Abstract
Transforming growth factor-β (TGF-β) increases or decreases nuclear factor kappa B (NFκB) signaling in a context-dependent manner through mechanisms that remain to be defined. The type III transforming growth factor-β receptor (TβRIII) is a TGF-β superfamily co-receptor with emerging roles in both mediating and regulating TGF-β superfamily signaling. We have previously reported a novel interaction of TβRIII with the scaffolding protein, β-arrestin2, which results in TβRIII internalization and downregulation of TGF-β signaling. β-arrestin2 also scaffolds interacting receptors with the mitogen-activated protein kinase and NFκB-signaling pathways. Here, we demonstrate that TβRIII, through its interaction with β-arrestin2, negatively regulates NFκB signaling in MCF10A breast epithelial and MDA-MB-231 breast cancer cells. Increasing TβRIII expression reduced NFκB-mediated transcriptional activation and IκBα degradation, whereas a TβRIII mutant unable to interact with β-arrestin2, TβRIII-T841A, had no effect. In a reciprocal manner, short hairpin RNA-mediated silencing of either TβRIII expression or β-arrestin2 expression increased NFκB-mediated transcriptional activation and IκBα degradation. Functionally, TβRIII-mediated repression of NFκB signaling is important for TβRIII-mediated inhibition of breast cancer cell migration. These studies define a mechanism through which TβRIII regulates NFκB signaling and expand the roles of this TGF-β superfamily co-receptor in regulating epithelial cell homeostasis.
Introduction
Transforming growth factor-β (TGF-β) is the founding member of a superfamily of homodimeric polypeptide growth factors that have essential roles in a variety of cellular processes including development, growth control, differentiation, migration and apoptosis (1–6). There are three TGF-β isoforms, TGF-β1, TGF-β2 and TGF-β3, which are encoded by distinct genes and expressed in both a tissue-specific and a developmentally regulated manner. TGF-β isoforms signals through binding to three high-affinity cell surface receptors, the TGF-β type I (TβRI or ALK5), type II (TβRII) and type III (TβRIII or betaglycan) receptors. Upon ligand binding, TβRII, a serine/threonine kinase, forms a complex with phosphorylates and activates TβRI to initiate intracellular signaling. The serine/threonine kinase TβRI then directly phosphorylates Smad2 and Smad3, which form a complex with Smad4, accumulates in the nucleus and regulates gene transcription (7). In addition to the Smad-dependent canonical pathway, cross talk and signaling through Smad-independent pathways, including mitogen-activated protein kinase, phosphatidylinositol 3-kinase/Akt and the nuclear factor kappa B (NFκB) enhancer-binding protein pathways, have been reported (8–14). Mechanisms for TGF-β signaling to these pathways remain to be fully defined.
In contrast to TβRII and TβRI, TβRIII is a TGF-β superfamily co-receptor, which binds TGF-β superfamily ligands including TGF-β isoforms (15), inhibin (16,17) and BMPs (18) and facilitates their interaction with the type II and type I TGF-β superfamily receptors. TβRIII is thought to function through binding TGF-β superfamily ligands and presenting them to the appropriate type II or type I TGF-β superfamily receptors. In addition, recent studies support essential, non-redundant roles for TβRIII in mediating TGF-β sensitivity in intestinal goblet cells (19), in valve formation during embryonic chick heart development (20) and in murine development (21). TβRIII has also been established as a suppressor of cancer progression, with frequent loss of TβRIII expression in cancers of the human kidney (22), breast (23), prostate (24), ovary (25), pancreas (26) and lung (27), loss of expression correlating with disease progression and a poorer patient prognosis and restoration of expression establishing a direct role for TβRIII in regulating cancer cell migration and invasion in vitro and angiogenesis and metastasis in vivo.
TβRIII has a short cytoplasmic domain without kinase activity. While this cytoplasmic domain is not essential for mediating the presentation role, it contributes to TβRIII/TGF-β-mediated inhibition of proliferation (28,29). We have also established specific functions of the cytoplasmic domain, including (i) binding the PDZ domain-containing protein, G alpha interacting protein (GAIP), C-terminus, to stabilize TβRIII on the cell surface and enhance TGF-β signaling (30) and (ii) binding the scaffolding protein β-arrestin2 to mediate the co-internalization of TβRII and TβRIII and downregulation of TGF-β signaling (31).
β-Arrestins are ubiquitous expressed scaffolding proteins that regulate G-protein-coupled receptor (GPCR) signaling by binding to the activated GPCR and causing receptor desensitization and internalization (32). In addition, β-arrestins also have important roles as adaptor proteins, linking GPCRs to clathrin-coated pits, interacting with Ral-GDS to regulate cytoskeleton rearrangement, scaffolding GPCRs with Src, Hck/c-Fgr, Yes and mediating GPCR activation of c-jun N-terminal kinase 3 and extracellular signal-regulated kinase 1/2 (33–35). Recent studies also support β-arrestin2 as a negative regulator of NFκB signaling. Here, β-arrestin2 functions by interacting with IκBα and preventing its phosphorylation by IκB kinase (IKK) (36–38). Although TβRIII has been shown to interact with β-arrestin2 through its cytoplasmic domain (31), whether this couples TβRIII to Smad-independent signaling cascades has not been established.
