Abstract
Objectives To examine the effectiveness of cytological surveillance in primary care compared with immediate referral for colposcopic examination in women with low grade abnormal results on cervical cytology tests.
Design Multicentre individually randomised controlled trial.
Setting NHS cervical screening programmes in Grampian, Tayside, and Nottingham.
Participants 4439 women, aged 20-59, with a cytology result showing borderline nuclear abnormalities or mild dyskaryosis, October 1999-October 2002.
Interventions Cytological screening every six months in primary care (n=2223) or referral for colposcopy and related interventions (n=2216). All women were followed for three years, concluding with an exit appointment at which colposcopic examination was undertaken. Colposcopists assessing outcome at this appointment were blinded to randomisation.
Main outcome measures Primary end point: cumulative incidence of cervical intraepithelial neoplasia grade II or more severe disease. Other end points: cervical intraepithelial neoplasia grade III or worse, clinically significant anxiety and depression, other self reported after effects, and rates of non-attendance. Analysis was by intention to treat; all those randomised were included.
Results The cumulative incidence of cervical intraepithelial neoplasia grade II or worse was 79 per 1000 person years in the colposcopy arm and 58 per 1000 person years in the cytological surveillance arm (relative risk 1.37, 95% confidence interval 1.19 to 1.57). This difference was less marked for cervical intraepithelial neoplasia grade III or more severe disease, but the incidence was still higher in the colposcopy arm (relative risk 1.26, 1.04 to 1.53). Among women randomised to immediate colposcopy, 79% (74.9% to 82.5%) of cases of cervical intraepithelial neoplasia grade II or worse were diagnosed at the time of the immediate colposcopy, while among women randomised to cytological surveillance, 77% (72.1% to 81.2%) of cases were detected by surveillance cytology and related interventions. Similar proportions of women were anxious or depressed in the two arms. A higher proportion of women in the colposcopy arm reported after effects, and these were of longer duration and more severe. Non-attendance was low in both arms.
Conclusion The more marked difference between the arms in the occurrence of cervical intraepithelial neoplasia grade II or worse than in the occurrence of grade III or worse can probably be accounted for by the spontaneous regression of some cases of grade II neoplasia. Compared with cytological surveillance, a policy of immediate colposcopy detects more cervical intraepithelial neoplasia grade II or worse, and some more grade III or worse, but might lead to overtreatment. Such a policy is associated with a higher rate of reported after effects, which are more severe and of longer duration than those associated with cytological surveillance.
Trial registration ISRCTN 34841617.
Introduction
Organised cervical cytological screening programmes have reduced mortality from cervical cancer,1 2 3 4 5 but they cannot eliminate risk. The challenge in the future development of these programmes is to reach an optimum balance between benefit, harm, and affordability.6 The initial cytological screening test detects changes in cervical cells that are common. For example, in the UK National Health Service (NHS) cervical screening programmes over a quarter of a million cytology tests showing low grade abnormalities are reported each year.7 The number of women with screen detected abnormal cytology is about 20-fold higher than the number expected to develop cervical cancer in the absence of screening, and about 60-fold higher than the number who would die from cervical cancer.8 Two methods of managing women with low grade abnormalities—that is, borderline nuclear abnormalities or mild dyskaryosis—are routinely used: cytological surveillance (repeat cytology tests in primary care) or immediate referral for colposcopic examination in a hospital outpatient clinic.9 There has been prolonged controversy as to which method is the more effective and efficient.7 10 11
Management by cytological surveillance has raised concern that some cases of high grade disease might escape detection because of non-attendance or limited sensitivity and that women might experience anxiety over a prolonged period.7 Moreover, an estimated 65% of women with mild dyskaryosis will eventually be referred for colposcopy.12 By contrast, a policy of immediate referral for colposcopy has raised concerns about possible overtreatment,13 14 complications,15 effect on subsequent pregnancy,16 high levels of anxiety,17 18 resource constraints in general,19 and waiting times.19 20 Consequently, there is considerable variation in practice between countries.21 The guidelines for the English NHS cervical screening programme reflect the uncertainty—“women should be referred for colposcopy after one test reported as mild dyskaryosis, but it is acceptable to recommend a repeat test”22—whereas Scottish guidelines continue to defer referral for colposcopy until after two mildly dyskaryotic results.23 In both jurisdictions, colposcopic referral for women with borderline nuclear abnormalities is recommended only after three such results are reported, but in recent years there has been an increasing tendency to refer sooner.24
There have been few direct comparisons of these alternative managements. The ALTS trial in the United States concluded that women whose index cytology showed low grade squamous intraepithelial lesions (broadly comparable with mild dyskaryosis) would best be managed by immediate colposcopy.25 For women whose index cytology showed atypical squamous cells of uncertain significance (broadly comparable with borderline nuclear abnormalities), cytological surveillance resulted in a similar level of detection of cervical intraepithelial neoplasia grade III or more severe disease as immediate colposcopy, while reducing the proportion of women referred for colposcopy.26 In two studies in the UK that included women with low grade abnormal cytology, women were randomised to immediate colposcopy or to surveillance, with different periods of follow-up.27 28 In one of these, the frequency of cervical intraepithelial neoplasia grade II or worse was somewhat higher in the group offered immediate colposcopy than in the three groups offered surveillance over different periods,27 and the other observed no difference between the proportions with cervical intraepithelial neoplasia grade II or worse at immediate colposcopy or after 24 months of cytological follow-up.28 In one other follow-up study of women with mild dyskaryosis in the UK, the proportion with cervical intraepithelial neoplasia II or worse was lower in women undergoing cytological surveillance than in those who underwent immediate colposcopy,29 but no adjustment was made for differences between the groups in sociodemographic characteristics and risk factors for cervical neoplasia.
