Abstract
Oxidative stress has been regarded as an important underlying cause for the delayed neuronal death after cerebral ischemia. In this study, the effects of short term oral administration of grape polyphenol extract (GPE) on ischemia/reperfusion (I/R) injury in a gerbil global ischemia model were determined. Ischemia was induced by occlusion of the common carotid arteries for 5 min. GPE (30 mg/ml)-containing formula or formula without GPE was administered daily via gavage for 4 days prior to and/or for 4 days after I/R. I/R resulted in hyperlocomotion, extensive delayed neuronal death (DND), oxidative and fragmented DNA damage, and an increase in reactive astrocytes and microglial cells in the hippocampal CA1 region. GPE administration for 4 days prior to I/R and for 4 days after I/R attenuated DND, DNA damage, and glial cell activation. However, neuroprotection was more pronounced when GPE was administered for 4 days after I/R than when administered for 4 days prior to I/R. GPE administration after I/R attenuated I/R-induced hyperlocomotion. These findings indicate that oral GPE intake may confer protection against I/R injury and emphasize that early intervention may be an effective therapeutic measure for ameliorating brain injury in stroke.
Keywords: Grape polyphenols, ischemia/reperfusion, oxidative stress, neuronal death, glial cell activation, locomotor activity
1. Introduction
Polyphenols are enriched in vegetables, fruits, grains, seeds, tea and wine and are recognized for their antioxidant properties [1-4]. Epidemiological studies have linked moderate wine consumption to a lower incidence of cardiovascular disease, the so-called “French paradox”. This effect seems to be attributed to the polyphenols such as resveratrol, in red wine [5-9]. In recent years, studies directed at elucidating the mechanism underlying the “French paradox” have stimulated interest in exploring whether polyphenolic antioxidants can also offer antioxidant benefit for the brain [7, 10, 11]. Our initial studies have found that grape polyphenols ameliorate oxidative and inflammatory damages that occur with chronic ethanol consumption [12-14]. A growing body of evidence also has demonstrated that polyphenols are neuroprotective in a variety of in vitro and in vivo models of neurodegenerative diseases and that these effects are primarily due to their antioxidative properties [15, 16].
Cerebral I/R is known to induce the generation of reactive oxygen species [9], which in turn, leads to oxidative damage to membrane lipids, proteins and nucleic acids [17-19]. In global cerebral ischemia, increased production of ROS has been regarded as an underlying factor for mediating delayed neuronal death (DND), especially to pyramidal neurons in the hippocampal CA1 area [20-24]. Our previous studies demonstrated the ability of resveratrol to ameliorate cerebral ischemia-induced neuronal death in gerbils [24]. Resveratrol also protected against excitotoxic damage induced by kainic acid in rats [25, 26]. These and other studies support the concept that resveratrol may represent a novel therapeutic intervention for neuroprotection in stroke [27]. Recently, we demonstrated that dietary supplement of grape powder for 2 months could offer protection against ischemic injury [28]. These studies on resveratrol and grape powder raised the questions: (1) Can a complex mixture of polyphenols from grapes provide better protection than a single ingredient such as resveratrol? (2) Does this grape mixture provide neuroprotective effects even when given after stroke?
In this study, a gerbil global cerebral ischemia model was used to assess the effects of orally administered grape polyphenol extract (GPE) on transient ischemia-induced neuronal damage, glial cell activation and behavioral outcomes. Comparisons were made to assess protective effects with regard to pre- and postischemic GPE administration and a 4 day regimen versus a more prolonged 8 day treatment protocol after I/R.
2. MATERIALS AND METHODS
2.1. Grape polyphenol extract (GPE)
Grape powder was prepared from freeze-dried California grapes kindly provided by the California Table Grape Commission (Fresno, CA). In their preparation, 100 g of fresh grapes yielded approximately 18.2 g grape powder. In the grape powder, selected phytochemical components based on 100 g of grape powder are: total phenols 0.58 g, flavans 0.41 g, anthocyanins 0.077 g, quercetin 10.2 micromol, myricetin 0.8 micromol, kaempferol 1.1 micromol and resveratrol 0.7 micromol (information from the California Table Grape Commission).
We extracted polyphenols from the grape powder with food grade ethyl acetate (Fisher, St. Louis, MO). Briefly, grape powder was placed in a flask containing ethyl acetate (1g per 10 ml). The mixture was placed in the hood and was stirred occasionally and after 24 h, the solution was filtered using Beckman filter papers and a glass funnel. The filtered extract was evaporated to dryness and stored at -20°C until use. Before administration, the GPE was mixed with a liquid formula (Sustacal) and adjusted to 30 mg GPE/ml of liquid diet. To verify the presence of resveratrol in the GPE, samples were analyzed by negative ion atmospheric pressure chemical ionization (APCI) LC-MS on a Finnegan MAT LCQ Duo ion trap, equipped with a Spectra System P4000 pump, UV6000 LP UV diode array detector and AP3000 autosampler. An Ace® C18 analytical column from MacMod Analytical, Inc. (Chadds Ford, PA), 10 cm × 2.1 mm i.d., 100 Angstrom pore C18, was used. Purified resveratrol (Sigma-Aldrich, St. Louis, MO) was eluted at the mobile phase with 90:10:1 methanol:water:acetic acid (v/v/v). Selected ion monitoring (SIM) with m/z = 227 showed a single peak (data not shown).
2.2. Animal protocols
Adult male Mongolian gerbils (60-80 g body wt) (Charles River, Wilmington, MA) were provided free access to water and lab chow and the colony was maintained at 22 ± 2° C with a constant humidity and a 12:12h light:dark cycle. Experiments were carried out according to the guidelines by the NIH Guide for the Care and Use of Laboratory Animals and have been approved by the University of Missouri-Columbia Animal Care and Use Committee (Protocol #1741).
