age used to define CVIDs has changed, with general consensus but without obvious recognition, to a minimum age of 4 years of age due to the need to exclude children with other immune defects.
An additional starting point for a diagnosis of CVID is to confirm the use of “ primary” antibody failure and to exclude other conditions known to cause failure of antibody production (Table 1), that may have added to confusion in previous series of “CVID” patients; however there will be only a few such patients in any large series. To avoid confusion with secondary antibody failure due to lymphoid malignancy, the definition insists on a period of 2 years following diagnosis of reduced serum immunoglobulin levels without development of a lymphoid malignancy before the diagnosis of a CVID is secure. Other known causes of primary antibody deficiency must also be eliminated (Geha 2007). Whilst this is simpler for common conditions with a known genetic defect, such as XLA, it is not always possible to test for mutations in all the genes currently known to be involved in antibody failure (such as CD40L, CD40, BLINK, μ chain, lambda 5, AID, UNG, SAP and XIAP, (Eastwood 2004)(Lopez-Granados – submitted), let alone the huge number of genes defects in which result in potentially leaky Severe Combined Immune Deficiencies (such as ADA RAG1 or RAG2 or Artemis) (Antony FC 2002) or those immune deficiencies combined with non-immunological conditions (Geha 2007). In practice boys with affected male relations or patients of either gender with a positive family history should be tested for the appropriate gene defect before a diagnosis of a CVID can be made; absence of B cells is no longer sufficient to exclude XLA.
Table 1.
Differential diagnosis of patients with recurrent bacterial infections by age group
|
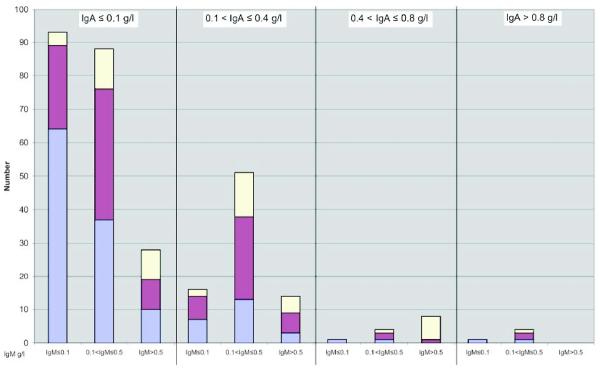
The hallmark of CVIDs is reduced serum levels of serum immune globulins, IgG, IgA and or IgM. Assay results depend on the methods used, the ethnicity, gender mix and age of the reference population. Since the distribution of serum immunoglobulin levels is non-parametric, the 97.5% centiles are used to define normal ranges for each age group. For CVIDs, the serum IgG levels must be below the minimum level found in the general population at that age, recognising that this will include 2.5% of normal subjects. Despite the wide range of IgG levels in CVID patients, the majority of patients (94.2%) had initial IgG levels <4.5 g/l at diagnosis in the European study, similar to those in the US where a cohort of 394 subjects followed at one medical centre, 85% had similar IgG baseline values (Figure 1). This suggests that a lower diagnostic level for serum IgG – potentially 4.5g/l – might provide more comparative cohorts of patients for studies. The small minority excluded from a diagnosis of a CVID by this definition could be classified as “possible CVID”.
Figure 1.
Figure 1(a): European Cohort: Serum Immunoglobulin isotypes at diagnosis.
Patients are divided into four boxes depending on their presenting IgA level. Each box is subdivided by the IgM level into three columns, with those with the lowest IgM levels (≤0.1 g/l) in the first column; those with the highest IgM (>0.5 g/l) in the third column and those with IgM levels between (0.11-0.5 g/l) in the second column. Each column is divided into three, depending on the IgG level; those in the lower part with IgG ≤ 1.0 g/l; those with IgG > 3.0≤ 6.5 g/l at the top and those with IgG 1.1-3 g/l in the middle. The number of patients in each group is in each box and plotted on the y-axis. (from Chapel et al 2008 with permission from Blood)
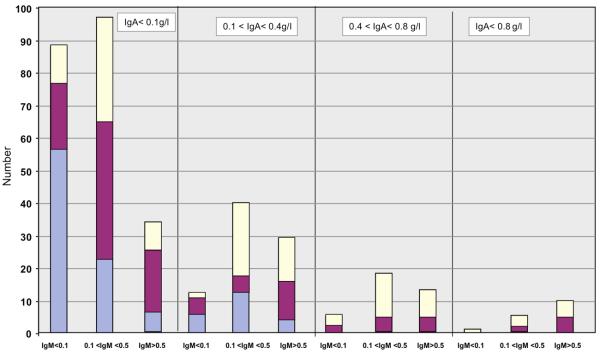
Figure 1(b): United States Cohort: Serum Immunoglobulin isotypes at diagnosis.
Similar to Figure 1a, 349 subjects from one medical centre (Mount Sinai, NYC) are divided into the same categories based on presenting serum immunoglobulin levels.
The range of serum IgM levels is also very variable; these may be low, normal or even raised (Figure 1). Some of these patients may have an unrecognised hyper-IgM syndrome or switch defect. The increasing recognition of new hyper-IgM syndromes (Durandy 2007) and the awareness that these can and do occur in females as well as males, should alert immunologists to a known immunoglobulin class-switch defect, since 18% of patients in the US study(Cunningham-Rundles 1999) and 12% in the European study(Chapel 2008) were in this category. Initial serum IgM levels are also important in prognosis, since there is a significant correlation between increased IgM and the development of polyclonal lymphocytic infiltration or a lymphoid malignancy, both also associated with hyper-IgM syndromes (Chapel 2008).
The number of B cells in patients with CVIDs is also highly variable (Chapel 2008). Whilst 12% have no detectable B cells, 12% have reduced B cells, 54% have in the normal range for B cells (6-16% of circulating lymphocytes), 19% have slightly increased proportions (17-24%) of B cells and 5% have markedly increased numbers. This reflects the range of potential B cell defects that might be involved. For subjects over 49 years of age without B cells, the presence of a thymoma must be excluded by computerised tomography. Raised numbers of B cells may indicate the presence of B cell monoclonality.
A potential misdiagnosis of a CVID is less likely, provided that those with a family history of a suspected immune deficiency, absent B cells, abnormal lymph-node architecture, raised serum IgM, raised B cell numbers or extensive lymphadenopathy are screened genetically (Eastwood 2004) for known single gene diseases
Failure of antibody production following known exposure or immunisation is the crucially important feature of CVIDs in the IUIS definition as well as in most national guidelines. The most commonly used, licensed vaccines include tetanus toxoid, diphtheria toxoid, hepatitis A subunit, hepatitis B surface antigen, rabies, tick-borne encephalitis, and pneumococcal polysaccharides (Pneumovax) and Haemophilus conjugate vaccines. Alternatively, failure of antibody production can be tested following documented infections such as chicken pox or shingles (VZV), measles or invasive Strep. pneumoniae infections. However there is a hierarchy to the strength of the antigens used in both vaccines and during exposure to environmental pathogens. For example tetanus toxoid, measles and VZV are strong immunogens whereas diphtheria toxoid is relatively weak; there are often low titre but detectable antibodies present to tetanus toxoid or measles antigens(Goldacker 2007). Transient antibodies were produced in patients infected with hepatitis C virus in 1994(Healey CJ 1996) thus it seems logical to check not only titre but persistence (that is still present 6 months after immunisation or exposure). Patients with moderately reduced immune globulin levels and some retained antibody production may have declining immune functions later and should be re-evaluated at intervals as they may meet the full criteria for immunoglobulin replacement in time. We propose that the minimum criterium for competence in antibody production is the demonstration of protective levels of specific IgG to two or more protein antigens after immunisation or exposure (Chapel 2007, Orange 2006). Patients with a CVID generally fail to produce a four-fold increase in specific IgG, or to have only transient antibodies, to protein antigens.
The production of antibodies after test immunisation to carbohydrate antigens, such as Pneumovax, is more problematic as there is huge variability in antibody responses amongst healthy individuals to existing panels of pneumococcal antigens (Hare ND 2008, Musher DM 2008 ), whilst a proportion of apparently healthy individuals do not appear to respond to some serotypes. The development of an assay to measure IgG antibodie following Typhum Vi (salmonella carbohydrates) may help to define abnormal responses (Ferry et al 20 ADD REF). The lack of such antibodies does support a diagnosis of a CVID and other forms of antibody failure including chronic lymphocytic leukaemia (CLL) (Chapel HM 1987, Wadhwa PD 2006).
Suggested definitions of CVIDs have occasionally included a requirement for significant infections. However, while the majority of patients who have low levels of serum immunoglobulins have had recurrent acute /unusual/ persistent or severe infections, 10% of patients with remarkably low levels of immunoglobulins may be infection free. These may present with ITP, AHA, a sarcoid-like picture (Arnold, et al 2008), minor infections or even no infections. It is important to check specific antibody production in such patients, as immunoglobulin therapy is not usually required for those patients who are able to produce antibodies.
As discussed, CVIDs remain diagnoses of exclusion, as outlined on Table 1. We have used the following laboratory definition of common variable immunodeficiency disorders (CVIDs); we suggest that subjects included in clinical studies meet these criteria:
4 years of age or older
Serum IgG levels < 4.5 g/l for adults or the 2.5th centile for age, usually with levels of serum IgA below the lower limit of normal for age but alternatively serum IgM below the lower limit of normal for age
Significant lack of antibody responses to protein antigens following immunisation or exposure antigens in at least two assays
Exclusion of all other known causes of failure of immunoglobulin production, as in the IUIS table (Geha 2007)
There are additional criteria that could be used, such as very low numbers of circulating switched or memory B cells, but these are not specific for CVIDs, as loss of these cells occurs in other forms of primary and secondary antibody deficiencies. The recommended investigations can be found in most national and international guidelines and are outlined in Table 2.
Table 2.
Investigations in patients with suspected CVIDs
|
Prevalence
There have been no real changes in the reported prevalence of these conditions in recent years. The overall prevalence of primary antibody deficiencies in Sweden is 1:230 which includes selective IgA deficiency and all IgG subclass deficiencies not usually included in other surveys (Björkander J 1984, Grumach AS 1997, Kirkpatrick P 2007, Matamoros Florí N 1997, Rezaei N 2006, Stray-Pedersen A 2000). These figures vary due to different levels of ascertainment in differing countries (see Table 3). The most reliable minimum figure for CVIDs is a minimum of 1:30,000 (Stray-Pedersen A 2000).
Table 3.
Prevalence of antibody deficiencies around the world (based on data for last two decades and population figures for 2000)
| Numbers of patients* |
Population in 2000 [×106] |
Prevalence | ||
|---|---|---|---|---|
| Sweden | Fasth 1982 | 100 | 5 | 1:50,000 |
| San Paulo, Brazil | Grumach 1997 | 101 | 157 | 1:79,000 |
| Spain | Matamoros Flori 1997 | 300 (213) | 35 | 1:117,000 |
| Norway | Stray-Pedersen 2000 | 303 (150) | 4.45 | 1:15,000 |
| Iran | Rezaei 2006 | 242 (195) | 22 | 1:91,000 |
| Australia & New Zealand |
Kirkpatrick 2007 | 930 | 23 | 1:25,000 |
(Numbers for CVIDs, where given, is in brackets).
