Abstract
Neisseria gonorrhoeae encodes a number of important genes that aid in survival during times of oxidative stress. The same immune cells capable of oxygen-dependent killing mechanisms also have the capacity to generate reactive nitrogen species (RNS) that may function antimicrobially. F62 and eight additional gonococcal strains displayed a high level of resistance to peroxynitrite, while Neisseria meningitidis and Escherichia coli showed a four- to seven-log and a four-log decrease in viability, respectively. Mutation of gonococcal orthologues that are known or suspected to be involved in RNS defence in other bacteria (ahpC, dnrN and msrA) resulted in no loss of viability, suggesting that N. gonorrhoeae has a novel mechanism of resistance to peroxynitrite. Whole-cell extracts of F62 prevented the oxidation of dihydrorhodamine, and decomposition of peroxynitrite was not dependent on ahpC, dnrN or msrA. F62 grown in co-culture with E. coli strain DH10B was shown to protect E. coli viability 10-fold. Also, peroxynitrite treatment of F62 did not result in accumulation of nitrated proteins, suggesting that an active peroxynitrite reductase is responsible for peroxynitrite decomposition rather than a protein sink for amino acid modification.
INTRODUCTION
Neisseria gonorrhoeae is a Gram-negative diplococcus and the causative agent of the sexually transmitted infection gonorrhoea. The US Centers for Disease Control (CDC) estimates that nearly 700 000 new cases of gonorrhoea occur each year in the USA, with a total of 65 million cases worldwide (www.cdc.gov). As an obligate human pathogen, the gonococcus is armed with a wide array of mechanisms that impede an appropriate immune response. Prolonged infection may allow bacterial spread from the genitourinary tract, leading to complications such as disseminated gonococcal infection (DGI) and pelvic inflammatory disease (PID) (Edwards & Apicella, 2004).
Efficient clearance of a bacterial infection often relies on the ability of the immune system to generate a variety of reactive oxygen and nitrogen species (ROS and RNS, respectively) (Bogdan et al., 2000). Monocytes, macrophages and neutrophils contain the NADPH phagocyte oxidase complex (PHOX), which is responsible for the generation of 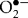 , which either spontaneously or enzymically is disproportionated to H2O2, or to hydroxyl radicals (OH•), by an iron-catalysed Haber–Weiss reaction (Bogdan et al., 2000). Proinflammatory cytokines produced in response to bacterial infection lead to activation of the inducible nitric oxide synthase (iNOS), resulting in de novo iNOS synthesis and micromolar production of nitric oxide (NO) (MacMicking et al., 1997). NO plays an important role in immunity. Through activation or inhibition of key receptors, enzymes and transcription factors, NO is capable of modulating the host immune response (Aktan, 2004; Bogdan, 2001). The NF-κB and mitogen-activated protein kinase pathways have both been shown to be controlled by NO (Pacher et al., 2007).
, which either spontaneously or enzymically is disproportionated to H2O2, or to hydroxyl radicals (OH•), by an iron-catalysed Haber–Weiss reaction (Bogdan et al., 2000). Proinflammatory cytokines produced in response to bacterial infection lead to activation of the inducible nitric oxide synthase (iNOS), resulting in de novo iNOS synthesis and micromolar production of nitric oxide (NO) (MacMicking et al., 1997). NO plays an important role in immunity. Through activation or inhibition of key receptors, enzymes and transcription factors, NO is capable of modulating the host immune response (Aktan, 2004; Bogdan, 2001). The NF-κB and mitogen-activated protein kinase pathways have both been shown to be controlled by NO (Pacher et al., 2007).
During the various stages of gonococcal infection there is an onslaught of both oxidative and nitrosative stress (Carreras et al., 1994; Hampton et al., 1998). Previous research has shown that multiple gonococcal gene products aid in survival under oxidative conditions, and 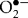 and H2O2 are largely ineffective at eliminating gonococcal infection (Alcorn et al., 1994; Archibald & Duong, 1986; Fu et al., 1989; Rest et al., 1982; Seib et al., 2003). Furthermore, in vitro studies have demonstrated that a subpopulation of N. gonorrhoeae survive within neutrophils, leukocytes that are armed with both ROS and RNS generation potential (Casey et al., 1979, 1986; Simons et al., 2005). This suggests that the organism is capable of persistent colonization within an activated immune system, and that oxygen-dependent killing mechanisms may not be completely effective at eradicating infection (Seib et al., 2006).
and H2O2 are largely ineffective at eliminating gonococcal infection (Alcorn et al., 1994; Archibald & Duong, 1986; Fu et al., 1989; Rest et al., 1982; Seib et al., 2003). Furthermore, in vitro studies have demonstrated that a subpopulation of N. gonorrhoeae survive within neutrophils, leukocytes that are armed with both ROS and RNS generation potential (Casey et al., 1979, 1986; Simons et al., 2005). This suggests that the organism is capable of persistent colonization within an activated immune system, and that oxygen-dependent killing mechanisms may not be completely effective at eradicating infection (Seib et al., 2006).
Pathogenic Neisseria spp. are capable of anaerobic growth using nitrite or NO as an alternative electron acceptor, and previous studies have examined the toxicity of NO within gonococcal and meningococcal strains (Dyet & Moir, 2006; Householder et al., 2000). Interestingly, a gonococcal strain incapable of reducing NO due to mutation of the nitric oxide reductase gene norB, which normally enzymically reduces NO to N2O, remains viable in the presence of nitrite anaerobically (Householder et al., 2000). This result was unexpected, as norB mutations in other denitrifying bacteria have been shown to be lethal under similar conditions (Braun & Zumft, 1991; Cramm et al., 1997).
The simultaneous presence of both 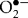 and NO allows for the generation of peroxynitrite (ONOO−), an RNS that is a stronger oxidant (Goldstein & Merenyi, 2008; Szabo et al., 2007) and is much more reactive than its parent molecules (Beckman & Koppenol, 1996). Peroxynitrite reactivity is highly pH-dependent, and both peroxynitrite anion (ONOO−) and peroxynitrous acid (ONOOH) can participate in one- and two-electron oxidation reactions (Beckman & Koppenol, 1996). Reactivity occurs through two distinct pathways, either direct (oxidative processes by peroxynitrite itself) or indirect (reactions initiated by the radicals formed via decomposition of peroxynitrite) mechanisms (Alvarez & Radi, 2003; Szabo et al., 2007). Reactivity with carbon dioxide leads to the formation of carbonate (
and NO allows for the generation of peroxynitrite (ONOO−), an RNS that is a stronger oxidant (Goldstein & Merenyi, 2008; Szabo et al., 2007) and is much more reactive than its parent molecules (Beckman & Koppenol, 1996). Peroxynitrite reactivity is highly pH-dependent, and both peroxynitrite anion (ONOO−) and peroxynitrous acid (ONOOH) can participate in one- and two-electron oxidation reactions (Beckman & Koppenol, 1996). Reactivity occurs through two distinct pathways, either direct (oxidative processes by peroxynitrite itself) or indirect (reactions initiated by the radicals formed via decomposition of peroxynitrite) mechanisms (Alvarez & Radi, 2003; Szabo et al., 2007). Reactivity with carbon dioxide leads to the formation of carbonate (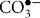 ) and nitrogen dioxide (
) and nitrogen dioxide (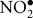 ) radicals, which are one-electron oxidants. Other reactions can result in peroxynitrite decomposition to nitrite (
) radicals, which are one-electron oxidants. Other reactions can result in peroxynitrite decomposition to nitrite (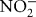 ) and nitrate (
) and nitrate (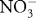 ) and sulphenic acid derivatives. Alternatively, ONOOH decomposes to hydroxyl (OH•) and
) and sulphenic acid derivatives. Alternatively, ONOOH decomposes to hydroxyl (OH•) and 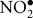 radicals. Peroxynitrite and its decomposition products are capable of oxidizing lipids (Radi et al., 1991b) and DNA (Burney et al., 1999), as well as modifying specific amino acid residues within peptide chains (Pacher et al., 2007). Protein modifications may include oxidation of thiol groups (Radi et al., 1991a) and nitration of tyrosine residues (Reiter et al., 2000).
radicals. Peroxynitrite and its decomposition products are capable of oxidizing lipids (Radi et al., 1991b) and DNA (Burney et al., 1999), as well as modifying specific amino acid residues within peptide chains (Pacher et al., 2007). Protein modifications may include oxidation of thiol groups (Radi et al., 1991a) and nitration of tyrosine residues (Reiter et al., 2000).
Peroxynitrite-mediated killing has been shown in a number of bacterial species (De Groote et al., 1995; Zhu et al., 1992). Incubation of Salmonella enterica serovars Typhimurium and Typhi with 21 μM ONOO− results in a six-log loss of viability (Alam et al., 2006). Escherichia coli and Helicobacter pylori have shown three- and four-log decreases in viability, respectively, when incubated with either molecular-generated ONOO− or directly added 1 mM ONOO− (Dyet & Moir, 2006; Kuwahara et al., 2000). Currently, only Mycobacterium tuberculosis has shown high levels of peroxynitrite resistance, whereby treatment at a 1 mM concentration results in nearly 100 % viability and is dependent on the strain being virulent (Yu et al., 1999).