NFκB is a dimeric transcription factor that regulates genes involved in immune regulation, cell migration, inflammation and apoptosis (39). There are five mammalian isoforms, RelA/p65, c-Rel, RelB, p50 and p52, which form hetero- or homodimers. Most NFκB dimers are bound to its potent inhibitor protein IκB and sequestered in the cytoplasm. When stimulated by appropriate extracellular signals, IκB is phosphorylated by IKK, which results in proteasome-mediated degradation of IκB. Once disassociated from IκB, NFκB translocates to the nucleus and activates specific target genes. Although there is growing evidence supporting increased/constitutive Rel/NFκB signaling in human tumors, mechanisms for increased activation of NFκB signaling remain to be established. Recently, short hairpin RNA (shRNA)-mediated silencing of TβRIII expression was reported to increase NFκB signaling in a murine mammary epithelial cell line (40), although the mechanism was not defined. Here, we investigate the role of TβRIII and β-arrestin2 in regulating NFκB activation during breast cancer progression.
Materials and methods
Materials and plasmids
Dulbecco's modified Eagle's medium,minimum essential medium and HAMS F12 were purchased from Invitrogen Co. (Carlsbad, CA). Defined fetal bovine serum and equine serum were from Hyclone (Logan, UT). Insulin, hydrocortisone and epidermal growth factor were from Invitrogen Co. and cholera toxin was from Calbiochem (La Jolla, CA). Fugene 6 was from Roche Molecular Biochemicals (Mannheim, Germany) and TGF-β1 and TGF-β2 were from R&D Systems (Minneapolis, MN). G418 was from Calbiochem. Mouse monoclonal antibody specific for Smad2 and for phospho-p65, rabbit polyclonal antibodies specific for phospho-Smad2, IκBα, phospho-IκBα, and horseradish peroxidase-conjugated anti-mouse and anti-rabbit antibodies were from Cell Signaling Technology (Beverly, MA). The polyclonal antibody recognizing TβRIII protein (820) has been described previously (23). Chemically synthesized small interfering RNAs (siRNA) corresponding to human β-arrestin2 with the sequence 5′-AAGGACCGCAAAGUGUUUGUG-3′ as described previously (31) were purchased from Dharmacon (Lafayette, CO).
Cell culture and adenoviral infection
MCF10A cells and MDA-MB-231 cells were purchased from the American Type Culture Collection (Manassas, VA). MCF10A cells were maintained in Dulbecco's modified Eagle's medium/F12 (Gibco, Karlsruhe, Germany) with 5% heat-inactivated equine serum, 20 ng/ml epidermal growth factor, 100 ng/ml cholera toxin, 0.002 U/ml insulin and 500 ng/ml hydrocortisone. MDA-MB-231 cells were maintained as monolayers in minimum essential medium supplemented with 10% heat-inactivated fetal bovine serum, non-essential amino acid and sodium pyruvate. MDA-MB-231-Neo and MDA-MB-231-TβRIII stable cell lines were selected in 0.6 mg/ml G418 and maintained in growth media containing 0.3 mg/ml G418 as described previously (23). All cells were grown in 5% CO2 at 37°C in a humidified atmosphere.
Binding and cross-linking assay
Radioligand binding and cross-linking of 125I-TGF-β1 to TGF-β receptors in each cell lines were performed by incubating subconfluent cells with Kerbs--Ringer--Hepes (KRH) buffer (50 mM HEPES, pH 7.5, 130 mM NaCl, 5 mM MgSO4, 1 mM CaCl2 and 5 mM KCl) containing 0.5% bovine serum albumin for 30 min at 37°C and then with 100 pM 125I-TGF-β1 for 3 h at 4°C. 125I-TGF-β1 was cross-linked with 0.5 mg/ml disuccinimidyl suberate and quenched with 20 mM glycine. Cells were then washed with KRH buffer, lysed in radioimmunoprecipitation assay buffer, immunoprecipitated with the indicated antibodies and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and phosphorimaging analysis of dried gels.
Transcription reporter luciferase assay
Cells (3 × 104) were grown in a 24-well plate and transfected with the p3TP vector containing the luciferase gene under the regulation of a promoter based on the TGF-β-inducible promoter (a part of the plasminogen activator inhibitor-1 promoter) (41–43) or the pNFkB-Luc vector (Stratagene, La Jolla, CA) containing five repeats of an NFκB-sensitive enhancer element upstream of the TATA box controlling luciferase gene expression (44) and the pRL-SV40 vector (Promega Corp., Madison, WI) expressing Renilla luciferase under the control of SV40 promoter to control for transfection efficiency using Fugene 6. Besides those reporter plasmids, some cells were transfected with pcDNA3, pcDNA3-TβRIII, pcDNA3-TβRIII-T841A, siRNA against β-arrestin2 or scrambled siRNA oligonucleotides. After 36 h, the cells were incubated with or without TGF-β1 (100 pM) or TGF-β2 (200 pM) for 12–24 h before harvest. Luciferase activities were measured in a multilabel counter (Perkin-Elmer, Waltham, MA) using the dual luciferase reporter assay system (Promega Corp.).
Western blotting
Protein samples were heated to 95°C for 5 min and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis on 8 or 10% acrylamide gels, followed by electrophoretic transfer to polyvinylidene difluoride membranes for 1 h at 350 mA with a NOVEX semi transfer unit. Membranes were then blocked for 1 h in Tris-buffered saline with 0.01% Tween 20 with 5% non-fat-dried milk, after which they were incubated for 2 h with primary antibody in Tris-buffered saline with 0.01% Tween 20 with 2% bovine serum albumin, followed by 1 h with horseradish peroxidase-conjugated anti-mouse or anti-rabbit antibody. The blots were developed with an enhanced chemiluminescence kit (Amersham Pharmacia Biotech, Piscataway, NJ), and quantification of band intensity on XAR-5 film (Eastman Kodak Co., Rochester, NY) was measured by Quantity one software (Bio-Rad, Chicago, IL).
shRNA-mediated silencing of human TβRIII in MCF10A or MDA-MB-231 cells
TβRIII knockdown was achieved by shRNA-mediated silencing of TβRIII via adenoviral transduction as described previously (29). For shRNA knockdown, MCF10A or MDA-MB-231 cells were infected with Adeno-X-shRIII or control-scrambed adenoviruses and the selective knockdown of TβRIII verified by 125I-TGF-β1 binding and cross-linking and western blotting.