Presentation
Several large series of patients have reported the tremendous variety of presenting symptoms in the past and the reader is referred to these (Cunningham-Rundles 1999, Hermaszewski 1993). There are no particular recent changes, other than awareness of the need to investigate those very few patients who develop systemic bacterial disease despite being immunised with appropriate bacterial vaccines (Heath 2000). Although most patients present with recurrent bacterial infections, some present with suggestive inflammatory or autoimmune complications (Arnold, et al 2008, Cunningham-Rundles 2002, Kalha and Sellin 2004, Knight and Cunningham-Rundles 2006).
Complications
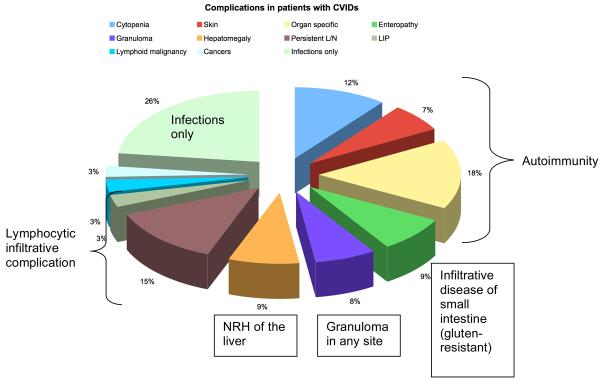
Most of the complications have been known for some years and clinical immunologists are well aware of them (Figure 2). They include conditions due to acute or chronic infections, characteristic inflammatory or autoimmune processes and occasionally neoplastic disease. These complications may be present at the onset or may appear later. As patients are living longer, the prevalence of these complications is increasing. A striking finding in the recent analysis of 334 European patients (representing 9,461 patient-years) showed that there were significant differences in the prevalences of these complications between countries (Chapel 2008). Since the analyses included only those of Caucasian origin, differences due to racial background were excluded. With increased reporting of such patients from newly established registers in countries such as Malaysia, Japan and Iran, new patterns of complications of CVIDs may emerge.
Figure 2. Types of complications in patients with CVIDs and proportions of patients affected.
Patients may have had more than one complication (Data from Chapel et al 2008) [Abbreviations: L/N lymphadenopathy;LIP lymphoid interstitial pneumonitis; NHR nodular regenerative hyperplasia].
While not easily dissected in every case, it is important to distinguish those complications due to previous infections from those associated with underlying immune dysregulation, a cardinal theme in the CVID syndromes. Thus complications can be divided into structural damage due to significant prior infections, particular and unusual infections, and the consequences of the underlying immune dysfunction.
Complications related to infections
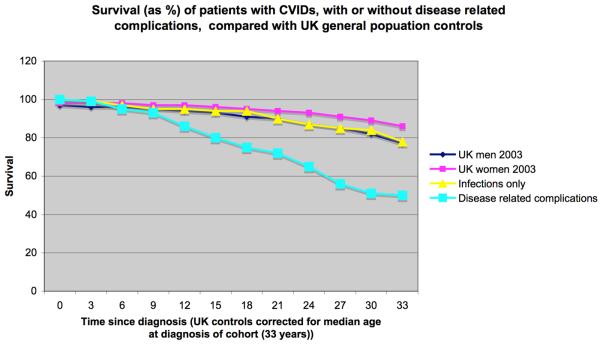
The particular infection risk for patients with any form of antibody failure is from extracellular bacteria, especially those that affect the respiratory tract (Hermaszewski and Webster 1993), namely Haemophilus influenzae (non-encapsulated) and Strep. pneumoniae. Recurrent infections in the upper respiratory tract result in chronic sinusitis. Recurrent infection in the lower respiratory tract were also thought to result in bronchiectasis, since bronchiectasis is a common finding in CVIDs patients at presentation (Kainulainen 2001, Kainulainen, et al 1999b). However in the large European study, bronchiectasis was only related to previous serious infections (pneumonia/septicaemia)(p=0.03) rather than recurrent moderate chest infections (Chapel 2008). Quite intriguing was the fact that these serious infections were not related to presenting levels of serum IgG < 1.5 g/l. Neither was bronchiectasis related to diagnostic delay (a surrogate for lack of immunoglobulin treatment), time since onset of symptoms (for length of disease) or smoking (Chapel 2008). Not surprisingly there was reduced survival in those with bronchiectasis (p=<0.001), as reported previously (Ilowite J 2008). These data suggest that selected serious infections initiate a destructive pulmonary process and that serum levels of IgG antibodies are not the sole determinant of this outcome. Recent data shows that those patients who only suffer from bacterial infections have markedly improved survival compared with those that have other disease related complications (Figure 4), again reflecting the heterogeneity of these conditions (Chapel 2008).
Figure 4. Survival of patients with CVIDs, by years since diagnosis and by clinical phenotype.
(a) Yellow line represents those patients without complications. (b) Turquoise line represents those patients with at least one disease related complication. Kaplan-Meier plot of survival. (Data from Chapel et al 2008)
Patients with CVIDs are also known to be susceptible to infections with particular organisms. Recurrent urinary tract infections due to Ureaplasma uraelyticum may result in a fibrotic bladder (Webster AD 1982). Joint infection with a Mycoplasma species (Bloom, et al 2008, Franz A. 1997, Lee, et al 1993) has resulted in severe destruction. Enteroviral infections causing meningo-encephalitis or dermatomyositis were largely restricted to antibody deficient patients (CVIDs and XLA) when lower doses (0.1 - 0.3 g/Kg/month) of therapeutic immunoglobulin were used (Halliday E 2003). The exact immunological deficiency that causes susceptibility to these particular organisms is unknown, though exploration of innate mechanisms is underway (Cunningham-Rundles, et al 2006). Susceptibility to gastrointestinal Giardia infections is also increased in antibody deficiencies and can cause both malabsorption and enteropathic histology (Onbaşi K 2005). Such selected infections occur more frequently in those with any form of severe antibody failure (e.g. CVIDs or XLA) in comparison with the general population; furthermore there has been a reduction in prevalence since higher doses of immunoglobulin have been used (Wood 2007), so it is likely that the immunopathogenesis involves a dysfunction of B cells.
Autoimmune/inflammatory/immunoproliferative complications
The non-infective, or disease related, complications of CVIDs have been known for some time (Cunningham-Rundles and Bodian 1999, Hermaszewski and Webster 1993) and yet for the most part are quite poorly understood. These intrinsic complications include autoimmune, inflammatory and immunoproliferative pathologies. The variation in cohorts from different countries suggests that these may be related to varying genetic backgrounds. There is some evidence that disease-related complications are more frequent in those with an earlier age of onset (Dalal 1998, Quartier P. 1998) and that these may be associated with a poorer prognosis. Whether or not such individuals represent a distinct antibody deficiency syndrome remains controversial.
Some of these complications can be divided into groups in terms of likely immunopathogenesis (Figure 3). In terms of prevalence, the commonest autoimmune conditions in CVID are the cytopenias, immune thrombocytopenia (ITP) in particular, also haemolytic anaemia or more rarely autoimmune neutropenia; all are much more frequent than in the general population. Cytopenias occur in 11-12% of patients (Chapel 2008, Wang and Cunningham-Rundles 2005), often before antibody deficiency is suspected; it is not uncommon for patients to have several episodes of acute thrombocytopenia or haemolytic anaemia, or both at different times and logistic regression showed no significant difference for autoimmunity when analysed for time since onset of symptoms. The ratio of females to males is 1.2 and there is no association with organ specific autoimmunity though there is a strong association with splenomegaly (p= <0.001) (Chapel 2008). Since a significant number of patients present with cytopenias, this has been used to define a particular clinical phenotype. The risk of early mortality in those patients with a CVID and an autoimmune disease was increased (p= 0.03; relative risk 2.5) (Table 5). There appear to be two separate immunologic features for subjects with these autoimmune manifestations, the presence of very low number of isotype switched memory B cells in peripheral blood, and the likelihood of having a mutation in the transmembrane activator and calcium-modulating cyclophilin ligand interactor, TACI. For example, of 14 individuals with TACI mutations (11 heterozygous and 3 compound heterozygotes, in a cohort of 199 CVID patients tested), 10 had significant splenomegaly. Comparing CVID subjects with and without mutations, these differences were significant (Zhang, et al 2007) and similar to those in a European cohort (Salzer, et al 2008). Whether or not these patients have a genetic predisposition, possibly shared with immunocompetent individuals, remains to be seen.
Figure 3. Individual complications associated with CVIDs across Europe.
Patients may have had more than one complication. (from Chapel et al 2008 with permission from Blood)
Table 5.
Prognostic data for different clinical phenotypes in patients with CVIDs (data from Chapel et al 2008)
| Phenotype | Significance for increased mortality rate compared with infections only group (p values) |
Relative risk |
|---|---|---|
| Enteropathy | <0.001 | 4.0 |
| Polyclonal lymphocytic infiltration |
<0.001 | 3.0 |
| Lymphoid malignancy | 0.002 | 5.5 |
| Autoimmunity | 0.03 | 2.5 |
The solid organ-specific autoimmune diseases, such as pernicious anaemia, thyroid diseases and vitiligo have prevalences in patients with CVIDs that are more than 5%, more common than in the general population. Other autoimmune/ inflammatory conditions include insulin dependent diabetes, psoriasis, systemic lupus, rheumatoid arthritis, juvenile rheumatoid arthritis and uveitis but the prevalence in comparison to other populations has not been clarified (Cunningham-Rundles 2008).
Another characteristic inflammatory phenotype in CVIDs is the presence of multisystem granuloma. The aetiology of these lesions remains a mystery; they may be a consequence of diminished cell mediated immunity resulting in persistent though undetected viral or other infection since they are significantly associated (p= <0.001) with, but not always present alongside, lymphoid interstitial pneumonitis or persistent unexplained lymphadenopathy. Such an infection could trigger an inflammatory response that fails to be down-regulated once the infection is resolved. Alternatively granuloma may represent an intrinsic autoimmune condition, since immune thrombocytopenia or haemolytic anaemia are commonly associated. Increased levels of TNF in serum (Aukrust, et al 1996) in patients with granuloma suggested chronic mononuclear cell activation, possibly with a predisposition to over production of TNF. We showed that the TNF 488A allele is strongly positively associated with granulomatous disease in CVIDs ((Mullighan, et al 1997); a later report suggested an association with particular IL-10 promoter haplotypes (Mullighan, et al 1997, Mullighan, et al 1999).
The aetiology of lymphoid interstitial pneumonitis (LIP) remains obscure. Attempts to demonstrate an infective cause by PCR, viral antigen staining or in situ hybridisation have failed. It is not clear whether or not LIP is the result of HHV-8 or an unknown viral or other agent (Wheat, et al 2005). Since treatment with low-dose oral corticosteroids does reverse the largely T cell (CD4+ or CD8+ cells) infiltration, chronic infection seems less likely. However, post-mortem studies have shown end-stage fibrosis in the lungs of those with LIP treated with steroids (Bhole – personal communication), maybe following cytokine-induced chronic inflammatory changes. This group has a high mortality rate (p= <0.001)(relative risk 3.0) (Table 5) (Chapel 2008).