Currently, the evaluation of peroxynitrite-mediated lethality in the gonococcus has been overlooked. Here we show that N. gonorrhoeae is highly resistant to peroxynitrite, while E. coli DH10B and Neisseria meningitidis strains show a significant degree of killing. Resistance to peroxynitrite-mediated killing occurs both when it is generated molecularly and when it is added directly to growing cultures. Insertional inactivation of gonococcal orthologues to genes known or suspected to be involved in RNS defence in other bacterial species resulted in wild-type levels of peroxynitrite resistance. This suggests that N. gonorrhoeae contains a novel mechanism for avoiding the bactericidal effects of peroxynitrite.
METHODS
Bacterial strains and growth conditions.
All gonococcal mutant strains were derived from laboratory strain F62. Other isolates were obtained from the Neisseria Reference Laboratory, CDC, Atlanta, GA. N. gonorrhoeae and N. meningitidis strains (Supplementary Table S1) were grown on Difco GC medium base (Becton Dickinson) plates with 1 % Kellogg's supplement (GCK) (Kellogg et al., 1963), aerobically in a 5 % CO2 incubator at 37 °C. For broth cultures, inoculum was taken from overnight-grown bacteria from GCK plates and added to GCK broth plus 0.042 % (w/v) NaHCO3 with shaking at 240 r.p.m. in a Gyrotory water bath shaker (New Brunswick Scientific) at 37 °C. E. coli DH10B was the typical cloning strain, grown on either GCK or Luria agar plates at 37 °C. Broth cultures were either in GCK or in Luria broth (LB) plus 0.042 % (w/v) NaHCO3.
Chemicals and reagents.
The long-term NO donor DETA/NO [2,2′-(hydroxynitrosohydrazono) bis-ethanimine], hypoxanthine and xanthine oxidase (Sigma) were used in these studies.
Insertional inactivation of genes potentially involved in ROS and RNS defence.
Gonococcal strain F62 was used to construct an aniA mutant strain with an erythromycin antibiotic cassette inserted within the aniA coding region, as previously described (Householder et al., 1999). Similarly, the norB coding region was interrupted by an erythromycin antibiotic cassette, as previously described (Householder et al., 2000). Gonococcal strain F62 was used to construct mutant strains that had ahpC, cycP, dnrN, gor, msrA or NG1184 insertionally inactivated. Each mutant strain had an antibiotic-resistance cassette inserted within the coding region of the respective gene, allowing for genetic screening of the appropriate clones.
Detailed strain construction was performed as follows. ahpC was insertionally inactivated by inserting a chloramphenicol-resistance cassette into the gene (NG0328). NG0328 is annotated within the STDGEN gonococcal database (http://stdgen.northwestern.edu/) as bacterioferritin comigratory protein (bcp). A Pfam search examining the protein domains placed NG0328 within the AhpC-TSA family of proteins, in agreement with experimental evidence (Jeong et al., 2000). Consequently we consider NG0328 to be ahpC. Two fragments were amplified using GC-Rich Taq (Roche). The 5′ fragment began 433 bp upstream from the ahpC start site and included 125 bp of the coding region, with a PstI restriction site on the 3′ end. The second fragment had an XhoI restriction site on the 5′ end, began 380 bp into the gene and ended 384 bp after the TGA stop codon. The two fragments were cut with either PstI or XhoI and ligated with a complementarily digested cat cassette, then transformed into strain F62, creating strain RUG 8000.
The cytochrome c′ gene (cycP) was inactivated by inserting a kanamycin (aph) resistance cassette into the gene (NG1080). Two fragments were amplified using AmpliTaq (Applied Biosystems). The 5′ fragment began 744 bp upstream of the cycP start site and included 28 bp of the coding region, with an XhoI restriction site on the 3′ end. The second fragment had a HindIII restriction site on the 5′ end, began 445 bp into the gene and ended 793 bp after the TAA stop codon. The two fragments were cut with either XhoI or HindIII and ligated with a complementarily digested aph cassette, then transformed into strain F62, creating strain RUG 8008.
The dnrN gene was inactivated by inserting an erythromycin-resistance (erm) cassette into the gene (NG0653). Two fragments were amplified using GC-rich Taq. The 5′ fragment began 500 bp upstream from the dnrN start site and included 151 bp of the coding region, with a HindIII restriction site at the 3′ end. The second fragment had an XhoI restriction site on the 5′ end, began 356 bp into the gene and ended 386 bp after the TAA stop codon. The two fragments were cut with either HindIII or XhoI and ligated to a complementarily digested erm cassette, then transformed into strain F62, creating strain RUG 8001.
The msrA gene was inactivated by inserting a kanamycin (aph) resistance cassette into the gene (NG2059). Two fragments were amplified using GC-rich Taq. The 5′ fragment began 317 bp upstream from the msrA start site and included 299 bp of the coding region, with a HindIII restriction site at the 3′ end. The second fragment had an XhoI restriction site on the 5′ end, began 1320 bp into the gene and ended 471 bp after the TGA stop codon. The two fragments were cut with either HindIII or XhoI and ligated to a complementarily digested aph cassette, then transformed into strain F62, creating strain RUG 8002.
The gor gene was inactivated by inserting an erythromycin-resistance cassette into the gene (NG0925). Two fragments were amplified using AmpliTaq. The 5′ fragment began 692 bp upstream from the gor start site and included 237 bp of the coding region, with a PstI restriction site at the 3′ end. The second fragment had an XhoI restriction site on the 5′ end, and began 88 bp upstream of the TGA stop codon. The two fragments were cut with PstI or XhoI and ligated to a complementarily digested erm cassette, then transformed into strain F62, creating strain RUG 8010.
NG1184 was inactivated by inserting an erythromycin-resistance cassette into the gene, creating strain RUG 8011. Two fragments were amplified using AmpliTaq. The 5′ fragment began 680 bp upstream from the transcriptional start site and included 263 bp of the coding region, with a PstI restriction site at the 3′ end. The second fragment had an XhoI restriction site on the 5′ end, began 1062 bp into the gene and ended 851 bp after the TAA stop codon. The two fragments were cut with either PstI or XhoI and ligated to a complementarily digested erm cassette, then transformed into strain F62. RUG 8012 was created by transforming this same DNA fragment into F62.
Strains that contained two or three insertionally inactivated genes (RUG 8003, RUG 8004, RUG 8005, RUG 8006, RUG 8012) were created using the same methods, except that the same strain was used for each transformation, thus generating multiple gene inactivations within the same genetic background. Cloning of DNA sequences was verified at ACGT, Inc.
Survival counts of bacteria exposed to RNS produced by molecular generation.
Gonococcal strain F62 and E. coli strain DH10B were grown in GCK and LB respectively, in a water bath shaker to OD600 0.5 and diluted to OD600 0.25 into fresh media containing a final concentration of either 2 mM DETA/NO, 0.1 units xanthine oxidase ml−1 and 250 μM hypoxanthine, or 2 mM DETA/NO and 0.1 units xanthine oxidase ml−1 and 250 μM hypoxanthine. At time points of 0, 30, 60, 90 and 120 min, bacteria were diluted and plated to determine viability. The extent of RNS-mediated killing or growth of the culture was calculated by dividing the measured c.f.u. by the initial c.f.u.
Survival counts with direct peroxynitrite addition.
The ability of peroxynitrite to kill various gonococcal and meningococcal strains, as well as E. coli DH10B, was tested by directly adding peroxynitrite (Calbiochem) at final concentrations of 1 or 2 mM, or no addition, to exponential-phase cultures diluted into fresh GCK broth plus NaHCO3. Peroxynitrite is stable in 4.7 % NaOH and begins to decompose at pH 8 or below. Peroxynitrite-mediated killing was examined by diluting bacteria and plating at time points of 0, 30, 60, 90 and 120 min to determine cell viability as described above. For experiments determining whether N. gonorrhoeae could protect E. coli from RNS-mediated killing, F62 and E. coli DH10B were added to cultures at an input ratio of either 1 : 1 or 5 : 1 with respect to OD600. E. coli DH10B was added at a constant OD600 of 0.125 for all conditions, while F62 was added at an OD600 of either 0.125 or 0.625. E. coli viable cell counts were obtained by dilution-plating of cultures with either no addition or addition of 1 mM peroxynitrite after 120 min of growth on Luria agar plates. This medium supports growth of E. coli but not N. gonorrhoeae.
The dose response of peroxynitrite-mediated killing was assayed by following the viability of strains when exposed to increasing concentrations of peroxynitrite, ranging from no addition to 1 mM. After 120 min, the cells were diluted and plated to determine viability. The c.f.u. at 120 min was divided by the initial c.f.u.
Prevention of dihydrorhodamine (DHR) oxidation by whole bacterial cells.