In experiment 1, gerbils were divided into 4 groups: (1) sham + formula (n=9), (2) sham + GPE in formula (n=9), (3) I/R + formula (n=8), and (4) I/R + GPE in formula (n=9). Each gerbil received 1 ml of the liquid formula with or without GPE (30 mg/ml) by gavage daily for 8 days, starting 4 days before ischemia and continued for 4 days after ischemia. The rationale for using a 4-4 day-paradigm is because in our previous studies, evaluation of neuronal death was carried out 4 days after I/R [24]. Animals were sacrificed 4 days after I/R, the brains were harvested for assessment of DND and the numbers of reactive astrocytes and microglial cells in the hippocampal CA1 region.
In experiment 2, GPE was administered either for 4 days prior to I/R or for 4 days after I/R. In this study, gerbils were divided into 4 groups (n=12 per group): (1) sham + formula for 4 days after surgery, (2) I/R + formula for 4 days after surgery, (3) I/R + GPE in formula for 4 days before surgery, and (4) I/R + GPE in formula for 4 days after surgery. Administration of formula with/without GPE was the same as described in the first experiment and animals were sacrificed 4 days after I/R.
In experiment 3, we examined whether the protective effects of GPE remain the same or decrease beyond 4 days after I/R by extending the treatment period to 8 days. In this study, gerbils were divided into five groups: (1) sham + formula daily for 4 days after surgery (n=11), (2) I/R + formula daily for 4 days after surgery (n=10), (3) I/R + formula daily for 8 days after surgery (n=9), (4) I/R + daily GPE in formula for 4 days after surgery (n=10), and (5) I/R + daily GPE in formula for 8 days after surgery (n=9). Administration of formula with/without GPE was the same as described in the first experiment. Groups 1, 2 and 4 were sacrificed 4 days after I/R and groups 3 and 5 were sacrificed 8 days after I/R.
2.3. Induction of global forebrain ischemia and preparation of brain samples
Surgical procedures were the same as described in our earlier reports [24]. Briefly, transient global cerebral ischemia was induced by occlusion of both common carotid arteries (CCA) for 5 min under anesthesia with isoflurane (2.5%), nitrous oxide (70%), and oxygen (30%). The sham-operated group underwent the same procedures, except for the occlusion of CCA. The presence of communicating arteries in gerbils was detected by monitoring the decrease in regional cerebral blood flow (rCBF) before and after clamping the bilateral CCA using a laser doppler blood flow monitor (MBF3D, Moor Instruments, Axminster, Devon, UK). Gerbils that showed a decrease in rCBF of less than 80% were excluded from subsequent analyses [24]. The animal numbers above (2.2) represent the numbers used for analyses; 10-15% were exluded because of the criteria on rCBF.
Gerbils were transcardially perfused with heparinized saline (30 ml) and then with 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4, 100 ml). The brains were then post-fixed in the same fixative for 3 days. Brain tissues were embedded in paraffin for histochemical and immunohistochemical examinations as desribed earlier [24]. Six micron thick coronal sections were cut at the dorsal hippocampal area.
2.4. Histochemical and immunohistochemical staining
Assessment of neurons, astrocytes, and microglial cells
Brain sections were stained with cresyl violet for neurons and immunohistochemical staining of glial fibrillary acidic protein (GFAP) for astrocytes according to protocol described earlier [24]. Microglial cells were identified using peroxidase labeled isolectin-B4 (Sigma-Aldrich, St. Louis, MO), according to the protocol of Streit [29] with modifications [24].
DAPI staining to assess nuclear DNA damage
The fluorescent dye, 4’, 6-diamidine-2’-phenylindole (DAPI, Roche Molecular Biochemicals, Mannheim, Germany), which intercalates specifically into the adenine-thymidine base pairs of DNA, was used to identify nuclear DNA in the cells [21]. The deparaffinized and hydrated sections were immersed with DAPI (0.1 microg/ml) in PBS for 20 min at room temperature and then examined under an absorbance maximum at 340 nm and an emission maximum at 448 nm.
8-OHdG immunohistochemistry to identify oxidized DNA
Formation of 8-OHdG (8-hydroxyl-deoxyguanosine) is regarded as a hallmark of oxidative DNA damage [30-32]. In this study, determination of 8-OHdG was carried out with brain sections obtained at 4 days after I/R as described previously [28]. Briefly, brain sections were immunostained with mouse anti-8-OHdG antibody (diluted at 5 microg/ml, OxisResearch, Portland, OR) followed by goat anti-mouse IgG labeled with horseradish peroxidase (dilution 1: 200, Sigma-Aldrich, St. Louis, MO).
TUNEL staining to identify fragmented DNA
For detecting apoptotic cell death, TUNEL staining was performed using an in situ cell death detection kit (Roche Molecular Biochemicals, Mannheim, Germany) as described in our earlier report [28]. Briefly, deparaffinized and rehydrated sections were incubated with 20 microg/ml proteinase K in 0.01M Tris-HCl (pH 7.4) and then permeabilized in a solution containing 0.1% Triton-X 100 and 0.1% sodium citrate. The sections were then incubated in TUNEL-reaction mixture containing terminal deoxynucleotidyl transferase.
2.5. Assessment of locomotor activity
Locomotor activity was monitored automatically using Med Associates (St. Albans, VT) Open Field Test Environment (ENV-515), comprised of a 16 × 16 horizontal grid of infrared sensors and a bank of 16 vertical sensors. Each monitor surrounded an acrylic cage (43.2 × 43.2 × 30.5 cm) housed in a large sound-resistant cubicle (ENV-017M). Data were collected at 5 min intervals using the Med Associates’ Open Field Activity Software (SOF-811) that records the number of sensor breaks and computes distance traveled.
At 48 h after surgery, gerbils were weighed and placed in the activity monitor for 30 min. After the session, gerbils were removed and the apparatus was cleaned with a mild soap solution. Gerbils from the third experiment were used. Data from gerbils that received I/R + formula daily for 4 days after surgery (Group 2) and from gerbils that received I/R + formula daily for 8 days after surgery (Group 3) were combined because statistical analysis did not reveal a significant difference between these groups (p>0.05). Similarly, data from gerbils that received I/R + daily GPE in formula for 4 days after surgery (Group 4) and from gerbils that received I/R + daily GPE in formula for 8 days after surgery (Group 5) were combined and no differences (p>0.05) were revealed between these groups. As such, in the design of the experiment, three conditions were formed, i.e., sham (n=11), I/R (n=19) and I/R+GPE (n=19).