Overexpression of wild-type or T841A mutant of human TβRIII by adenoviral infection
Cells were seeded on 12- or 6-well plates, grown for 24 h in each specific media and infected with Adeno-TβRIII or Adeno-TβRIII-T841A or GFP-adenoviruses. After 2 days, cells were starved for 12 h and exposed to TGF-β for 1 h, after which cells were harvested, separated by gel electrophoresis and subjected to western blot analysis.
Migration assay
Cells (3 × 104) were seeded in the upper chamber of a transwell filter (8 μm pore transwell insert, Costar, Cambridge, MA), coated both at the top and at the bottom with 50 μg/ml fibronectin (Calbiochem) to assess cell migration. Cells were allowed to migrate for 24 h at 37°C through the fibronectin toward the lower chamber containing media plus 10% fetal bovine serum. Cells on the upper surface of the filter were removed and the cells that migrated to the underside of the filter were fixed and stained using the three Step Stain Set (Richard-Allan Scientific, Kalamazoo, MI). Each assay was conducted at least four times with five random fields from a ×20 magnification analyzed for each membrane.
Statistical analysis
All data are expressed as percentages of control and shown as means ± SDs, unless otherwise indicated. Statistical comparisons between groups were made using Student's t-tests.
Results
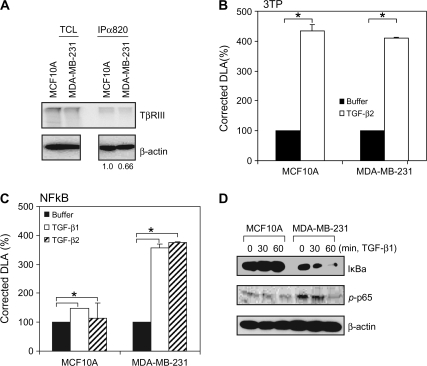
We have previously reported that TβRIII interacts with β-arrestin2 through its cytoplasmic domain in a TβRII-mediated phosphorylation-dependent manner, with this interaction regulating receptor internalization and Smad-dependent signaling (31). As TGF-β has been reported to either activate (45–48) or inhibit (49–52) NFκB signaling through mechanisms yet to be fully defined and β-arrestin2 has been shown to have a role in linking interacting receptors to NFκB regulation (36–38,53), and shRNA-mediated silencing of TβRIII expression was reported to increase NFκB signaling in a murine mammary epithelial cell line (40), we investigated whether TβRIII was involved in NFκB regulation via its interaction with β-arrestin2. As we and others have demonstrated that TβRIII expression is frequently decreased in human breast cancer cell lines and specimens relative to normal breast cancer epithelial cells (23,54), we first examined the effect of TGF-β on NFκB activation in MCF10A cells, immortalized human mammary epithelial cells with basal levels of TβRIII expression, and MDA-MB-231 cells, malignant human breast cancer cells, with decreased TβRIII expression (Figure 1A). Although TGF-β induced a Smad-dependent promoter (3TP) in both MCF10A and MDA-MB-231 cells to similar extent (Figure 1B), TGF-β only induced a NFκB-dependent promoter in MDA-MB-231 cells and not in MCF10A cells (Figure 1C). As NFκB activation should be accompanied by proteasome-mediated degradation of IκBα, we also investigated basal IκBα protein expression and IκBα protein expression in response to TGF-β stimulation. Basal IκBα protein expression was lower in MDA-MB-231 cells than in MCF10A cells (Figure 1D). In addition, IκBα protein was degraded in response to TGF-β in MDA-MB-231 cells (Figure 1D), whereas there was no decrease in IκBα expression in MCF10A cells in response to TGF-β (Figure 1D). Activation of RelA/p65 was examined by following its phosphorylation on Ser536 (55,56) basally and in response to TGF-β. Consistent with the NFκB-dependent promoter and IκBα expression results, phosphorylation of p65 was increased in MDA-MB-231 cells relative to MCF10A cells (Figure 1D), although TGF-β stimulation did not further stimulate p65 phosphorylation in either cell line. Taken together, these studies suggested that decreased TβRIII expression could be restoring NFκB activation in breast cancer cells.
Fig. 1.
TβRIII expression correlates with NFκB signaling in MCF10A and MDA-MB-231 cells. (A) Cell surface expression of TβRIII in MCF10A and MDA-MB-231 cells was demonstrated by binding and cross-linking assay as described in Materials and Methods. Both total cell lysate and immunoprecipitates with anti-TβRIII antibody (820) were analyzed. For the immunoprecipitates, densitometry of TβRIII expression controlled for β-actin is presented below. (B and C) Cells were transfected with pNFκB-Luc (B) or p3TP-Luc (C) and pRL-SV40 using Fugene 6 and grown for 24 h. Cells were treated with buffer, TGF-β1 (100 pM) or TGF-β2 (200 pM) for 24 h and harvested for luciferase activity assay. Data are expressed as means ± SDs of at least three independent experiments. Statistical significance of differences was assessed using unpaired Student's t-tests (*P < 0.01). (D) Subconfluent cells were treated with TGF-β1 (200 pM) for the indicated times and harvested for western blotting with the indicated antibodies. The results shown are representative of at least three independent experiments.