To assay for apparent peroxynitrite reductase activity, cells were tested for their ability to block peroxynitrite-mediated oxidation of DHR (Sigma). Gonococcal strains and E. coli DH10B were grown to OD600 1.0. The cells were then washed and resuspended in a solution of DHR (50 μM) in 0.1 M phosphate buffer, pH 7.3, containing 0.1 mM diethylenetriamine-pentaacetic acid and 5 mM glucose. The cells were concentrated 10-fold to increase the probability of cells reacting with peroxynitrite before it reacted with DHR. At time 0, peroxynitrite in 4.7 % NaOH was added to a final concentration of 100 μM under conditions of intense stirring. Conversion of DHR to rhodamine was determined spectrophotometrically (ε=78 800 M−1cm−1), with the background and final absorbance at 500 nm determined before and 1 min after peroxynitrite addition.
SDS-PAGE and Western blotting for protein nitration.
A control reaction of purified BSA (Promega) at 5 mg ml−1, or bacterial cultures grown to OD600 0.5 in GCK broth plus NaHCO3 and diluted to OD600 0.25, were exposed to three treatments with peroxynitrite to a final concentration of 1 mM with continuous mixing. Bacterial cultures were concentrated by spinning down 1 ml cells and resuspending in 50 μl Tris-buffered saline prior to peroxynitrite addition, or by harvesting cells at 5 and 30 min following the outgrowth stage while in a water shaker bath after peroxynitrite treatment. Samples of purified BSA were taken prior to and after peroxynitrite addition. The cells were lysed with SDS sample buffer lacking DTT and the proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with anti-nitrotyrosine mAb (clone 1AG, Upstate Biotechnology). A sheep anti-mouse IgG horseradish peroxidase-linked antibody was used for secondary probing. Antibody binding was detected by ECL (Amersham Pharmacia Biotech).
RESULTS
Resistance to RNS and ROS
We examined the effects of oxidative and nitrosative stress on the growth and viability of gonococcal strain F62 by adding molecular generators to growing cultures that produced 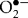 (xanthine oxidase), NO (DETA/NO; long-term NO donor) or a combination of both to produce peroxynitrite (De Groote et al., 1997). It should be noted that the xanthine oxidase/hypoxanthine enzymic system generates equimolar amounts of
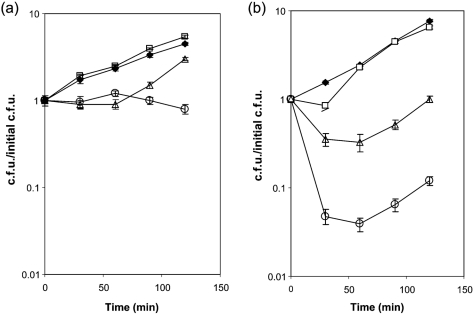
(xanthine oxidase), NO (DETA/NO; long-term NO donor) or a combination of both to produce peroxynitrite (De Groote et al., 1997). It should be noted that the xanthine oxidase/hypoxanthine enzymic system generates equimolar amounts of 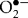 and H2O2; however, catalase activity is constitutive and is nearly 100 times higher in N. gonorrhoeae than in E. coli (Hassett et al., 1990). Gonococci grew normally in the presence of DETA/NO (Fig. 1a). In the presence of xanthine oxidase, gonococcal growth was arrested for 60 min, after which time growth resumed normally. Surprisingly, gonococci still survived in the presence of both the NO and
and H2O2; however, catalase activity is constitutive and is nearly 100 times higher in N. gonorrhoeae than in E. coli (Hassett et al., 1990). Gonococci grew normally in the presence of DETA/NO (Fig. 1a). In the presence of xanthine oxidase, gonococcal growth was arrested for 60 min, after which time growth resumed normally. Surprisingly, gonococci still survived in the presence of both the NO and 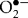 generator, though growth was inhibited. It is important to note that F62 showed no reduction in viability under any condition. Conversely, E. coli DH10B showed 10-fold reduced viability in the presence of
generator, though growth was inhibited. It is important to note that F62 showed no reduction in viability under any condition. Conversely, E. coli DH10B showed 10-fold reduced viability in the presence of 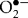 and 100-fold reduced viability under conditions of ONOO− generation (Fig. 1b).
and 100-fold reduced viability under conditions of ONOO− generation (Fig. 1b).
Fig. 1.
Effects of ROS and RNS on N. gonorrhoeae F62 (a) and E. coli DH10B (b) viability. Exponential-phase bacterial cultures were diluted to OD600 0.25 into fresh media containing the following final concentrations: no addition (⧫); 2 mM DETA/NO (□); 0.1 units xanthine oxidase ml−1 and 250 μM hypoxanthine (▵); 2 mM DETA/NO, 0.1 units xanthine oxidase ml−1 and 250 μM hypoxanthine (○). At the indicated times, bacteria were diluted and plated to determine viable counts. Results represent the mean±se of three independent determinations.
Resistance of N. gonorrhoeae but not E. coli to peroxynitrite
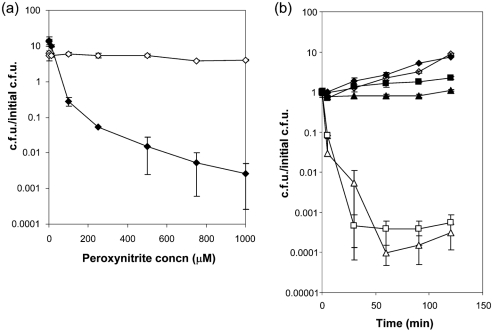
The use of molecular generators to produce peroxynitrite raises concerns because it is not possible to determine the effects of each reactive species within the culture (NO, 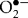 and ONOO−). To address this, experiments were modified to use the direct addition of peroxynitrite. As seen in Fig. 2(a), N. gonorrhoeae displayed significant resistance to the direct addition of peroxynitrite over a range of 1 μM to 1 mM. Even at the highest concentration of RNS, there was no loss in gonococcal viability. Conversely, E. coli was highly susceptible to RNS-mediated killing, displaying an approximate four-log decrease in viability at a concentration of 1 mM ONOO− (Fig. 2a).
and ONOO−). To address this, experiments were modified to use the direct addition of peroxynitrite. As seen in Fig. 2(a), N. gonorrhoeae displayed significant resistance to the direct addition of peroxynitrite over a range of 1 μM to 1 mM. Even at the highest concentration of RNS, there was no loss in gonococcal viability. Conversely, E. coli was highly susceptible to RNS-mediated killing, displaying an approximate four-log decrease in viability at a concentration of 1 mM ONOO− (Fig. 2a).
Fig. 2.
Dose response and time course of peroxynitrite treatment. (a) Resistance of N. gonorrhoeae to direct peroxynitrite addition, while E. coli shows a dose response to peroxynitrite-mediated killing. Exponential-phase cultures of gonococcal strain F62 (◊) and E. coli DH10B (⧫) were diluted into fresh GCK media and the indicated final concentration of peroxynitrite was added at time 0. Following 120 min outgrowth, the cells were diluted and plated for viability. Values represent the mean±se of three determinations of the calculated c.f.u./initial c.f.u. (b) Growth of N. gonorrhoeae in the presence of high concentrations of peroxynitrite. Exponential-phase gonococcal strain F62 (filled symbols) or E. coli DH10B (open symbols) was diluted into fresh GCK medium and peroxynitrite was added at a final concentration of 0 (diamonds), 1 mM (squares) or 2 mM (triangles). At the indicated times, samples were taken to determine viable counts. Values represent the mean±se of three determinations of the calculated c.f.u./initial c.f.u.
Growth of N. gonorrhoeae with peroxynitrite treatment
We next monitored the viability of F62 over time after direct addition of 1 mM ONOO− to growing cultures. F62 not only maintained cell viability, but was actually capable of growth over a 120 min time period (Fig. 2b). These data show that N. gonorrhoeae is highly resistant to peroxynitrite. In contrast to this finding, E. coli viability was significantly affected, with a decrease of three to four logs. Since peroxynitrite has an extremely short half-life of approximately 1 s, the initial burst of peroxynitrite presumably results in killing of E. coli, while it appears to only cause growth arrest in N. gonorrhoeae. When the RNS is no longer present, growth resumes after a period of time, dependent on peroxynitrite concentration. This suggests that the gonococcus has a mechanism either to escape the RNS or to repair RNS damage, rather than to adapt to RNS addition.
Peroxynitrite resistance is common to other gonococcal strains
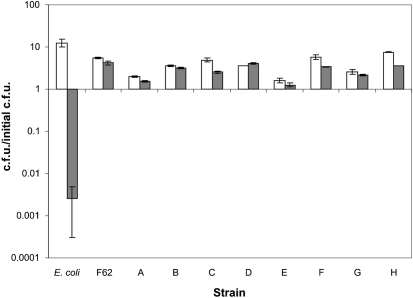
To ensure that the resistance of F62 to ONOO− was not unique, eight additional gonococcal strains were assayed for ONOO− resistance. Though there was some period of growth cessation in the presence of the RNS, as seen previously, all eight strains were capable of growth in the presence of ONOO− (Fig. 3), suggesting that ONOO− resistance is common in this species. The data for E. coli were included for comparison, illustrating the effects of peroxynitrite on the viability of a sensitive organism.