2.6. Quantitative assessment and data analysis
Neuronal damage, glial activation, DNA damage, oxidized DNA and apoptosis were quantified by counting the number of surviving neurons and fluorescent or immunopositive cells for GFAP, isolectin-B4, DAPI, 8-OHdG, or TUNEL in the middle of a defined CA1 region (100 microm × 300 microm) in both sides of the hippocampus (magnification 400X) using the Bioquant Image Analysis System (Bioquant True Color Windows 95 Software Version 2.50, Nashville, TN) attached to a Nikon Eclipse E600 microscope equipped with Nikon digital still camera and the MATA Imaging Serials (Version 6.1, Molecular Devices Corp., Downingtown, PA). In each brain section, the average values from both sides of the hippocampus were obtained [33].
Data are expressed as mean ± SEM and were analyzed by either two-way or one-way ANOVA followed by pairwise comparisons (Newman-Keuls posttest) using Graph Pad Prism program version 4.0 (Graph Pad Software Inc., San Diego, CA). For the behavioral study, distance traveled [34] data were analyzed via two-way repeated measures ANOVA with treatment group as a between-groups factor and session time as a within-subject factor. Where appropriate, Tukey post hoc tests were performed. P values of <0.05 were considered statistically significant.
3. Results
3.1. Analysis of resveratrol in GPE by LC-MS
Although many polyphenolic and heterocyclic compounds are extracted in the GPE, our analysis was pertaining to verify the presence of resveratrol in the preparation. Using HPLC-UV analysis together with standard, we observed a peak similar to resveratrol standard with retention time at 8.6 min for the GPE (data not shown).
3.2. GPE is neuroprotective against I/R-induced DND and glial cell activation
Four days after a 5 min CCA occlusion, extensive neuronal death was observed in the hippocampal CA1 area (Fig. 1C vs. 1A and 1B). Administration of GPE for 8 days (4 days before and 4 days after ischemia) resulted in a marked reduction in DND (Fig. 1D vs. 1C). After ischemia, GFAP staining showed an increase in astrocytes with small cell bodies and fine cytoplasmic processes distributed around the hippocampal CA1 area (Fig. 1G vs. 1E and 1F). Treatment with GPE attenuated reactive astrogliosis (Fig. 1H vs. 1G). Using isolectin-B4 as marker, very few microglial cells were found in the sham control groups (with or without GPE) (Fig. 1I and 1J). I/R induced a substantial increase in microglial cells in the hippocampal CA1 region, especially in the areas where pyramidal neuronal death was apparent (Fig. 1K vs. 1I and 1J). Administration of GPE resulted in a decrease in microglial cells as compared to that in the group subjected to I/R alone (Fig. 1L vs. 1K).
Figure 1.
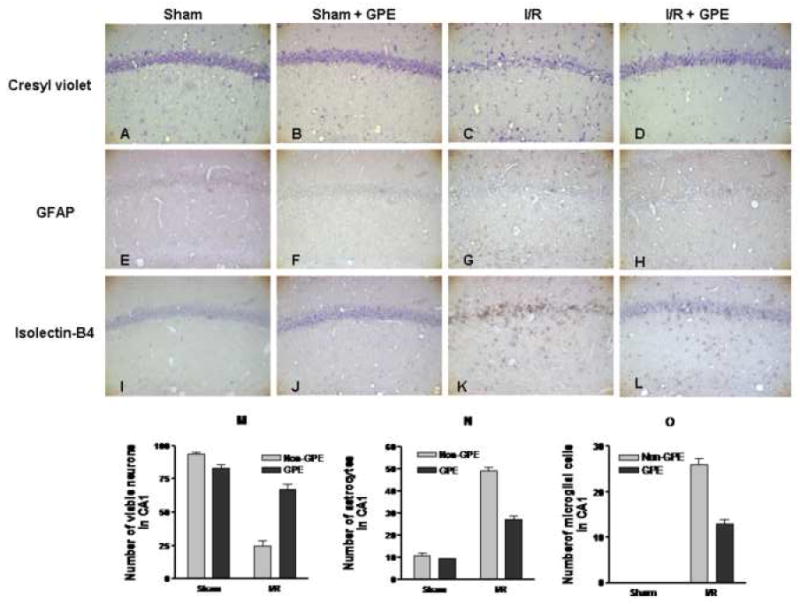
The effects of GPE on neuronal survival, astrocytic and microglial activation in the hippocampal CA1 area at 4 days after ischemia. GPE was administered orally for 8 days, from 4 days before through 4 days after I/R. Representative photomicrographs depicting neurons (cresyl violet), astrocytes (GFAP), and microglial cells (isolectin-B4). The experimental design and staining procedures were described in Methods. A, B, C, D - cresyl violet staining for neurons; E, F, G, H - GFAP staining for astrocytes; I, J, K, L - isolectin-B4 staining for microglial cells. A, E, I - sham control; B, F, J – sham control with GPE; C, G, K - ischemia; D, H, L – ischemia with GPE. (Magnification, 200X). Histograms depicting the number of neurons (M), astrocytes (N), microglial cells (O) in hippocampal CA1 area in sham (n = 9), sham+GPE (n = 9), ischemia (n = 8) and ischemia+GPE (n = 9) groups. See Methods for description of counting cells. Data represent means ± SEM. Two-way ANOVA revealed significant interactions between ischemia and GPE for each parameter. The main effects of ischemia and GPE on these parameters were also significant. See text for details in analyses.