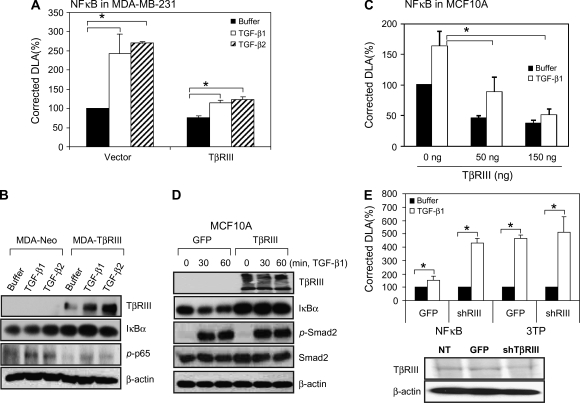
To directly investigate the role of TβRIII on NFκB activation, human TβRIII expression was restored in MDA-MB-231 cells and the effects on NFκB activation assessed. Consistent with a direct effect of TβRIII on NFκB activation, restoring TβRIII expression suppressed TGF-β-induced NFκB-dependent promoter activity (Figure 2A), increased basal and TGF-β-stimulated IκBα expression and decreased basal and TGF-β-stimulated phosphorylation of p65 (Figure 2B) in MDA-MB-231 breast cancer cells. Similar effects were observed in MCF10A cells, where transiently increasing TβRIII expression decreased both TGF-β-induced and basal NFκB-dependent promoter activity in a dose-dependent fashion (Figure 2C) and increased IκBα expression (Figure 2D). These effects were specific, as TGF-β-induced Smad2 phosphorylation was not altered (Figure 2D). In a reciprocal fashion, shRNA-mediated silencing of TβRIII expression, as confirmed by iodinated TGF-β1 binding and cross-linking (Figure 2E, bottom), increased TGF-β-induced NFκB-dependent promoter activity (Figure 2E, upper), without altering activation of the Smad-dependent promoter (3TP), consistent with recent reports (40,57). Taken together, these results support a direct role for TβRIII in negatively regulating both basal- and TGF-β-induced NFκB activation.
Fig. 2.
TβRIII negatively regulates NFκB signaling. (A and C) MCF10A and MDA-MB-231 cells were transfected with pNFκB-Luc, pRL-SV40 and TβRIII (300 ng per well in a 24-well plate for MDA-MB-231 cells, indicated amounts for MCF10A cells) using Fugene 6 and grown for 24 h. Cells were treated with buffer, TGF-β1 (100 pM) or TGF-β2 (200 pM) for 24 h and harvested for luciferase activity assay. Data are expressed as means ± SDs of at least three independent experiments. Statistical significance of differences was assessed using unpaired Student's t-tests (*P < 0.01). (B) MDA-MB-231-Neo and MDA-MB-231-TβRIII cells were treated with TGF-β1 or TGF-β2 for 1 h, harvested and subjected to western blotting. (D) Subconfluent MCF10A cells were infected with Adeno-TβRIII or GFP-adenovirus. After 2 days, cells were treated with TGF-β1 (200 pM) for the indicated times and harvested for western blotting with the indicated antibodies. The results shown are representative of at least three independent experiments. (E) MCF10A cells were infected with GFP or shRNA of TβRIII adenovirus and transfected with pNFκB and pRL-SV40 for reporter gene assay. After 1 day, cells were treated with TGF-β1 or TGF-β2 for 24 h and subjected to reporter gene assay and binding and cross-linking assay. Data are expressed as means ± SDs of at least three independent experiments. Statistical significance of differences was assessed using unpaired Student's t-tests (*P < 0.01).
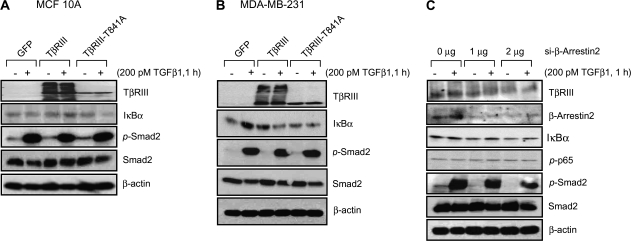
As β-arrestin2 interacts with TβRIII (31) and β-arrestin2 has been demonstrated to negatively regulate NFκB signaling by binding IκBα (38,53), we investigated the role of β-arrestin2 in negative regulation of NFκB activation by TβRIII. We first examined the effect of a TβRIII mutant unable to interact with β-arrestin2, TβRIII-T841A (31). In MCF10A cells, while TβRIII was able to increase TGF-β-induced IκBα expression, TβRIII-T841A was unable to do so (Figure 3A). In MDA-MB-231 cells, TβRIII increased basal IκBα expression, whereas TβRIII-T841A was unable to do so (Figure 3B). These differences were not due to a fundamental defect in TβRIII-T841A, as TβRIII-T841A was able to traffic to the cell surface and bind TGF-β1 (data not shown). These data suggest a role for β-arrestin2 binding in mediating TβRIIIs effects on NFκB regulation. To further investigate a role for β-arrestin2 in TβRIII-mediated suppression of NFκB signaling, we transfected MDA-MB-231 cells with siRNA against β-arrestin2 (Figure 3C). siRNA to β-arrestin2 decreased β-arrestin2 in a dose-dependent fashion and resulted in a progressive loss of IκBα expression and a progressive increase in phosphorylation of p65, independent of TGF-β stimulation (Figure 3C). In all cases, the effects of TβRIII were specific as TGF-β-induced Smad2 phosphorylation was not altered (Figure 3A–C). Taken together, these results support a role for β-arrestin2 in mediating the effects of TβRIII in suppressing NFκB signaling.