The psychological effects of alternative managements, and women’s preferences, are largely unknown.7 In a UK trial assessing the psychological effects of cytological surveillance compared with those of having a choice between this form of management and immediate colposcopy in women with recurrent borderline nuclear abnormalities or mild dyskaryosis, there was no difference in psychological morbidity over a 12 month period.30 Studies of hypothetical scenarios in the US suggest that women prefer colposcopy when the index cytology is more severe, but otherwise prefer cytological surveillance.31 32
There is a need for comprehensive evaluation of the effects of alternative managements, comparing clinical effectiveness, psychological and other after effects, and cost effectiveness. The Trial Of Management of Borderline and Other Low grade Abnormal smears (TOMBOLA), a pragmatic randomised controlled trial set within the cervical screening programmes in Scotland and England, compared the effectiveness and efficiency of cytological surveillance in primary care and immediate colposcopy for women with low grade cervical abnormalities and considered whether testing for human papillomavirus infection of types associated with a high risk for cervical cancer33 might help appropriate management decisions.24 We present data on the detection of cervical intraepithelial neoplasia grade II or worse, anxiety and depression, and other after effects. Other aspects of the study are presented elsewhere.34
Methods
Design, participants, and procedures
Full details of the design, eligibility criteria, recruitment methods, human papillomavirus testing, interventions, follow-up, and outcome assessment have been described previously.24 Briefly, the trial invited women aged 20-59 living in Grampian, Tayside, and Nottingham, whose index cytology indicated mild dyskaryosis or borderline nuclear abnormality in October 1999-October 2002 to take part. There were two phases of recruitment. During the first phase (October 1999-March 2001), women whose index cytology indicated borderline nuclear abnormality were eligible for inclusion only when a cytology test six months later indicated borderline nuclear abnormality or mild dyskaryosis; this was not required in the second phase (March 2001-October 2002). This change was made primarily because of increasing pressure in the UK to refer women with a single result indicating borderline nuclear abnormality to colposcopy, so it was important that the value of such a change be evaluated before there was widespread change in practice.24
Women were invited to attend a recruitment clinic and, after informed consent, completed sociodemographic and lifestyle questionnaires. An endocervical swab was taken for human papillomavirus testing (further details elsewhere).24 Randomisation to cytological surveillance or immediate colposcopy was undertaken after the endocervical sample was analysed (one to four weeks after recruitment), with a dedicated touch-tone telephone randomisation service provided by the health services research unit of Aberdeen University. Randomisation was stratified by age group, index cytology result, result of test for human papillomavirus infection, and recruitment centre to ensure balance between the arms.
Cytological surveillance involved repeat cytology tests every six months in primary care. After three consecutive normal results a woman returned to “routine recall” (that is, cytology tests at three or five year intervals, according to guidelines at that time). If a woman had moderate dyskaryosis or worse, or had three consecutive inadequate results, she was referred to an NHS colposcopy clinic, according to cervical screening programme guidelines.22 23 Otherwise, she remained on recall for cytology tests every six months for the trial duration.
The immediate colposcopy arm involved colposcopic examination of the cervix at a hospital outpatient clinic. Women who attended were invited to participate in a second randomisation, described in detail in a companion paper.35 Those who consented were assigned to either immediate large loop excision of the transformation zone (henceforth large loop excision) or immediate biopsies with selective recall for large loop excision.
No additional procedures were carried out if the transformation zone appeared normal at colposcopy. If colposcopy was inadequate, the woman was treated according to local NHS protocols. Women with an adequate colposcopy and an abnormal transformation zone received the treatment assigned in the second randomisation. For the women undergoing biopsy, up to four targeted punch biopsies were taken; the number of biopsies has been identified as the most important factor to identify disease.36 37 For a minority of women in whom the result of the biopsy or large loop excision histology was worse than cervical intraepithelial neoplasia grade III, or in whom there was an indication of involved margins, postcolposcopy follow-up was according to local NHS protocols. For most women follow-up involved cytology tests annually or every six months either in primary care or colposcopy clinics, the results of which determined the date for the next cytology test or re-referral to colposcopy. Women who declined the second randomisation underwent a colposcopic examination and any requisite procedures (according to local practice) and returned to primary care for follow-up. Women who did not attend either of the two colposcopy appointments offered according to trial protocol were managed according to local NHS protocols. To avoid bias, all women were invited to the exit examination (see below) and included in the intention to treat analysis.
Women were followed up for three years. During this time, the results of any cytology or colposcopies undertaken in the NHS were captured on the trial database. Three years after recruitment, all women were invited back to the trial clinic for an exit examination that included a colposcopy, with large loop excision if the transformation zone was abnormal. The colposcopist was blinded to the woman’s initial cytology status, her randomisation(s), and any clinical outcomes to minimise potential bias in assessment of the transformation zone. In addition, around the scheduled time of the exit appointment and irrespective of attendance at this appointment, the woman’s medical records, together with hospital and pathology databases, were reviewed to ascertain details of any additional relevant events and procedures, such as referral with cervical intraepithelial neoplasia.
Quality assurance
Trial colposcopists were accredited by the British Society for Colposcopy and Cervical Pathology. The cytopathology and histopathology laboratories in the trial centres participate in national quality assurance schemes. To assess consistency in cytological grading, slides of different cytological grade were circulated between trial cytopathologists. One or two independent pathologists who were not aware of the original histopathological results centrally reviewed histology samples, including those from large loop excision and punch biopsy, from a random sample of 272 participants. In 252 cases, the review diagnosis was identical to the original diagnosis, yielding a κ of 0.9, indicating good agreement.
