Abstract
Certain adult diseases may have their origin early in life, and perinatal exposures may contribute to cancers both during childhood and later in life. We recently demonstrated that mainstream cigarette smoke (MCS) induces a potent carcinogenic response in mice when exposure starts soon after birth. We also showed that the antioxidant N-acetylcysteine (NAC) prevents the extensive nucleotide and gene expression alterations that occur ‘physiologically’ at birth in mouse lung. The present study was designed to evaluate whether administration of NAC during pregnancy may affect the yield of tumors in mice exposed to MCS, starting after birth and continuing for 120 days. The results obtained showed that 210 days after birth, one adenoma only was detectable in sham-exposed mice. In contrast, as much as the 61.1% (33/54) of MCS-exposed mice born from untreated dams had lung tumors, including both benign tumors and bronchoalveolar carcinomas. Treatment with NAC during pregnancy strikingly inhibited the formation of benign lung tumors and totally prevented occurrence of carcinomas. In addition, prenatal NAC inhibited the MCS-induced hyperplasia of the urinary bladder epithelium. These findings demonstrate for the first time that treatment during pregnancy with an antioxidant chemopreventive agent can affect the induction of tumors consequent to exposure to a carcinogen after birth.
Introduction
Several lines of evidence suggest that certain adult diseases may have their origin early in life (1) and that perinatal exposures may contribute to cancers both during childhood and later in life (2). We recently demonstrated that mainstream cigarette smoke (MCS) induces a potent carcinogenic response in mice when exposure starts soon after birth (3). One of the mechanisms accounting for the high susceptibility at birth is that the sudden transition from the maternal-mediated respiration to the autonomous pulmonary respiration generates a tremendous oxidative stress, triggering extensive DNA damage and overexpression of genes in mouse lung (4,5). Administration during pregnancy of N-acetylcysteine (NAC), an antioxidant that has been in the clinical practice for almost 50 years (6) and is also available as a dietary supplement, prevented all birth-related genomic and postgenomic changes in mouse lung (4).
The evidence that birth represents a critical event of life, during which the organism is particularly vulnerable, prompted us to evaluate whether NAC, administered orally during pregnancy, may affect the yield of lung tumors in mice exposed to MCS during the postnatal life, starting at birth and continuing daily for 120 consecutive days. The results obtained showed that treatment with NAC during pregnancy strikingly inhibits the formation of benign lung tumors and totally prevents lung cancer in mice exposed to MCS for 120 days, starting immediately after birth. In addition, prenatal NAC prevented the MCS-induced hyperplasia of the urinary bladder epithelium. This demonstrates for the first time that the protection afforded by an antioxidant chemopreventive agent during pregnancy can affect the induction of tumors consequent to exposure to a carcinogen after birth.
Material and methods
Mice
We used 14 pregnant H mice, originated from Swiss albino mice, aged 14–16 weeks and weighing 27–30 g. Housing at the Animal Laboratory of the National Center of Oncology (Sofia, Bulgaria), breeding and treatment of mice were in accordance with national and institutional guidelines. The mice were kept in Makrolon cages on sawdust bedding and maintained on rodent chow (Kostinbrod, Sofia, Bulgaria) and tap water ad libitum, at a temperature of 23 ± 2°C, relative humidity of 55% and a 12 h day–night cycle.
Treatment with NAC during pregnancy
While nine pregnant mice were kept untreated, five mice received, throughout duration of pregnancy, NAC in the form of a pharmacological preparation (Fluimucil, Zambon, Vicenza, Italy), added to the drinking water at a concentration accounting for a calculated daily intake of 1 g/kg body wt.
Exposure of neonatal mice to MCS
Within 12 h after birth, 54 newborns (29 males and 25 females) born from untreated dams and 47 newborns (26 males and 21 females) born from NAC-treated dams started to be exposed to MCS. Forty newborns (23 males and 17 females) born from untreated dams were kept in filtered air (sham-exposed mice). A whole-body exposure of mice to MCS was achieved as described previously (3).
Evaluation of lung tumors and other histopathological alterations
After 4 months of daily exposure to MCS, treatment was discontinued, and all mice were kept in filtered air for an additional 3 months. The ‘spontaneously’ moribund mice and all those mice that survived after 7 months were deeply anesthesized with diethyl ether, killed by cervical dislocation and subjected to complete necropsy. The lungs (six standardized sections), liver and urinary bladder (two sections each) and all organs with suspected lesions were processed by standard histopathological analysis.