Fig. 3.
TβRIII negatively regulates NFκB signaling via β-arrestin2. MCF10A (A) and MDA-MB-231 (B) cells were infected with adenovirus expressing GFP, TβRIII or TβRIII-T841A and grown for 48 h. Cells were then treated with TGF-β1, 200 pM for 1 h, harvested and subjected to western blotting with the indicated antibodies. (C) MDA-MB-231 cells were transfected with siRNA against β-arrestin2 for 2 days and treated with TGF-β1 (200 pM, 1 h), after which cells were harvested for western blotting. The results shown are representative of at least three independent experiments.
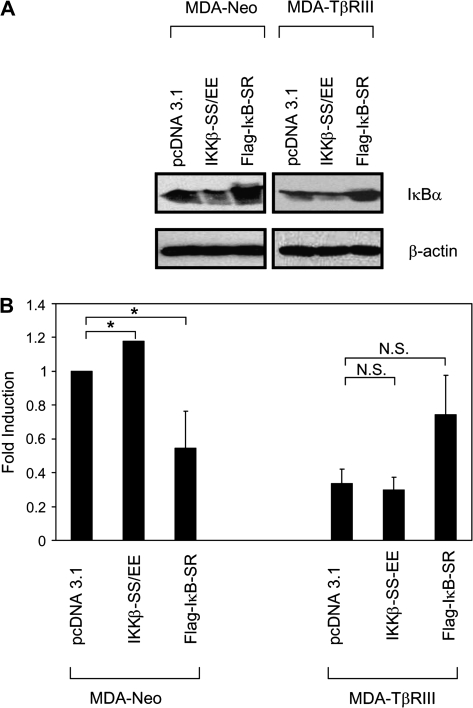
We have previously demonstrated a role for TβRIII in suppressing breast cancer progression, primarily through inhibition of breast cancer cell migration and invasion (23). To investigate the role of TβRIII/β-arrestin2-mediated inhibition of NFκB signaling on TβRIII-mediated inhibition of cell migration, we investigated the effects of increasing and decreasing NFκB signaling on breast cancer cell migration in the presence and absence of exogenous TβRIII expression in the MDA-MB-231 model. NFκB signaling was increased by expression of constitutively active IKKβ (IKKβ-SS/EE), which successfully increased NFκB signaling as assessed by decreased IκBα levels in both MDA-MB-231 and MDA-MB-231-TβRIII cells (Figure 4A), whereas NFκB signaling was decreased by expression of the IκB super-repressor (IκB-S32/36A), which successfully decreased NFκB signaling as assessed by increased IκBα levels in both MDA-MB-231 and MDA-MB-231-TβRIII cells (Figure 4A). We then assessed the effects of increasing or decreasing NFκB signaling on breast cancer cell migration. Consistent with our prior results, restoration of TβRIII expression decreased the migration of MDA-MB-231 cells ∼60% (Figure 4B). In addition, while IKKβ-SS/EE consistently increased and IκB-S32/36A decreased the migration of MDA-MB-231 cells, in the presence of TβRIII, neither IKKβ-SS/EE nor IκB-S32/36A was able to alter the migration of MDA-MB-231 cells (Figure 4B). These results suggest that inhibition of NFκB signaling represents a potential mechanism for TβRIII-mediated inhibition of cell migration.
Fig. 4.
TβRIII suppresses cell migration, in part, by negatively regulating the NFκB pathway. MDA-MB-231 cells were transfected with pcDNA3.1, IKKβ-SS/EE or IκB-super-repressor (SR) and grown for 48 h. Cells were then harvested for western blotting (A) or migration assays (B). The results shown are the average of four independent experiments ± SEM, NS, not significant, *P< 0.05.
Discussion
While the interaction of the cytoplasmic domain of TβRIII with scaffolding proteins including GAIP-interacting protein, C-terminus (30) and β-arrestin2 (31) had been defined, to date these interactions had been demonstrated to alter the trafficking and internalization of receptors to regulate canonical TGF-β signaling (31,58,59). In addition, we had demonstrated a role for TβRIII in signaling through the p38 mitogen-activated protein kinase pathway to regulate cellular proliferation, which was at least partially dependent on the cytoplasmic domain of TβRIII (29). The current studies expand the function of TβRIII, defining a novel function for the conserved cytoplasmic domain of TβRIII in negatively regulating NFκB signaling through its interaction with β-arrestin2.
How might TβRIII negatively regulate NFκB signaling through its interaction with β-arrestin2? Previous studies have supported a mechanism by which β-arrestin2 directly binds IκBα (37,38), preventing phosphorylation of IκBα and blocking IκBα degradation (37). Our current results are consistent with this model, as increasing TβRIII expression increases IκBα protein levels, whereas decreasing β-arrestin2 decreases IκBα protein levels. Although TGF-β signaling can either increase (45–48) or decrease (49–52) NFκB signaling depending on the cellular context, the mechanism coupling the TGF-β-signaling pathway and TGF-β superfamily signaling components to NFκB signaling as well the mechanism for the differential response of cells to TGF-β signaling in terms of NFκB signaling remains to be fully defined. In the context of mammary carcinogenesis, the ability of TGF-β to activate NFκB signaling has been linked to the ability to form TGF-β-activated kinase 1 (TAK1)-binding protein 1 (TAB1):IKKβ complexes, which activate the TAK1:IKKβ:p65 pathway (60). The current studies suggest that differential expression of either TβRIII and/or β-arrestin2 could partially explain this divergent response as well, with the absence of either being generally permissive and allowing activation of NFκB signaling and the presence of both resulting in inhibition of NFκB signaling. The inhibition of NFκB signaling by TβRIII and β-arrestin2 occurs both in the presence (Figure 1C and D and Figure 2A, B and C) and in the absence (Figure 1D and Figure 2A, B and C) of exogenous TGF-β stimulation. While it is difficult to entirely rule out the contribution of endogenous TGF-β production, this suggests that TβRIII via β-arrestin2 might be responsible for tonically repressing NFκB signaling independent of its role in regulating TGF-β signaling. However, in the context of TGF-β signaling, the TβRIII/β-arrestin2 axis might function to repress NFκB signaling by blocking the TAB1:TAK1:IKKβ:IκBα:p65 NFκB signaling axis, either by binding and sequestering IκBα or potentially by interfering with other components of this pathway. Whether the TβRIII/β-arrestin2-mediated regulation of NFκB signaling is operative in other cell contexts is currently being investigated.