Outcomes
Cervical intraepithelial neoplasia grade II or worse—The primary outcome was the cumulative incidence of cervical intraepithelial neoplasia grade II or worse over the period from recruitment up to and including the three year exit examination. As cervical screening programmes aim to detect and treat premalignant cervical lesions, cervical intraepithelial neoplasia grade II or more severe disease is a widely accepted surrogate. We also considered the point prevalence of cervical intraepithelial neoplasia grade II or worse detected at the exit examination. This could have been a result of failure to detect cervical intraepithelial neoplasia grade II or worse earlier, failure to treat it adequately, or newly incident disease that had developed during the interval since the last examination or treatment. We also considered cervical intraepithelial neoplasia grade III or worse as this has been considered in previous studies.25 26 Similar to ALTS,25 26 we defined “management success” for a policy of immediate colposcopy as the proportion of cervical intraepithelial neoplasia grade II or worse detected at the time of the immediate colposcopy (as distinct from during follow-up or at exit) and management success for a policy of cytological surveillance as the proportion of cervical intraepithelial neoplasia grade II or worse detected during the surveillance period (as distinct from detected at exit).
Referral for colposcopy during follow-up—In view of a previous estimate that a high proportion of women with mild dyskaryosis will eventually be referred for colposcopy,12 we examined the proportions of women referred for colposcopy in the cytological surveillance arm during follow-up (that is, excluding the exit appointment) and the proportion in the immediate colposcopy arm (excluding the immediate colposcopy and related appointments, and the exit appointment). This was important in relation to anxiety, depression, and other after effects, as well as in relation to impact on resources and wait times.
Anxiety, depression, and other after effects—In the second phase of the trial, information on psychosocial sequelae was collected at multiple time points. The hospital anxiety and depression scale evaluated anxiety or depression, or both, associated with the alternative managements.38 Following established practice, we categorised women as possibly having depression if they had a score of 8 or more on the depression scale and as possibly having anxiety if they had a score of 11 or more on the anxiety scale.39 Also in the second phase, women completed postal questionnaires about short term after effects of management, specifically pain, bleeding, and discharge together with the duration (in number of days) and severity. Severity of pain was recorded on a five point scale (very mild, mild, moderate, severe, or very severe) and severity of bleeding and discharge also on a five point scale (ranging from very light to very heavy). The postal questionnaires were scheduled six weeks after the first surveillance cytology test in the cytology arm and six weeks after the colposcopy or any related procedures or appointments in the colposcopy arm. Full details of psychosocial and other after effects are reported elsewhere.40
Non-attendance—We counted women as non-attenders if they did not attend for a cytology test or attended more than six months after it was due. In the immediate colposcopy arm, non-attenders failed to attend the two appointments offered.
Statistical analysis
Analyses were conducted on an intention to treat basis. We excluded from all analyses women who were randomised and subsequently found to be ineligible for the trial.41 To take account of variation between women in length of follow-up, each woman accrued person years from the randomisation date until the date of their exit examination for those who attended or, for others, the date the exit appointment was scheduled, they requested to leave the trial, had a hysterectomy, died, or moved out of the area. We compared the cumulative incidence of cervical intraepithelial neoplasia grade II or worse for immediate colposcopy versus cytological surveillance using relative risks and associated 95% confidence intervals computed by Poisson regression. Risk estimates were adjusted for the stratification factors (age group, index cytology, human papillomavirus result, and recruitment centre). Adjustment for additional sociodemographic and lifestyle factors, including deprivation level, reproductive history, smoking status, use of hormonal contraception, marital status, ethnic group, and level of physical activity, had little effect on the risk estimates. We repeated the analysis stratified by age (20-29, 30-59) and index cytology (borderline nuclear abnormality, mild dyskaryosis). In addition, we restricted an analysis to women who had no previous abnormal results on cytology. We compared time to disease detection since randomisation between the arms using Kaplan-Meier curves and log rank tests (women without disease were censored at the end of their follow-up).
We compared the proportions of women with clinically significant anxiety or depression, or both, and the proportions reporting pain, bleeding, or discharge between the trial arms, using tests for differences in proportions. The binomial distribution was used to compute exact confidence intervals for proportions. The medians and associated interquartile ranges for duration of reported pain, bleeding, and discharge were calculated.
Statistical power
We estimated that a sample size of about 4500 women would have 80% power to detect a relative risk of 1.15 in cumulative incidence of cervical intraepithelial neoplasia grade II or worse and 95% power to detect a relative risk of 1.2 (assuming an overall cumulative incidence of cervical intraepithelial neoplasia grade II or worse of 15% and α=0.05 for a two sided test). We calculated that this sample would have 80% power to detect a difference of 6% in the cumulative proportions of clinically significant anxiety or depression, or both, and 90% power to detect a difference of 7% (assuming an overall cumulative proportion of clinically significant anxiety and depression, or both, of 30% and α=0.05 for a two sided test).
Results
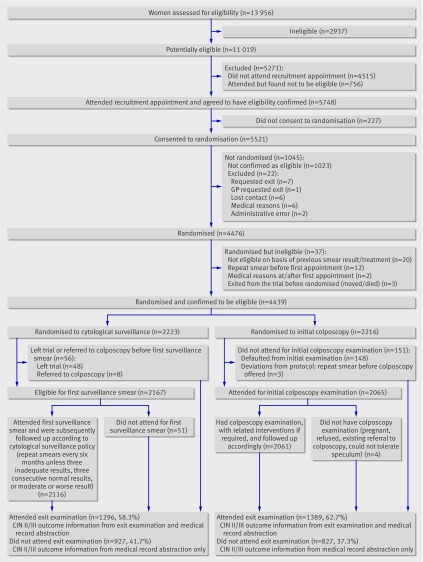
Figure 1 summarises recruitment, randomisation, and follow-up of this part of the trial. (A flow chart of the entire trial is on bmj.com.) Of the 11 019 women identified as potentially eligible to participate, 6504 (59.0%) attended an appointment to have initial eligibility assessed, of whom 5521 proved to be initially eligible and consented to randomisation. Of these, 4439 were confirmed as eligible and randomised, 2223 (50.1%) to cytological surveillance in primary care and 2216 (49.9%) to immediate colposcopy. Of these, 1296 (58.3%) women in the cytological surveillance arm and 1389 (62.7%) in the colposcopy arm attended the exit examination.