A poorly characterised gastrointestinal inflammatory complication, unexplained (or idiopathic) enteropathy, also occurs in CVIDs. While the pathology is similar to that of coeliac disease, unlike coeliac disease this enteropathy is not responsive to gluten withdrawal. (Arnold, et al 2008, Aslam A 2004, Daniels JA 2007, Knight and Cunningham-Rundles 2006, Mannon, et al 2006). The infiltrative lymphocytes are commonly T cells but this does not necessarily indicate an autoimmune aetiology. anti-Enterocyte antibodies found in a 13-year-old boy with common variable hypogammaglobulinemia and type I diabetes (ref Catassi 1988) could be responsible, suggesting an autoimmune pathogenesis. However these have also been reported in IPEX where they are thought to be of a secondary nature and not immunopathogenic (Ruemmelea 2004)
There are associations between this gastrointestinal phenotype and persistent unexplained lymphadenopathy (p= 0.01) or hepatomegaly (p= 0.03), which might reflect a common aetiology. This complication has the highest rate of non-malignant mortality (p= <0.001)(relative risk 4.0) (Table 5) (Chapel 2008), possibly due to the resulting malabsorption. It is important to distinguish the secondary effects of particular complications. For example enteropathy was associated with iron deficiency (p= <0.001)(Chapel 2008) and failure to absorbed fat soluble vitamin E is a complication of enteropathy even in patients without obvious symptoms of malabsorption (Aslam A 2004). In contrast low levels of vitamins A and D are found more generally in CVIDs patients, including in those without enteropathy or malabsorption.
Splenomegaly is common in patients with CVIDs (30%), though there are a variety of potential causes (hepatomegaly, infiltration, expansion due to cytopenias, excessive macrophage activity due to persistent infection). Hepatomegaly also has a variety of common causes, including infection with extrinsic agents (such as hepatitis C or alcohol), autoimmune liver disease (chronic active hepatitis or primary biliary cirrrhosis) or infiltrative conditions (malignant metastases). Recently unexplained hepatomegaly in CVIDs has been shown to be due to nodular regenerative hyperplasia (Ward, et al 2008) (Malamut G 2008). There is no evidence of inflammation on liver histology and it is likely to be a non-specific tissue response to varied blood flow rather than being a specific pathological process (Wanless 1990), though there were associations with cytopenias and non-gluten sensitive enteropathy. It was reassuring that there was no correlation with length of time on immunoglobulin therapy, despite higher IgG trough levels (Ward, et al 2008), suggesting that transmission of an unknown pathogen is unlikely. There was only moderate fibrosis in our series though portal hypertension secondary to cirrhosis was common in the French patients (Malamut G 2008).
Continuing the theme of lymphoid hypertrophy, symptomatic lymphoid hyperplasia is another common feature in CVIDs, often in concert with splenomegaly. Investigations for the presence of lymphoma are inevitable. In most cases, benign lymphoid hyperplasia is found on biopsy. Clonal analysis can be misleading (Gompels MM 2003) as oligoclonal lymphocyte populations were demonstrated in biopsies irrespective of histology in a UK study of 29 such CVIDs patients, suggesting that isolated evidence of clonality in biopsy material is insufficient to determine malignancy.
Lymphoid malignancies
About 2-8% of subjects with CVID are diagnosed with a non-Hodgkin's lymphoma, particularly now that patients are living longer. In most cases these are B cell in type, are usually EBV negative and in many cases the B cells are well differentiated.(Gompels MM 2003) These are most likely to occur in the 6th or 7th decades of life but have been reported in younger patients too. These lymphomas almost always extra-nodal, and have a tendency to locate in mucosal regions.(Cunningham-Rundles, et al 2002). The development of lymphoma has been variously associated with the presence of lymphoid hyperplasia, granulomatous disease (Le Guern 2003) and the retention of serum IgM (Chapel 2008). As BCL-6 mutations have commonly been found in CVID lymphomas, a germinal centre origin has been suggested (Ariatti 2000). An earlier report noted a female preponderance; in a European cohort, in that 3 of the 4 lymphomas in 176 subjects were found in women ((Mellemkjaer 2002).
Due to the frequency of recurrent infections associated with low serum immunoglobulin levels in patients with proven chronic lymphocytic leukaemia or non-Hodgkin's lymphoma (NHL), it is important that such a malignancy is distinguished from lymphoma complicating a long standing CVID, particularly as all result in accompanying antibody production failure. The inclusion of a period of two years after a finding of primary antibody failure before a diagnosis of a CVID is confirmed aids the distinction between primary failure and a lymphoid malignancy complicating a CVID. The histology can be indistinguishable from a primary tumour, including absence of EBV antigens on immunohistology; this may be an important distinction from those lymphoid tumours associated with another condition confused with CVIDs, namely X-linked lymphoproliferative disease in which EBV driven tumours are seen. The pathogenesis of tumours associated with CVIDs remains unknown. Treatment however does not differ from spontaneous NHL.
While stomach cancer has been noted in patients with a CVID, it is usually associated with pre-existing pernicious anaemia and appears to be rare in large cohorts nowadays.
Complications due to therapies
Complications due to transmission of infectious agents, such as hepatitis B or hepatitis C, are now extremely rare (Bresee JS 1996, Chapel 2001), due to improvements in the elimination of viruses in the extraction processes of immunoglobulins from plasma. Although concerns remain about new or unknown infectious agents, there is no data to suggest that transmission is a current risk. However, it is still important to continue to monitor patients on replacement therapy.
The side effects of splenectomy, corticosteroid and immune suppressive therapies are well known and are particularly relevant in those who are already immune deficient.
Clinical Phenotypes, Outcomes and Prognosis
The recent analysis of a large cohort of 334 European patients (largely British and Swedish) showed that it is possible to use the clinical and immunologic characteristics described above, to sort out the various different syndromes that make up the group of CVIDs and define the distinct clinical phenotypes (Chapel 2008). This data relates mainly to adults since relatively few children presented with a CVID in this or indeed any other series.
The aim was to develop criteria for clinical phenotyping to help in the interpretation of disease causing and disease-modifying genes, a search was made for significant associations between the various characteristic complications. Only intrinsically related disease complications were used for clinical phenotyping and the criteria chosen were shown to be independent of each other. Common or secondary features, such as splenomegaly and bronchiectasis respectively, were not included. The criteria developed resulted in 83% of patients exhibiting features of one clinical phenotype only; 12.6% of patients showed criteria for 2 clinical phenotypes. Only 8.4% of the whole cohort had features of both lymphocytic infiltration and autoimmunity. The proportion of those suffering only breakthrough infections and or the structural consequences of previous infections was 35%.
Later onset of symptoms was associated with the “infections only” phenotype (p= <0.001) and also with autoimmunity (p= 0.01). Surprisingly the age at onset of symptoms was not predictive of polyclonal lymphocytic infiltration, enteropathy or malignancy. The phenotypic characteristics of individual subjects were stable over a period of years as there were no differences in the prevalences of these clinical phenotypes at 5, 10 or 15 years after diagnosis. This suggests that it may be possible to detect the likely ongoing phenotype within 5 years of diagnosis, though this needs to be confirmed. It is clear from the data that a considerable number of patients have not developed infiltrative, autoimmune or other inflammatory complications over 25 years, whereas other subjects are found to have these problems at the time of initial diagnosis.
The use of clinical phenotyping for prognosis, as well as to clarify the several conditions that make up CVIDs, was demonstrated by the different survival rates in individuals with different phenotypes (Figure 4), suggesting there is biological validity to clinical phenotyping. It also confirmed the clinical suspicion that the particular complications making up the polyclonal lymphocytic infiltrative phenotype are associated with reduced survival (Bates, et al 2004, Quinti, et al 2007) as each clinical phenotype was significantly associated with reduced survival. Not surprisingly those patients who developed a lymphoid malignancy had the worst prognosis (Table 5) and those with infections only had the longest survival, with life expectancy almost equalling that of the general UK population (Figure 4).
Immunophenotyping and clinical outcomes
The role of immunophenotyping has also demonstrated that CVIDs are a group of syndromes. The variability of the numbers and proportions of T, B, NK cells in the circulation of patients with CVIDs has been known for many years (Spickett 1990). The first attempt to classify patients depended on the ability of B cells to secrete different classes of immunoglobulins in vitro after appropriate stimulation (Bryant, et al 1990). This was successful in dividing patients with the most severe hypogammaglobulinaemia from those with more normal serum immunoglobulin levels. There were some correlations of more severe forms with an enlarged spleen but it was not possible at this time to correlate with clinical severity or with other complications.
More recently classifications based on the numbers of B memory cells have been proposed and these show some associations with splenomegaly or autoimmunity (Piqueras, et al 2003, Sanchez-Ramon, et al 2008, Warnatz, et al 2002, Wehr, et al 2008). Since low or absent B memory cell numbers is a feature of most antibody failure syndromes, the practical usefulness of these classifications remains to be determined. However they do provide useful pointers in studies of immunopathogeneses and have been shown to supplement the in vitro secretion classification of Bryant et al. (Ferry BL 2005, Livaditi O 2007).
The use of immunological markers to predict complications was attempted as part of the European study but only significant correlations between serum IgM levels at presentation and the development of polyclonal lymphocytic infiltration (p= 0.018) or lymphoid malignancy (p= 0.02) were found (Chapel 2008). In relation to cellular immunophenotyping, baseline CD8 proportions were lower in those that developed autoimmunity (p= 0.04). Higher proportions of total CD4+ cells were associated with lower serum IgG levels at presentation but since these were not associated with more severe infections, this was not of prognostic significance though possibly interesting in terms of pathogeneses.
Genetics
Patients with CVIDs can be divided into those with a positive family history for some form of primary antibody failure (usually selective IgA deficiency) and those that do not; the latter group are known as spontaneous CVIDs and represent about 90% in Caucasian CVID populations (Cunningham-Rundles 1999). Linkage studies may not be revealing for these patients except for a few non-redundant genes. Comparison of this incidence of inheritance with that in Iran (20% in consanguineous families) (Aghamohammadi A 2008) may suggest autosomal recessive defects in this population. In rare families, perhaps accounting for 1% of CVIDs patients, a pattern of autosomal dominant inheritance involving chromosomes 4q, 16q or 6 appears to be associated with immunoglobulin deficiencies (Vorechovsky I 1995). However for most patients, a mixture of polymorphisms in conjunction with environmental factors are more likely to be involved since these diseases are not familial and are of late onset. In addition, genes that act in a disease modifying capacity, and so contribute to the varying clinical phenotypes, have been described (Tables 6 and 7).
Table 6.
Types of disease causing genes involved in CVIDs
| Single genes |
Mutations in which lead to disease on an AD or an AR basis
|
| Polymorphisms |
Associated with disease susceptibility
|
Table 7.
