Abstract
Haem in red meat (RM) stimulates the endogenous production of mutagenic nitroso compounds (NOC). Processed (nitrite-preserved red) meat additionally contains high concentrations of preformed NOC. In two studies, of a fresh RM versus a vegetarian (VEG) diet (six males and six females) and of a nitrite-preserved red meat (PM) versus a VEG diet (5 males and 11 females), we investigated whether processing of meat might increase colorectal cancer risk by stimulating nitrosation and DNA damage. Meat diets contained 420 g (males) or 366 g (females) meat/per day. Faecal homogenates from day 10 onwards were analysed for haem and NOC and associated supernatants for genotoxicity. Means are adjusted for differences in male to female ratios between studies. Faecal NOC concentrations on VEG diets were low (2.6 and 3.5 mmol/g) but significantly higher on meat diets (PM 175 ± 19 nmol/g versus RM 185 ± 22 nmol/g; P = 0.75). The RM diet resulted in a larger proportion of nitrosyl iron (RM 78% versus PM 54%; P < 0.0001) and less nitrosothiols (RM 12% versus PM 19%; P < 0.01) and other NOC (RM 10% versus PM 27%; P < 0.0001). There was no statistically significant difference in DNA breaks induced by faecal water (FW) following PM and RM diets (P = 0.80). However, PM resulted in higher levels of oxidized pyrimidines (P < 0.05). Surprisingly, VEG diets resulted in significantly more FW-induced DNA strand breaks than the meat diets (P < 0.05), which needs to be clarified in further studies. Meats cured with nitrite have the same effect as fresh RM on endogenous nitrosation but show increased FW-induced oxidative DNA damage.
Introduction
Red and processed (nitrite-preserved red) meat (PM) increase the risk of colorectal cancer with PM showing higher risk estimates per gram of intake than red meat (RM) (1–3). Heterocyclic amines—known carcinogens (4)—are formed during cooking of meat at high temperatures, but this is not specific for red and PM (5).
RM intake shows a dose–response relation with the endogenous formation of nitroso compounds (NOC), whereas there is no such relation for white meat (6,7). This is probably due to the abundant presence of haem in RM (8,9), which can readily become nitrosylated and act as a nitrosating agent (10). Recently, we confirmed that nitrosyl iron (FeNO) is the main contributor to high RM diet-induced endogenous formation of NOC (11). In addition, nitrosothiols (RSNO) are rapidly formed from nitrite and thiol groups at low pH in the stomach and can be precursors for the endogenous formation of nitrosyl haem and other NOC, such as N- and O-NOC, in the small and large bowel (11,12).
Many NOC are known carcinogens and the alkylation of DNA can induce G to A transitions in genes mutated in colorectal cancer such as ras (13). The NOC-specific DNA adduct O6-carboxymethyl-2′-deoxy-guanosine increases in exfoliated colonic cells from volunteers fed a high RM diet (14). O6-carboxymethyl-2′-deoxy-guanosine is not repaired by O6-alkylguanine transferase in in vitro assays and may at least partly explain the link between meat consumption and colorectal cancer.
PM is a mixed category of meats that are preserved by a variety of mechanical, chemical or enzymatic procedures. Different preservatives, such as nitrite and nitrate in ham and sulphur dioxide in sausages, may be used. Minced products such as hamburgers may or may not be classified as PM. In most populations on which existing epidemiological findings are based, PM mainly consists of processed RMs such as beef and pork (3,15).
Supplements of nitrate have been shown to increase faecal NOC levels (16), but it is not clear whether preservation by this method would result in an increased endogenous NOC production, and hence explain some of the higher colorectal cancer risk with processed as compared with RM. In two studies, of a fresh RM (beef and pork) versus a vegetarian (VEG) diet—referred to as the RM study—and of a nitrite-preserved red meat (bacon, ham, luncheon meat and corned beef) versus a VEG diet—referred to as the PM study—we investigated whether this form of processing might increase colorectal cancer risk by stimulating nitrosation and DNA damage.
Methods
Subjects
Healthy males and females from Cambridgeshire were recruited through local advertisements. Participants had to be between 20 and 85 years of age, non-smokers, free from diabetes and bowel disease, not taking medication affecting the gut for at least 3 months prior to the study, not pregnant and not participating in another biochemical intervention study at the same time. Prior to the study, subjects were examined by a medical practitioner.
All subjects received verbal and written information and signed a written consent form. The studies were approved by the Cambridge Local Research Ethics Committee.
Sixteen volunteers were included in the PM study (5 males and 11 females); 12 volunteers participated in the RM study (six males and six females), which has been described previously (14).
Study design
The studies had a randomized crossover design of a high meat versus a VEG period: PM versus VEG in the PM study and RM versus VEG in the RM study. Each dietary period lasted at least 14 days. Subjects lived in the volunteer suite of the MRC Dunn Human Nutrition Unit, where all food was provided and carefully controlled and all specimens could be collected and processed immediately. Subjects followed their normal routine but were only allowed to consume foods and drinks prepared by the diet technicians. Body weights were monitored to ensure a constant weight throughout. Faecal samples were collected and weighed daily and radio opaque marker capsules were taken throughout to check compliance and for the measurement of transit time (17). After subjects had consumed each diet for 10 days, stools were collected on dry ice for analysis of NOC, haem and genotoxicity.