To compare the extent of DND and glial cell activation in the hippocampal CA1 subfield across treatment conditions, quantitative analysis of the numbers of neurons, astrocytes and microglial cells was performed. Two-way ANOVA analysis of the numbers of surviving neurons in CA1 revealed a significant interaction between GPE and ischemia (F1,31=74.99, p<0.0001), and significant main effects of GPE (F1,31=28.04, p<0.0001) and ischemia (F1,31=192.11, p<0.0001) (Fig. 1M). Analysis of the numbers of astrocytes indicated a significant interaction between GPE and ischemia (F1,31=62.39, p<0.0001), and significant main effect of GPE (F1,31=77.32, p<0.0001) as well as of ischemia (F1,31=454.39, p<0.0001) (Fig. 1N). Analysis of the microglia numbers in CA1 revealed a significant interaction between GPE and ischemia (F1,31=52.50, p<0.0001). The main effect of ischemia (F1,31=467.79, p<0.0001) and GPE (F1,31=52.50, p<0.0001) was also significant (Fig. 1O).
3.3. GPE administration attenuated I/R induced DNA oxidation and fragmentation
DAPI staining was used to observe alterations in nuclear morphology after ischemic insult. In brain sections from control sham and GPE sham, the CA1 pyramidal neurons show large round nuclei with clear nucleolus (Fig. 2A and 2B). However, 4 days after I/R, most of the nuclei of CA1 pyramidal neurons changed into fragmented, irregular and condensed shape (Fig. 2C). These changes were inhibited by GPE treatment for 8 days, 4 days prior and 4 days after I/R (Fig. 2D vs. 2C). Oxidized DNA was assessed by immunohistochemistry of 8-OHdG in the hippocampal CA1 region. In the control and GPE sham groups, very weak 8-OHdG immunoreactivity was observed (Fig. 2E and 2F). Four days after I/R, 8-OHdG immunoreactivity was increased in the CA1 region (Fig. 2G vs. 2E and 2F), and this increase was diminished by GPE administration (Fig. 2H vs. 2G). In the control and GPE sham-operated groups, very few TUNEL positive cells were observed (Fig. 2I and 2J). However, TUNEL positive cells increased 4 days after I/R in the hippocampal CA1 region (Fig. 2K vs. 2I and 2J). GPE supplement reduced the number and intensity of TUNEL-positive cells as compared to those found in the ischemic brain (Fig. 2L vs. 2K).
Figure 2.
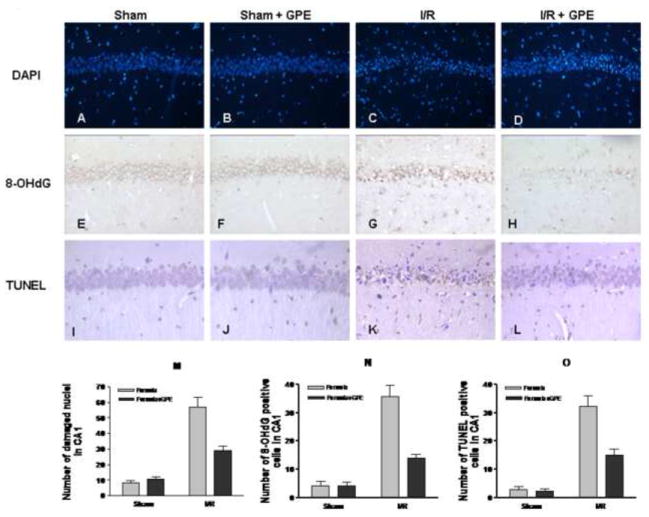
The effects of GPE on nuclear damage, DNA oxidation and fragmentation in the hippocampal CA1 area at 4 days after ischemia. GPE was administered orally for 4 days before I/R and continued for 4 days after I/R. Representative photomicrographs depicting nuclear staining (DAPI), oxidized DNA (8-OHdG), and fragmented DNA (TUNEL). The experimental design and staining procedures were described in Methods. A, B, C, D - DAPI staining; E, F, G, H - 8-OHdG staining; I, J, K, L - TUNEL staining. A, E, I - sham control; B, F, J - sham control with GPE; C, G, K - ischemia; D, H, L – ischemia with GPE. (Magnifications: DAPI, 200X; 8-OHdG and TUNEL, 400X). Histograms depicting the number of DAPI (M), 8-OHdG (N), and TUNEL (O) staining in hippocampal CA1 area in sham (n = 9), sham+GPE (n = 9), ischemia (n = 8) and ischemia+GPE (n = 9) groups. See Methods for description of counting cells. Data represent means ± SEM. Two-way ANOVA revealed significant interactions between ischemia and GPE for each parameter. The main effects of ischemia and GPE on these parameters were also significant. See text for details in analyses.
To compare the extent of nuclear damage, oxidized and fragmented DNA in the hippocampal CA1 subfield across treatment conditions, quantitative analysis of the staining was performed. Two-way ANOVA revealed a significant interactions between GPE and ischemia for nuclear damage (F1,31=19.95, p<0.0001), and significant main effects of GPE (F1,31=14.30, p=0.0007) and ischemia (F1,31=98.16, p<0.0001) (Fig. 2M). Analysis of the 8-OHdG staining indicated a significant interaction between GPE and ischemia (F1,31=24.22, p<0.0001), and significant main effect of GPE (F1,31=24.47, p<0.0001) as well as of ischemia (F1,31=87.58, p<0.0001) (Fig. 2N). Analysis of TUNEL staining revealed a significant interaction between GPE and ischemia (F1,31=14.36, p=0.0007). The main effect of ischemia (F1,31=94.48, p<0.0001) and GPE (F1,31=16.36, p=0.0003) was also significant (Fig. 2O).