The role for TβRIII in negatively regulating NFκB signaling through its interaction with β-arrestin2 provides another mechanism by which TβRIII can function as a suppressor of cancer progression in breast cancer. We had previously identified a role for TβRIII in mediating inhibition of cell proliferation through both Smad-dependent and Smad-independent pathways (29). Thus, loss of TβRIII could partially mediate the increased proliferation observed during mammary carcinogenesis. We had also identified a role for TβRIII in negatively regulating invasion, largely through production of soluble TβRIII (23). Most recently, we have defined a role for TβRIII in negatively regulating cell migration through a TβRI/Smad2-independent mechanism (M.Karthikeyan and G.C.Blobe, in preparation). The identification of multiple mechanisms by which TβRIII normally regulates the homeostasis of mammary epithelial cells underscores the physiological significance of TβRIII and further supports the significance of loss of TβRIII expression during breast cancer progression. The relative contribution of these distinct pathways to the suppressor of cancer progression role of TβRIII in human cancers is currently under investigation.
In addition to functioning as a suppressor of cancer progression in breast cancer, TβRIII expression is also decreased in lung, ovarian, pancreatic and prostate cancer. Although constitutive activation of NFκB signaling has been reported in cancers of the breast (61), lung (62), pancreas (63) and prostate (64), mechanisms by which this constitutive activation occurs have not been established. The current studies suggest that loss of TβRIII expression could be one such mechanism. The role of loss of TβRIII expression in the constitutive activation of NFκB signaling in cancers of the lung, ovary, pancreas and prostate remains to be explored.
In summary, here, we establish the TβRIII/β-arrestin2 axis as an important negative regulator of NFκB signaling in breast cancer and a potential mechanism for TβRIII-mediated inhibition of breast cancer cell migration and breast cancer progression.
Funding
National Institute of Health/National Cancer Institute (R01-CA106307) to G.C.B.; Susan G.Komen Breast Cancer Foundation to H.J.Y.
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- GPCR
G-protein-coupled receptor
- IKK
IκB kinase
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- TGF-β
transforming growth factor-β
- TβRIII
type III transforming growth factor-β receptor
References
- 1.Bierie B, et al. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 2.Elliott RL, et al. Role of transforming growth factor Beta in human cancer. J. Clin. Oncol. 2005;23:2078–2093. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 3.Gordon KJ, et al. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim. Biophys. Acta. 2008;1782:197–228. doi: 10.1016/j.bbadis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Kirkbride KC, et al. Cell-surface co-receptors: emerging roles in signaling and human disease. Trends Biochem. Sci. 2005;30:611–621. doi: 10.1016/j.tibs.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Massague J, et al. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 6.Piek E, et al. Specificity, diversity, and regulation in TGF-beta superfamily signaling. FASEB J. 1999;13:2105–2124. [PubMed] [Google Scholar]
- 7.Macias-Silva M, et al. MADR2 is a substrate of the TGFbeta receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell. 1996;87:1215–1224. doi: 10.1016/s0092-8674(00)81817-6. [DOI] [PubMed] [Google Scholar]
- 8.Kamaraju AK, et al. Role of Rho/ROCK and p38 MAP kinase pathways in transforming growth factor-beta-mediated Smad-dependent growth inhibition of human breast carcinoma cells in vivo. J. Biol. Chem. 2005;280:1024–1036. doi: 10.1074/jbc.M403960200. [DOI] [PubMed] [Google Scholar]
- 9.Moustakas A, et al. Non-Smad TGF-beta signals. J. Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 10.Remy I, et al. PKB/Akt modulates TGF-beta signalling through a direct interaction with Smad3. Nat. Cell Biol. 2004;6:358–365. doi: 10.1038/ncb1113. [DOI] [PubMed] [Google Scholar]
- 11.Song K, et al. Novel roles of Akt and mTOR in suppressing TGF-beta/ALK5-mediated Smad3 activation. EMBO J. 2006;25:58–69. doi: 10.1038/sj.emboj.7600917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian F, et al. Smad-binding defective mutant of transforming growth factor beta type I receptor enhances tumorigenesis but suppresses metastasis of breast cancer cell lines. Cancer Res. 2004;64:4523–4530. doi: 10.1158/0008-5472.CAN-04-0030. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe H, et al. Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-beta-induced aggrecan gene expression in chondrogenic ATDC5 cells. J. Biol. Chem. 2001;276:14466–14473. doi: 10.1074/jbc.M005724200. [DOI] [PubMed] [Google Scholar]
- 14.Yu L, et al. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002;21:3749–3759. doi: 10.1093/emboj/cdf366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blobe GC, et al. Role of transforming growth factor beta in human disease. N. Engl. J. Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 16.Lewis KA, et al. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature. 2000;404:411–414. doi: 10.1038/35006129. [DOI] [PubMed] [Google Scholar]