Statistical analysis
Body weights and multiplicity data were expressed as means ± SEs of the mice composing each experimental group, and comparisons between groups were made by Student's t-test for unpaired data. Comparisons between groups regarding survival and incidence data were made by χ2 analysis.
Results
Treatment with NAC during pregnancy did not affect the number of mice in the offspring, with 9.4 newborns/litter from NAC-treated dams versus 10.4 newborns/litter from untreated dams. The body weights at birth (means ± SEs) were similar in newborns from untreated dams (1.3 ± 0.03 g) and in newborns from NAC-treated mice (1.2 ± 0.02 g). Survival after 210 days was 31/40 (77.5%) in sham-exposed mice, 42/54 (77.8%) in MCS-exposed mice born from untreated dams and 40/47 (85.1%) in MCS-exposed mice born from NAC-treated dams. Premature deaths were mainly due to cases of pneumonia and leukemia, whose prevalence was not affected by treatments. Table I reports the body weights of the mice measured after 120 days, at the end of the exposure period to MCS, and after 210 days, at the end of the experiment. Exposure to MCS significantly decreased the body weights in both genders after 120 days, a trend that was significantly attenuated in MCS-exposed females born from NAC-treated dams. After 210 days, no difference was detectable among the variously treated mice.
Table I.
Body weights of variously treated mice at the end of the exposure period to MCS (120 days) and at the end of the experiment (210 days)
| Treatment of mice | Gender | Body weights (g) |
|
| 120 Days | 210 Days | ||
| Sham-exposed mice | M | 31.9 ± 0.91 | 32.4 ± 1.36 |
| F | 27.2 ± 0.79 | 29.5 ± 1.17 | |
| MCS-exposed micea | M | 27.3 ± 0.78b | 30.9 ± 0.78 |
| F | 23.5 ± 0.96b | 26.8 ± 1.11 | |
| MCS-exposed micec | M | 25.5 ± 0.70d | 32.5 ± 0.91 |
| F | 25.9 ± 0.69e | 26.6 ± 0.87 | |
All data are means ± SEs within each experimental group. F, female; M, male.
Born from untreated dams.
P < 0.05, as compared with sham-exposed mice of the same gender.
Born from NAC-treated dams.
P < 0.01, as compared with sham-exposed mice of the same gender.
P < 0.05, as compared with MCS-exposed females born from untreated dams.
A total of 57 lung tumors were detected in the 141 mice examined, including 28 microadenomas, 23 adenomas and six malignant tumors, all of them identified as bronchoalveolar carcinomas. Some of the carcinomas had a big size, occupying a whole lobe or a whole lung. We refer to our previous paper (3) for examples of microscopical appearance of these lung tumors.
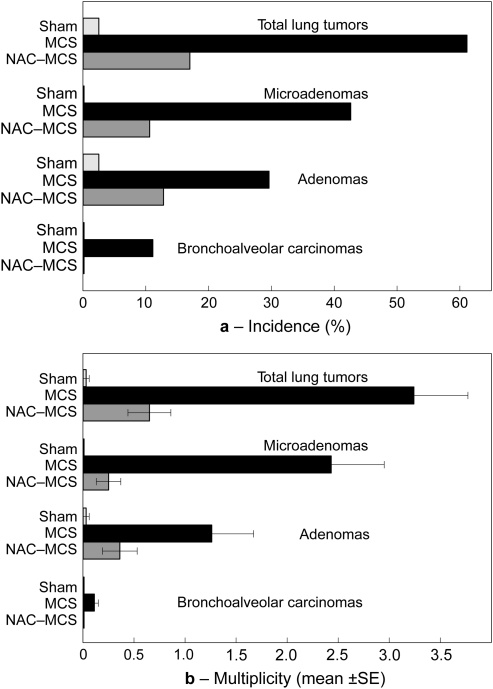
Figure 1 shows the incidence and multiplicity of lung tumors in the three experimental groups. In sham-exposed mice, only one adenoma was detected, with an overall multiplicity of 0.03 ± 0.03 tumors per mouse (mean ± SE). In contrast, as much as the 61.1% of MCS-exposed mice born from untreated dams (18 males and 15 females) had lung tumors (P < 0.0001 versus sham-exposed mice), with a multiplicity of 3.24 ± 0.53 (P < 0.0001). In particular, 23 mice in this group (13 males and 10 females) had microadenomas (P < 0.0001), 16 mice (10 males and six females) had adenomas (P < 0.05) and six of them (three males and three females) had bronchoalveolar carcinomas (P < 0.05), with multiplicities of 2.43 ± 0.52 (P < 0.0001), 1.24 ± 0.40 (P = 0.01) and 0.1 ± 0.04 (P < 0.05), respectively.