3.4. Comparing the effects of GPE administered before or after I/R
In the second experiment, we compared the effects of GPE administered 4 days before or 4 days after I/R. The number of viable neurons in CA1 was 76.1± 3.5 (mean± SEM) in the sham group (n=12), 8.8± 3.7 in the I/R group (n=12), 23.8± 2.6 in the GPE+I/R group treated before ischemia (n=12), and 39.1± 6.1 in the I/R+GPE group treated after ischemia (n=12). One-way ANOVA revealed significant differences among groups (F3,44=47.86, p<0.0001). Newman-Keuls Multiple Comparison Tests revealed significant differences between sham and I/R (p<0.001) or I/R groups treated with GPE independent of the time of treatment (p<0.001). Comparison of I/R vs. I/R treated with GPE also revealed significant differences (p<0.05 between I/R and I/R with GPE pre-treatment and p<0.001 between I/R and I/R with GPE post-treatment). However, neuronal survival was significantly higher in the post-treatment group (p<0.05) as compared with the pre-treatment group. These results indicate GPE is more effective when administered immediately after the ischemic injury as compared to that before ischemia.
3.5. The effects of prolonged administration of GPE after I/R
In the third experiment, we compared the effects of GPE administered daily for 4 or 8 days after I/R. One-way ANOVA analysis of the numbers of surviving CA1 neurons showed significant differences among groups (F4,44=43.18, p<0.0001, Fig. 3A). There was no significant difference for surviving neurons between 4 and 8 days post-ischemia but GPE-treated ischemic groups differed significantly from formula-treated ischemic groups (p<0.01 for both 4 and 8 days, Fig. 3A). Analysis of microglia numbers has also revealed the same pattern (F4,44=24.71, p<0.0001, p<0.001 between I/R and I/R+GPE for both 4 and 8 days, Fig. 3C). However, there was a significant increase (p<0.01) in astrocytes at 8 days post-ischemia as compared to 4 days post-ischemia (F4,44=59.65, p<0.0001, Fig. 3B). Furthermore, there was a significant decrease (p<0.05) in the numbers of astrocytes between 4 and 8 days after GPE treatment, indicating that GPE is able to further reduce glial activation.
Figure 3.
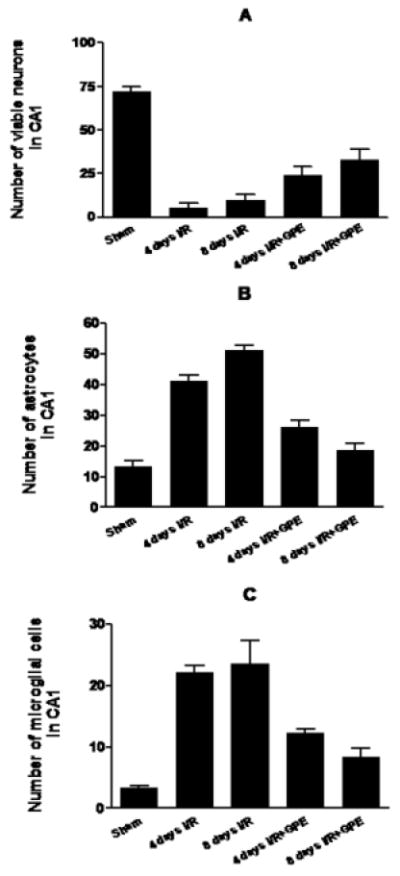
The effects of GPE on neuronal survival, astrocytic and microglial activation in the hippocampal CA1 area at 4 or 8 days after ischemia. GPE was administered daily for 4 or 8 days after I/R. Histograms depict the number of surviving neurons (A), activated astrocytes (B) and microglial cells (C) in hippocampal CA1 area among sham (n = 11), 4 days I/R (n = 10) or 8 days I/R (n = 9), and 4 days I/R+GPE (n = 10) or 8 days I/R+GPE (n = 9). See Methods for description of counting cells and experimental protocol. Data are expressed as means ± SEM. One-way ANOVA revealed significant differences among groups (p<0.0001 for neurons, astrocytes and microglia). Neuwman-Keuls Multiple Comparison revealed significant differences between I/R+GPE groups as compared to their respective I/R groups but comparison of 4 days I/R+GPE and 8 days I/R+GPE showed a significant difference only for astrocytes (p<0.05). See text for details on pairwise comparisons.
3.6. GPE administration attenuated I/R-induced hyperactivity in gerbils
Analysis of distance traveled data over a 30 min session revealed a significant main effect of treatment group (F2,48=4.08, p<0.05) and a significant treatment group by session time interaction (F10,461=2.35, p<0.05). The time course is presented in Fig. 4. Overall, there was greater activity for gerbils in the ischemia group than for gerbils pretreated with GPE or for the sham group. Regarding the time course, there was greater activity for gerbils in the ischemia group than those in the GPE or sham groups at the 5-30 min time points. Thus, treatment with GPE attenuated ischemia-induced hyperactivity.
Figure 4.
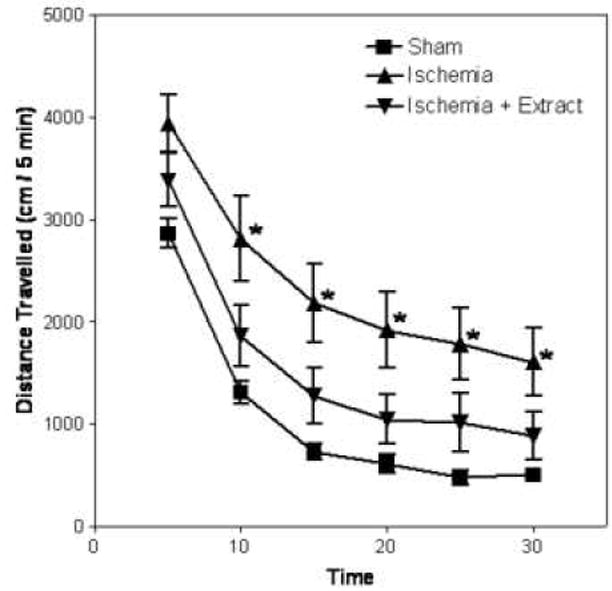
Effects of GPE administration on the spontaneous locomotor activity of gerbils at 48 hours after I/R (n = 11 for sham, n = 19 for I/R group, and n = 19 for I/R+GPE group). Data represent means ± SEM. Procedures are described in Methods. Two-way repeated measures ANOVA revealed a significant main effect of group (p<0.05) and a group by time interaction (p<0.05). Asterisks denote a significant difference from the sham group at the respective time point.