Disease modifying genes - polymorphisms studied for associations with different clinical complications
| Gene | Clinical phenotype | Association | Reference |
|---|---|---|---|
| TNF | Granuloma | TNF 488A allele, (p = 0.0009) |
(Mullighan, et al 1997, Mullighan, et al 1999) |
| IL-10 | Granuloma | IL-10 a-t- (p = 0.002) | (Mullighan, et al 1999) |
| NOD2 | Granuloma | ? | Hackwood submitted |
| Alpha 1 antitrypsin |
Bronchiectasis | No | (Fazlollahi MR 2006, Sansom ME 2002) |
| MBL | Bronchiectasis Lung fibrosis |
Yes (p = 0.009) Yes (p = 0.037 |
(Litzman J 2008, Mullighan, et al 2000) |
| CTLA4 | Autoimmunity | No | Knight et al 2007 |
| IL-12, IFN | Acute phase responses |
No | (Martinez-Pomar N 2006) |
| Vitamin D receptors | VDR ApaI AA | Yes (p = 0.002), | (Mullighan, et al 1999) |
Possible disease causing mutations
Using a candidate gene approach within families with several affected members, one form of CVID was shown to be caused by homozygous ICOS deficiency in 13 members of 9 families. A large deletion in ICOS was the same in all subjects and is likely to be a founder effect (Grimbacher 2003). This deficiency may be better classified as a hyper-IgM syndrome, being a defect in a T cell related protein involved in immunoglobulin class switching. Several patients with XLP, due to defects in the SAP gene, and gradual onset of antibody failure have been shown to have defects in ICOS and to have a CVID-like clinical syndrome (Eastwood 2004). Mutations in the CD19 gene have also been shown to cause a CVID phenotype; three siblings in a family from Columbia (van Zelm MC 2006) and other subjects identified subsequently (Kanegane, et al 2007). These observations demonstrate that selected mutations in non-redundant genes can result in B cells that are incapable of antibody production.
In contrast, polymorphisms in genes that have more subtle effects on B cell function are also associated with CVIDs. These include TACI and BAFFR. The products of these genes are essential for B cell survival and differentiation. TACI homozygous null mutations or compound heterozygous states are associated in some patients with reduced or absent binding of B cell activating factors BAFF or APRIL though not necessarily with a CVID-like disease (Castigli, et al 2005, Garibyan, et al 2007, Salzer 2005). However there are multiple polymorphisms in TACI, of which some are also found in antibody sufficient individuals in the heterozygous state (Pan-Hammarstrom, et al 2007). These polymorphisms may contribute to susceptibility to antibody failure in our out-bred species. In addition, as discussed above, heterozygous mutations in TACI have been found to be significantly associated with an increased likelihood of developing autoimmunity and lymphoid hyperplasia in patients with a CVID. Whether or not there are mutations that are involved in plasma cell differentiation in those patients with some residual but low levels of antibodies remains to be seen (Mantchev, et al 2007). Since mutations in BAFF or BAFF-R cause B-cell lymphopenia and antibody deficiency in mice, defects of these were sought in patients with CVIDs. One patient and his apparently healthy elderly sibling were identified with identical homozygous deletions in BAFFR (Warnatz K 2005); the significance of this mutation is not yet clear.
Twelve percent of patients with a CVID are likely to have an immunoglobulin class switch defect. The DNA repair protein, Msh5, has been shown to be involved switch regulation and two single nucleotide polymorphisms in the MSH5 gene have been associated with CVID and with selective IgA deficiency (Sekine H 2007). This may also represent a disease susceptibility polymorphism (see Table 6).
Possible disease modifying polymorphisms
Genes that may modify the clinical picture in a patient with a CVID include those for the cytokine TNF, the intracellular non-specific pattern recognition receptor NOD2, the enzyme inhibitor α1 antitrypsin, complement activator mannose binding lectin MBL, the regulator gene for IL-10, interleukins IL-12 and Interferon as well as Vitamin D receptors and CTLA4. These are the candidates that have been studied so far and the results are summarised in Table 7 (Fazlollahi MR 2006, Knight, et al 2007, Litzman J 2008, Martinez-Pomar N 2006, Mullighan, et al 1997, Mullighan, et al 1999, Mullighan, et al 2000, Sansom 2002).
The low levels of vitamins A and D are associated with reduced receptors for retinoic acid (Aukrust P 2000, Kilic SS 2005). Since not all CVIDs patients have these findings, it is may be possible to distinguish a disease modifying polymorphism. There are also patients with unexpected Vitamin D deficiency in the absence of enteropathy. The mechanism of this deficiency is unknown, though vitamin D receptor expression was found to be lower in the peripheral blood mononuclear cells and hair follicles of CVIDs patients when compared to a control group (Ardeniz O 2008); searches continue for disease modifying polymorphisms in the genes for these receptors.
Management
There is new data on survival of patients. Mortality was high in patients in an earlier series (Cunningham-Rundles 1999) and patients with selected complications still have shortened life (Chapel 2008) (Figure 4) but in general patients are surviving longer (Figure 4), maybe due to higher doses of therapeutic protective antibodies and more liberal use of antibiotics (Wood 2007).
However other factors also play a role. With the growth of patient organisations to assist with patient & physician education, patients understand that recurrent or untreated infections can result in either the development of bronchiectasis or worsening of pre-existing disease. This results in prompt treatment of breakthrough infections and easier access to wider range of antibiotics from their medical advisers; many patients hold their own reserves at home with careful instructions for use. One outstanding issue is the role of prophylactic antibiotics in those with pre-existing bronchiectasis.
Patients are followed regularly by clinicians with experience of primary immune deficiencies for the variety of common and rare complications. Careful monitoring of CVID patients to detect autoimmune conditions (including cytopenias), enteropathy or lymphoproliferation is undertaken, since those with such clinical phenotypes have reduced survival. NRH usually develops slowly in patients with chronic systemic illness and regular monitoring of liver function, originally undertaken to detect transmission of HCV, should be continued and endoscopy instigated if portal hypertension is suspected. CVIDs patients with a liver alkaline phosphatase level more than 1.5 times the upper limit of normal for more than 6 months should have a liver biopsy (including reticulin staining) to look for NRH and granulomata (Ward, et al 2008). Immunologists should have a low threshold for obtaining biopsy material from other sites to confirm other treatable complications.
As with other immune deficient or suppressed patients, it is important that advice about travel and everyday risks is available. Provided there is good compliance with immunoglobulin replacement therapy and prompt treatment of any intercurrent infection, patients are not at any known risk relating to travel, life-style, employment or pregnancy.
Treatment with replacement immunoglobulin
Basic treatment for CVIDs is the replacement immunoglobulin in adequate doses. This has been standard therapy for over 30 years, commencing with intramuscular products but abandoned once safe intravenous (IVIg) or rapid subcutaneous (SCIg) administration was possible. The aim of therapy is a significant reduction of bacterial infections and prevention of all serious, life-threatening infections. This has been largely achieved by the IgG trough level reaching normal levels, with data to show that use of larger doses of immunoglobulin (usually 0.4-0.6 g/Kg/month in divided doses) does obviate breakthrough infections (Eijkhout HW 2001, Riofman C. 2003, Roifman C. 1987). A few individual patients require higher doses but these are exceptions. It is important to realise that individual patients require very variable doses to prevent infections and that simply relying on IgG trough levels is not sufficient.
There have been few recent advances in the methods for replacement immunoglobulin therapy. Both IVIg and SCIg have been proven to be efficacious in infection prevention, to be safe in terms of infusion related adverse events (Brennan V. 2003) and to result in improved quality of life for affected patients(Gardulf A 1995a, Gardulf A 1995b) Whilst self-infusion by the IV route at home in the UK, US and now France, has accepted for many years, the use of SCIg (Gardulf A 1995b) has increased the availability of home treatment programmes in the rest of Europe and world-wide.
Management of complications
Not all complications require therapy and the instigation of additional treatments aside from standard measures, depends on a risk assessment in each patient. However some complications do necessitate therapy, such as cortico-steroid therapy (with or without steroid sparing measures) for significant autoimmunity, substantial granulomatous or lymphoid infiltrations in essential organs (Bates, et al 2004, Meyer, et al 2005, Misbah, et al 1992, Park 2005, Thatayatikom, et al 2005), including acute ITP, AIHA or reduced lung function (Bates, et al 2004, Mechanic, et al 1997, Morimoto and Routes 2005). The course of such treatment is a matter of clinical judgement; higher doses tapering in a few weeks or low doses for several months will depend on other factors such as the condition being treated, complications such as secondary candidiasis, herpes simplex infection or the presence of pre-existing osteopenia.
In general moderate doses of corticosteroids have been safe in CVIDs patients and should be used as in immunocompetent individuals, though there are a few reports of intestinal cytomegalovirus infection after long-term high dose steroids in CVIDs (Daniels JA 2007, Kainulainen, et al 1999a, Medlicott SA 2006, Raeiszadeh M 2006). The use of antiviral agents, such as Gancliclovir, appears to be as safe (as in patients with HIV) and effective but again the data is anecdotal (Medlicott SA 2006). Greater use of biopsy material with immunostaining for CMV antigens and in situ hybridisation as well as blood tests such as PCR to determine CMV status, should be used to monitor those in whom prolonged corticosteroids are used. Unfortunately neither Pleconaril or Ribavirin were not found to be useful against enteroviruses in those few that developed enteroviral meningo-encephalitis (Rotbart HA 2001).
A controversial area is that of new therapies for granuloma in CVIDs. As for sarcoidosis, there is international agreement (Arnold, et al 2008) that if therapy is to be used, corticosteroids are the first line of therapy. The use of steroid sparing agents, such as azathioprine, 6 mercaptopurine, cyclosporine A, mycophenolate mofetil, or methotrexate have been used successfully in anecdotal reports but the low prevalence of this complication makes controlled or open trials difficult. The same is true for the use of hydroxychloroquine for the treatment of granuloma and lymphoid interstitial pneumonitis. Reports of the use of TNF antagonists are sparse (Bates, et al 2004, Chua I 2007, Hatab and Ballas 2005, Lin, et al 2006, Smith and Skelton 2001, Thatayatikom, et al 2005) and the outcomes not established. An international treatment register for the rare use of anti-TNF monoclonal antibodies (or other therapies in due course) is needed urgently, along with agreed methods for monitoring, in order to capture successful and unsuccessful courses of treatment.
Therapies for the lymphoid infiltration resulting in gluten-insensitive enteropathy have been largely unsuccessful, though the non-absorbable corticosteroid, budesonide, or low dose 6 mercaptopurine have been found to be useful. The malabsorption resulting from the enteropathy can be extremely debilitating and potentially fatal. Since most patients are not screened for nutrient deficiencies, including vitamins A, D and E, deficiencies are often missed. All patients with unexplained malabsorption should be screened initially for low levels of fat-soluble vitamins and have a bone scan even if they are free of osteomalacia or gastrointestinal related symptoms (Ardeniz O 2008).
The use of splenectomy for those patients with ITP or AHA remains controversial in the absence of data. There is an international audit underway in Europe, though local audits in Oxford and Mount Sinai found that patients were not at increased risk of serious or life-threatening infection with encapsulated organisms if the patients remained on replacement immunoglobulin therapy. Data on the use of Rituximab (anti CD20 monoclonal antibody) therapy in CVIDs patients is limited to anecdotal experience (Cunningham-Rundles 2008); the proven low risk and apparent efficacy for ITP and AIHA in immunocompetent patients is reassuring.