Fig. 3.
Resistance of gonococcal strains to peroxynitrite. Exponential-phase bacterial strains were diluted into fresh GCK medium. Bacteria were diluted and plated for viable cell counts at time 0. Peroxynitrite was added at a final concentration of 0 (white bars) or 1 mM (grey bars). Viable counts were determined following 120 min incubation with or without peroxynitrite. Values represent the mean±se of the calculated c.f.u./initial c.f.u. of three independent determinations. Strains are: E. coli DH10B, N. gonorrhoeae F62, NRL 905 (A), RUN 4002 (B), RUN 4383 (C), RUN 5632 (D), RUN 5635 (E), RUN 5636 (F), RUN 5638 (G) and RUN 5640 (H).
N. meningitidis is highly susceptible to peroxynitrite-mediated killing
Since high levels of ONOO− resistance were common to a variety of gonococcal strains, we wished to determine whether the other pathogenic Neisseria species, N. meningitidis, displayed a similar level of resistance. In contrast to what was observed in the gonococcal strains, meningococcal strains that were members of serogroups A, B and C all displayed high levels of peroxynitrite sensitivity (Fig. 4). The effect of peroxynitrite on meningococcal cell viability ranged from a four-log loss in viability (serogroup A) to nearly 100 % cell death, with a loss of viability of six to seven logs (serogroups B and C). This suggests that ONOO− resistance is not common among Neisseria spp.
Fig. 4.
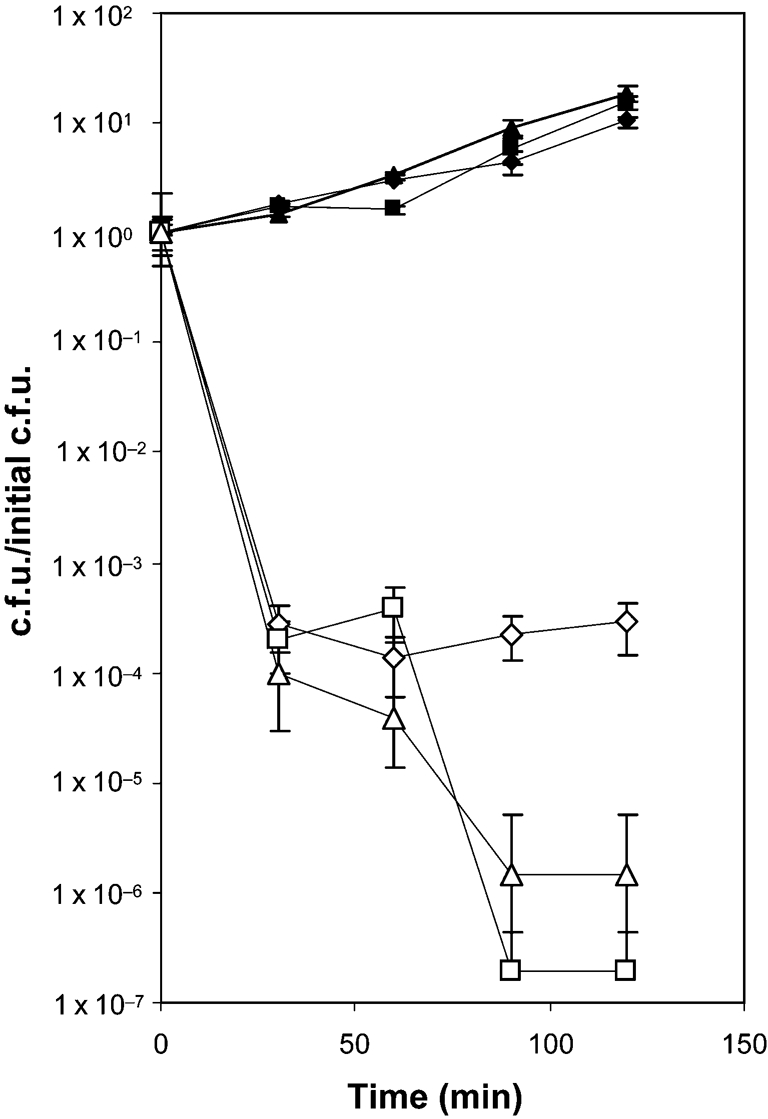
High-level peroxynitrite-mediated killing in N. meningitidis serogroups A, B and C. Exponential-phase N. meningitidis strains NRL-9205 (serogroup A; diamonds), NRL-9206 (serogroup B; triangles) and NRL-9207 (serogroup C; squares) were diluted into fresh GCK medium and peroxynitrite was added at a final concentration of 0 (filled symbols) or 1 mM (open symbols). At the indicated times, bacteria were diluted and plated to determine viable counts. The data are the mean±se of the calculated c.f.u./initial c.f.u. of three independent determinations.
It has been reported that N. meningitidis strain MC58 shows a limited amount of cell death when 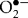 and NO generators are added to cells (Dyet & Moir, 2006). Since peroxynitrite forms from
and NO generators are added to cells (Dyet & Moir, 2006). Since peroxynitrite forms from 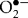 and NO, removal of either or both of these species will preclude formation of peroxynitrite. Both
and NO, removal of either or both of these species will preclude formation of peroxynitrite. Both 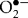 and NO can be removed from the system by meningococcal superoxide dismutase and nitric oxide reductase, respectively. Consequently, one is unaware of how much if any ONOO− is produced, especially since the NO donor 2-(N,N-diethylamino)-diazenolate-2-oxide (DEA/NO) has a half-life of only 90 s. Thus, we have evaluated N. meningitidis viability during direct ONOO− addition in order to eliminate any interference by the bacterium in the process of ONOO− generation. Comparison of peroxynitrite-mediated killing when peroxynitrite was added directly or generated via molecular generators gave significant differences in the viability of E. coli (compare Fig. 1b and Fig. 2b).
and NO can be removed from the system by meningococcal superoxide dismutase and nitric oxide reductase, respectively. Consequently, one is unaware of how much if any ONOO− is produced, especially since the NO donor 2-(N,N-diethylamino)-diazenolate-2-oxide (DEA/NO) has a half-life of only 90 s. Thus, we have evaluated N. meningitidis viability during direct ONOO− addition in order to eliminate any interference by the bacterium in the process of ONOO− generation. Comparison of peroxynitrite-mediated killing when peroxynitrite was added directly or generated via molecular generators gave significant differences in the viability of E. coli (compare Fig. 1b and Fig. 2b).
Effect on resistance to peroxynitrite of mutation in gonococcal genes orthologous to known or putative RNS resistance genes
Resistance to ROS and RNS has been studied in a number of bacterial species, especially members of the Enterobacteriaceae and Mycobacterium spp. (Alam et al., 2006; Chan et al., 2001; De Groote et al., 1995; Dyet & Moir, 2006; Kuwahara et al., 2000). As a result of this work, a number of ROS/RNS resistance genes have been identified (Table 1). The sequences of these known genes were used to blast-search the gonococcal genome for orthologues that may have a similar function. N. gonorrhoeae contained genes orthologous to ahpC (NG0328), encoding a protein shown to act as an alkyl hydroperoxidase and peroxynitrite reductase, dnrN (NG0653), a gene induced by NO and encoding a protein shown to be involved in Fe–S cluster repair following oxidative or nitrosative stress, and msrA (NG2059), encoding a protein involved in repair of oxidized methionines (Bryk et al., 2000; Overton et al., 2006, 2008; Poole, 2005; Skaar et al., 2002). Interestingly, the gene for the highly conserved NO-scavenging protein, hmp, as well as the genes found to confer resistance to RNS in M. tuberculosis (noxR1, noxR3, cysH) (Ehrt et al., 1997; Ruan et al., 1999; Senaratne et al., 2006) and Mycobacterium marinum (mel2) (Subbian et al., 2007), were not found in the gonococcus.
Table 1.
Bacterial genes identified or proposed to be involved in resistance to reactive nitrogen and oxygen species
*Annotation number from N. gonorrhoeae FA1090 genome on STDGEN database (http://stdgen.northwestern.edu/).
†Annotation number from N. meningitidis Z2491 genome on National Center for Biotechnology Information database (http://www.ncbi.nlm.nih.gov/).
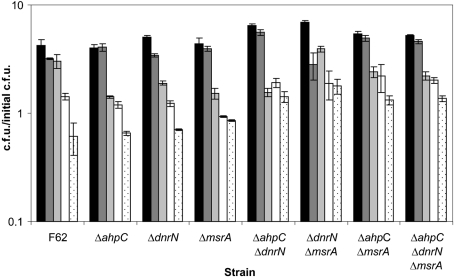
To determine whether any of these genes function in peroxynitrite resistance, we constructed ahpC, dnrN and msrA mutants and assayed these strains as described above. Mutations in ahpC, dnrN or msrA, alone or in combination, had little effect on peroxynitrite resistance. The addition of increasing concentrations of peroxynitrite extended the period in which the RNS caused growth cessation. All strains eventually recovered and resumed growth unless peroxynitrite reached a 2 mM concentration (Fig. 5).