Study diets
All diets were provided as similar menus on a 3 day rotating schedule. Male PM diets contained 420 g PM per day [given as 100 g bacon (days 1 and 2) or pork luncheon meat (day 3) at lunch and 320 g corned beef (days 1 and 2) or gammon (day 3) at dinner] and female PM diets contained 366 g PM per day (given as 60 g bacon or pork luncheon meat at lunch and 306 g corned beef or gammon at dinner). RM diets contained the same amount of meat, but lunch was roast beef (days 1, 2 and 3) and dinner beef mince (days 1 and 3) or pork (day 2). Over the course of the study, each volunteer consumed the same amount of each type of meat.
Energy and macronutrient composition of the diets (Appendix) were calculated using Data Into Nutrients for Epidemiological Research (DINER) (18). Fat content of the diets was kept constant by exchanging protein for carbohydrates. Energy requirements were estimated from body weight and physical activity using standard equations for basal metabolic rate and estimates of physical activity level (19). The energy intake of each participant was matched to estimated energy requirement with 1 MJ standardized increments (chocolate bar, shortbread or a combination of white bread, butter and marmalade) added to 8 MJ/day (female) or 10 MJ/day (males) basal diets.
Meat was not overcooked to minimize the formation of heterocyclic amines. Purified water was given throughout for drinking and used for cooking and low-nitrate vegetables were used to keep nitrate intake at constant low levels. Tea, coffee and an aspartame-based sweetener were provided in the suite and consumed freely, but subjects were asked to keep their intake constant during the study.
Dietary and faecal NOC and haem
Duplicates for each daily diet were prepared. All foods consumed on 1 day were prepared as normal, added together and diluted 1:2 in ultrapure water. The mixture was homogenized with a food processor, snap frozen on dry ice and stored at −20°C until analysis. Results are presented per gram of diet after correction for dilution. For analysis of the separate nitrite-preserved red meats consumed in the PM study, meats were cooked as normal and homogenized and frozen separately as described above. Results are presented per gram of cooked weight after correction for dilution.
For stool analysis, ∼40 g of frozen stool was thawed, diluted 1:5 in ultrapure water and homogenized for 20 min in a stomacher (Colworth 3500, Seward Medical, London, UK). The faecal homogenates were snap frozen on dry ice and stored at −20°C until analysis. Results are presented per gram of faeces after correction for dilution.
NOC were analysed using a modification of the method previously used (11), using an Ecomedics CLD 88 Exhalyzer (Ecomedics, Duernten, Switzerland). Approximately 100 μl of faecal homogenates or 500 μl of homogenized diet or meat were weighed and incubated briefly with 100 μl of an aqueous solution of N-ethylmaleimide (50 mM) and diethylene triamine pentaacetic acid (100 μM) to protect RSNO. Thereafter, 500 μl of a 5% (wt/vol) sulfamic acid solution was added to remove nitrite and samples were injected into a purge vessel kept at 60°C and filled with a standard tri-iodide reagent (20) (38 mg I2 was added to a solution of 108 mg KI in 1 ml water; to this mixture, 13.5 ml glacial acetic acid was added) to determine total NOC. To determine mercury(II) stable compounds, 100 μl 10 mM aqueous HgCl2 was added prior to analysis; to determine mercury(II) and ferricyanide stable compounds, 100 μl each of 10 mM aqueous HgCl2 and 10 mM aqueous K3Fe(CN)6 solution were added prior to analysis. RSNO were determined as the difference between total NOC and mercury(II) stable NOC; FeNO was determined as difference between mercury(II) stable NOC and mercury(II) and K3Fe(CN)6 stable compounds. Other NOC were determined as mercury(II) and K3Fe(CN)6 stable compounds.
Haem was analysed using the HemoQuant assay (8) as described previously (11). Briefly, 400 μl hot oxalic acid reagent (3 M oxalic acid, 0.1 M FeSO4, 60 mM uric acid and 60 mM mannitol) was added to ∼500 mg dietary or faecal homogenate, mixed and incubated for 30 min at 100°C. After cooling, 1 ml 3 M KAc and 3 ml acetate/acetic acid (10/1 vol/vol) were added. To 2 ml of the organic phase, 0.8 ml n-butanol and 6 ml 3 M KAc in 1 M KOH was added, mixed and 1 ml of the organic phase subsequently extracted with 4 ml phosphoric acid/acetic acid (2 M phosphoric acid:glacial acetic acid, 9:1 vol/vol). Fluorescence (excitation, 402 nm; fluorescence, 600 nm) of the aqueous phase was determined using a Spectramax Gemini XS fluorimeter (Molecular Devices, Sunnyvale, CA). To quantify samples, dietary and faecal homogenates were spiked with haemoglobin to obtain a calibration curve. Results are expressed as mmol/d for diets or nmol/g for faeces.
Faecal water genotoxicity with the comet assay
Faecal water (FW) was prepared by centrifuging ∼25 g of thawed faecal homogenate at 50 000g for 2 h at 4°C. The clear supernatant was aliquoted, snap frozen on dry ice and stored at −80°C until analysis.