Fig 1 Flowchart of recruitment, randomisation, and follow-up of women (CIN=cervical intraepithelial neoplasia)
The women in the two arms were similar in terms of the factors on which randomisation was stratified (centre, age group, index cytology, and human papillomavirus status at recruitment) and in sociodemographic characteristics (table 1). About a third had mild dyskaryosis, and 43.5% of those providing a sample had infection with human papillomavirus of a type associated with high risk for cervical cancer. Most women were white, had been born in the UK, and were in full or part time employment. About two thirds had ever been pregnant, and the age at first pregnancy was similar between trial arms (data not shown). Among current and former smokers, the distributions by pack years and years of smoking were similar between the groups (data not shown).
Table 1.
Characteristics of women at enrolment, by trial arm. Figures are numbers (percentages) of women
| Cytological surveillance | Colposcopy | |
|---|---|---|
| Total | 2223 | 2216 |
| Cytology status at enrolment: | ||
| Mild, with previous borderline nuclear abnormalities | 57 (2.6) | 60 (2.7) |
| Mild, no previous borderline nuclear abnormalities | 732 (32.9) | 727 (32.8) |
| Borderline nuclear abnormalities, with previous borderline nuclear abnormalities | 329 (14.8) | 317 (14.3) |
| Borderline nuclear abnormalities, no previous borderline nuclear abnormalities | 1105 (49.7) | 1112 (50.1) |
| Age (years): | ||
| 20-29 | 982 (44.2) | 978 (44.1) |
| 30-39 | 596 (26.8) | 596 (26.9) |
| 40-49 | 459 (20.6) | 455 (20.5) |
| 50-59 | 186 (8.4) | 187 (8.4) |
| Human papillomavirus (HPV) status: | ||
| High risk HPV | 875 (39.4) | 880 (39.7) |
| No high risk HPV | 1147 (51.6) | 1129 (50.9) |
| Not known* | 201 (9.0) | 207 (9.3) |
| Centre: | ||
| A | 738 (33.2) | 739 (33.3) |
| B | 553 (24.9) | 548 (24.7) |
| C | 932 (41.9) | 929 (41.9) |
| Ethnic group†: | ||
| White | 2112 (95.0) | 2108 (95.1) |
| Other‡ | 98 (4.4) | 91 (4.1) |
| Not stated | 13 (0.6) | 17 (0.8) |
| Post school education and training†: | ||
| None | 620 (27.9) | 615 (27.8) |
| Through work with formal qualifications | 448 (20.2) | 424 (19.1) |
| Qualification other than degree from college or university | 634 (28.5) | 627 (28.3) |
| Degree | 502 (22.6) | 537 (24.2) |
| Not stated | 19 (0.9) | 13 (0.6) |
| Employment status†: | ||
| Full time paid employment | 1096 (49.3) | 1082 (48.8) |
| Part time paid employment | 512 (23.0) | 508 (22.9) |
| Student | 207 (9.3) | 216 (9.7) |
| Not in paid employment | 397 (17.9) | 400 (18.1) |
| Not stated | 11 (0.5) | 10 (0.5) |
| Marital status†: | ||
| Married/living as married | 1235 (55.6) | 1177 (53.1) |
| Divorced/separated/widowed | 298 (13.4) | 303 (13.7) |
| Single | 660 (29.7) | 711 (32.1) |
| Not stated | 30 (1.4) | 25 (1.1) |
| Ever been pregnant†: | ||
| Yes | 1459 (65.6) | 1480 (66.8) |
| No | 740 (33.3) | 721 (32.5) |
| Not stated | 24 (1.1) | 15 (0.7) |
| Parity: | ||
| 0 | 953 (42.9) | 972 (43.9) |
| 1 | 370 (16.6) | 341 (15.4) |
| ≥2 | 835 (37.6) | 855 (38.6) |
| Not stated | 65 (2.9) | 48 (2.2) |
| Contraception†: | ||
| Use of pill or other hormonal contraceptives only | 840 (37.8) | 795 (35.9) |
| Use of barrier contraceptive only | 272 (12.2) | 306 (13.8) |
| Use of hormonal and barrier contraceptives | 83 (3.7) | 83 (3.7) |
| None | 1018 (45.8) | 1023 (46.2) |
| Not stated | 10 (0.5) | 9 (0.4) |
| Physical activity†: | ||
| <1 time/week | 879 (39.5) | 885 (39.9) |
| 1-3 times/week | 534 (24.0) | 491 (22.2) |
| >3 times/week | 770 (34.6) | 803 (36.2) |
| Not stated | 40 (1.8) | 37 (1.7) |
| Smoking status†: | ||
| Never smoker | 1027 (46.2) | 1016 (45.8) |
| Former smoker | 383 (17.2) | 368 (16.6) |
| Current smoker | 783 (35.2) | 810 (36.6) |
| Not stated | 30 (1.4) | 22 (1.0) |
*Includes women whose samples were inadequate for analysis (n=28) and women who did not have human papillomavirus test at recruitment (n=380), most of whom declined to provide sample because they were menstruating at time of recruitment appointment.
†Enrolment questionnaires on sociodemographic characteristics not completed by 10 women in cytological surveillance arm and 9 in colposcopy arm.