Treatment of malignancies in patients with a CVID is no different from that in an otherwise immunocompetent patient, though it is particularly important to monitor adequacy of replacement immunoglobulin therapy by the serum trough IgG level and to increase the dose of immunoglobulin if needed.
Current controversies
Definition
There are many aspects to this group of primary antibody failure syndromes that are not understood at present but the first responsibility of those who diagnose and manage these patients is to agree on a revised international set of diagnostic criteria. For example, once the age of onset is agreed, i.e. that late onset (>4 years) is a more practical cut-off, registers can use standardised data for diagnostic validation (Chapel 2008). The inclusion of patients with evidence of recurrent or severe infections should distinguish patients with CVIDs from those healthy individuals with low serum immunoglobulin levels but normal antibody production. Whether or not the serum level of IgA should be low, and if so how low, is also unresolved. This is variable depending on the population studied: 49% of European patients had undetectable serum IgA (<0.07g/l) (Chapel 2008) compared with 70% in the US study (Cunningham-Rundles 1999). This might be explained by the difference in gender distribution (58% and 41% males in the studies respectively), since normal levels of serum IgA are higher in women with a CVID (Cunningham-Rundles 1999). The 1999 diagnostic criteria stated that IgG and one of the other 2 major immunoglobulin levels needed to be below the normal range; those with normal IgA but low IgM are still included, though they may form a separate subgroup with a distinct immunopathogenesis.
Diagnostic delay and age of onset
Although there is greater medical and public awareness of immune defects, the length of diagnostic delay for subjects with a CVID has not changed significantly over 20 years (Quinti, et al 2007, Seymour 2005). Since it has been difficult to be certain of the date of onset of characteristic symptoms, greater attention to the patient's past history is essential, particularly if data relating to potential triggers is to be determined. The ages of patients at diagnosis varies markedly as in other late-onset diseases such as insulin dependant diabetes (now known to be polygenic in nature). Whether or not there are particular age peaks for presentation will depend on the use of precise definitions and larger groups of patients than those reported so far. Cunningham-Rundles suggested two age peaks of diagnosis (Cunningham-Rundles 1999) but statistical analysis on the largest group (Chapel 2008) showed no significant variation with age and confirmed the range of age of diagnosis to be 4 – 80+years. Once national registers are truly representative of both adult and paediatric populations, this will become clear.
Causes of complications
Unfortunately nothing definite is known of the causes of enteropathy or lymphoid interstitial pneumonitis. Nor are the aetiologies of the widespread granuloma or the nodular regenerative hyperplasia in the liver known. Granuloma may be due to intrinsic lymphocyte or macrophage activation in patients with an increased genetic susceptibility due to polymorphisms in genes such as TNF or NOD-2. NRH may have a vascular pathogenesis but this is speculative.
Immunopathogenesis
Finally the role of genetics in terms of causative mutations, susceptibility polymorphisms or both, remains controversial and poorly understood at present. It is also problematic to attempt to correlate the numerous studies of selected cell types reported in the literature. This is due to the small numbers of patients, often with poorly characterised disease, studied in any one report. Searching for a single aetiology or even pathogenesis is unlikely to yield significant results in view of the diverse nature of CVIDs. However failure to demonstrate vertical or horizontal transmission of CVIDs makes a transmissible infective agent an unlikely cause.
Defects in pathways involving dendritic cells, T cells and B cells have been reported in patients with CVIDs but usually without reference to the clinical phenotype of these patients. Clinical evidence that failure of antibody production is due to T cells, at least in some patients, comes from reversion to full antibody production with HIV infection (Branda, et al 1996, Webster AD 1989, Wright JJ 1987), suggesting that B cell function is reversible in some situations. On the other hand irreversible intrinsic B cell differentiation defects probably account for those cases with very low numbers of B cells. Since there are different pathways for the production of antibodies to viruses, bacterial cell walls and polysaccharide capsules, involving different cell types, many B cell pathways could be dysfunctional in the majority of patients with normal B cell numbers. Furthermore, presentation of auto-antigens is quite different from that of extrinsic antigens, so that those patients with autoimmune defects as well as primary antibody failure may have more than one defect.
Abnormalities in T cell numbers and function, reviewed in (Giovannetti, et al 2007), have been associated with granuloma and only rarely with unusual viral infections (Narula S 2007). Furthermore enteroviral infections occur in XLA patients in whom T cells are thought to be normal. It is important to distinguish primary T cell abnormalities from those secondary to disease or therapy. Abnormalities of T cells in 19 CVIDs patients with raised inflammatory markers showed enhanced CXCR3 serum levels as well as CXCR3 receptor expression when compared with 44 CVIDs patients with normal acute phase proteins (Fevang B 2008). This suggests that ongoing inflammation does affect immunophenotyping, making identification of the various immunopathogeneses even more complicated.
Future work
Progress in understanding the causes of antibody failure will be limited until the separation of this heterogeneous group of diseases into distinct phenotypes is confirmed and clinical and immunological markers are found.
There is a need to improve and standardise further the criteria for each phenotype, so that these phenotypes can be tested in other populations, particularly in view of the country variations in the rates of complications.
Since the European analysis was published, the majority of patients with CVIDs and unexplained hepatomegaly have been shown by two independent groups to have nodular regenerative hyperplasia. Since these patients have few other complications, it seems sensible to move them to a new subgroup if NRH is biopsy proven, particularly as the aetiology is likely to be different.
Disease related polymorphisms should be sought using the new techniques such as DNA microarrays and RNA expression arrays but only on well-defined, large populations
The search for disease modifying mutations continues, especially for complications such as bronchiectasis, granuloma, polyclonal T or B cell proliferation, the development of lymphoid malignancy or autoimmunity
Animal models, by knock out or knock in methods, are unlikely to provide useful information as CVIDs are not single gene diseases; furthermore mice may have rather different B cell physiology
Table 4.
Correlations between complications (Numbers and p values for associations) (data from Chapel et al 2008)
| Granuloma | Enteropathy -unexplained |
Hepatomegaly - unexplained |
Lymphadenopathy - persistent |
Iron deficiency |
Splenomegaly | |
|---|---|---|---|---|---|---|
| Lymphoid interstitial pneumonitis (n=10) |
5 p=0.0005 |
1 p=1.0 |
2 p=0.21 |
8 p=4.5 ×10−6 |
6 p=0.04 |
7 p=0.009 |
| Granuloma (n=28) | - | 6 p=0.09 |
6 p=0.09 |
13 p=2.3 ×10−5 |
13 p=0.047 |
17 p=0.0004 |
| Enteropathy – unexplained (n=29) |
- | 6 p=0.03 |
9 p=0.01 |
17 p=0.0004 |
14 p=0.03 |
|
| Hepatomegaly – unexplained (n=29) |
- | 11 p=0.001 |
16 p=0.002 |
22 p=1.1 ×10−7 |
||
| Lymphadenopathy – persistent (n=49) |
- | 23 p=0.003 |
35 p=7.5 ×10−11 |
|||
| Iron deficiency (n=96) |
- | 36 p=0.06 |
||||
| Splenomegaly (n=100) |
- |
Acknowledgements
We thank Dr Siraj Misbah for reading the manuscript critically and for helpful suggestions.
We are grateful to the following funding bodies for their support:
NIHR Oxford Biomedical Centre, EU 7th Framework Programme (PADnet), the Primary Immunodeficiency Association, Baxter Healthcare, the Jeffrey Modell Foundation (HC) and grants from the National Institutes of Health, AI 101093, AI-467320, AI-48693 and NIAID Contract 03-22 (CCR)
REFERENCES
- Aghamohammadi A,SL, Saeed SE, Kouhkan A, Heydarzadeh M, Pourpak Z. Alterations in humoral immunity in relatives of patients with common variable immunodeficiency. J Investig Allergol Clin Immunol. 2008;18:266–271. [PubMed] [Google Scholar]
- Antony FC,WA, Bain MD, Harland CC. Recalcitrant palmoplantar warts associated with adult-onset adenosine deaminase deficiency. Br J Dermatol. 2002;147:182–183. doi: 10.1046/j.1365-2133.2002.47562.x. [DOI] [PubMed] [Google Scholar]
- Ardeniz O,AC, Sin A, Ozgen G, Gunsar F, Mete N, Gulbahar O, Kokuludag A. Vitamin D deficiency in the absence of enteropathy in three cases with common variable immunodeficiency. Int Arch Allergy Immunol. 2008;147:74–83. doi: 10.1159/000128661. [DOI] [PubMed] [Google Scholar]
- Ariatti C, Vivenza D, Capello D, Migliazza A, Parvis G, Fassone L, Buonaiuto D, Savinelli F, Rossi D, Saglio G, Gaidano G. Common-variable immunodeficiency-related lymphomas associate with mutations and rearrangements of BCL-6: pathogenetic and histogenetic implications. Hum Pathol. 2000;31:871–873. doi: 10.1053/hupa.2000.7626. [DOI] [PubMed] [Google Scholar]
- Arnold DF, Wiggins J, Cunningham-Rundles C, Misbah SA, Chapel HM. Granulomatous disease: Distinguishing primary antibody disease from sarcoidosis. Clin Immunol. 2008 doi: 10.1016/j.clim.2008.03.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam A,MS, Talbot K, Chapel H. Vitamin E deficiency induced neurological disease in common variable immunodeficiency: two cases and a review of the literature of vitamin E deficiency. Clin Immunol. 2004;112:24–29. doi: 10.1016/j.clim.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Aukrust P, Lien E, Kristoffersen AK, Muller F, Haug CJ, Espevik T, Froland SS. Persistent activation of the tumor necrosis factor system in a subgroup of patients with common variable immunodeficiency--possible immunologic and clinical consequences. Blood. 1996;87:674–681. [PubMed] [Google Scholar]