Fig. 5.
Mutation of RNS defence genes does not alter the high level of resistance to peroxynitrite. Exponential-phase gonococcal strains were diluted into fresh GCK medium and peroxynitrite was added at a final concentration of 0 (black bars), 0.25 mM (dark grey bars), 1 mM (light grey bars), 1.5 mM (white bars) or 2 mM (stippled bars). Viable counts were determined before RNS addition and after 120 min incubation with peroxynitrite to determine growth. Results are represented as the mean±se of the calculated c.f.u./initial c.f.u. of three determinations.
Effect on resistance to peroxynitrite of mutation in gonococcal genes involved in nitric oxide or oxidative stress
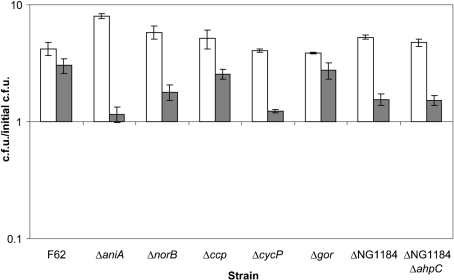
We broadened our analysis to examine not only genes involved in nitrosative stress, but also those that are important in oxidative stress, in addition to genes that are involved in NO metabolism. The genes of interest were aniA and norB, considering their involvement with the RNS nitric oxide. It is not uncommon to have protective proteins show interactions with more than one type of reactive species. The ability of a single protein to be involved in multiple protective systems, such as ROS and RNS defence, is however much more uncommon. Cytochrome c′ (cycP) and cytochrome c peroxidase (ccp) have been suggested to be involved in defence against oxidative and nitrosative stress (Seib et al., 2004; Stevanin et al., 2005; Turner et al., 2003, 2005). A gonococcal glutathione reductase (gor) was examined, since alterations in its function to maintain high levels of reduced glutathione could greatly limit any protective effect of glutathione against peroxynitrite. Gonococcal ORF NG1184 was examined because of its annotation as an NADH oxidoreductase that is completely absent from the meningococcal genome. Lastly, a ΔNG1184 ΔahpC double mutant strain was examined, since the two gene products may have an active role in directly detoxifying peroxynitrite. Mutations in any of the genes examined showed no peroxynitrite-induced killing, although there were slight changes to the amount of growth after peroxynitrite treatment compared with wild-type F62 (Fig. 6). These data suggest that gonococcal resistance to peroxynitrite is the result of redundant systems or a novel gene product.
Fig. 6.
Gonococcal mutants having inactivated genes involved with nitric oxide and oxidative stress remain resistant to peroxynitrite. Exponential-phase gonococcal strains were diluted into fresh GCK medium. Bacteria were diluted and plated for viable cell counts at time 0. Peroxynitrite was added at a final concentration of 0 (white bars) or 1 mM (grey bars). Viable counts were determined following 120 min incubation with or without peroxynitrite. Values represent the mean±se of the calculated c.f.u./initial c.f.u. of three independent determinations.
Peroxynitrite reductase assay
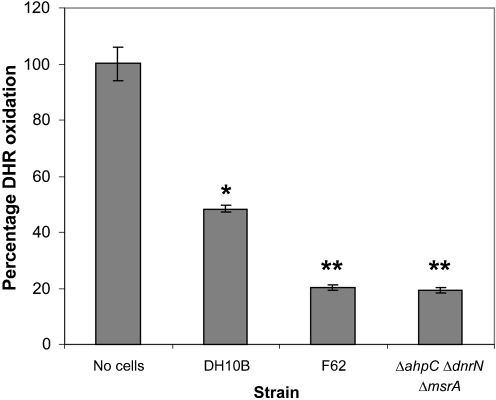
To determine whether gonococcal cells contain peroxynitrite reductase activity, we examined the ability of F62 to block peroxynitrite-mediated oxidation of DHR (Ischiropoulos et al., 1999; Kooy et al., 1994). Both the parental strain F62 and a strain containing mutations in putative RNS resistance genes, F62 ΔahpC ΔdnrN ΔmsrA, effectively prevented the oxidation of DHR (Fig. 7), suggesting that a novel peroxynitrite reductase exists in N. gonorrhoeae. Both gonococcal strains also showed an enhanced ability to prevent DHR oxidation compared with E. coli DH10B (Fig. 7).
Fig. 7.
N. gonorrhoeae peroxynitrite reductase activity. Washed cells of gonococcal strain F62, the triple mutant F62 ΔahpC ΔdnrN ΔmsrA, and E. coli DH10B were concentrated 10-fold and tested for ability to convert DHR to rhodamine, as described in Methods. Background and final A500 were determined before and 1 min after addition of peroxynitrite. Assays were performed in triplicate and the values represent the mean±se percentage DHR oxidation. Highly significant differences comparing E. coli DH10B with the no-cells control are indicated by an asterisk (P<0.001), while significant differences comparing either F62 or F62 ΔahpC ΔdnrN ΔmsrA with E. coli DH10B are indicated by double asterisks (P<0.00005). Statistical analysis included a Student's t test assuming equal variance.
There exists a possibility that other gonococcal components compete with DHR for ONOO−-induced oxidation. To address this concern, we investigated the nitration of proteins as a result of ONOO− treatment. Gonococcal cells were exposed to three treatments with ONOO− to a final concentration of 1 mM. The cells were then lysed, and whole-cell extracts were assayed by Western analysis using anti-nitrotyrosine antibody as a probe. There was no detectable nitration of gonococcal proteins under conditions in which BSA was heavily nitrated by peroxynitrite (data not shown). These results would be expected if peroxynitrite is reduced before protein nitration can occur.
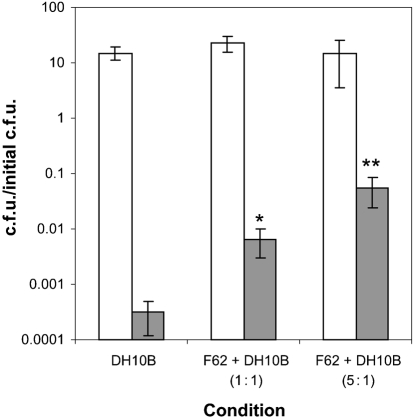
A second approach was used in order to establish that an active mechanism is responsible for peroxynitrite resistance. When N. gonorrhoeae was added to a culture of E. coli prior to peroxynitrite treatment, there was significant protection from RNS-mediated killing. If N. gonorrhoeae was present within the culture at a 1 : 1 ratio with respect to OD600, there was a 10-fold level of protection, while a 5 : 1 ratio (N. gonorrhoeae : E. coli) caused an increase in E. coli viability 100-fold (Fig. 8). This suggests that a peroxynitrite reductase activity is present in exponential-phase N. gonorrhoeae cultures, and is capable of preventing peroxynitrite-induced killing in E. coli.
Fig. 8.
N. gonorrhoeae protects E. coli from RNS-mediated killing. F62 and E. coli were grown separately in GCK broth then diluted into fresh medium with either E. coli DH10B alone, F62+DH10B (1 : 1 ratio) or F62+DH10B (5 : 1 ratio). Cells were treated with either no addition (white bars) or 1 mM peroxynitrite (grey bars). After 120 min the cells were diluted and plated for viability. The data are the mean±se of three independent determinations. Highly significant differences compared with E. coli DH10B culture alone in the presence of 1 mM peroxynitrite are indicated by an asterisk (P<0.02) or double asterisks (P<0.007). Statistical analysis included a Student's t test assuming equal variance.
DISCUSSION
The importance of ROS in host defence is apparent when the consequences of deficient production are examined. Chronic granulomatous disease (CGD) is a hereditary disease in which patients can have mutations in any of the four required subunits of PHOX (gp91-phox, p47-phox, p22-phox, p67-phox), with the gp91-phox gene being the most commonly mutated (Roos et al., 1996). Phagocytes of CGD patients are incapable of ROS generation, and subsequent effects include recurring bouts of infection by Staphylococcus aureus, Klebsiella spp., Aspergillus fumigatus and Candida albicans (Fang, 2004).
The production of reactive nitrogen intermediates has only recently become appreciated with respect to its role in host defence. Although there is no identified primary genetic deficiency of iNOS in humans, studies using isolated macrophages or knockout mice have demonstrated an instrumental contribution to pathogen control (Bogdan, 2001). Mice with single deficiencies in iNOS show increased susceptibility to a number of parasitic, bacterial and viral infections, including Leishmania major and M. turberculosis (Chan et al., 2001; Green et al., 1990). In infections caused by Listeria monocytogenes, Salmonella spp. and Chlamydia pneumoniae, iNOS has been shown to contribute to host defence but not to be essential (Alam et al., 2002; MacMicking et al., 1995; Mayer et al., 1993; Umezawa et al., 1997).