Caco2 cells (European Collection of Cell Cultures, Salisbury, UK) were cultured as monolayers in Eagle's Minimum Essential Medium containing 10% foetal bovine serum, 2 mM L-glutamine, 1% non-essential amino acids and 100 U/l penicillin–streptomycin (all Sigma–Aldrich, Gillingham, UK). Cells were harvested after a 3 min incubation at 37°C with 0.25% trypsin–ethylenediaminetetraacetic acid (EDTA) (Sigma–Aldrich) and resuspended in non-supplemented Eagle's Minimum Essential Medium. Three hundred microlitres of cell suspension (∼12 × 104 cells) was incubated with 300 μl FW (50%) for 30 min at 37°C. A quality control was included in each experiment. Samples were measured in duplicate in separate experiments.
Cells were centrifuged at 200g for 3 min at 4°C, the supernatant was discarded and cells were resuspended in 420 μl 1% low melting point agarose made in phosphate-buffered saline. Cells from one treatment were divided as roughly equal drops of ∼70 μl over three microscope slides (two gels per slide), a 22 × 22 mm coverslip was applied and gels were allowed to set at 4°C. Coverslips were removed and cells were lysed in 2.5 M NaCl; 0.1 M Na2EDTA; 10 mM Tris–HCl, pH 10 and 1% Triton X-100 for 1 h at 4°C. Microscope slides had been precoated with 1% normal melting point agarose and left to dry before use.
Slides were rinsed in enzyme buffer (40 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 0.1 M KCl, 0.5 mM EDTA and 0.2 mg/ml bovine serum albumin, pH 8) three times for 5 min each at 4°C. Per treatment, one slide was then incubated with enzyme buffer alone, one with endonuclease III (EndoIII) in buffer and one with formamidopyrimidine (FPG) in buffer for 30 min at 37°C. EndoIII recognizes oxidized pyrimidines and FPG recognizes altered purines, creating additional DNA strand breaks at these sites.
Subsequently, the DNA was allowed to unwind in electrophoresis solution (0.3 M NaOH and 1 mM Na2EDTA, pH 13) for 40 min at 4°C before electrophoresis at 0.9 V/cm, 300 mA for 30 min at 4°C. Slides were neutralized in phosphate-buffered saline and rinsed in ultrapure water for 10 min each at 4°C and left to dry overnight at room temperature.
Gels were stained with 4′,6-diamidino-2-phenylindole and viewed by fluorescence microscopy. Comets were classified with visual scoring (21); 100 randomly selected comets were classified into five classes (0–4) according to the extent of tail DNA to give an overall score of between 0 and 400 arbitrary units per treatment.
Statistical analysis
Faecal nitrite values and transit times were 10log transformed to normalize the distribution and are presented as geometric means and antilogged 95% confidence intervals (CIs). Other results are presented as mean ± SE. Meat versus VEG results were compared within each study using a paired Student's t-test (two tailed). Statistical differences between PM and RM diets (between studies) were analysed by analysis of covariance with type of meat (PM or RM) as independent variable and gender as dichotomous covariate to adjust for differences in male to female ratios between the two studies. Where PM and RM results are compared (between studies), results are presented as adjusted mean ± SE. Where meat and VEG results are compared (within studies), results are presented as unadjusted means.
Associations between continuous variables were tested using Pearson's correlation coefficient (r). P <0.05 was considered statistically significant. SPSS 16 for Macintosh (SPSS, Chicago, IL, 2008) was used for the analysis.
Results
Dietary NOC and haem
Only male diets were analysed as they contained the largest amount of meat. PM diets contained 58 ± 15 mmol/d total NOC, composed of 24 ± 8 mmol/d RSNO, 20 ± 6 mmol/d FeNO and 14 ± 2 mmol/d other NOC and 17 ± 10 μmol/d nitrite. The NOC composition of the processed meats, i.e. bacon, corned beef, gammon and pork luncheon meat, is shown in Table I. RM and VEG diets contained negligible amounts of NOC and nitrite.
Table I.
NOC composition (nmol/g cooked weight) of the nitrite-preserved red meats consumed in the PM study
| Total NOC | RSNO | FeNO | Other NOC | |
| Bacon | 428 | 357 | 45 | 26 |
| Corned beef | 110 | 26 | 42 | 42 |
| Gammon | 84 | 15 | 50 | 19 |
| Pork luncheon meat | 58 | 17 | 27 | 14 |
PM diets contained similar amounts of haem (86 mmol/d) to RM diets (110 mmol/d). The haem content of the VEG diets was negligible.
Faecal NOC and haem
Faecal levels of NOC on VEG diets were very low but increased significantly on both PM and RM diets (Table II). There was no statistically significant difference in total NOC concentrations on the PM and RM diets (adjusted means PM 175 ± 19 nmol/g versus RM 185 ± 22 nmol/g; P = 0.75). FeNO was the main contributor to total NOC on both meat diets although the RM diet resulted in a larger proportion of FeNO (adjusted means RM 78% versus PM 54%; P < 0.0001) and less RSNO (adjusted means RM 12% versus PM 19%; P < 0.01) and other NOC (adjusted means RM 10% versus PM 27%; P < 0.0001) compared with PM. Similar results were obtained when absolute concentrations were used.
Table II.