‡Comprises women in following categories defined by 1991 Census: black-Caribbean (n=65), black-African (n=14), black-other (n=16), Indian (n=23), Pakistani (n=23), Bangladeshi (n=2), Chinese (n=16), other ethnic group (n=30).
Cervical intraepithelial neoplasia grade II or worse and grade III or worse
Over the full period of follow-up, including the exit examination, the cumulative incidence of cervical intraepithelial neoplasia grade II or worse was higher in the colposcopy arm (79/1000 person years) than in the cytological surveillance arm (58/1000 person years) (table 2). The adjusted relative risk for the risk of cervical intraepithelial neoplasia grade II or worse in the colposcopy arm compared to the other arm was 1.37 (95% confidence interval 1.19 to 1.57). Similarly, for cervical intraepithelial neoplasia grade III or worse, the cumulative incidence was higher in the immediate colposcopy arm, although the difference between the arms was less marked (adjusted relative risk 1.26, 1.04 to 1.53) (table 2). These patterns were also apparent when we stratified analysis by age and index cytology and restricted it to women with no previous abnormal cytology (table 3). The difference in cumulative incidence between the trial arms was more pronounced in younger women and in those whose index cytology showed mild dyskaryosis (relative risk 1.54) rather than borderline nuclear abnormalities (relative risk 1.17) (table 3). In younger women and those with mild dyskaryosis at recruitment, the relative risks were markedly higher for cervical intraepithelial neoplasia grade II or worse than for grade III or worse. These patterns were similar when we restricted the analysis to women who attended for the exit examination (data not shown).
Table 2.
Incidence of cervical intraepithelial neoplasia II, III, or worse, by trial arm and follow-up period
| Cytological surveillance | Immediate colposcopy | ||||
|---|---|---|---|---|---|
| No (%) | % of disease detected | No (%) | % of disease detected | ||
| Total No of women randomised | 2223 | — | 2216 | — | |
| Total person years of observation | 6003 | — | 5906 | — | |
| Cervical intraepithelial neoplasia II, III, or worse* | |||||
| Immediate colposcopy | — | — | 369 (16.7) | 78.8 | |
| Follow-up | 269 (12.1) | 76.9 | 66 (3.0) | 14.1 | |
| Exit | 81 (3.7) | 23.1 | 33 (1.5) | 7.1 | |
| Total | 350† (15.7) | 100 | 468‡ (21.1) | 100 | |
| Cumulative incidence per 1000 person years: | |||||
| Excluding cases detected at exit | 45 | — | 74 | — | |
| Including cases detected at exit | 58 | — | 79 | — | |
| Point prevalence at exit (%)§ | 6.3 | — | 2.4 | — | |
| Cervical intraepithelial neoplasia III or worse* | |||||
| Immediate colposcopy | — | — | 188 (8.5) | 79.0 | |
| Follow-up | 168 (7.6) | 87.0 | 43 (1.9) | 18.1 | |
| Exit | 25 (1.1) | 13.0 | 7 (0.3) | 2.9 | |
| Total | 193† (8.7) | 100 | 238‡ (10.7) | 100 | |
| Cumulative incidence per 1000 person years: | |||||
| Excluding cases detected at exit | 28 | — | 39 | — | |
| Including cases detected at exit | 32 | — | 40 | — | |
| Point prevalence at exit (%)§ | 1.9 | — | 0.5 | — | |
*Relative risk (with cytology surveillance as reference) is 1.37 (1.19 to 1.57) for grade II, III, or worse and 1.26 (1.04 to 1.53) for grade III or III+. Relates to total cases. Adjusted for stratification variables (age group, index cytology, human papillomavirus result, and recruitment centre).
†Seven cases in cytological surveillance arm had more severe disease (see text).
‡Four cases in colposcopy arm had more severe disease (see text).
§Denominator is 1296 women who attended exit examination in cytological surveillance arm, and 1389 women in immediate colposcopy arm.
Table 3.
Cumulative incidence per 1000 person years of cervical intraepithelial neoplasia II, III, or worse, by trial arm and age, enrolment cytology, human papillomavirus status at enrolment and trial centre
| Subgroup | Cytological surveillance | Immediate colposcopy | Relative risk (95% CI)* for colposcopy v
surveillance |
|||||
|---|---|---|---|---|---|---|---|---|
| No of cases | Cumulative incidence | No of cases | Cumulative incidence | |||||
| Grade II and worse | CIN III+ | |||||||
| Age (years): | ||||||||
| 20-29 | 203 | 78 | 286 | 113 | 1.45 (1.21 to 1.74) | 1.29 (1.02 to 1.65) | ||
| 30-39 | 98 | 59 | 131 | 81 | 1.36 (1.05 to 1.77) | 1.40 (0.98 to 2.00) | ||
| 40-49 | 36 | 29 | 41 | 33 | 1.10 (0.70 to 1.73) | 0.76 (0.37 to 1.54) | ||
| 50-59 | 13 | 25 | 10 | 19 | 0.79 (0.34 to 1.80) | 0.79 (0.21 to 3.04) | ||
| Enrolment cytology: | ||||||||
| Borderline nuclear abnormalities | 171 | 44 | 201 | 53 | 1.17 (0.95 to 1.43) | 1.22 (0.91 to 1.64) | ||
| Mild dyskaryosis | 179 | 84 | 267 | 128 | 1.54 (1.28 to 1.86) | 1.28 (1.00 to 1.64) | ||
| Analysis restricted to women with no previous abnormal cytology | 301 | 61 | 393 | 80 | 1.34 (1.16 to 1.56) | 1.29 (1.05 to 1.58) | ||
*Adjusted for stratification variables (age group, index cytology, human papillomavirus result, and recruitment centre).