Fig. 1.
Incidence (a) and multiplicity (b) of lung tumors of varying histopathological nature in sham-exposed mice (Sham) or in mice exposed to MCS since birth, born either from untreated dams (MCS) or from NAC-treated dams (NAC-MCS).
Treatment with NAC during pregnancy exerted a striking protective effect on the yield of MCS-induced lung tumors after birth (Figure 1). In fact, only the 17.0% of these mice (six males and two females) developed lung tumors, with an overall multiplicity of 0.65 ± 0.21. The reduction of both incidence and multiplicity due to the prenatal exposure to NAC was statistically significant (P < 0.0001). In particular, only five mice in this group (four males and one female) had microadenomas (P < 0.0001 for both incidence and multiplicity, as compared with MCS-exposed mice born from untreated mice), six mice (four males and two females) had adenomas (P < 0.05 for incidence and P < 0.001 for multiplicity) and none of them was affected by malignant lung tumors (P < 0.05 for both incidence for multiplicity).
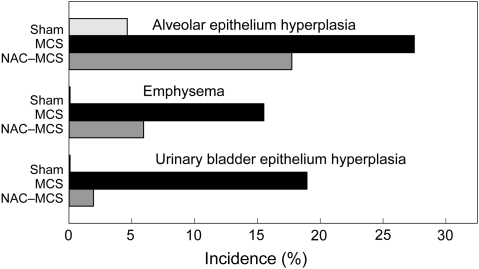
In addition, as shown in Figure 2, two sham-exposed mice (5.0%) exhibited proliferation or hyperplasia of the alveolar epithelium, a lesion that was more frequent in MCS-exposed mice born either from untreated dams (29.6%, P < 0.01) or from NAC-treated mice (19.1%; P = 0.05). No emphysema was detected in sham-exposed mice, versus nine cases (16.7%, P < 0.01) and three cases (6.4%, not significant) in the two MCS-exposed groups, respectively. Thus, there was a consistent trend to an attenuation of these pulmonary alterations following treatment with NAC during pregnancy, although the differences between the two groups of MCS-exposed mice did not reach the statistical significance threshold. Another MCS-related alteration was hyperplasia of the urinary bladder epithelium, which was absent in sham-exposed mice (Figure 2). In particular, there were 11 cases (20.4%, P < 0.01) in MCS-exposed mice born from untreated dams and one case (2.1%) in MCS-exposed mice born from NAC-treated mice. The protective effect of NAC toward this lesion was statistically significant (P < 0.01).
Fig. 2.
Incidence of alveolar epithelium hyperplasia, emphysema and urinary bladder epithelium hyperplasia in sham-exposed mice (Sham) or in mice exposed to MCS since birth, born either from untreated dams (MCS) or from NAC-treated dams (NAC-MCS).
Discussion
Experimental studies evaluating the ability of chemopreventive agents, given during pregnancy, to inhibit transplacental carcinogenesis are available in the literature (7–9). The present data provide the first demonstration that it is possible to inhibit postnatally induced cancer by administering a chemopreventive agent during pregnancy.