Faecal NOC concentrations (nmol/g) (a) PM diet versus VEG diet (n = 16); (b) RM diet versus VEG diet (n = 12) (unadjusted means, comparison within studies)
| Intake |
Excretion |
Intake |
Excretion |
|||
| mmol/d | nmol/g | mmol/d | mmol/d | nmol/g | mmol/d | |
| (a) | PM | VEG | ||||
| Haem | 86 | 329 ± 41 | 0.05 ± 0.007 | a | 61 ± 5* | 0.01 ± 0.001* |
| Total NOC | 58 | 181 ± 20 | 28.6 ± 2.9 | a | 2.6 ± 0.3* | 0.6 ± 0.06* |
| RSNO | 24 | 33 ± 4 | 5.2 ± 0.6 | a | 0.2 ± 0.1* | 0.05 ± 0.02* |
| FeNO | 20 | 95 ± 9 | 15.3 ± 1.6 | a | 2.0 ± 0.3* | 0.4 ± 0.05* |
| Other NOC | 14 | 53 ± 8 | 8.1 ± 1.2 | a | 0.5 ± 0.1* | 0.1 ± 0.02* |
| (b) | RM | VEG | ||||
| Heam (n = 11) | 110 | 1028 ± 109 | 0.13 ± 0.02 | a | 63 ± 11* | 0.02 ± 0.005* |
| Total NOC | a | 177 ± 26 | 21.7 ± 2.2 | a | 3.5 ± 0.7* | 1.0 ± 0.2* |
| RSNO | a | 19 ± 4 | 2.3 ± 0.3 | a | 0.4 ± 0.1* | 0.1 ± 0.02* |
| FeNO | a | 140 ± 20 | 17.1 ± 1.9 | a | 1.8 ± 0.3* | 0.5 ± 0.1* |
| Other NOC | a | 18 ± 4 | 2.2 ± 0.5 | a | 1.2 ± 0.4** | 0.3 ± 0.08*** |
Only male diets analysed. Excretion measured in faecal homogenates and adjusted for dilution. P values for paired Students’ t-test of meat versus VEG, *P < 0.0001, **P = 0.001, ***P < 0.01.
Negligible amounts.
PM, processed (nitrite-preserved red) meat.
NOC concentrations in FW used in the comet assay were ∼1.7% of the concentration in homogenates before correction for dilution (PM 0.65 nmol/g and RM 0.41 nmol/g).
There was no statistically significant difference between adjusted mean faecal nitrite concentrations on the PM diet (19 nmol/g, 95% CI 11–35) and those on the RM diet (28 nmol/g, 95% CI 14–56, P = 0.41). Concentrations on both meat diets were significantly higher than after VEG diets (3 nmol/g, 95% CI 2–6, P < 0.0001). There was no statistically significant correlation between concentrations of faecal nitrite and total NOC.
Faecal haem was significantly higher when the meat diets were consumed as compared with the VEG diets (P < 0.0001, Table II) although to a lesser extent on the PM diet compared with the RM diet (adjusted means PM 326 ± 69 nmol/g versus RM 1033 ± 84 nmol/g, n = 11; P < 0.0001). Haem concentrations were positively related with FeNO concentrations on the RM diet (r = 0.63, P < 0.05; n = 11) and marginally on the PM diet (r = 0.45, P = 0.08). Haem concentrations were positively related to RSNO on the RM diet (r = 0.64, P < 0.05; n = 11) but not on the PM diet (r = 0.15, P = 0.58).
FW genotoxicity with the comet assay
There was no statistically significant difference in DNA breaks induced in Caco2 cells by FW from both PM and RM diets (adjusted means PM 167 ± 12 versus RM 163 ± 13; P = 0.80). However, PM resulted in higher levels of EndoIII-sensitive sites (adjusted means PM 24 ± 5 versus RM 9 ± 5; P < 0.05) and a larger variation in FPG-sensitive sites compared with RM (Figure 1A). Surprisingly, FW from both dietary studies induced significantly more DNA strand breaks on the VEG diets compared with the meat diets (P < 0.05; Table III).
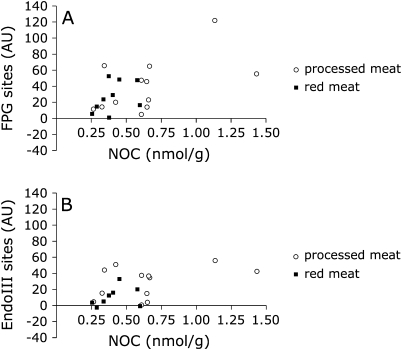
Fig. 1.
FW-induced FPG-sensitive sites (A) and EndoIII-sensitive sites (B) in Caco2 cells measured with the comet assay versus total NOC concentrations in FW. Processed meat diet (n = 13, open circles) and RM diet (n = 9, filled squares).
Table III.
Level of FW-induced DNA strand breaks, EndoIII- and FPG-sensitive sites in Caco2 cells measured with the comet assay (a) PM diet versus VEG diet (n = 16); (b) RM diet versus VEG diet (n = 12) (unadjusted means, comparison within studies)
| Mean | SE | Mean | SE | Mean | SE | |
| (a) | Control slides | PM | VEG | |||
| Strand breaks (AU) | 113 | 3 | 164 | 13 | 206* | 17 |
| EndoIII sites (AU) | 8 | 2 | 24 | 5 | 26 | 6 |
| FPG sites (AU) | 25 | 5 | 39 | 8 | 42 | 7 |
| (b) | Control slides | RM | VEG | |||
| Strand breaks (AU) | 113 | 3 | 167 | 13 | 196* | 15 |
| EndoIII sites (AU) | 8 | 2 | 9 | 4 | 15 | 6 |
| FPG sites (AU) | 25 | 5 | 24 | 6 | 28 | 4 |
AU, arbitrary units; PM, processed (nitrite-preserved red) meat. P value for paired Students’ t-test of meat versus VEG, *P < 0.05. Control slides were incubated with phosphate-buffered saline and represent the level of background DNA strand breaks.