- Aukrust P,MF, Ueland T, Svardal AM, Berge RK, Frøland SS. Decreased vitamin A levels in common variable immunodeficiency: vitamin A supplementation in vivo enhances immunoglobulin production and downregulates inflammatory responses. Eur J Clin Invest. 2000;30:252–259. doi: 10.1046/j.1365-2362.2000.00619.x. [DOI] [PubMed] [Google Scholar]
- Bates CA, Ellison MC, Lynch DA, Cool CD, Brown KK, Routes JM. Granulomatous-lymphocytic lung disease shortens survival in common variable immunodeficiency. J Allergy Clin Immunol. 2004;114:415–421. doi: 10.1016/j.jaci.2004.05.057. [DOI] [PubMed] [Google Scholar]
- Björkander J,BB, Hanson LA. Primary hypogammaglobulinaemia: impaired lung function and body growth with delayed diagnosis and inadequate treatment. Eur J Respir Dis. 1984;65:529–536. [PubMed] [Google Scholar]
- Bloom KA, Chung D, Cunningham-Rundles C. Osteoarticular infectious complications in patients with primary immunodeficiencies. Curr Opin Rheumatol. 2008;20:480–485. doi: 10.1097/BOR.0b013e3282fd6e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda RF, Moore AL, Hong R, McCormack JJ, Zon G, Cunningham-Rundles C. B-cell proliferation and differentiation in common variable immunodeficiency patients produced by an antisense oligomer to the rev gene of HIV-1. Clin Immunol Immunopathol. 1996;79:115–121. doi: 10.1006/clin.1996.0058. [DOI] [PubMed] [Google Scholar]
- Brennan V,S-BN, Chapel H. Prospective audit of adverse reactions occurring in 459 PAD patients receiving IVIg. Clin Exp Immunol. 2003;133:247–251. doi: 10.1046/j.1365-2249.2003.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresee JS,ME, Coleman PJ, Baron MJ, Schonberger LB, Alter MJ, Jonas MM, Yu MY, Renzi PM, Schneider LC. Hepatitis C virus infection associated with administration of intravenous immune globulin. A cohort study. JAMA. 1996;276:1563–1567. [PubMed] [Google Scholar]
- Bryant A, Calver NC, Toubi E, Webster AD, Farrant J. Classification of patients with common variable immunodeficiency by B cell secretion of IgM and IgG in response to anti-IgM and interleukin-2. Clin Immunol Immunopathol. 1990;56:239–248. doi: 10.1016/0090-1229(90)90145-g. [DOI] [PubMed] [Google Scholar]
- Castigli E, Wilson SA, Garibyan L, Rachid R, Bonilla F, Schneider L, Geha RS. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37:829–834. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- Chapel H, Christie J, Peach V, Chapman R. Five year followup of patients with primary antibody deficiencies following an outbreak of acute hepatitis C. Clin Immunol. 2001;99:320–324. doi: 10.1006/clim.2001.5036. [DOI] [PubMed] [Google Scholar]
- Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, Fieschi C, Thon V, Abedi MR, Hammarstrom L. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112:277–286. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- Chapel H, Misbah SM, Webster ADB. Ochs H, Smith CIE, Puck JM, editors. Assesment of the immune system. Primary Immunodeficiency Diseases - a molecular and genetic approach. 2007;2e:611–632. [Google Scholar]
- Chapel HM,BC. Mechanisms of infection in chronic lymphocytic leukemia. Semin Hematol. 1987;24:291–296. [PubMed] [Google Scholar]
- Chua I,SR, Lear S, Harbord M, Eren E, Raeiszadeh M, Workman S, Webster D. Anti-tumour necrosis factor-alpha therapy for severe enteropathy in patients with common variable immunodeficiency (CVID) Clin Exp Immunol. 2007;150:306–311. doi: 10.1111/j.1365-2249.2007.03481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley M, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies) Clin Immunol. 1999;93:190–197. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles C. Hematologic complications of primary immune deficiencies. Blood Rev. 2002;16:61–64. doi: 10.1054/blre.2001.0185. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles C. Autoimmune manifestations in common variable immunodeficiency. J Clin Immunol. 2008;28 Suppl 1:S42–45. doi: 10.1007/s10875-008-9182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles C, Cooper DL, Duffy TP, Strauchen J. Lymphomas of mucosal-associated lymphoid tissue in common variable immunodeficiency. Am J Hematol. 2002;69:171–178. doi: 10.1002/ajh.10050. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles C, Radigan L, Knight AK, Zhang L, Bauer L, Nakazawa A. TLR9 activation is defective in common variable immune deficiency. J Immunol. 2006;176:1978–1987. doi: 10.4049/jimmunol.176.3.1978. [DOI] [PubMed] [Google Scholar]
- Dalal I, Reid B, Nisbet-Brown E, Roifman C. The outcome of patients with hypogammaglobulinaemia in infancy & early childhood. J Pediatr. 1998;133:144–146. doi: 10.1016/s0022-3476(98)70195-7. [DOI] [PubMed] [Google Scholar]
- Daniels JA,LH, Maitra A, Montgomery EA. Gastrointestinal tract pathology in patients with common variable immunodeficiency (CVID): a clinicopathologic study and review. Am J Surg Pathol. 2007;31:1800–1812. doi: 10.1097/PAS.0b013e3180cab60c. [DOI] [PubMed] [Google Scholar]
- Durandy A, Revy P, Fischer A. Ochs H, Smith CIE, Puck JM, editors. Autosomal hyper_IgM syndromes caused by an intrinsic B cell defect. Primary Immunodeficiency Diseases - a molecular and genetic approach. 2007;2e:269–278. [Google Scholar]
- Eastwood D, Gilmour KC, Nistala K, Meaney C, Chapel H, Sherrell Z, Webster ADB, Davies G, Jones A, Gaspar HB. Prevalence of SAP gene defects in male patients diagnosed with common variable immunodeficiency. Clinical & Experimental Immunology. 2004;137:584–588. doi: 10.1111/j.1365-2249.2004.02581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkhout HW,D-MJ, Kallenberg CG, Weening RS, van Dissel JT, Sanders LA, et al. The effect of two different dosages of intravenous immunoglobulin on the incidence of recurrent infections in patients with primary hypogammaglobulinemia. A randomized, double-blind, multicenter crossover trial. Annals of Internal Medicine. 2001;135:165–174. doi: 10.7326/0003-4819-135-3-200108070-00008. [DOI] [PubMed] [Google Scholar]
- Fazlollahi MR,AA, Hosseini RF, Lotfi AS, Khoshdel A, Farhoudi A, Movahedi M, Gharagozlou M, Mozaffari H, Zandieh F, Mansouri M, Ghaffari J, Rezaei N. Study of alpha1-antitrypsin phenotypes frequencies in patients with primary antibody deficiency. Iran J Allergy Asthma Immunol. 2006;5:69–74. [PubMed] [Google Scholar]
- Ferry BL,JJ, Bateman EA, Woodham N, Warnatz K, Schlesier M, Misbah SA, Peter HH, Chapel HM. Measurement of peripheral B cell subpopulations in common variable immunodeficiency (CVID) using a whole blood method. Clin Exp Immunol. 2005;140:532–539. doi: 10.1111/j.1365-2249.2005.02793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevang B,YA, Damås JK, Bjerkeli V, Ueland T, Holm AM, Beiske K, Aukrust P, Frøland SS. Chemokines and common variable immunodeficiency; possible contribution of the fractalkine system (CX3CL1/CX3CR1) to chronic inflammation. Clin Immunol. 2008 doi: 10.1016/j.clim.2008.09.002. in press. [DOI] [PubMed] [Google Scholar]
- Franz A,W,ADB, Furr PM, Taylor-Robinson D. Mycoplasmal arthritis in patients with primary immunoglobulin deficiency: clinical features and outcome in 18 patients. Brit. J. Rheumatol. 1997;36:661–668. doi: 10.1093/rheumatology/36.6.661. [DOI] [PubMed] [Google Scholar]
- Fudenberg H, Good RA, Goodman HC, Hitzig W, Kunkel HG, Roitt IM, Rosen FS, Rowe DS, Seligmann M, Soothill JR. Primary immunodeficiencies-report of a World Health Organization Committee. Pediatrics. 1971;47:927. [PubMed] [Google Scholar]
- Gardulf A,AV, Bjorkander J, et al. Subcutaneous immunoglobulin replacement in patients with primary antibody deficiencies: safety and costs. Lancet. 1995a;345:365. doi: 10.1016/s0140-6736(95)90346-1. [DOI] [PubMed] [Google Scholar]
- Gardulf A,BH, Andersen V, et al. Lifelong treatment with gammaglobulin for primary antibody deficiencies: the patients' experiences of subcutaneous self-infusions and home therapy. J Adv Nurs. 1995b;21:917. doi: 10.1046/j.1365-2648.1995.21050917.x. [DOI] [PubMed] [Google Scholar]
- Garibyan L, Lobito AA, Siegel RM, Call ME, Wucherpfennig KW, Geha RS. Dominant-negative effect of the heterozygous C104R TACI mutation in common variable immunodeficiency (CVID) J Clin Invest. 2007;117:1550–1557. doi: 10.1172/JCI31023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha R, Notarangelo LD, Casanova JL, Chapel H, Conley ME, Fischer A, Hammarström L, Nonoyama S, Ochs HD, Puck JM, Roifman C, Seger R, Wedgwood J, International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee Primary immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee. J Allergy Clin Immunol. 2007;120:776–794. doi: 10.1016/j.jaci.2007.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannetti A, Pierdominici M, Mazzetta F, Marziali M, Renzi C, Mileo AM, De Felice M, Mora B, Esposito A, Carello R, Pizzuti A, Paggi MG, Paganelli R, Malorni W, Aiuti F. Unravelling the complexity of T cell abnormalities in common variable immunodeficiency. J Immunol. 2007;178:3932–3943. doi: 10.4049/jimmunol.178.6.3932. [DOI] [PubMed] [Google Scholar]
- Goldacker S, Draeger R, Warnatz K, Huzly D, Salzer U, Thiel A, Eibel H, Schlesier M, Peter HH. Active vaccination in patients with common variable immunodeficiency (CVID) Clinical Immunology. 2007;124:294–303. doi: 10.1016/j.clim.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Gompels MM,HE, Lock RJ, Angus B, White H, Larkin A, Chapel HM, Spickett GP, Misbah SA, Smith JL, Associated Study Group Lymphoproliferative disease in antibody deficiency: a multi-centre study. Clin Exp Immunol. 2003;134:314–320. doi: 10.1046/j.1365-2249.2003.02253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Drager R, Eibel H, Fischer B, Schaffer AA, Mages HW, Kroczek RA, Peter HH. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4:261–268. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- Grumach AS,DA, Bellinati-Pires R, Pastorino AC, Jacob CM, Diogo CL, Condino-Neto A, Kirschfink M, Carneiro-Sampaio MM. Brazilian report on primary immunodeficiencies in children: 166 cases studied over a follow-up time of 15 years. J Clin Immunol. 1997;17:340–345. doi: 10.1023/a:1027335000994. [DOI] [PubMed] [Google Scholar]
- Halliday E,WJ, Webster AD. Enteroviral infections in primary immunodeficiency (PID): a survey of morbidity and mortality. J Infect. 2003;46:1–8. doi: 10.1053/jinf.2002.1066. [DOI] [PubMed] [Google Scholar]