With regard to more severe disease than cervical intraepithelial neoplasia grade III, there were seven cases in the cytological surveillance arm and four in the immediate colposcopy arm (relative risk colposcopy v surveillance 0.58, 0.12 to 2.28). In the surveillance arm, the specific diagnoses were adenocarcinoma with cervical glandular intraepithelial neoplasia and lymphatic space invasion (one case); adenocarcinoma stage 1B1 (one case); squamous carcinoma in large loop excision (one case); cervical intraepithelial neoplasia grade III with stromal invasion in large loop excision (one case); and microinvasion (three cases). In the colposcopy arm, the diagnoses were adenocarcinoma in situ (one case); squamous carcinoma (one case); microinvasion (two cases).
Timing of detection of cervical intraepithelial neoplasia grade II or worse
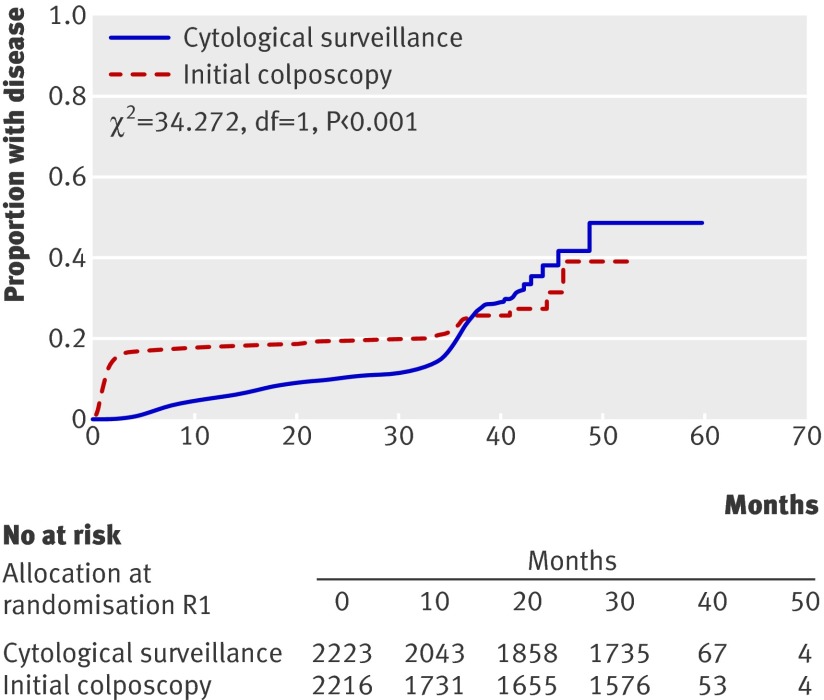
Cervical intraepithelial neoplasia grade II or worse was detected earlier in the colposcopy arm than in the surveillance arm (fig 2; log rank P<0.001); this was also evident when we repeated the analysis for cervical intraepithelial neoplasia grade III or more severe disease (log rank P=0.005).
Fig 2 Comparison of proportion of women developing cervical intraepithelial neoplasia II, III, or more severe disease over time between trial arms
Among women randomised to immediate colposcopy, the proportion of “management success,”—that is, the proportion of cervical intraepithelial neoplasia grade II or worse detected at the immediate colposcopy—was 78.8% (74.9% to 82.5%); for grade III or worse, the proportion was similar (79.0%, 73.3% to 84.0%) (table 2). For cytological surveillance, the proportion of “management success,”—that is, the proportion of cervical intraepithelial neoplasia grade II or worse detected during follow-up—was 76.9% (72.1% to 81.2%); for grade III, it was 87.0% (81.5% to 91.4%).
A higher proportion of disease was detected at exit in the surveillance arm compared with the immediate colposcopy arm (grade II or worse 23.1% v 7.1%; grade III or worse 13.0% v 2.9%). Within the surveillance arm, there was no difference in the proportions by age or severity of index cytology of grade III or worse detected at exit, but the proportion of women with grade III or worse who had this detected at exit was higher for those who did not have infection with human papillomavirus of a type associated with high risk for cervical cancer at recruitment than who had such an infection (data not shown). Of the 25 cases of cervical intraepithelial neoplasia grade III or worse detected at exit in the cytology arm, 10 were in women who did not have a high risk human papillomavirus infection at enrolment, 12 were in women with such an infection, and three were in women of unknown human papillomavirus status. Thus, about half of the cases of cervical intraepithelial neoplasia grade III or worse detected at exit in the cytology arm might have been a result of new infection during follow-up. Again within the surveillance arm, the proportion of cervical intraepithelial neoplasia grade II or worse detected at exit was higher either when the index cytology showed borderline nuclear abnormalities or when the woman did not have a high risk human papillomavirus infection at recruitment.
Referral to colposcopy
In the cytological surveillance arm 19.1% (17.5% to 20.8%) women were referred to colposcopy. In the colposcopy arm, 8.0% (6.9% to 9.2%) were re-referred to colposcopy during follow-up.
Anxiety and depression
There was no significant difference in the proportion of women classified as likely to have depression six weeks after the initial surveillance cytology test or the immediate colposcopy (table 4). The proportion classified as likely to be anxious at this time point was significantly higher in the surveillance arm than in the immediate colposcopy arm. At subsequent time points, the proportions of women who were anxious or depressed were similar between the arms.
Table 4.