In the comet assay, FPG detects oxidatively damaged purines but also seems to have a high sensitivity towards N-7 alkylation of guanine (ring opened) (22). There was no statistically significant correlation between FPG-sensitive sites and NOC concentrations in faecal homogenates on both meat diets, but NOC concentrations in FW—despite being relatively low—trended towards a positive association with FPG-sensitive sites on the PM (r = 0.55, P = 0.06; n = 12, three samples not analysed, one sample with no peak in assay) but not on the RM diet (r = 0.25, P = 0.49; n = 10, two samples no peak in assay) (Figure 1A). There were no significant associations with DNA strand breaks and EndoIII-sensitive sites (Figure 1B). No NOC could be detected in FW on the VEG diets. Nitrite concentrations in FW were ∼18% of those in faecal homogenates (PM study only, RM not analysed); there were no statistically significant associations with DNA strand breaks or enzyme-sensitive sites.
Mean transit time
Mean transit time (MTT) on the PM diet (adjusted geometric mean 44 h, 95% CI 35–55; n = 15) was significantly lower than on the RM diet (adjusted geometric mean 70 h, 95% CI 55–90; P < 0.01). MTT during both VEG periods were similar (VEG in PM study 44 h, 95% CI 35–55; n = 15 versus VEG in RM study 55 h, 95% CI 43–70; P = 0.18) and not significantly different from their respective meat periods (PM study P = 0.80 and RM study P = 0.07).
MTT was positively related with total NOC concentrations (r = 0.63, P = 0.01; n = 15), FeNO (r = 0.60, P = 0.02; n = 15) and other NOC (r = 0.74, P < 0.01; n = 15) but not with RSNO (r = 0.13, P = 0.64; n = 15) on the PM diet. In contrast, there were no statistically significant correlations between MTT and NOC concentrations on the RM diet (P > 0.25).
Daily faecal weight, calculated as the mean faecal weight over the last 4 days of the diet, was significantly higher on the VEG diet compared with the meat diet in both studies (PM 167.5 ± 11.9 g/d versus VEG 223.8 ± 24.1 g/d; P < 0.05 and RM 148.1 ± 19.5 g/d versus VEG 308.3 ± 41.4 g/d; P < 0.0001). There were no statistically significant differences between studies (P > 0.12). Daily faecal weight was not statistically significantly associated with MTT on any of the diets or for both studies in total (P > 0.09).
Discussion
Recently, we implicated haem as a facilitator of the endogenous formation of NOC from dietary nitrite or nitrate and nitrite from inducible and endogenous nitric oxide synthases (9,11,23). Similar to our previous studies, faecal NOC levels were low (3–4 nmol/g) on diets containing no meat and a negligible amount of haem. On nitrite-preserved (PM) and fresh RM diets containing similar amounts of haem (86–110 mmol/d), faecal NOC levels increase significantly to ∼180 nmol/g.
Dietary NOC in the PM diet (58 mmol/d) was in the same order of magnitude or greater as excretion, whereas NOC content of the RM diet was negligible but resulted in similar levels of excretion in faeces as on the PM diet (adjusted means PM 28.4 ± 2.6 mmol/d and RM 22.0 ± 3.0 mmol/d). However, on a RM diet, faecal FeNO represent a greater proportion of total NOC compared with the PM diet and concentrations are positively associated with haem concentrations. In addition, on both meat diets, concentrations of FeNO were significantly higher than those of RSNO, which confirms the important contribution of haem to endogenous NOC production (9).
Mirvish et al. (24) studied mice fed hot dogs with or without NaNO2 and concluded that faecal NOC excretion represents mainly excretion of ingested NOC and of NOC formed by nitrosation of precursor NOC by nitrite. In contrast, our results suggest that faecal NOC are unlikely to be simply derived from the diet and are mainly formed endogenously. However, the preformed NOC present in our study diets are not to be confused with the precursor NOC analysed in faeces by Mirvish et al. (24), which were defined as NOC that were formed after in vitro nitrosation of faecal supernatant.
The endogenous formation of NOC is likely to begin with the formation of RSNO in the stomach, as acidic conditions facilitate the formation of these compounds (11). These compounds can then promote the formation of other NOC, in particular nitrosyl haem, in the anaerobic and reducing environment of the gastrointestinal tract. Considering the importance of NOC production along the gastrointestinal tract, the association between MTT and faecal NOC concentrations was investigated in the meat diets. The statistically significant association between MTT and faecal NOC concentrations on the PM diet—in particular for FeNO compounds–supports the hypothesis that these compounds are formed in the anaerobic conditions of the small and large intestines. However, the lack of association following the RM diet with a significantly longer transit times suggests a threshold effect.