- 17.Wiater E, et al. Inhibin is an antagonist of bone morphogenetic protein signaling. J. Biol. Chem. 2003;278:7934–7941. doi: 10.1074/jbc.M209710200. [DOI] [PubMed] [Google Scholar]
- 18.Kirkbride KC, et al. Bone morphogenetic proteins signal through the transforming growth factor-beta type III receptor. J. Biol. Chem. 2008;283:7628–7637. doi: 10.1074/jbc.M704883200. [DOI] [PubMed] [Google Scholar]
- 19.Deng X, et al. Differential responsiveness to autocrine and exogenous transforming growth factor (TGF) beta1 in cells with nonfunctional TGF-beta receptor type III. Cell Growth Differ. 1999;10:11–18. [PubMed] [Google Scholar]
- 20.Brown CB, et al. Requirement of type III TGF-beta receptor for endocardial cell transformation in the heart. Science. 1999;283:2080–2082. doi: 10.1126/science.283.5410.2080. [DOI] [PubMed] [Google Scholar]
- 21.Stenvers KL, et al. Heart and liver defects and reduced transforming growth factor beta2 sensitivity in transforming growth factor beta type III receptor-deficient embryos. Mol. Cell. Biol. 2003;23:4371–4385. doi: 10.1128/MCB.23.12.4371-4385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Copland JA, et al. Genomic profiling identifies alterations in TGFbeta signaling through loss of TGFbeta receptor expression in human renal cell carcinogenesis and progression. Oncogene. 2003;22:8053–8062. doi: 10.1038/sj.onc.1206835. [DOI] [PubMed] [Google Scholar]
- 23.Dong M, et al. The type III TGF-beta receptor suppresses breast cancer progression. J. Clin. Invest. 2007;117:206–217. doi: 10.1172/JCI29293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turley RS, et al. The type III transforming growth factor-beta receptor as a novel tumor suppressor gene in prostate cancer. Cancer Res. 2007;67:1090–1098. doi: 10.1158/0008-5472.CAN-06-3117. [DOI] [PubMed] [Google Scholar]
- 25.Hempel N, et al. Loss of betaglycan expression in ovarian cancer: role in motility and invasion. Cancer Res. 2007;67:5231–5238. doi: 10.1158/0008-5472.CAN-07-0035. [DOI] [PubMed] [Google Scholar]
- 26.Gordon KJ, et al. Loss of type III transforming growth factor beta receptor expression increases motility and invasiveness associated with epithelial to mesenchymal transition during pancreatic cancer progression. Carcinogenesis. 2008;29:252–262. doi: 10.1093/carcin/bgm249. [DOI] [PubMed] [Google Scholar]
- 27.Finger EC, et al. TbetaRIII suppresses non-small cell lung cancer invasiveness and tumorigenicity. Carcinogenesis. 2008;29:528–535. doi: 10.1093/carcin/bgm289. [DOI] [PubMed] [Google Scholar]
- 28.Blobe GC, et al. Functional roles for the cytoplasmic domain of the type III transforming growth factor beta receptor in regulating transforming growth factor beta signaling. J. Biol. Chem. 2001;276:24627–24637. doi: 10.1074/jbc.M100188200. [DOI] [PubMed] [Google Scholar]
- 29.You HJ, et al. The type III TGF-beta receptor signals through both Smad3 and the p38 MAP kinase pathways to contribute to inhibition of cell proliferation. Carcinogenesis. 2007;28:2491–2500. doi: 10.1093/carcin/bgm195. [DOI] [PubMed] [Google Scholar]
- 30.Blobe GC, et al. A novel mechanism for regulating transforming growth factor beta (TGF-beta) signaling. Functional modulation of type III TGF-beta receptor expression through interaction with the PDZ domain protein, GIPC. J. Biol. Chem. 2001;276:39608–39617. doi: 10.1074/jbc.M106831200. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, et al. Beta-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science. 2003;301:1394–1397. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- 32.Luttrell LM, et al. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 33.Goodman OB, Jr, et al. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 34.Perry SJ, et al. Arresting developments in heptahelical receptor signaling and regulation. Trends Cell Biol. 2002;12:130–138. doi: 10.1016/s0962-8924(01)02239-5. [DOI] [PubMed] [Google Scholar]
- 35.Bhattacharya M, et al. Beta-arrestins regulate a Ral-GDS Ral effector pathway that mediates cytoskeletal reorganization. Nat. Cell Biol. 2002;4:547–555. doi: 10.1038/ncb821. [DOI] [PubMed] [Google Scholar]
- 36.Chen F. Arresting NF-kappaB by beta-arrestin2. Cell Death Differ. 2004;11:1155–1156. doi: 10.1038/sj.cdd.4401496. [DOI] [PubMed] [Google Scholar]
- 37.Gao H, et al. Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol. Cell. 2004;14:303–317. doi: 10.1016/s1097-2765(04)00216-3. [DOI] [PubMed] [Google Scholar]
- 38.Witherow DS, et al. Beta-arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha. Proc. Natl Acad. Sci. USA. 2004;101:8603–8607. doi: 10.1073/pnas.0402851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 40.Criswell TL, et al. Modulation of NFkappaB activity and E-cadherin by the type III transforming growth factor beta receptor regulates cell growth and motility. J. Biol. Chem. 2007;282:32491–32500. doi: 10.1074/jbc.M704434200. [DOI] [PubMed] [Google Scholar]
- 41.Keeton MR, et al. Identification of regulatory sequences in the type 1 plasminogen activator inhibitor gene responsive to transforming growth factor beta. J. Biol. Chem. 1991;266:23048–23052. [PubMed] [Google Scholar]