4. Discussion
Mongolian gerbils have been widely used as an animal model to study the effects of transient global cerebral I/R. With this model, extensive and selective damage occurs in neurons located within the hippocampal CA1 region at 4 days after a 5 min CCA occlusion [35], a phenomenon known as DND [20]. After ischemia, re-oxygenation during reperfusion provides an excess supply of oxygen that not only sustains neuronal viability but also contributes to the formation of ROS. In turn, ROS are directly involved in oxidative damage of macromolecules including lipids, proteins and DNA [19, 34, 36, 37]. Our previous studies with this model have demonstrated DND and glial cell activation in the hippocampal CA1 area after global cerebral I/R, and the protective effects of botanical antioxidants [24]. DND and glial activation were preceded by increases in lipid peroxidation and cytochrome C release, suggesting the involvement of ROS production in the apoptotic pathway [38]. Our recent study further demonstrated a role of NADPH oxidase in mediating I/R-induced lipid peroxidation and DND [33].
Many plant polyphenols exhibit a variety of biochemical and pharmacological properties that offer neuroprotective effects against I/R injury [27]. Considerable interest has been focused on polyphenols in grapes, with a particular interest in resveratrol from grape skin [39]. Dietary supplementation of grape powder or oral administration of grape seed extract have been shown to be effective in limiting neuronal damage induced by cerebral I/R [24, 32, 40]. In our earlier studies, we demonstrated neuroprotective effects of resveratrol administered by i.p. injection using a global cerebral ischemia model in gerbils [24]. Similar protective effects were observed with intravenous injection of resveratrol using a focal ischemia model in rats [28]. Studies including ours have shown that resveratrol is readily converted to its glycoconjugate, reaching a high level in plasma within hour of administration and capable of penetrating the blood-brain barrier [24, 41]. It is also important to mention that other grape polyphenols, such as quercetin, can influence resveratrol metabolism [41]. Furthermore, in vitro studies have indicated the synergistic effects of polyphenols which may explain why relatively low doses could provide a considerable health benefit [reviewed by 41].
In this study, we expanded on our earlier work [25] by demonstrating that the protective effects of GPE through oral administration prior to and after I/R. In our earlier study, a 50% protection could be observed upon dietary supplementation of a low dose (0.5g/100g diet) of grape extract for 2 months [25]. However, no further protective effect was observed with a higher dose (5.0g/100g diet) [25]. In this study, more than 80% of neurons survived upon oral administration with GPE 4 days before ischemia and continued for 4 days after I/R. GPE also attenuated activation of both astrocytes and microglial cells. When the effects of GPE administered for 4 days prior to I/R were compared with 4 days after I/R, our results showed that the post-ischemic treatment produced greater neuroprotection. These results are in agreement with the notion that early reperfusion is critical for stroke treatment, which is in agreement with the time window concept of stroke therapy clinically [42, 43].
In gerbils, global cerebral ischemia induces locomotor hyperactivity several hours after ischemia, and it persists for several days after insult [44-46]. Administration of different neuroprotective drugs ameliorated both hippocampal DND and ischemia-induced locomotor hyperactivity, and the two effects were closely correlated, suggesting that ischemia-induced CA1 injury and hypermotility share some common mechanisms [45]. In this study, the I/R-induced increase in locomotor activity was partially attenuated by GPE. The ability for GPE administration to ameliorate behavioral changes, in addition to our morphological findings, suggests a positive correlation between hippocampal function and neurological outcome. Other studies have also reported similar beneficial effects of plant extracts and polyphenolic compounds [28, 38].
Although extensive DND occurs by 4 days after a 5 min CCA occlusion, few studies have examined whether neuronal cell death progresses after 4 days of reperfusion and whether pharmacological treatments continue to protect against cell death beyond the initial 4 days after I/R. In this study, we did not observe obvious differences in neuronal death or microglial activation between groups reperfused for 4 or 8 days after I/R. However, there was an increased amount of astrocytes in the ischemic group at 8 days after I/R as compared to those at 4 days, and GPE administration resulted a further decrease in GFAP positive astrocytes. At 8 days after I/R, astrocytes also tend to show large cell bodies and longer cytoplasmic processes, effects that may be related to astrocytic hypertrophy [47]. Inflammatory activation of astrocytes is known to cause secondary neurodegeneration and distinct polyphenolic compounds have been shown to inhibit these processes in cell culture systems [48, 49].
Cerebral ischemia triggers a number of pathophysiological and biochemical changes in the brain that present multiple targets for therapeutic intervention [50]. Free radicals have long been thought to contribute to early brain damage following stroke or I/R [51]. A possible explanation for our results is that GPE administered at the early stage of I/R may intervene the oxidative stress pathways leading to DND. In addition, the repeated administration of GPE may increase their beneficial effects since it is likely that different biological activities of GPE contribute to multiple mechanisms in different types of cells. Results from this study will pave the way for future investigations not only for protective effects of GPE but also other polyphenolic compounds against stroke damage.
Acknowledgments
This work was supported by NIH P01 AG018357, DK 43785 and R01 AA14945, and a grant from the California Table Grape Commission.