The alkaline comet assay detects DNA strand breaks and alkali-labile sites, i.e. apurinic or apyrimidinic sites or baseless sugars. These can result from a variety of damage and might also represent intermediates in the repair process. Including DNA glycosylase enzymes results in excision of oxidatively damaged nucleobases from the DNA strand, which leaves additional strand breaks (21). Despite the different composition of faecal NOC on PM and RM diets, there was no clear effect on FW-induced DNA strand breaks, but PM resulted in significantly more FW-induced EndoIII-sensitive sites. However, due to the very low concentrations of total NOC in the FW, we were not able to analyse NOC composition and hence cannot relate NOC composition of the FW and the faecal homogenates. We have currently no explanation for the unexpected higher level of DNA strand breaks on the VEG diets as compared with the high meat diets, which is intriguing given a recent study showing a higher colorectal cancer incidence in vegetarians than in meat eaters (25). Nevertheless, further studies are underway exchanging red or PM with white meat or fish to eliminate possible interfering plant constituents, e.g. polyphenols. Dietary analysis of total phenols using the Folin–Ciolcalteu method showed similar levels of phenols in our meat and VEG study diets (data not shown), but this test does not distinguish between different kinds of polyphenolic compounds. However, we have not been able as yet to obtain reliable estimates for FW levels with this method.
The aqueous fraction of faeces, prepared by either direct ultracentrifugation, dilution in phosphate-buffered saline or ultrapure water or reconstitution of freeze-dried faeces, has been shown to be genotoxic in several colonic cell lines (26–30). Previous studies, the most recent ones using nuclear magnetic resonance profiling, have identified a number of compounds in FW including short chain fatty acids, organic acids, phenolic compounds and amino acids. Inter- and intra-individual differences are related to variation in concentrations rather than composition, which in turn seem to be related to diet (31,32). However, still little is known about the nature of FW, which may explain inconsistent results when investigating the effect of diet on DNA damage using the comet assay (26,27,29).
Colonic cells have been shown to be susceptible to NOC-induced damage. Potassium diazoacetate, a stable nitrosated derivative of glycine, shows a dose–response effect on genotoxicity in Caco2 cells, human lymphocytes and rat colonocytes (33) and N-nitrosomorpholine causes a dose-dependent increase in DNA strand breaks in Caco2 cells, all of which originated from alkali-labile sites (34). No oxidative damage was observed, although pretreatment with vitamins E and C reduced the formation of strand breaks (34). Our results showed the presence of altered purines and pyrimidines induced by FW, with higher levels of oxidized pyrimidines on the PM diet compared with the RM diet. Despite the NOC levels in FW being very low compared with the faecal homogenates, they tended to be positively related to altered purines on the PM diet indicating that they are related to oxidative damage.
Nitrite intake from PM was 0.8 mg/d (17 μmol/d) in our study. Intake of NaNO2 by PM consumption in the UK, Spain and Germany reported in the European Prospective Study of Cancer and Nutrition was 0.1–3.3 mg/d for women and 0.8–5.4 mg/d for men (adjusted for energy intake, age, weekday and season), which would be somehow lower when nitrite alone is calculated (15). The link between dietary nitrite and cancers of the gastrointestinal tract is inconclusive (35,36), which may be because most nitrite is absorbed early and the majority does not reach the colon (37). There was no association between FW nitrite and FW-induced DNA damage on our PM diet.
In conclusion, meats cured with nitrite have the same effect as fresh RM on endogenous nitrosation but show increased FW-induced oxidative DNA damage, which could be a result of pro-oxidative compounds derived from both dietary factors or endogenous immune response. The higher level of DNA strand breaks on the VEG diets is an intriguing finding that needs to be clarified in further studies.
Acknowledgments
We thank Valerie Church, Hilary Slack and Judith Wills for preparing the study diets and taking care of the volunteers and Marleen Lentjes for help in using DINER.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CI
confidence interval
- EDTA
ethylenediaminetetraacetic acid
- EndoIII
endonuclease III
- FW
faecal water
- FeNO
nitrosyl iron
- FPG
formamidopyrimidine
- MTT
mean transit time
- NOC
nitroso compounds
- PM
processed (nitrite-preserved red) meat
- RM
red meat
- RSNO
nitrosothiols
- VEG
vegetarian
Appendix
Appendix.
| PM |
VEG |
|||||
| All (n = 16) | Female (n = 11) | Male (n = 5) | All (n = 16) | Female (n = 11) | Male (n = 5) | |
| Energy intake (MJ) | 9 | 8 | 11 | 9 | 8 | 12 |
| Protein (g/d) | 145 (27) | 137 (29) | 163 (25) | 77 (14) | 73 (15) | 85 (12) |
| Fat (g/d) | 80 (33) | 71 (32) | 101 (33) | 79 (31) | 66 (29) | 107 (34) |
| SFA (g/d) | 30 (12) | 27 (12) | 37 (12) | 33 (12) | 24 (11) | 51 (16) |
| MUFA (g/d) | 27 (11) | 24 (11) | 33 (11) | 23 (9) | 20 (9) | 32 (10) |
| PUFA (g/d) | 16 (6) | 13 (6) | 22 (7) | 14 (6) | 15 (7) | 11 (4) |
| Carbohydrates (g/d) | 230 (43) | 200 (42) | 296 (45) | 321 (59) | 289 (59) | 389 (57) |
| Fibre (g/d) | 19 | 16 | 23 | 30 | 28 | 34 |
| Calcium (mg/d) | 811 | 762 | 918 | 1187 | 1115 | 1348 |
| Iron (mg/d) | 18 | 17 | 22 | 14 | 13 | 17 |
| Folate (μg/d) | 189 | 168 | 236 | 244 | 229 | 277 |
| Vitamin C (mg/d) | 74 | 73 | 77 | 112 | 100 | 138 |
| RM |
VEG |
|||||
| All (n = 12) | Female (n = 6) | Male (n = 6) | All (n = 12) | Female (n = 6) | Male (n = 6) | |
| Energy intake (MJ) | 10 | 9 | 11 | 9 | 8 | 10 |
| Protein (g/d) | 147 (25) | 133 (25) | 161 (25) | 77 (14) | 73 (15) | 80 (13) |
| Fat (g/d) | 80 (29) | 70 (29) | 90 (30) | 81 (31) | 68 (29) | 95 (34) |
| SFA (g/d) | 34 (12) | 26 (11) | 41 (14) | 33 (13) | 24 (10) | 42 (15) |
| MUFA (g/d) | 27 (10) | 24 (10) | 30 (10) | 25 (10) | 21 (9) | 29 (10) |
| PUFA (g/d) | 10 (4) | 13 (5) | 7 (2) | 14 (5) | 16 (7) | 12 (4) |
| Carbohydrates (g/d) | 293 (49) | 265 (50) | 321 (49) | 323 (58) | 296 (59) | 351 (57) |
| Fibre (g/d) | 12 | 11 | 13 | 30 | 28 | 33 |
| Calcium (mg/d) | 895 | 875 | 915 | 1093 | 1058 | 1128 |
| Iron (mg/d) | 16 | 15 | 18 | 16 | 15 | 18 |
| Folate (μg/d) | 318 | 274 | 361 | 291 | 267 | 315 |
| Vitamin C (mg/d) | 93 | 90 | 95 | 86 | 71 | 100 |
SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PM, processed (nitrite-preserved red) meat; PUFA, polyunsaturated fatty acids.