Women’s reports of after effects, anxiety and depression, by trial arm: intention to treat analysis. Figures are percentages (numbers) unless stated otherwise
| Cytological surveillance | Immediate colposcopy | P value | |
|---|---|---|---|
| Pain*: | |||
| Any pain | 15.0 (145/968) | 38.9 (304/782) | <0.001 |
| Median (IQR) duration (days) | 1 (1-2) | 2 (1-3) | |
| Moderate or more severe | 5.8 (56/965) | 18.6 (144/774) | <0.001 |
| Bleeding*: | |||
| Any bleeding | 17.2 (166/967) | 46.9 (366/781) | <0.001 |
| Median (IQR) duration (days) | 1 (1-2) | 5 (2-10) | |
| Moderate or more severe | 1.6 (16/961) | 18.6 (144/772) | <0.001 |
| Discharge*: | |||
| Any discharge | 8.6 (83/964) | 34.2 (267/780) | <0.001 |
| Median (IQR) duration (days) | 2 (1-4) | 5 (2-12) | |
| Moderate or more severe | 3.7 (36/962) | 17.1 (133/777) | <0.001 |
| Anxiety†: | |||
| 6 weeks after procedure | 13.4 (121/900) | 7.9 (59/751) | <0.001 |
| 12 months after randomisation | 19.3 (218/1130) | 16.4 (190/1161) | 0.067 |
| 18 months after randomisation | 17.6 (177/1008) | 15.4 (162/1050) | 0.193 |
| 24 months after randomisation | 18.4 (177/962) | 17.9 (179/1001) | 0.766 |
| 30 months after randomisation | 16.1 (143/887) | 15.4 (146/949) | 0.665 |
| Depression‡: | |||
| 6 weeks after procedure | 7.5 (68/902) | 6.6 (50/757) | 0.461 |
| 12 months after randomisation | 11.6 (132/1136) | 9.5 (110/1162) | 0.093 |
| 18 months after randomisation | 11.2 (114/1016) | 10.1 (106/1052) | 0.399 |
| 24 months after randomisation | 10.8 (104/964) | 11.1 (111/1001) | 0.831 |
| 30 months after randomisation | 12.2 (108/887) | 10.7 (101/948) | 0.305 |
IQR=interquartile range.
*Determined by questionnaire six weeks after first surveillance cytology in cytological surveillance arm and six weeks after colposcopy or any related procedures or appointments in colposcopy arm. Proportion of women returning questionnaires on short term after effects was 78.4% in cytological surveillance arm and 84.7% in colposcopy arm.
†≥11 on hospital anxiety and depression scale anxiety subscale.
‡≥8 on hospital anxiety and depression scale depression subscale.
Other after effects
Significantly higher proportions of women randomised to immediate colposcopy reported pain, bleeding, or discharge compared with women in the surveillance arm. Women in the colposcopy arm also reported after effects that lasted longer and were more severe (table 4).
Non-attendance
In the cytological surveillance arm, 229 (10.6%) of women did not attend, whereas in the other arm, 151 (6.8%) of women failed to attend for immediate colposcopy. Within the surveillance arm, 51 (2.4%) of women failed to attend for the first surveillance cytology, while 178 (8.2%) attended later than six months after it was due. The proportions failing to attend or attending late for surveillance cytology increased slightly for the second (3.9% and 8.6%, respectively) and third (5.8% and 7.9%, respectively) tests, but declined for subsequent tests (about 3-4% and 1-4%, respectively).
Discussion
In this randomised controlled trial, the cumulative incidence of cervical intraepithelial neoplasia grade II or worse in women referred for immediate colposcopy was higher than in those randomised to cytological surveillance. This difference was less marked for cervical intraepithelial neoplasia grade III or worse and was accounted for by grade II or worse detected at the immediate colposcopy. As would be expected, disease was detected earlier in the colposcopy arm than in the cytology arm. There was little difference between the arms in the proportions of women who were anxious or depressed. A higher proportion of women in the colposcopy arm reported after effects, and these were of longer duration and of greater severity. Non-attendance was low in both arms.
Cervical intraepithelial neoplasia grade II or worse
The more marked difference between the arms in the occurrence of cervical intraepithelial neoplasia grade II or worse than in the occurrence of grade III or worse is probably because of spontaneous regression of some cases of grade II in the surveillance arm. A similar pattern was observed both in the ALTS trial25 26 and in UK studies of women with mild dyskaryosis29 or either moderate or mild dyskaryosis.27 This suggests that a policy of immediate colposcopy can lead to overtreatment, an increasingly recognised problem.42 The pattern of a more marked difference between the trial arms for cervical intraepithelial neoplasia grade II or worse than grade III or worse was apparent only for women aged under 40. For this reason, we do not support the recent suggestion that women aged 35 or less would benefit from immediate referral,43 particularly because of concerns regarding overtreatment and after effects and effects on subsequent pregnancy outcome16 in women who have not completed their families. Giannopoulos et al also concluded that it is not possible to prioritise women for colposcopy on the basis of age.44
There was a marked difference between the arms in the detection of cervical intraepithelial neoplasia grade II or worse for women whose index cytology indicated mild dyskaryosis, but a much less marked difference for cervical intraepithelial neoplasia grade III or worse. For women whose index cytology showed borderline nuclear abnormalities, the magnitude of the difference between trial arms was similar for cervical intraepithelial neoplasia grade II or worse and cervical intraepithelial neoplasia grade III or worse. This suggests that following the most recent guidelines for England22 and referring for colposcopy after a cytology test showing mild dyskaryosis, a policy that is recommended in several other countries,21 could result in substantial overtreatment.