- Hare ND,SB, Ballas ZK. Antibody response to pneumococcal vaccination as a function of preimmunization titer. J Allergy Clin Immunol. 2008 doi: 10.1016/j.jaci.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatab AZ, Ballas ZK. Caseating granulomatous disease in common variable immunodeficiency treated with infliximab. J Allergy Clin Immunol. 2005;116:1161–1162. doi: 10.1016/j.jaci.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Healey CJ,SN, Daub J, Davidson F, Yap PL, Fleming KA, Chapman RWG, Simmonds P, Chapel HM. Outbreak of acute hepatitis C following the use of anti-hepatitis C virus screened intravenous immunoglobulin therapy. Gastroenterology. 1996;110:1120–1126. doi: 10.1053/gast.1996.v110.pm8613001. [DOI] [PubMed] [Google Scholar]
- Heath P, Booy R, Griffiths H, Clutterbuck E, Azzopardi HJ, Slack MP, Fogarty J, Moloney AC, Moxon ER. Clinical and immunological risk factors associated with Haemophilus influenzae type b conjugate vaccine failure in childhood. Clin Infect Dis. 2000;31:973–980. doi: 10.1086/318132. [DOI] [PubMed] [Google Scholar]
- Hermaszewski R, Webster ABD. Primary hypogammaglobulinaemia: a survery of clinical manifestations & complications. QJM. 1993;86:31–42. [PubMed] [Google Scholar]
- Hermaszewski RA, Webster AD. Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications. Q J Med. 1993;86:31–42. [PubMed] [Google Scholar]
- Ilowite J,SP, Chawla S. Bronchiectasis: new findings in the pathogenesis and treatment of this disease. Curr Opin Infect Dis. 2008;21:163–167. doi: 10.1097/QCO.0b013e3282f4f237. [DOI] [PubMed] [Google Scholar]
- Kainulainen L, Nikoskelainen J, Vuorinen T, Tevola K, Liippo K, Ruuskanen O. Viruses and bacteria in bronchial samples from patients with primary hypogammaglobulinemia. Am J Respir Crit Care Med. 1999a;159:1199–1204. doi: 10.1164/ajrccm.159.4.9807067. [DOI] [PubMed] [Google Scholar]
- Kainulainen L, Nikoskelainen J, Ruuskanen O. Diagnostic findings in 95 Finnish patients with CVID. J Clin Immunol. 2001;21:145–149. doi: 10.1023/a:1011012023616. [DOI] [PubMed] [Google Scholar]
- Kainulainen L, Varpula M, Liippo K, Svedstrom E, Nikoskelainen J, Ruuskanen O. Pulmonary abnormalities in patients with primary hypogammaglobulinemia. J Allergy Clin Immunol. 1999b;104:1031–1036. doi: 10.1016/s0091-6749(99)70085-0. [DOI] [PubMed] [Google Scholar]
- Kalha I, Sellin JH. Common variable immunodeficiency and the gastrointestinal tract. Curr Gastroenterol Rep. 2004;6:377–383. doi: 10.1007/s11894-004-0053-y. [DOI] [PubMed] [Google Scholar]
- Kanegane H, Agematsu K, Futatani T, Sira MM, Suga K, Sekiguchi T, van Zelm MC, Miyawaki T. Novel mutations in a Japanese patient with CD19 deficiency. Genes Immun. 2007;8:663–670. doi: 10.1038/sj.gene.6364431. [DOI] [PubMed] [Google Scholar]
- Kilic SS,KE, Ilcol YO, Yakut T, Aydin S, Ulus IH. Vitamin a deficiency in patients with common variable immunodeficiency. J Clin Immunol. 2005;25:275–280. doi: 10.1007/s10875-005-4090-6. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick P,RS. Primary immunodeficiency diseases in Australia and New Zealand. J Clin Immunol. 2007;27:517–524. doi: 10.1007/s10875-007-9105-z. [DOI] [PubMed] [Google Scholar]
- Knight AK, Cunningham-Rundles C. Inflammatory and autoimmune complications of common variable immune deficiency. Autoimmun Rev. 2006;5:156–159. doi: 10.1016/j.autrev.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Knight AK, Serrano D, Tomer Y, Cunningham-Rundles C. CTLA-4 gene exon-1 +49 A/G polymorphism: lack of association with autoimmune disease in patients with common variable immune deficiency. J Clin Immunol. 2007;27:95–100. doi: 10.1007/s10875-006-9049-8. [DOI] [PubMed] [Google Scholar]
- Le Guern V, Le Roux G, Martin A, Feuillard J, Cohen P, Poirel H, Raphaël M, Guillevin L, Mouthon L. Lymphoma complicating common variable immunodeficiency with granulomatous disease: report of two cases. Eur J Haematol. 2003;71:459–463. doi: 10.1046/j.0902-4441.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- Lee AH, Levinson AI, Schumacher HR., Jr. Hypogammaglobulinemia and rheumatic disease. Semin Arthritis Rheum. 1993;22:252–264. doi: 10.1016/0049-0172(93)80073-o. [DOI] [PubMed] [Google Scholar]
- Lin JH, Liebhaber M, Roberts RL, Dyer Z, Stiehm ER. Etanercept treatment of cutaneous granulomas in common variable immunodeficiency. J Allergy Clin Immunol. 2006;117:878–882. doi: 10.1016/j.jaci.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Litzman J,FT, Grimbacher B, Gathmann B, Salzer U, Pavlík T, Vlcek J, Postránecká V, Trávnícková Z, Thon V. Mannose-binding lectin gene polymorphic variants predispose to the development of bronchopulmonary complications but have no influence on other clinical and laboratory symptoms or signs of common variable immunodeficiency. Clin Exp Immunol. 2008;153:324–330. doi: 10.1111/j.1365-2249.2008.03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livaditi O,G-BE, Kakkas I, Kapsimali V, Lymberi P, Papastariades C, Douzinas EE. Grouping of patients with common variable immunodeficiency based on immunoglobulin biosynthesis: comparison with a classification system on CD4-naïve cells. Immunol Lett. 2007;114:103–109. doi: 10.1016/j.imlet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Malamut G,ZM, Suarez F, Beaugrand M, Viallard JF, Lascaux AS, Verkarre V, Bechade D, Poynard T, Hermine O, Cellier C. Nodular regenerative hyperplasia: the main liver disease in patients with primary hypogammaglobulinemia and hepatic abnormalities. J Hepatol. 2008;48:74–82. doi: 10.1016/j.jhep.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Mannon PJ, Fuss IJ, Dill S, Friend J, Groden C, Hornung R, Yang Z, Yi C, Quezado M, Brown M, Strober W. Excess IL-12 but not IL-23 accompanies the inflammatory bowel disease associated with common variable immunodeficiency. Gastroenterology. 2006;131:748–756. doi: 10.1053/j.gastro.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Mantchev GT, Cortesao CS, Rebrovich M, Cascalho M, Bram RJ. TACI is required for efficient plasma cell differentiation in response to T-independent type 2 antigens. J Immunol. 2007;179:2282–2288. doi: 10.4049/jimmunol.179.4.2282. [DOI] [PubMed] [Google Scholar]
- Martinez-Pomar N,RS, Ferrer J, Pons J, Munoz-Saa I, Julia MR, de Gracia J, Matamoros N. Elevated serum interleukin (IL)-12p40 levels in common variable immunodeficiency disease and decreased peripheral blood dendritic cells: analysis of IL-12p40 and interferon-gamma gene. Clin Exp Immunol. 2006;144:233–238. doi: 10.1111/j.1365-2249.2006.03063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamoros Florí N,MLJ, Español Boren T, Raga Borja S, Fontan Casariego G. Primary immunodeficiency syndrome in Spain: first report of the National Registry in Children and Adults. J Clin Immunol. 1997;17:333–339. doi: 10.1023/a:1027382916924. [DOI] [PubMed] [Google Scholar]
- Mechanic LJ, Dikman S, Cunningham-Rundles C. Granulomatous disease in common variable immunodeficiency. Ann Intern Med. 1997;127:613–617. doi: 10.7326/0003-4819-127-8_part_1-199710150-00005. [DOI] [PubMed] [Google Scholar]
- Medlicott SA,CS, Horne G, Panaccione R. Multimodal immunosuppressant therapy in steroid-refractory common variable immunodeficiency sprue: a case report complicating cytomegalovirus infection. Int J Surg Pathol. 2006;14:101–106. doi: 10.1177/106689690601400120. [DOI] [PubMed] [Google Scholar]
- Mellemkjaer L, Hammarstrom L, Andersen V, Yuen J, Heilmann C, Barington T, Bjorkander J, Olsen JH. Cancer risk among patients with IgA deficiency or common variable immunodeficiency and their relatives: a combined Danish and Swedish study. Clin Exp Immunol. 2002;130:495–500. doi: 10.1046/j.1365-2249.2002.02004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Lachmann HJ, Webster AD, Burns A, Thway K. Hypercalcemia in a patient with common variable immunodeficiency and renal granulomas. Am J Kidney Dis. 2005;45:e90–93. doi: 10.1053/j.ajkd.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Misbah SA, Spickett GP, Esiri MM, Hughes JT, Matthews WB, Thompson RA, Chapel HM. Recurrent intra-cranial granulomata presenting as space-occupying lesions in a patient with common variable immunodeficiency. Postgrad Med J. 1992;68:359–362. doi: 10.1136/pgmj.68.799.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto Y, Routes JM. Granulomatous disease in common variable immunodeficiency. Curr Allergy Asthma Rep. 2005;5:370–375. doi: 10.1007/s11882-005-0008-x. [DOI] [PubMed] [Google Scholar]
- Mullighan CG, Fanning GC, Chapel HM, Welsh KI. TNF and lymphotoxin-alpha polymorphisms associated with common variable immunodeficiency: role in the pathogenesis of granulomatous disease. J Immunol. 1997;159:6236–6241. [PubMed] [Google Scholar]
- Mullighan CG, Marshall SE, Bunce M, Welsh KI. Variation in immunoregulatory genes determines the clinical phenotype of common variable immunodeficiency. Genes Immun. 1999;1:137–148. doi: 10.1038/sj.gene.6363653. [DOI] [PubMed] [Google Scholar]
- Mullighan CG, Marshall SE, Welsh KI. Mannose binding lectin polymorphisms are associated with early age of disease onset and autoimmunity in common variable immunodeficiency. Scand J Immunol. 2000;51:111–122. doi: 10.1046/j.1365-3083.2000.00697.x. [DOI] [PubMed] [Google Scholar]
- Musher DM,RA, Nahm MH, Graviss EA, Rodriguez-Barradas MC. Initial and subsequent response to pneumococcal polysaccharide and protein-conjugate vaccines administered sequentially to adults who have recovered from pneumococcal pneumonia. J Infect Dis. 2008;198:1019–1027. doi: 10.1086/591629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narula S,LD, Kamoun M, Dalmau J, Levinson AI. Progressive multifocal leukoencephalopathy in a patient with common variable immunodeficiency and abnormal CD8+ T-cell subset distribution. Ann Allergy Asthma Immunol. 2007;98:483–489. doi: 10.1016/S1081-1206(10)60764-8. [DOI] [PubMed] [Google Scholar]
- Onbaşi K,GF, Sin AZ, Ardeniz O, Kokuludağ A, Sebik F. Common variable immunodeficiency (CVID) presenting with malabsorption due to giardiasis. Turk J Gastroenterol. 2005;16:111–113. [PubMed] [Google Scholar]