Mean nutrient intake.
Values between parentheses represent %energy.
References
- 1.Cross AJ, et al. A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med. 2007;4:e325. doi: 10.1371/journal.pmed.0040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsson SC, et al. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int. J. Cancer. 2006;119:2657–2664. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- 3.Norat T, et al. Meat, fish, and colorectal cancer risk: the European Prospective Investigation into cancer and nutrition. J. Natl Cancer Inst. 2005;97:906–916. doi: 10.1093/jnci/dji164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugimura T. Overview of carcinogenic heterocyclic amines. Mutat. Res. 1997;376:211–219. doi: 10.1016/s0027-5107(97)00045-6. [DOI] [PubMed] [Google Scholar]
- 5.Rohrmann S, et al. Intake of heterocyclic aromatic amines from meat in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Heidelberg cohort. Br. J. Nutr. 2007;98:1112–1115. doi: 10.1017/s000711450778145x. [DOI] [PubMed] [Google Scholar]
- 6.Bingham SA, et al. Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response. J. Nutr. 2002;132:3522S–3525S. doi: 10.1093/jn/132.11.3522S. [DOI] [PubMed] [Google Scholar]
- 7.Hughes R, et al. Dose-dependent effect of dietary meat on endogenous colonic N-nitrosation. Carcinogenesis. 2001;22:199–202. doi: 10.1093/carcin/22.1.199. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz S, et al. Quantitative fecal recovery of ingested hemoglobin-heme in blood: comparisons by HemoQuant assay with ingested meat and fish. Gastroenterology. 1985;89:19–26. doi: 10.1016/0016-5085(85)90740-1. [DOI] [PubMed] [Google Scholar]
- 9.Cross AJ, et al. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003;63:2358–2360. [PubMed] [Google Scholar]
- 10.Bonnett R, et al. Reactions of nitrous acid and nitric oxide with porphyrins and haems. Nitrosylhaems as nitrosating agents. J. Chem. Soc. Chem. Commun. 1975:884–885. [Google Scholar]
- 11.Kuhnle GG, et al. Diet-induced endogenous formation of nitroso compounds in the GI tract. Free Radic. Biol. Med. 2007;43:1040–1047. doi: 10.1016/j.freeradbiomed.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Kuhnle GG, et al. Dietary meat, endogenous nitrosation and colorectal cancer. Biochem. Soc. Trans. 2007;35:1355–1357. doi: 10.1042/BST0351355. [DOI] [PubMed] [Google Scholar]
- 13.Povey AC, et al. DNA alkylation and repair in the large bowel: animal and human studies. J. Nutr. 2002;132:3518S–3521S. doi: 10.1093/jn/132.11.3518S. [DOI] [PubMed] [Google Scholar]
- 14.Lewin MH, et al. Red meat enhances the colonic formation of the DNA adduct O6-carboxymethyl guanine: implications for colorectal cancer risk. Cancer Res. 2006;66:1859–1865. doi: 10.1158/0008-5472.CAN-05-2237. [DOI] [PubMed] [Google Scholar]
- 15.Linseisen J, et al. Dietary intake of different types and characteristics of processed meat which might be associated with cancer risk—results from the 24-hour diet recalls in the European Prospective Investigation into Cancer and Nutrition (EPIC) Public Health Nutr. 2006;9:449–464. doi: 10.1079/phn2005861. [DOI] [PubMed] [Google Scholar]
- 16.Rowland IR, et al. Endogenous N-nitrosation in man assessed by measurement of apparent total N-nitroso compounds in faeces. Carcinogenesis. 1991;12:1395–1401. doi: 10.1093/carcin/12.8.1395. [DOI] [PubMed] [Google Scholar]
- 17.Cummings JH, et al. Measurement of the mean transit time of dietary residue through the human gut. Gut. 1976;17:210–218. doi: 10.1136/gut.17.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welch AA, et al. DINER (Data Into Nutrients for Epidemiological Research)—a new data-entry program for nutritional analysis in the EPIC-Norfolk cohort and the 7-day diary method. Public Health Nutr. 2001;4:1253–1265. doi: 10.1079/phn2001196. [DOI] [PubMed] [Google Scholar]
- 19.Department of Health. Dietary Reference Values for Food, Energy and Nutrients for the United Kingdom (Report on Health and Social Subjects No. 41) London, UK: HMSO; 1991. [PubMed] [Google Scholar]