Abbreviations
- CCA
common carotid arteries
- I/R
ischemia/reperfusion
- DND
delayed neuronal death
- GFAP
glial fibrillary acidic protein
- GPE
grape polyphenol extract
- LC-MS
liquid chromatography-mass spectrometry
- rCBF
regional cerebral blood flow
- ROS
reactive oxygen species
- DAPI
4’,6-diamidine-2’-phenylindole
- 8-OHdG
8-hydroxyl-deoxyguanosine
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998 Nov;56(11):317–33. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 2.Deschamps V, Barberger-Gateau P, Peuchant E, Orgogozo JM. Nutritional factors in cerebral aging and dementia: epidemiological arguments for a role of oxidative stress. Neuroepidemiology. 2001 Feb;20(1):7–15. doi: 10.1159/000054752. [DOI] [PubMed] [Google Scholar]
- 3.Fremont L. Biological effects of resveratrol. Life Sci. 2000 Jan 14;66(8):663–73. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 4.Youdim KA, Joseph JA. A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: a multiplicity of effects. Free Radic Biol Med. 2001 Mar 15;30(6):583–94. doi: 10.1016/s0891-5849(00)00510-4. [DOI] [PubMed] [Google Scholar]
- 5.Ray PS, Maulik G, Cordis GA, et al. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic Biol Med. 1999 Jul;27(12):160–9. doi: 10.1016/s0891-5849(99)00063-5. [DOI] [PubMed] [Google Scholar]
- 6.Cui J, Juhasz B, Tosaki A, et al. Cardioprotection with grapes. J Cardiovasc Pharmacol. 2002 Nov;40(5):762–9. doi: 10.1097/00005344-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Sun AY, Simonyi A, Sun GY. The “French Paradox” and beyond: neuroprotective effects of polyphenols. Free Radic Biol Med. 2002 Feb 15;32(4):314–8. doi: 10.1016/s0891-5849(01)00803-6. [DOI] [PubMed] [Google Scholar]
- 8.Shigematsu S, Ishida S, Hara M, et al. Resveratrol, a red wine constituent polyphenol, prevents superoxide-dependent inflammatory responses induced by ischemia/reperfusion, platelet-activating factor, or oxidants. Free Radic Biol Med. 2003 Apr 1;34(7):810–7. doi: 10.1016/s0891-5849(02)01430-2. [DOI] [PubMed] [Google Scholar]
- 9.Aldini G, Carini M, Piccoli A, et al. Procyanidins from grape seeds protect endothelial cells from peroxynitrite damage and enhance endothelium-dependent relaxation in human artery: new evidences for cardio-protection. Life Sci. 2003 Oct 17;73(22):2883–98. doi: 10.1016/s0024-3205(03)00697-0. [DOI] [PubMed] [Google Scholar]
- 10.Esposito E, Rotilio D, Di Matteo V, et al. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol Aging. 2002 Sep-Oct;23(5):719–35. doi: 10.1016/s0197-4580(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 11.Sinha K, Chaudhary G, Gupta YK. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci. 2002 Jun 28;71(6):655–65. doi: 10.1016/s0024-3205(02)01691-0. [DOI] [PubMed] [Google Scholar]
- 12.Sun GY, Xia J, Draczynska-Lusiak B, et al. Grape polyphenols protect neurodegenerative changes induced by chronic ethanol administration. Neuroreport. 1999 Jan 18;10(1):93–6. doi: 10.1097/00001756-199901180-00018. [DOI] [PubMed] [Google Scholar]
- 13.Sun GY, Xia J, Xu J, et al. Dietary supplementation of grape polyphenols to rats ameliorates chronic ethanol-induced changes in hepatic morphology without altering changes in hepatic lipids. J Nutr. 1999 Oct;129(10):1814–9. doi: 10.1093/jn/129.10.1814. [DOI] [PubMed] [Google Scholar]
- 14.Simonyi A, Woods D, Sun AY, Sun GY. Grape polyphenols inhibit chronic ethanol-induced COX-2 mRNA expression in rat brain. Alcohol Clin Exp Res. 2002 Mar;26(3):352–7. [PubMed] [Google Scholar]
- 15.Butterfield D, Castegna A, Pocernich C, et al. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J Nutr Biochem. 2002 Aug;13(8):444. doi: 10.1016/s0955-2863(02)00205-x. [DOI] [PubMed] [Google Scholar]
- 16.Mandel S, Youdim MB. Catechin polyphenols: neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic Biol Med. 2004 Aug 1;37(3):304–17. doi: 10.1016/j.freeradbiomed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Sun AY, Chen YM. Oxidative stress and neurodegenerative disorders. J Biomed Sci. 1998 Nov-Dec;5(6):401–14. doi: 10.1007/BF02255928. [DOI] [PubMed] [Google Scholar]
- 18.Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999 Jan;9(1):119–31. doi: 10.1111/j.1750-3639.1999.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001 Jan;21(1):2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Kirino T. Delayed neuronal death. Neuropathology. 2000 Sep;20(Suppl):S95–7. doi: 10.1046/j.1440-1789.2000.00306.x. [DOI] [PubMed] [Google Scholar]
- 21.Hara A, Niwa M, Iwai T, et al. Neuronal apoptosis studied by a sequential TUNEL technique: a method for tract-tracing. Brain Res Brain Res Protoc. 1999 Jul;4(2):140–6. doi: 10.1016/s1385-299x(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 22.Hara A, Mori H, Niwa M. Novel apoptotic evidence for delayed neuronal death in the hippocampal CA1 pyramidal cells after transient ischemia. Stroke. 2000 Jan;31(1):236–8. doi: 10.1161/01.str.31.1.231-e. [DOI] [PubMed] [Google Scholar]
- 23.Graham SH, Chen J. Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2001 Feb;21(2):99–109. doi: 10.1097/00004647-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Wang Q, Xu J, Rottinghaus GE, et al. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002 Dec 27;958(2):439–47. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Yu S, Simonyi A, et al. Resveratrol protects against neurotoxicity induced by kainic acid. Neurochem Res. 2004 Nov;29(11):2105–12. doi: 10.1007/s11064-004-6883-z. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Yu S, Simonyi A, et al. Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol Neurobiol. 2005;31(13):3–16. doi: 10.1385/MN:31:1-3:003. [DOI] [PubMed] [Google Scholar]