- 42.Hua X, et al. Synergistic cooperation of TFE3 and smad proteins in TGF-beta-induced transcription of the plasminogen activator inhibitor-1 gene. Genes Dev. 1998;12:3084–3095. doi: 10.1101/gad.12.19.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wrana JL, et al. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 44.You HJ, et al. A pathway involving protein kinase Cdelta up-regulates cytosolic phospholipase A(2)alpha in airway epithelium. Biochem. Biophys. Res. Commun. 2004;321:657–664. doi: 10.1016/j.bbrc.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 45.Arsura M, et al. Transient activation of NF-kappaB through a TAK1/IKK kinase pathway by TGF-beta1 inhibits AP-1/SMAD signaling and apoptosis: implications in liver tumor formation. Oncogene. 2003;22:412–425. doi: 10.1038/sj.onc.1206132. [DOI] [PubMed] [Google Scholar]
- 46.Huber MA, et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Invest. 2004;114:569–581. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konig HG, et al. TGF-{beta}1 activates two distinct type I receptors in neurons: implications for neuronal NF-{kappa}B signaling. J. Cell Biol. 2005;168:1077–1086. doi: 10.1083/jcb.200407027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu T, et al. Dose-dependent cross-talk between the transforming growth factor-beta and interleukin-1 signaling pathways. Proc. Natl Acad. Sci. USA. 2007;104:4365–4370. doi: 10.1073/pnas.0700118104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arsura M, et al. TGF beta 1 inhibits NF-kappa B/Rel activity inducing apoptosis of B cells: transcriptional activation of I kappa B alpha. Immunity. 1996;5:31–40. doi: 10.1016/s1074-7613(00)80307-6. [DOI] [PubMed] [Google Scholar]
- 50.Arsura M, et al. Nuclear factor-kappaB/Rel blocks transforming growth factor beta1-induced apoptosis of murine hepatocyte cell lines. Cell Growth Differ. 1997;8:1049–1059. [PubMed] [Google Scholar]
- 51.Nagarajan RP, et al. Repression of transforming-growth-factor-beta-mediated transcription by nuclear factor kappaB. Biochem. J. 2000;348(Pt 3):591–596. [PMC free article] [PubMed] [Google Scholar]
- 52.Monteleone G, et al. A failure of transforming growth factor-beta1 negative regulation maintains sustained NF-kappaB activation in gut inflammation. J. Biol. Chem. 2004;279:3925–3932. doi: 10.1074/jbc.M303654200. [DOI] [PubMed] [Google Scholar]
- 53.Luan B, et al. Beta-arrestin2 functions as a phosphorylation-regulated suppressor of UV-induced NF-kappaB activation. EMBO J. 2005;24:4237–4246. doi: 10.1038/sj.emboj.7600882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hempel N, et al. Expression of the type III TGF-beta receptor is negatively regulated by TGF-beta. Carcinogenesis. 2008;29:905–912. doi: 10.1093/carcin/bgn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin CC, et al. Transforming growth factor-beta1 stimulates heme oxygenase-1 expression via the PI3K/Akt and NF-kappaB pathways in human lung epithelial cells. Eur. J. Pharmacol. 2007;560:101–109. doi: 10.1016/j.ejphar.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 56.Sizemore N, et al. Distinct roles of the Ikappa B kinase alpha and beta subunits in liberating nuclear factor kappa B (NF-kappa B) from Ikappa B and in phosphorylating the p65 subunit of NF-kappa B. J. Biol. Chem. 2002;277:3863–3869. doi: 10.1074/jbc.M110572200. [DOI] [PubMed] [Google Scholar]
- 57.Criswell TL, et al. Knockdown of the transforming growth factor-beta type III receptor impairs motility and invasion of metastatic cancer cells. Cancer Res. 2008;68:7304–7312. doi: 10.1158/0008-5472.CAN-07-6777. [DOI] [PubMed] [Google Scholar]
- 58.Finger EC, et al. Endocytosis of the type III TGF-beta receptor through the clathrin-independent/lipid raft pathway regulates TGF-betasignaling and receptor downregulation. J. Biol. Chem. 2008;283:34808–34818. doi: 10.1074/jbc.M804741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee NY, et al. Endoglin promotes transforming growth factor-beta-mediated smad 1/5/8 signaling and inhibits endothelial cell migration through its association with GIPC. J. Biol. Chem. 2008;283:32527–32533. doi: 10.1074/jbc.M803059200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neil JR, et al. Altered TAB1:I kappaB kinase interaction promotes transforming growth factor beta-mediated nuclear factor-kappaB activation during breast cancer progression. Cancer Res. 2008;68:1462–1470. doi: 10.1158/0008-5472.CAN-07-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sovak MA, et al. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J. Clin. Invest. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mukhopadhyay T, et al. Altered expression of the p50 subunit of the NF-kappa B transcription factor complex in non-small cell lung carcinoma. Oncogene. 1995;11:999–1003. [PubMed] [Google Scholar]
- 63.Wang W, et al. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin. Cancer Res. 1999;5:119–127. [PubMed] [Google Scholar]
- 64.Suh J, et al. Mechanisms of constitutive NF-kappaB activation in human prostate cancer cells. Prostate. 2002;52:183–200. doi: 10.1002/pros.10082. [DOI] [PubMed] [Google Scholar]