- 20.Feelisch M, et al. Concomitant S-, N-, and heme-nitros(yl)ation in biological tissues and fluids: implications for the fate of NO in vivo. FASEB J. 2002;16:1775–1785. doi: 10.1096/fj.02-0363com. [DOI] [PubMed] [Google Scholar]
- 21.Collins AR. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol. Biotechnol. 2004;26:249–261. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]
- 22.Speit G, et al. Sensitivity of the FPG protein towards alkylation damage in the comet assay. Toxicol. Lett. 2004;146:151–158. doi: 10.1016/j.toxlet.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Roediger WE, et al. Nitrite from inflammatory cells–a cancer risk factor in ulcerative colitis? Dis. Colon Rectum. 1990;33:1034–1036. doi: 10.1007/BF02139219. [DOI] [PubMed] [Google Scholar]
- 24.Mirvish SS, et al. Effect of feeding nitrite, ascorbate, hemin, and omeprazole on excretion of fecal total apparent N-nitroso compounds in mice. Chem. Res. Toxicol. 2008;21:2344–2351. doi: 10.1021/tx8001884. [DOI] [PubMed] [Google Scholar]
- 25.Key TJ, et al. Cancer incidence in vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford) Am. J. Clin. Nutr. 2009;89:1620S–1626S. doi: 10.3945/ajcn.2009.26736M. [DOI] [PubMed] [Google Scholar]
- 26.Cross AJ, et al. Variability in fecal water genotoxicity, determined using the Comet assay, is independent of endogenous N-nitroso compound formation attributed to red meat consumption. Environ. Mol. Mutagen. 2006;47:179–184. doi: 10.1002/em.20181. [DOI] [PubMed] [Google Scholar]
- 27.Glinghammar B, et al. Shift from a dairy product-rich to a dairy product-free diet: influence on cytotoxicity and genotoxicity of fecal water—potential risk factors for colon cancer. Am. J. Clin. Nutr. 1997;66:1277–1282. doi: 10.1093/ajcn/66.5.1277. [DOI] [PubMed] [Google Scholar]
- 28.Klinder A, et al. Fecal water as a non-invasive biomarker in nutritional intervention: comparison of preparation methods and refinement of different endpoints. Nutr. Cancer. 2007;57:158–167. doi: 10.1080/01635580701274848. [DOI] [PubMed] [Google Scholar]
- 29.Rieger MA, et al. A diet high in fat and meat but low in dietary fibre increases the genotoxic potential of ‘faecal water’. Carcinogenesis. 1999;20:2311–2316. doi: 10.1093/carcin/20.12.2311. [DOI] [PubMed] [Google Scholar]
- 30.Venturi M, et al. Genotoxic activity in human faecal water and the role of bile acids: a study using the alkaline comet assay. Carcinogenesis. 1997;18:2353–2359. doi: 10.1093/carcin/18.12.2353. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs DM, et al. (1)H NMR metabolite profiling of feces as a tool to assess the impact of nutrition on the human microbiome. NMR Biomed. 2008;21:615–626. doi: 10.1002/nbm.1233. [DOI] [PubMed] [Google Scholar]
- 32.Pettersson J, et al. NMR metabolomic analysis of fecal water from subjects on a vegetarian diet. Biol. Pharm. Bull. 2008;31:1192–1198. doi: 10.1248/bpb.31.1192. [DOI] [PubMed] [Google Scholar]
- 33.Anderson D, et al. The effect of potassium diazoacetate on human peripheral lymphocytes, human adenocarcinoma Colon caco-2 cells, and rat primary colon cells in the comet assay. Teratog. Carcinog. Mutagen. 1999;19:137–146. doi: 10.1002/(sici)1520-6866(1999)19:2<137::aid-tcm6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 34.Robichova S, et al. Study of N-nitrosomorpholine-induced DNA strand breaks in Caco-2 cells by the classical and modified comet assay: influence of vitamins E and C. Nutr. Cancer. 2001;39:267–272. doi: 10.1207/S15327914nc392_17. [DOI] [PubMed] [Google Scholar]
- 35.Jakszyn P, et al. Endogenous versus exogenous exposure to N-nitroso compounds and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study. Carcinogenesis. 2006;27:1497–1501. doi: 10.1093/carcin/bgl019. [DOI] [PubMed] [Google Scholar]
- 36.Knekt P, et al. Risk of colorectal and other gastro-intestinal cancers after exposure to nitrate, nitrite and N-nitroso compounds: a follow-up study. Int. J. Cancer. 1999;80:852–856. doi: 10.1002/(sici)1097-0215(19990315)80:6<852::aid-ijc9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 37.Florin TH, et al. The effect of dietary nitrate on nitrate and nitrite excretion in man. Br. J. Nutr. 1990;64:387–397. doi: 10.1079/bjn19900040. [DOI] [PubMed] [Google Scholar]