Pathogenic micro-organisms utilize a broad range of strategies to counteract the effects of RNS. Such strategies may include tightly regulated systems of oxidant avoidance or scavenging, as well as the repair of stress-damaged biomolecules (Chakravortty & Hensel, 2003). Detoxification of RNS to less toxic species is a common defensive tactic (Fang, 2004). S. enterica serovar Typhimurium utilizes flavohaemoglobin, Hmp, to convert NO to nitrate or nitrous oxide, depending on the concentration of oxygen present (Poole & Hughes, 2000). Organisms that contain elements of the denitrification pathway can directly reduce NO to N2O, e.g. by the nitric oxide reductase in N. gonorrhoeae (Cardinale & Clark, 2005; Householder et al., 2000). Alkyl hydroperoxide reductase (ahpC) is widespread among bacteria and has been shown to be capable of reducing peroxynitrite to nitrite (Bryk et al., 2000), though loss of this activity did not increase gonococcal sensitivity to peroxynitrite (Fig. 5).
RNS avoidance may also be considered an important survival mechanism. Components capable of eliminating ROS, such as superoxide dismutase (SOD) and catalase (Kat), indirectly lower the potential to generate more damaging nitrogen oxide radicals, including peroxynitrite (Bogdan et al., 2000; Pacher et al., 2007). Gonococci display unusually high catalase and peroxidase activities, while also maintaining very high glutathione levels (Archibald & Duong, 1986). This may account for the inability of oxidative stresses to kill the organism. Francisella tularensis utilizes phase variation to alter LPS structure so that it no longer activates iNOS, leading to intracellular replication of the pathogen in rat macrophages (Cowley et al., 1996). H. pylori encodes an arginase that degrades the substrate for iNOS activity, l-arginine, preventing NO concentrations from reaching bactericidal levels (Gobert et al., 2001). S. enterica is capable of disrupting eukaryotic cell trafficking in such a manner that an insufficient amount of iNOS reaches the mature phagosome. As a result, peroxynitrite has been shown to be absent from vacuoles containing bacteria (Chakravortty et al., 2002). Even without affecting peroxynitrite resistance, gonococcal cycP may be important in binding NO in vivo, thus preventing peroxynitrite generation (Turner et al., 2005).
In the event that damage occurs as a result of oxidant production, many bacteria encode machinery that repairs damaged biomolecules. Viability can be directly related to the ability to repair DNA damage. The activity of RecBC is essential in S. enterica, and strains deficient in RecBC are more susceptible to killing by RNS (Shiloh et al., 1999). A gonococcal recN mutant shows increased sensitivity to hydrogen peroxide and polymorphonuclear leukocyte killing (Stohl et al., 2005). Proteins are susceptible to RNS-mediated damage, especially at specific amino acid residues, motifs or cofactors. Such damage includes cysteine oxidation, tyrosine nitration and changes to transition metal centres, all of which can greatly modify protein function and/or activity (Pacher et al., 2007). Methionine sulfoxide reductase (Msr) is widespread among bacterial species and functions to reduce oxidized methionines, thus preventing excessive accumulation of non-functional proteins (Moskovitz, 2005). N. gonorrhoeae has a unique genetic organization, in which msrA and msrB are translationally fused to form a single polypeptide, unlike E. coli, which has two distinct transcription units (Ezraty et al., 2005). However, we have shown that msrA is dispensable for peroxynitrite resistance in the gonococcus (Fig. 5).
The search for the gene products responsible for the high levels of gonococcal peroxynitrite resistance has eliminated ahpC, ccp, cycP, dnrN, msrA and NG1184. The gonococcus also encodes a peroxiredoxin (prx), having 98 % sequence identity to Prx of N. meningitidis, which is able to reduce H2O2 in the presence of GSH (Rouhier & Jacquot, 2003). It is unlikely that Prx is involved in detoxification of peroxynitrite, since it has been identified as a member of the OxyR regulon, in which its expression is upregulated in the presence of H2O2 (Seib et al., 2007), comprising a component of oxidative stress defence. Since peroxynitrite has such a short half-life, our experimental setup prevents the involvement of those protective mechanisms that require induction. In addition, the decomposition products of peroxynitrite do not include hydrogen peroxide, the OxyR-requiring species that allows for gene induction of Prx. However, another member of the OxyR regulon is glutathione reductase (gor). This enzyme converts oxidized glutathione to its reduced form. Reduced glutathione can serve as an antioxidant for either ROS or RNS. Mutation of gor had no effect on peroxynitrite resistance (Fig. 6).
Co-infections are common among sexually transmitted diseases, especially co-infection by N. gonorrhoeae and Chlamydia trachomatis (Hillis et al., 1994). C. trachomatis may benefit from the defensive mechanisms employed by the gonococcus to cope with oxidative and nitrosative stress, considering that RNS inhibit intracellular multiplication of C. trachomatis (Igietseme et al., 1997). Studies have shown that local genital secretions from women infected with N. gonorrhoeae and concurrently with Trichomonas vaginalis and C. trachomatis have normal cytokine levels rather than the elevated levels normally associated with infection (Hedges et al., 1998). This indicates that N. gonorrhoeae may be able to establish an immunosuppressive environment capable of protecting other infectious bacteria. The effect can be twofold, including the reduction of peroxynitrite and subsequent increase in bacterial viability (Fig. 8), as well as the ability of N. gonorrhoeae to reduce high levels of nitric oxide to an anti-inflammatory level, preventing NF-κB activation (Barth & Clark, 2008; Cardinale & Clark, 2005; Liaudet et al., 2000).
In summary, the host immune system is capable of eliciting an assault on invading pathogens through the activity of a large collection of antimicrobial products. Some of these result in the generation of ROS and RNS, and the gonococcus shows high levels of resistance to both nitric oxide and peroxynitrite. Peroxynitrite resistance is a common mechanism within various gonococcal isolates and is not dependent on individual genes known to be involved in RNS defence in other bacterial species, suggesting a number of alternative possibilities. The fact that N. gonorrhoeae encodes such an expansive number of antioxidants means that creating mutants of each coding region is not a feasible technique to rapidly identify the mechanism of peroxynitrite resistance. This method would not be able to identify a novel mechanism. Furthermore, a change to a phenotype of peroxynitrite sensitivity may not be attainable without eliminating all of the resistance mechanisms. The hyperresistance of N. gonorrhoeae to peroxynitrite may be the result of a variety of systems. These may include biomolecules capable of scavenging peroxynitrite (i.e. glutathione), the enzymic reduction of the RNS (i.e. a novel reductase that has redundant activity with ahpC), repair of RNS-mediated damage (msrA, recN), or a combination of these mechanisms. Because high levels of peroxynitrite resistance do not occur in N. meningitidis, the gonococcal mechanism(s) responsible could be a function of variations in protein expression and/or genetic regulation between the two species or the result of a complete absence of a novel protective mechanism within the meningococcus. Current studies involve constructing a gonococcal library within N. meningitidis, where we will examine clones that give a significant increase in resistance to peroxynitrite.
Acknowledgments
This study was supported by Public Health Service grant R01 AI 11709 from the National Institutes of Health. Additionally, V. M. I. was supported by NIH grant T32 AI07362.
Abbreviations
DETA/NO, 2,2′-(hydroxynitrosohydrazono) bis-ethanimine
DHR, dihydrorhodamine
iNOS, inducible nitric oxide synthase
RNS, reactive nitrogen species
ROS, reactive oxygen species
Footnotes
A supplementary table, listing bacterial strains used in this study, with accompanying supplementary references, is available with the online version of this paper.