- 27.Simonyi A, Wang Q, Miller RL, et al. Polyphenols in cerebral ischemia: novel targets for neuroprotection. Mol Neurobiol. 2005;31(13):135–47. doi: 10.1385/MN:31:1-3:135. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Simonyi A, Li W, et al. Dietary grape supplement ameliorates cerebral ischemia-induced neuronal death in gerbils. Mol Nutr Food Res. 2005 May;49(5):443–51. doi: 10.1002/mnfr.200500019. [DOI] [PubMed] [Google Scholar]
- 29.Streit WJ. An improved staining method for rat microglial cells using the lectin from Griffonia simplicifolia (GSA I-B4) J Histochem Cytochem. 1990 Nov;38(11):1683–6. doi: 10.1177/38.11.2212623. [DOI] [PubMed] [Google Scholar]
- 30.Kim GW, Lewen A, Copin J, et al. The cytosolic antioxidant, copper/zinc superoxide dismutase, attenuates blood-brain barrier disruption and oxidative cellular injury after photothrombotic cortical ischemia in mice. Neuroscience. 2001;105(4):1007–18. doi: 10.1016/s0306-4522(01)00237-8. [DOI] [PubMed] [Google Scholar]
- 31.Won MH, Kang TC, Jeon GS, et al. Immunohistochemical detection of oxidative DNA damage induced by ischemia-reperfusion insults in gerbil hippocampus in vivo. Brain Res. 1999 Jul 31;836(12):70–8. doi: 10.1016/s0006-8993(99)01611-x. [DOI] [PubMed] [Google Scholar]
- 32.Hwang IK, Yoo KY, Kim DS, et al. Neuroprotective effects of grape seed extract on neuronal injury by inhibiting DNA damage in the gerbil hippocampus after transient forebrain ischemia. Life Sci. 2004 Sep 3;75(16):1989–2001. doi: 10.1016/j.lfs.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Tompkins KD, Simonyi A, et al. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006 May 23;1090(1):182–9. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 34.Hou ST, MacManus JP. Molecular mechanisms of cerebral ischemia-induced neuronal death. Int Rev Cytol. 2002;221:93–148. doi: 10.1016/s0074-7696(02)21011-6. [DOI] [PubMed] [Google Scholar]
- 35.Ginsberg MD, Busto R. Rodent models of cerebral ischemia. Stroke. 1989 Dec;20(12):1627–42. doi: 10.1161/01.str.20.12.1627. [DOI] [PubMed] [Google Scholar]
- 36.Juurlink BH, Sweeney MI. Mechanisms that result in damage during and following cerebral ischemia. Neurosci Biobehav Rev. 1997 Mar;21(2):121–8. doi: 10.1016/s0149-7634(96)00001-2. [DOI] [PubMed] [Google Scholar]
- 37.Yamato M, Egashira T, Utsumi H. Application of in vivo ESR spectroscopy to measurement of cerebrovascular ROS generation in stroke. Free Radic Biol Med. 2003 Dec 15;35(12):1619–31. doi: 10.1016/j.freeradbiomed.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q, Sun AY, Simonyi A, et al. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J Neurosci Res. 2005 Oct 1;82(1):138–48. doi: 10.1002/jnr.20610. [DOI] [PubMed] [Google Scholar]
- 39.Das DK, Sato M, Ray PS, et al. Cardioprotection of red wine: role of polyphenolic antioxidants. Drugs Exp Clin Res. 1999;25(23):115–20. [PubMed] [Google Scholar]
- 40.Huang SS, Tsai MC, Chih CL, et al. Resveratrol reduction of infarct size in Long-Evans rats subjected to focal cerebral ischemia. Life Sci. 2001 Jul 20;69(9):1057–65. doi: 10.1016/s0024-3205(01)01195-x. [DOI] [PubMed] [Google Scholar]
- 41.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006 Jun;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 42.Dyker AG, Lees KR. Duration of neuroprotective treatment for ischemic stroke. Stroke. 1998 Feb;29(2):535–42. doi: 10.1161/01.str.29.2.535. [DOI] [PubMed] [Google Scholar]
- 43.Fisher M. Developing therapy for acute ischemic stroke. Therapie. 2002 Nov-Dec;57(6):564–8. [PubMed] [Google Scholar]
- 44.Wang D, Corbett D. Cerebral ischemia, locomotor activity and spatial mapping. Brain Res. 1990 Nov 12;533(1):78–82. doi: 10.1016/0006-8993(90)91798-l. [DOI] [PubMed] [Google Scholar]
- 45.Katsuta K, Umemura K, Ueyama N, Matsuoka N. Pharmacological evidence for a correlation between hippocampal CA1 cell damage and hyperlocomotion following global cerebral ischemia in gerbils. Eur J Pharmacol. 2003 Apr 25;467(13):103–9. doi: 10.1016/s0014-2999(03)01573-5. [DOI] [PubMed] [Google Scholar]
- 46.Janac B, Radenovic L, Selakovic V, Prolic Z. Time course of motor behavior changes in Mongolian gerbils submitted to different durations of cerebral ischemia. Behav Brain Res. 2006 Dec 15;175(2):362–73. doi: 10.1016/j.bbr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Kielian T, Esen N. Effects of neuroinflammation on glia-glia gap junctional intercellular communication: a perspective. Neurochem Int. 2004 Jul-Aug;45(23):429–36. doi: 10.1016/j.neuint.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol. 2006 Sep 1;545(1):51–64. doi: 10.1016/j.ejphar.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 49.Weinreb O, Mandel S, Amit T, Youdim MB. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. J Nutr Biochem. 2004 Sep;15(9):506–16. doi: 10.1016/j.jnutbio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Chen SD, Lee JM, Yang DI, et al. Combination therapy for ischemic stroke: potential of neuroprotectants plus thrombolytics. Am J Cardiovasc Drugs. 2002;2(5):303–13. doi: 10.2165/00129784-200202050-00003. [DOI] [PubMed] [Google Scholar]
- 51.Urabe T, Yamasaki Y, Hattori N, et al. Accumulation of 4-hydroxynonenal-modified proteins in hippocampal CA1 pyramidal neurons precedes delayed neuronal damage in the gerbil brain. Neuroscience. 2000;100(2):241–50. doi: 10.1016/s0306-4522(00)00264-5. [DOI] [PubMed] [Google Scholar]