The results of our previous studies give a mechanistic base to the herein reported findings. In fact, the particular vulnerability at birth is amenable to the paraphysiological nucleotide alterations consequent to oxidative stress in the lung (4). The high sensitivity of the newborn to carcinogens may also depend on additional factors, such as an increased proliferation rate in neonatal organs, also including the lung (10), alterations of xenobiotic metabolism (11) and a lower efficiency of certain DNA repair mechanisms (12). Indeed, 60 years of research have supported the view that tobacco smoke is negative or weakly carcinogenic in rodents (13,14), in contrast with the overwhelming epidemiological evidence showing that smoke is the most important human carcinogen. Yet, when exposure starts immediately after birth, as confirmed in the present study, MCS induces a potent carcinogenic response, which is characterized by a high incidence of benign lung tumors and occurrence of malignant tumors, within a short latency time, as well as by other degenerative changes in the lung and even in extra-respiratory tissues (3). It should be noted that a neonatal tumorigenicity bioassay was proposed for the first time 50 years ago (15). Although the liver is the main target in this model, encouraging results were also obtained with pulmonary carcinogens, also including smoke components (16,17).
The impressive protection afforded by treatment with NAC throughout pregnancy demonstrates that the oxidative stress occurring at birth in the lung renders the organism particularly vulnerable to the action of carcinogens. Interestingly, prenatal NAC also prevented MCS-induced preneoplastic damage in the urinary bladder, which shows that treatment with this chemopreventive agent during pregnancy protects not only the lung but also another typical target of cigarette smoke. It is obvious that the dose of NAC used in the present study, which is the same dose used in a number of experimental studies with this agent (6), is high if compared with a realistic intake by humans. Yet, this dose is not toxic to rodents (6), and the need for using high doses both of carcinogens and protective agents is an inevitable drawback of all animal studies.
Parallel studies with environmental cigarette smoke (ECS) showed that exposure of mice since birth, for 5 weeks, results in a variety of early alterations, including cytogenetic damage in bone marrow and peripheral blood, formation of lipid peroxidation products in lung, increase of bulky DNA adducts and oxidatively generated DNA damage in lung, heart and aorta, overexpression of OGG1 in lung, stimulation of apoptosis, hyperproliferation and loss of Fhit protein in bronchial epithelial cells and pulmonary alveolar macrophages (18,19). Interestingly, nucleotide alterations were even more pronounced when the mice were exposed to ECS during the first 5 weeks of life rather than during adulthood for an equivalent period of time (18,19). It is also noteworthy that the expression levels of stem cell antigen-1 (Sca-1) gene are significantly higher in newborns than in adult mice, and exposure to ECS during the first 5 weeks of life significantly upregulated Sca-1 in lung, whereas no effect was observed in adults (20). In vitro, NAC inhibited the formation of DNA adducts and oxidatively generated DNA damage in transformed human mammary epithelial stem cells treated with 7,12-dimethylbenz(a)anthracene (21). In addition, exposure of neonatal mice to ECS produced, after 330 days, histopathological alterations in both lung and liver, which were not observed when exposure started 8 days later (22). These findings highlight the crucial susceptibility soon after birth.
NAC and its derivatives have a variety of protective mechanisms in carcinogenesis, the primary one being the ability of their thiol molecules to scavenge reactive oxygen species and other free radicals (6). It is probably that the protective environment created by NAC during pregnancy and at birth may both counteract the physiological birth-related oxidative stress and block the nucleophilic and oxidative species consequent to exposure to MCS immediately after birth. Moreover, we cannot rule out the hypothesis that prenatal NAC may have some ‘imprinting’ effect by influencing reduced glutathione metabolism in the offspring. Our previous studies have demonstrated that, in adult mice and rats, oral NAC is able to inhibit an impressive array of smoke-induced alterations of molecular, biochemical and cytogenetic endpoints (reviewed in ref. 23). When given during pregnancy to ECS-exposed mice, NAC protected the fetus liver against the formation of bulky DNA adducts, oxidatively generated DNA damage, cytogenetic damage and overexpression of a number of genes (24). Treatment during pregnancy of dams with NAC inhibited the increase of reduced glutathione and malondialdehyde in fetus lung (25), and administration of NAC to dams prevented oxidative stress associated with birth in neonatal rats (26). Moreover, administration of NAC during pregnancy protected Ku86-deficient mice from developmental cell death in the liver (27). Although the issue of the transplacental transport of NAC has been questioned by using an ovine model (28), all the above data lend support to the conclusion that this drug can indeed protect the fetus and the newborn from paraphysiological and toxicological alterations. A broad clinical experience shows that NAC is safe in pregnancy and can be administered, either orally or intravenously, according to the standard protocols in case of acetaminophen overdose during pregnancy (29).