References
- Aktan, F. (2004). iNOS-mediated nitric oxide production and its regulation. Life Sci 75, 639–653. [DOI] [PubMed] [Google Scholar]
- Alam, M. S., Akaike, T., Okamoto, S., Kubota, T., Yoshitake, J., Sawa, T., Miyamoto, Y., Tamura, F. & Maeda, H. (2002). Role of nitric oxide in host defense in murine salmonellosis as a function of its antibacterial and antiapoptotic activities. Infect Immun 70, 3130–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam, M. S., Zaki, M. H., Yoshitake, J., Akuta, T., Ezaki, T. & Akaike, T. (2006). Involvement of Salmonella enterica serovar Typhi RpoS in resistance to NO-mediated host defense against serovar Typhi infection. Microb Pathog 40, 116–125. [DOI] [PubMed] [Google Scholar]
- Alcorn, T. M., Zheng, H. Y., Gunther, M. R., Hassett, D. J. & Cohen, M. S. (1994). Variation in hydrogen peroxide sensitivity between different strains of Neisseria gonorrhoeae is dependent on factors in addition to catalase activity. Infect Immun 62, 2138–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez, B. & Radi, R. (2003). Peroxynitrite reactivity with amino acids and proteins. Amino Acids 25, 295–311. [DOI] [PubMed] [Google Scholar]
- Archibald, F. S. & Duong, M. N. (1986). Superoxide dismutase and oxygen toxicity defenses in the genus Neisseria. Infect Immun 51, 631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth, K. & Clark, V. L. (2008). Differences in nitric oxide steady states between arginine, hypoxanthine, uracil auxotrophs (AHU) and non-AHU strains of Neisseria gonorrhoeae during anaerobic respiration in the presence of nitrite. Can J Microbiol 54, 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman, J. S. & Koppenol, W. H. (1996). Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 271, C1424–C1437. [DOI] [PubMed] [Google Scholar]
- Bogdan, C. (2001). Nitric oxide and the immune response. Nat Immunol 2, 907–916. [DOI] [PubMed] [Google Scholar]
- Bogdan, C., Rollinghoff, M. & Diefenbach, A. (2000). Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol 12, 64–76. [DOI] [PubMed] [Google Scholar]
- Braun, C. & Zumft, W. G. (1991). Marker exchange of the structural genes for nitric oxide reductase blocks the denitrification pathway of Pseudomonas stutzeri at nitric oxide. J Biol Chem 266, 22785–22788. [PubMed] [Google Scholar]
- Bryk, R., Griffin, P. & Nathan, C. (2000). Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 407, 211–215. [DOI] [PubMed] [Google Scholar]
- Burney, S., Caulfield, J. L., Niles, J. C., Wishnok, J. S. & Tannenbaum, S. R. (1999). The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat Res 424, 37–49. [DOI] [PubMed] [Google Scholar]
- Cardinale, J. A. & Clark, V. L. (2005). Determinants of nitric oxide steady-state levels during anaerobic respiration by Neisseria gonorrhoeae. Mol Microbiol 58, 177–188. [DOI] [PubMed] [Google Scholar]
- Carreras, M. C., Pargament, G. A., Catz, S. D., Poderoso, J. J. & Boveris, A. (1994). Kinetics of nitric oxide and hydrogen peroxide production and formation of peroxynitrite during the respiratory burst of human neutrophils. FEBS Lett 341, 65–68. [DOI] [PubMed] [Google Scholar]
- Casey, S. G., Veale, D. R. & Smith, H. (1979). Demonstration of intracellular growth of gonococci in human phagocytes using spectinomycin to kill extracellular organisms. J Gen Microbiol 113, 395–398. [DOI] [PubMed] [Google Scholar]
- Casey, S. G., Shafer, W. M. & Spitznagel, J. K. (1986). Neisseria gonorrhoeae survive intraleukocytic oxygen-independent antimicrobial capacities of anaerobic and aerobic granulocytes in the presence of pyocin lethal for extracellular gonococci. Infect Immun 52, 384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravortty, D. & Hensel, M. (2003). Inducible nitric oxide synthase and control of intracellular bacterial pathogens. Microbes Infect 5, 621–627. [DOI] [PubMed] [Google Scholar]
- Chakravortty, D., Hansen-Wester, I. & Hensel, M. (2002). Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates. J Exp Med 195, 1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, E. D., Chan, J. & Schluger, N. W. (2001). What is the role of nitric oxide in murine and human host defense against tuberculosis? Current knowledge. Am J Respir Cell Mol Biol 25, 606–612. [DOI] [PubMed] [Google Scholar]
- Cowley, S. C., Myltseva, S. V. & Nano, F. E. (1996). Phase variation in Francisella tularensis affecting intracellular growth, lipopolysaccharide antigenicity and nitric oxide production. Mol Microbiol 20, 867–874. [DOI] [PubMed] [Google Scholar]
- Cramm, R., Siddiqui, R. A. & Friedrich, B. (1997). Two isofunctional nitric oxide reductases in Alcaligenes eutrophus H16. J Bacteriol 179, 6769–6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groote, M. A., Granger, D., Xu, Y., Campbell, G., Prince, R. & Fang, F. C. (1995). Genetic and redox determinants of nitric oxide cytotoxicity in a Salmonella typhimurium model. Proc Natl Acad Sci U S A 92, 6399–6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groote, M. A., Ochsner, U. A., Shiloh, M. U., Nathan, C., McCord, J. M., Dinauer, M. C., Libby, S. J., Vazquez-Torres, A., Xu, Y. & Fang, F. C. (1997). Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc Natl Acad Sci U S A 94, 13997–14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyet, K. & Moir, J. (2006). Effect of combined oxidative and nitrosative stress on Neisseria meningitidis. Biochem Soc Trans 34, 197–199. [DOI] [PubMed] [Google Scholar]
- Edwards, J. L. & Apicella, M. A. (2004). The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin Microbiol Rev 17, 965–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrt, S., Shiloh, M. U., Ruan, J., Choi, M., Gunzburg, S., Nathan, C., Xie, Q. & Riley, L. W. (1997). A novel antioxidant gene from Mycobacterium tuberculosis. J Exp Med 186, 1885–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezraty, B., Aussel, L. & Barras, F. (2005). Methionine sulfoxide reductases in prokaryotes. Biochim Biophys Acta 1703, 221–229. [DOI] [PubMed] [Google Scholar]
- Fang, F. C. (2004). Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol 2, 820–832. [DOI] [PubMed] [Google Scholar]
- Fu, H. S., Hassett, D. J. & Cohen, M. S. (1989). Oxidant stress in Neisseria gonorrhoeae: adaptation and effects on l-(+)-lactate dehydrogenase activity. Infect Immun 57, 2173–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert, A. P., McGee, D. J., Akhtar, M., Mendz, G. L., Newton, J. C., Cheng, Y., Mobley, H. L. & Wilson, K. T. (2001). Helicobacter pylori arginase inhibits nitric oxide production by eukaryotic cells: a strategy for bacterial survival. Proc Natl Acad Sci U S A 98, 13844–13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, S. & Merenyi, G. (2008). The chemistry of peroxynitrite: implications for biological activity. Methods Enzymol 436, 49–61. [DOI] [PubMed] [Google Scholar]
- Green, S. J., Meltzer, M. S., Hibbs, J. B., Jr & Nacy, C. A. (1990). Activated macrophages destroy intracellular Leishmania major amastigotes by an l-arginine-dependent killing mechanism. J Immunol 144, 278–283. [PubMed] [Google Scholar]
- Hampton, M. B., Kettle, A. J. & Winterbourn, C. C. (1998). Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92, 3007–3017. [PubMed] [Google Scholar]
- Hassett, D. J., Charniga, L. & Cohen, M. S. (1990). recA and catalase in H2O2-mediated toxicity in Neisseria gonorrhoeae. J Bacteriol 172, 7293–7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges, S. R., Sibley, D. A., Mayo, M. S., Hook, E. W., III & Russell, M. W. (1998). Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. J Infect Dis 178, 742–751. [DOI] [PubMed] [Google Scholar]
- Hillis, S. D., Nakashima, A., Marchbanks, P. A., Addiss, D. G. & Davis, J. P. (1994). Risk factors for recurrent Chlamydia trachomatis infections in women. Am J Obstet Gynecol 170, 801–806. [DOI] [PubMed] [Google Scholar]
- Householder, T. C., Belli, W. A., Lissenden, S., Cole, J. A. & Clark, V. L. (1999). cis- and trans-acting elements involved in regulation of aniA, the gene encoding the major anaerobically induced outer membrane protein in Neisseria gonorrhoeae. J Bacteriol 181, 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Householder, T. C., Fozo, E. M., Cardinale, J. A. & Clark, V. L. (2000). Gonococcal nitric oxide reductase is encoded by a single gene, norB, which is required for anaerobic growth and is induced by nitric oxide. Infect Immun 68, 5241–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igietseme, J. U., Uriri, I. M., Chow, M., Abe, E. & Rank, R. G. (1997). Inhibition of intracellular multiplication of human strains of Chlamydia trachomatis by nitric oxide. Biochem Biophys Res Commun 232, 595–601. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos, H., Gow, A., Thom, S. R., Kooy, N. W., Royall, J. A. & Crow, J. P. (1999). Detection of reactive nitrogen species using 2,7-dichlorodihydrofluorescein and dihydrorhodamine 123. Methods Enzymol 301, 367–373. [DOI] [PubMed] [Google Scholar]