- Orange J, Hossny EM, Weiler CR, Ballow M, Berger M, Bonilla FA, Buckley R, Chinen J, El-Gamal Y, Mazer BD, Nelson RP, Patel DD, Secord D, Sorensen RU, Wasserman RL, Cunningham-Rundles C. Use of intravenous immunoglobulin in human disease: A review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunology. 2006;117:S525–553. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Pan-Hammarstrom Q, Salzer U, Du L, Bjorkander J, Cunningham-Rundles C, Nelson DL, Bacchelli C, Gaspar HB, Offer S, Behrens TW, Grimbacher B, Hammarstrom L. Reexamining the role of TACI coding variants in common variable immunodeficiency and selective IgA deficiency. Nat Genet. 2007;39:429–430. doi: 10.1038/ng0407-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JE, Beal I, Dilworth JP, Tormey V, Haddock J. The HRCT appearances of granulomatous pulmonary disease in common variable immune deficiency. Eur J Radiol. 2005;54:359–364. doi: 10.1016/j.ejrad.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Piqueras B, Lavenu-Bombled C, Galicier L, Bergeron-van der Cruyssen F, Mouthon L, Chevret S, Debre P, Schmitt C, Oksenhendler E. Common variable immunodeficiency patient classification based on impaired B cell memory differentiation correlates with clinical aspects. J Clin Immunol. 2003;23:385–400. doi: 10.1023/a:1025373601374. [DOI] [PubMed] [Google Scholar]
- Quartier P,DM, de Blic J, Sauverzac R, Sayegh N, Jabado N, Haddad E, Blanche S, Casanova JL, Smith E, Le Diest F, de Saint Basile G, Fischer A. Early and prolonged Intravenous replacement therapy in childhood agammaglobulinaemia: a retrospective survey of 31 patients. J Pediatr. 1998;134:589–596. doi: 10.1016/s0022-3476(99)70246-5. [DOI] [PubMed] [Google Scholar]
- Quinti I, Soresina A, Spadaro G, Martino S, Donnanno S, Agostini C, Claudio P, Franco D, Maria Pesce A, Borghese F, Guerra A, Rondelli R, Plebani A. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol. 2007;27:308–316. doi: 10.1007/s10875-007-9075-1. [DOI] [PubMed] [Google Scholar]
- Raeiszadeh M,KJ, Paston SJ, Diss T, Lowdell M, Hardy GA, Hislop AD, Workman S, Dodi A, Emery V, Webster AD. The T cell response to persistent herpes virus infections in common variable immunodeficiency. Clin Exp Immunol. 2006;146:234–242. doi: 10.1111/j.1365-2249.2006.03209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei N,AA, Moin M, Pourpak Z, Movahedi M, Gharagozlou M, Atarod L, Ghazi BM, Isaeian A, Mahmoudi M, Abolmaali K, Mansouri D, Arshi S, Tarash NJ, Sherkat R, Akbari H, Amin R, Alborzi A, Kashef S, Farid R, Mohammadzadeh I, Shabestari MS, Nabavi M, Farhoudi A. Frequency and clinical manifestations of patients with primary immunodeficiency disorders in Iran: update from the Iranian Primary Immunodeficiency Registry. J Clin Immunol. 2006;26:519–532. doi: 10.1007/s10875-006-9047-x. [DOI] [PubMed] [Google Scholar]
- Riofman C,SH, Berger M, Sorensen R, Ballow M, Buckley R, et al. Comparison of the efficacy of IGIV-C (10%) and IGIV-SD (10%) as replacement in primary immune deficiency: a randomized double blind trial. International Immunopharmacology. 2003;3:1325–1333. doi: 10.1016/s1567-5769(03)00134-6. [DOI] [PubMed] [Google Scholar]
- Roifman C,LH, Gelfand E. High dose versus low dose intravenous immunoglobulin in hypogammaglobulinaemia and chronic lung disease. lancet. 1987;8541:1075–1077. doi: 10.1016/s0140-6736(87)90494-6. [DOI] [PubMed] [Google Scholar]
- Rotbart HA,WAPTRG. Treatment of potentially life-threatening enterovirus infections with pleconaril. Clin Infect Dis. 2001;32:228–235. doi: 10.1086/318452. [DOI] [PubMed] [Google Scholar]
- Ruemmelea R, Brousseb N, Gouleta O. Autoimmune enteropathy: molecular concepts. Curr Opin Gastroenterololgy. 2004;20:587–591. doi: 10.1097/00001574-200411000-00014. [DOI] [PubMed] [Google Scholar]
- Salzer U, Bacchelli C, Buckridge S, Pan-Hammarstrom Q, Jennings S, Lougaris V, Bergbreiter A, Hagena T, Birmelin J, Plebani A, Webster AD, Peter HH, Suez D, Chapel H, McLean-Tooke A, Spickett GP, Anover-Sombke S, Ochs HD, Urschel S, Belohradsky BH, Ugrinovic S, Kumararatne DS, Lawrence TC, Holm AM, Franco JL, Schulze I, Schneider P, Gertz EM, Schaffer AA, Hammarstrom L, Thrasher AJ, Gaspar HB, Grimbacher B. Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Blood. 2008 doi: 10.1182/blood-2008-02-141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer U, Chapel HM, Webster AD, Pan-Hammarstrom Q, Schmitt-Graeff A, Schlesier M, Peter HH, Rockstroh JK, Schneider P, Schaffer AA, Hammarstrom L, Grimbacher B. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nature Genetics. 2005;37:793–794. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramon S, Radigan L, Yu JE, Bard S, Cunningham-Rundles C. Memory B cells in common variable immunodeficiency: clinical associations and sex differences. Clin Immunol. 2008;128:314–321. doi: 10.1016/j.clim.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom ME,FB, Sherrell ZP, Chapel HM. A preliminary assessment of alpha-1 antitrypsin S and Z deficiency allele frequencies in common variable immunodeficiency patients with and without bronchiectasis. Clin Exp Immunol. 2002;130:489–494. doi: 10.1046/j.1365-2249.2002.01995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom ME, Ferry BL, Sherrell ZP, Chapel HM. A preliminary assessment of alpha-1 antitrypsin S and Z deficiency allele frequencies in common variable immunodeficiency patients with and without bronchiectasis. Clin Exp Immunol. 2002;130:489–494. doi: 10.1046/j.1365-2249.2002.01995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine H,FR, Pan-Hammarström Q, et al. Role for Msh5 in the regulation of Ig class switch recombination. Proc. Natl. Acad. Sci. U S A. 2007;104:7193–7198. doi: 10.1073/pnas.0700815104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B, Miles J, Haeney M. Primary antibody deficiency and diagnostic delay. J. Clin. Path. 2005;58:546–547. doi: 10.1136/jcp.2004.016204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Skelton H. Common variable immunodeficiency treated with a recombinant human IgG, tumour necrosis factor-alpha receptor fusion protein. Br J Dermatol. 2001;144:597–600. doi: 10.1046/j.1365-2133.2001.04092.x. [DOI] [PubMed] [Google Scholar]
- Spickett GP, Webster AD, Farrant J. Cellular abnormalities in common variable immunodeficiency. Immunodefic Rev. 1990;2:199–219. [PubMed] [Google Scholar]
- Stray-Pedersen A,AT, Frøland SS. Primary immunodeficiency diseases in Norway. J Clin Immunol. 2000;20:477–485. doi: 10.1023/a:1026416017763. [DOI] [PubMed] [Google Scholar]
- Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Ann Allergy Asthma Immunol. 2005;95:293–300. doi: 10.1016/S1081-1206(10)61228-8. [DOI] [PubMed] [Google Scholar]
- van Zelm MC,RI, van der Burg M, et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N. Engl. J. Med. 2006;354:1901–1912. doi: 10.1056/NEJMoa051568. [DOI] [PubMed] [Google Scholar]
- Vorechovsky I,ZH, Paganelli R, et al. Family and linkage study of selective IgA deficiency and common variable immunodeficiency. Clin. Immunol. Immunopathol. 1995;77:185–192. doi: 10.1006/clin.1995.1142. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD,MV. Infectious complications of chronic lymphocytic leukemia. Semin Oncol. 2006;33:240–249. doi: 10.1053/j.seminoncol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Wang J, Cunningham-Rundles C. Treatment and outcome of autoimmune hematologic disease in common variable immunodeficiency (CVID) J Autoimmun. 2005;25:57–62. doi: 10.1016/j.jaut.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Wanless I. Micronodular transformation (nodular regenerative hyperplasia) of the liver: A report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology. 1990;11:787–797. doi: 10.1002/hep.1840110512. [DOI] [PubMed] [Google Scholar]
- Ward C, Lucas M, Piris J, Collier J, Chapel H. Abnormal liver function in common variable immunodeficiency disorders due to nodular regenerative hyperplasia. Clin Exp Immunol. 2008 doi: 10.1111/j.1365-2249.2008.03711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnatz K, Denz A, Drager R, Braun M, Groth C, Wolff-Vorbeck G, Eibel H, Schlesier M, Peter HH. Severe deficiency of switched memory B cells (CD27(+)IgM(-)IgD(-)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99:1544–1551. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- Warnatz K,SU, Gutenberger S. Finally found: human BAFF-R deficiency causes hypogammaglobulinemia. Clin. Immunol. 2005;115:820. [Google Scholar]
- Webster AD,LA, Spickett G, Beattie R, North M, Thorpe R. Recovery of antibody production after HIV infection in ‘common’ variable hypogammaglobulinaemia. Clin Exp Immunol. 1989;77:309–313. [PMC free article] [PubMed] [Google Scholar]
- Webster AD,T-RD, Furr PM, Asherson GL. Chronic cystitis and urethritis associated with ureaplasmal and mycoplasmal infection in primary hypogammaglobulinaemia. Br J Urol. 1982;54:287–291. doi: 10.1111/j.1464-410x.1982.tb06977.x. [DOI] [PubMed] [Google Scholar]
- Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, Vlkova M, Hernandez M, Detkova D, Bos PR, Poerksen G, von Bernuth H, Baumann U, Goldacker S, Gutenberger S, Schlesier M, Bergeron-van der Cruyssen F, Le Garff M, Debre P, Jacobs R, Jones J, Bateman E, Litzman J, van Hagen PM, Plebani A, Schmidt RE, Thon V, Quinti I, Espanol T, Webster AD, Chapel H, Vihinen M, Oksenhendler E, Peter HH, Warnatz K. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- Wheat WH, Cool CD, Morimoto Y, Rai PR, Kirkpatrick CH, Lindenbaum BA, Bates CA, Ellison MC, Serls AE, Brown KK, Routes JM. Possible role of human herpesvirus 8 in the lymphoproliferative disorders in common variable immunodeficiency. J Exp Med. 2005;202:479–484. doi: 10.1084/jem.20050381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P, Stanworth S, Burton J, Jones A, Peckham DG, Green T, Hyde C, Chapel H. Recognition, clinical diagnosis and management of patients with primary antibody defciencies: a systematic review. Clin Exp Immunol. 2007 doi: 10.1111/j.1365-2249.2007.03432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JJ,BD, Wagner DK, Waldmann TA, Blaese RM, Fleisher TA. Normalization of antibody responsiveness in a patient with common variable hypogammaglobulinemia and HIV infection. N Engl J Med. 1987;317:1516–1520. doi: 10.1056/NEJM198712103172406. [DOI] [PubMed] [Google Scholar]
- Zhang L, Radigan L, Salzer U, Behrens TW, Grimbacher B, Diaz G, Bussel J, Cunningham-Rundles C. Transmembrane activator and calcium-modulating cyclophilin ligand interactor mutations in common variable immunodeficiency: clinical and immunologic outcomes in heterozygotes. J Allergy Clin Immunol. 2007;120:1178–1185. doi: 10.1016/j.jaci.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]