In conclusion, administration of NAC and presumably of other antioxidant drugs or foods during pregnancy defends the newborn both from oxidatively generated nucleotide alterations (4) and, as shown here, from susceptibility to noxious exposures occurring soon after birth, including inhibition of smoke-induced lung cancer. Maternal diet and supplements should be taken into account as confounding factors in epidemiological studies evaluating the effects of involuntary smoking in children. The results of the present study have obvious implications in preventive medicine and may be the premise for practical applications in maternal child health care and cancer prevention.
Funding
Bulgarian Ministry of Education and Science; US National Cancer Institute (contract N01-CN53301).
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- ECS
environmental cigarette smoke
- MCS
mainstream cigarette smoke
- NAC
N-acetylcysteine
References
- 1.Agrawal AK, et al. Neonatal phenobarbital imprints overexpression of cytochromes P450 with associated increase in tumorigenesis and reduced life span. FASEB J. 2005;19:470–472. doi: 10.1096/fj.04-2550fje. [DOI] [PubMed] [Google Scholar]
- 2.Anderson LM. Introduction and overview. Perinatal carcinogenesis: growing a node for epidemiology, risk management, and animal studies. Toxicol. Appl. Pharmacol. 2004;199:85–90. doi: 10.1016/j.taap.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Balansky R, et al. Potent carcinogenicity of cigarette smoke in mice exposed early in life. Carcinogenesis. 2007;28:2236–2243. doi: 10.1093/carcin/bgm122. [DOI] [PubMed] [Google Scholar]
- 4.Izzotti A, et al. Birth-related genomic and transcriptional changes in mouse lung. Modulation by transplacental N-acetylcysteine. Mutat. Res. 2003;544:441–449. doi: 10.1016/j.mrrev.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Bonner AE, et al. Molecular profiling of mouse lung tumors: association with tumor progression, lung development, and human lung adenocarcinomas. Oncogene. 2004;23:1166–1176. doi: 10.1038/sj.onc.1207234. [DOI] [PubMed] [Google Scholar]
- 6.De Flora S, et al. Mechanisms of N-acetylcysteine in the prevention of DNA damage and cancer, with special reference to smoking-related end-points. Carcinogenesis. 2001;22:999–1013. doi: 10.1093/carcin/22.7.999. [DOI] [PubMed] [Google Scholar]
- 7.Yu Z, et al. Indole-3-carbinol in the maternal diet provides chemoprotection for the fetus against transplacental carcinogenesis by the polycyclic aromatic hydrocarbon dibenzo(a,l)pyrene. Carcinogenesis. 2006;27:2116–2123. doi: 10.1093/carcin/bgl072. [DOI] [PubMed] [Google Scholar]
- 8.Castro DJ, et al. Chemoprevention of dibenzo(a,l)pyrene transplacental carcinogenesis in mice born to mothers administered green tea: primary role of caffeine. Carcinogenesis. 2008;29:1581–1586. doi: 10.1093/carcin/bgm237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castro DJ, et al. Identifying efficacious approaches to chemoprevention with chlorophyllin, purified chlorophylls, and freeze-dried spinach in a mouse model of transplacental carcinogenesis. Carcinogenesis. 2008;30:315–320. doi: 10.1093/carcin/bgn280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yee M, et al. Type II epithelial cells are critical target for hyperoxia-mediated impairment of postnatal lung development. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:L1101–L1111. doi: 10.1152/ajplung.00126.2006. [DOI] [PubMed] [Google Scholar]
- 11.Shupe T, et al. Low hepatic glutathione S-transferase and increased hepatic DNA adduction contribute to increased tumorigenicity of aflatoxin B1 in newborn and partially hepatectomized mice. Toxicol. Lett. 2004;148:1–9. doi: 10.1016/j.toxlet.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Coccia P, et al. Liver DNA alkylation after a single carcinogenic dose of dimethylnitrosamine to newborn and adult CFW Swiss mice. Chem. Biol. Interact. 1988;68:259–271. doi: 10.1016/0009-2797(88)90020-8. [DOI] [PubMed] [Google Scholar]