- Jeong, W., Cha, M. K. & Kim, I. H. (2000). Thioredoxin-dependent hydroperoxide peroxidase activity of bacterioferritin comigratory protein (BCP) as a new member of the thiol-specific antioxidant protein (TSA)/alkyl hydroperoxide peroxidase C (AhpC) family. J Biol Chem 275, 2924–2930. [DOI] [PubMed] [Google Scholar]
- Kellogg, D. S., Jr, Peacock, W. L., Jr, Deacon, W. E., Brown, L. & Pirkle, D. I. (1963). Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J Bacteriol 85, 1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooy, N. W., Royall, J. A., Ischiropoulos, H. & Beckman, J. S. (1994). Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic Biol Med 16, 149–156. [DOI] [PubMed] [Google Scholar]
- Kuwahara, H., Miyamoto, Y., Akaike, T., Kubota, T., Sawa, T., Okamoto, S. & Maeda, H. (2000). Helicobacter pylori urease suppresses bactericidal activity of peroxynitrite via carbon dioxide production. Infect Immun 68, 4378–4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaudet, L., Soriano, F. G. & Szabo, C. (2000). Biology of nitric oxide signaling. Crit Care Med 28, N37–N52. [DOI] [PubMed] [Google Scholar]
- MacMicking, J. D., Nathan, C., Hom, G., Chartrain, N., Fletcher, D. S., Trumbauer, M., Stevens, K., Xie, Q. W., Sokol, K. & other authors (1995). Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81, 641–650. [DOI] [PubMed] [Google Scholar]
- MacMicking, J., Xie, Q. W. & Nathan, C. (1997). Nitric oxide and macrophage function. Annu Rev Immunol 15, 323–350. [DOI] [PubMed] [Google Scholar]
- Mayer, J., Woods, M. L., Vavrin, Z. & Hibbs, J. B., Jr (1993). Gamma interferon-induced nitric oxide production reduces Chlamydia trachomatis infectivity in McCoy cells. Infect Immun 61, 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz, J. (2005). Methionine sulfoxide reductases: ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim Biophys Acta 1703, 213–219. [DOI] [PubMed] [Google Scholar]
- Overton, T. W., Whitehead, R., Li, Y., Snyder, L. A., Saunders, N. J., Smith, H. & Cole, J. A. (2006). Coordinated regulation of the Neisseria gonorrhoeae-truncated denitrification pathway by the nitric oxide-sensitive repressor, NsrR, and nitrite-insensitive NarQ–NarP. J Biol Chem 281, 33115–33126. [DOI] [PubMed] [Google Scholar]
- Overton, T. W., Justino, M. C., Li, Y., Baptista, J. M., Melo, A. M., Cole, J. A. & Saraiva, L. M. (2008). Widespread distribution in pathogenic bacteria of di-iron proteins that repair oxidative and nitrosative damage to iron-sulfur centers. J Bacteriol 190, 2004–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher, P., Beckman, J. S. & Liaudet, L. (2007). Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87, 315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole, L. B. (2005). Bacterial defenses against oxidants: mechanistic features of cysteine-based peroxidases and their flavoprotein reductases. Arch Biochem Biophys 433, 240–254. [DOI] [PubMed] [Google Scholar]
- Poole, R. K. & Hughes, M. N. (2000). New functions for the ancient globin family: bacterial responses to nitric oxide and nitrosative stress. Mol Microbiol 36, 775–783. [DOI] [PubMed] [Google Scholar]
- Radi, R., Beckman, J. S., Bush, K. M. & Freeman, B. A. (1991a). Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem 266, 4244–4250. [PubMed] [Google Scholar]
- Radi, R., Beckman, J. S., Bush, K. M. & Freeman, B. A. (1991b). Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 288, 481–487. [DOI] [PubMed] [Google Scholar]
- Reiter, C. D., Teng, R. J. & Beckman, J. S. (2000). Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. J Biol Chem 275, 32460–32466. [DOI] [PubMed] [Google Scholar]
- Rest, R. F., Fischer, S. H., Ingham, Z. Z. & Jones, J. F. (1982). Interactions of Neisseria gonorrhoeae with human neutrophils: effects of serum and gonococcal opacity on phagocyte killing and chemiluminescence. Infect Immun 36, 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos, D., de Boer, M., Kuribayashi, F., Meischl, C., Weening, R. S., Segal, A. W., Ahlin, A., Nemet, K., Hossle, J. P. & other authors (1996). Mutations in the X-linked and autosomal recessive forms of chronic granulomatous disease. Blood 87, 1663–1681. [PubMed] [Google Scholar]
- Rouhier, N. & Jacquot, J. P. (2003). Molecular and catalytic properties of a peroxiredoxin–glutaredoxin hybrid from Neisseria meningitidis. FEBS Lett 554, 149–153. [DOI] [PubMed] [Google Scholar]
- Ruan, J., St John, G., Ehrt, S., Riley, L. & Nathan, C. (1999). noxR3, a novel gene from Mycobacterium tuberculosis, protects Salmonella typhimurium from nitrosative and oxidative stress. Infect Immun 67, 3276–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seib, K. L., Jennings, M. P. & McEwan, A. G. (2003). A Sco homologue plays a role in defence against oxidative stress in pathogenic Neisseria. FEBS Lett 546, 411–415. [DOI] [PubMed] [Google Scholar]
- Seib, K. L., Tseng, H. J., McEwan, A. G., Apicella, M. A. & Jennings, M. P. (2004). Defenses against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: distinctive systems for different lifestyles. J Infect Dis 190, 136–147. [DOI] [PubMed] [Google Scholar]
- Seib, K. L., Wu, H. J., Kidd, S. P., Apicella, M. A., Jennings, M. P. & McEwan, A. G. (2006). Defenses against oxidative stress in Neisseria gonorrhoeae: a system tailored for a challenging environment. Microbiol Mol Biol Rev 70, 344–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seib, K. L., Wu, H. J., Srikhanta, Y. N., Edwards, J. L., Falsetta, M. L., Hamilton, A. J., Maguire, T. L., Grimmond, S. M., Apicella, M. A. & other authors (2007). Characterization of the OxyR regulon of Neisseria gonorrhoeae. Mol Microbiol 63, 54–68. [DOI] [PubMed] [Google Scholar]
- Senaratne, R. H., De Silva, A. D., Williams, S. J., Mougous, J. D., Reader, J. R., Zhang, T., Chan, S., Sidders, B., Lee, D. H. & other authors (2006). 5′-Adenosinephosphosulphate reductase (CysH) protects Mycobacterium tuberculosis against free radicals during chronic infection phase in mice. Mol Microbiol 59, 1744–1753. [DOI] [PubMed] [Google Scholar]
- Shiloh, M. U., MacMicking, J. D., Nicholson, S., Brause, J. E., Potter, S., Marino, M., Fang, F., Dinauer, M. & Nathan, C. (1999). Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity 10, 29–38. [DOI] [PubMed] [Google Scholar]
- Simons, M. P., Nauseef, W. M. & Apicella, M. A. (2005). Interactions of Neisseria gonorrhoeae with adherent polymorphonuclear leukocytes. Infect Immun 73, 1971–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaar, E. P., Tobiason, D. M., Quick, J., Judd, R. C., Weissbach, H., Etienne, F., Brot, N. & Seifert, H. S. (2002). The outer membrane localization of the Neisseria gonorrhoeae MsrA/B is involved in survival against reactive oxygen species. Proc Natl Acad Sci U S A 99, 10108–10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevanin, T. M., Moir, J. W. & Read, R. C. (2005). Nitric oxide detoxification systems enhance survival of Neisseria meningitidis in human macrophages and in nasopharyngeal mucosa. Infect Immun 73, 3322–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl, E. A., Criss, A. K. & Seifert, H. S. (2005). The transcriptome response of Neisseria gonorrhoeae to hydrogen peroxide reveals genes with previously uncharacterized roles in oxidative damage protection. Mol Microbiol 58, 520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbian, S., Mehta, P. K., Cirillo, S. L. & Cirillo, J. D. (2007). The Mycobacterium marinum mel2 locus displays similarity to bacterial bioluminescence systems and plays a role in defense against reactive oxygen and nitrogen species. BMC Microbiol 7, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo, C., Ischiropoulos, H. & Radi, R. (2007). Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6, 662–680. [DOI] [PubMed] [Google Scholar]
- Turner, S., Reid, E., Smith, H. & Cole, J. (2003). A novel cytochrome c peroxidase from Neisseria gonorrhoeae: a lipoprotein from a Gram-negative bacterium. Biochem J 373, 865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, S. M., Moir, J. W., Griffiths, L., Overton, T. W., Smith, H. & Cole, J. A. (2005). Mutational and biochemical analysis of cytochrome c′, a nitric oxide-binding lipoprotein important for adaptation of Neisseria gonorrhoeae to oxygen-limited growth. Biochem J 388, 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa, K., Akaike, T., Fujii, S., Suga, M., Setoguchi, K., Ozawa, A. & Maeda, H. (1997). Induction of nitric oxide synthesis and xanthine oxidase and their roles in the antimicrobial mechanism against Salmonella typhimurium infection in mice. Infect Immun 65, 2932–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, K., Mitchell, C., Xing, Y., Magliozzo, R. S., Bloom, B. R. & Chan, J. (1999). Toxicity of nitrogen oxides and related oxidants on mycobacteria: M. tuberculosis is resistant to peroxynitrite anion. Tuber Lung Dis 79, 191–198. [DOI] [PubMed] [Google Scholar]
- Zhu, L., Gunn, C. & Beckman, J. S. (1992). Bactericidal activity of peroxynitrite. Arch Biochem Biophys 298, 452–457. [DOI] [PubMed] [Google Scholar]