- 13.Hecht SS. Carcinogenicity studies of inhaled cigarette smoke in laboratory animals: old and new. Carcinogenesis. 2005;26:1488–1492. doi: 10.1093/carcin/bgi148. [DOI] [PubMed] [Google Scholar]
- 14.Witschi H. A/J mouse as a model for lung tumorigenesis caused by tobacco smoke: strengths and weaknesses. Exp. Lung Res. 2005;31:3–18. doi: 10.1080/01902140490494959. [DOI] [PubMed] [Google Scholar]
- 15.Pietra G, et al. Response of newly born mice to a chemical carcinogen. Nature. 1959;183:1689. doi: 10.1038/1831689a0. [DOI] [PubMed] [Google Scholar]
- 16.Beebe LE, et al. Comparison of transplacental and neonatal initiation of mouse lung and liver tumors by N-nitrosodimethylamine (NDMA) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and promotability by a polychlorinated biphenyls mixture (Aroclor 1254) Carcinogenesis. 1993;14:1545–1548. doi: 10.1093/carcin/14.8.1545. [DOI] [PubMed] [Google Scholar]
- 17.Yun TK, et al. Trial of a new medium-term model using benzo(a)pyrene induced lung tumor in newborn mice. Anticancer Res. 1995;15:839–845. [PubMed] [Google Scholar]
- 18.De Flora S, et al. High susceptibility of neonatal mice to molecular, biochemical and cytogenetic alterations induced by environmental cigarette smoke and light. Mutat. Res. Rev. 2008;659:137–146. doi: 10.1016/j.mrrev.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Izzotti A, et al. Exposure of mice to cigarette smoke and/or light causes DNA alterations in heart and aorta. Mutat. Res. 2008;644:38–42. doi: 10.1016/j.mrfmmm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Izzotti A, et al. Upregulation of stem cell antigen-1 in the lung of neonatal mice exposed to environmental cigarette smoke. Oncol. Rep. 2009 doi: 10.3892/or_00000458. in press. [DOI] [PubMed] [Google Scholar]
- 21.De Flora S, et al. Induction by 7,12-dimethylbenz(a)anthracene of molecular and biochemical alterations in transformed human mammary epithelial stem cells, and protection by N-acetylcysteine. Int. J. Oncol. 2006;29:521–529. [PubMed] [Google Scholar]
- 22.D'Agostini F, et al. Preneoplastic and neoplastic lesions in the lung, liver and urinary tract of mice exposed to environmental cigarette smoke and UV light since birth. Int. J. Cancer. 2008;123:2497–2502. doi: 10.1002/ijc.23836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Flora S, et al. Molecular and cytogenetical alterations induced by environmental cigarette smoke in mice heterozygous for Fhit. Cancer Res. 2007;67:1001–1006. doi: 10.1158/0008-5472.CAN-06-3882. [DOI] [PubMed] [Google Scholar]
- 24.Izzotti A, et al. Genomic and transcriptional alterations in mouse fetus liver after transplacental exposure to cigarette smoke. FASEB J. 2003;17:1127–1129. doi: 10.1096/fj.02-0967fje. [DOI] [PubMed] [Google Scholar]
- 25.Basyigit I, et al. Protective effects of N-acetylcysteine on peroxidative changes of the fetal rat lungs whose mothers were exposed to cigarette smoke. Hum. Exp. Toxicol. 2007;26:99–103. doi: 10.1177/0960327107071917. [DOI] [PubMed] [Google Scholar]
- 26.Sastre J, et al. Antioxidant administration to the mother prevents oxidative stress associated with birth in the neonatal rat. Life Sci. 1994;54:2055–2059. doi: 10.1016/0024-3205(94)00714-4. [DOI] [PubMed] [Google Scholar]
- 27.Reliene R, et al. Developmental cell death in the liver and newborn lethality of Ku86 deficient mice suppressed by antioxidant N-acetylcysteine. DNA Repair. 2006;5:1392–1397. doi: 10.1016/j.dnarep.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Selden BS, et al. Transplacental transport of N-acetylcysteine in an ovine model. Ann. Emerg. Med. 1991;20:1069–1072. doi: 10.1016/s0196-0644(05)81354-x. [DOI] [PubMed] [Google Scholar]
- 29.Wilkes JM, et al. Acetaminophen overdose in pregnancy. South. Med. J. 2005;98:1118–1122. doi: 10.1097/01.smj.0000184792.15407.51. [DOI] [PubMed] [Google Scholar]