As an approach to considering the public health importance of any delay in detection associated with cytological surveillance, we followed the ALTS trial in defining management as successful for immediate colposcopy when grade III or more severe disease was detected at immediate colposcopy, and for cytological surveillance when grade III or worse was detected either at recruitment or during follow-up.25 26 Applying these definitions in our trial, in the immediate colposcopy arm, just under 80% of the total cases of grade III or worse were detected at recruitment; these proportions were substantially higher than those observed in ALTS. One possible explanation for this difference is that in our trial, more than 90% of women undergoing biopsy received a minimum of two punch biopsies, whereas in ALTS, among those who had a biopsy taken during immediate colposcopy, 68% underwent a single biopsy.36 The number of punch biopsies has been found to be a key factor in identifying disease.36 37 In the cytological surveillance arm in our trial, the proportion of management successes for cervical intraepithelial neoplasia grade III or worse was 87.0%, again higher than in ALTS, reflecting differences in the nature of surveillance, the proportions of women with high risk human papillomavirus infection, and age distribution.25 26
Anxiety, depression, and other after effects
The lack of difference in proportions of women with anxiety or depression between the trial arms after the initial six week assessment is consistent with a previous UK trial.30 In our intention to treat analyses more women randomised to immediate colposcopy than cytological surveillance reported pain, bleeding, or discharge and after effects of greater severity and longer duration. Almost 20% of women on cytological surveillance were referred for colposcopy and so the comparisons between the arms are likely to be conservative. There has been no previous direct comparison of after effects between different management policies. In the colposcopy arm, 39% reported pain lasting for a median of two days, similar to a previous study of women undergoing large loop excision (proportion 40%, median duration three days).45 The proportions reporting discharge and bleeding were about half those in the large loop excision study,45 and the duration of these complications was shorter.
Non-attendance
The level of non-attendance we observed in the cytological surveillance arm (2% of women failed to attend for the first surveillance cytology, and 8% attended more than six months after it was due) was lower than that observed in previous studies,27 28 but in those studies surveillance required attendance at a colposcopy clinic. It is also lower than the 15.7% reported for women with low grade changes on cytology in the areas in which human papillomavirus testing was piloted in the UK46 and might reflect the tendency for trial participants to be more health conscious than the general screening population. Some 7% of women failed to attend for the immediate colposcopy, which is higher than the 3% (2.1% to 4.1%) estimated in a systematic review.47
Strengths and limitations
The trial was population based. All eligible women were invited to participate. The participation rate of 52% compares favourably with population based epidemiological studies,48 49 especially in view of concerns about barriers to women’s participation in trials.50 51 As reported elsewhere, the commonest reasons for non-participation were that women preferred follow-up by their own general practitioner and logistical issues (such as timing of recruitment clinics).51 The most common reasons for participation were altruism and worries about the cytology result. Although the participation rate was higher in older women and those living in the least deprived areas, the distributions of these factors did not differ between the trial arms and so will not have affected the randomised comparison.
With regards to generalisibility in the colposcopy arm, 30% of women (26.7% to 33.5%; 214/713) with mild dyskaryosis were found to have cervical intraepithelial neoplasia grade II or worse at the time of the immediate colposcopy. This is similar to the 29% observed in another UK series with a broadly comparable age distribution during a similar time period.44
Despite strenuous attempts to maximise attendance,24 a third of participants did not attend the exit examination. Those who did not attend were more likely to be young, be from non-white ethnic groups, have cytology result at recruitment indicating mild dyskaryosis, live in areas in the two most deprived categories, and be smokers at recruitment. As several of these are risk factors for cervical intraepithelial neoplasia grade II/III and cervical cancer,52 53 54 the overall cumulative incidence of grade II or worse was probably underestimated. The extent of the underestimation is probably small, however, because the proportion of previously undetected grade II or worse in women who attended the exit examination was low (6.3% in the cytological surveillance arm and 2.4% in the immediate colposcopy arm had grade II or worse; 1.9% and 0.5%, respectively, had grade III or worse). In addition, in the colposcopy arm the frequencies of cervical intraepithelial neoplasia grade II or worse detected up to, but not including, the exit examination (14% for women with borderline nuclear abnormalities and 32% for those with mild dyskaryosis) were similar to figures from two studies that followed participants in the NHS cervical screening programme with borderline nuclear abnormalities or mild dyskaryosis for five years (13% and 28%,10 13% and 36%55). When we restricted our analysis to women who had attended the exit examination, the difference between the trial arms was attenuated (relative risk 1.23, 1.03 to 1.47).
Conclusion
In women with low grade cytological abnormalities, a policy of immediate colposcopy detects more cervical intraepithelial neoplasia grade II or more severe disease and some more cervical intraepithelial neoplasia grade III or worse compared with cytological surveillance but can lead to overtreatment. This pattern is more apparent when the index cytology result indicates mild dyskaryosis rather than borderline nuclear abnormalities. Furthermore, such a policy is associated with a higher rate of reported after effects, which are more severe and of longer duration than those associated with cytological surveillance. We conclude that there is no clear benefit of a policy of immediate colposcopy as although it detects more cervical intraepithelial neoplasia grade II or more severe disease, it leads to a large number of referrals with no high grade cervical intraepithelial neoplasia, overtreatment with associated after effects in young women, and no clear psychological benefit.
What is already known on this topic
Cervical screening has reduced mortality from cervical cancer but at the cost of detecting low grade abnormalities in large numbers of women
There is uncertainty about the best form of follow-up: early referral for colposcopy or continued cytological surveillance
Cytological surveillance might result in some cases of high grade disease being missed because of non-attendance or limited sensitivity
Immediate referral for colposcopy might lead to overtreatment, complications, and later adverse outcomes of pregnancy
What this study adds
Immediate referral for colposcopy detects more cervical intraepithelial neoplasia grade II or worse at baseline, but there is little difference in its cumulative incidence by three years
Initial colposcopy leads to a large number of referrals where no cervical intraepithelial neoplasia grade II or worse is found and to more problems with side effects than cytology
A policy of referral for colposcopy after low grade cervical abnormalities confers no clear benefit compared with cytological surveillance and causes more side effects
We are grateful for the cooperation and assistance that we received from NHS staff in the coordinating centres and clinical sites. We thank the women who participated in TOMBOLA. We thank Paul Boyle and Zhiqiang Feng for providing data on deprivation categories and Valery L’Heureux for administrative assistance.
The TOMBOLA Group
Grant holders: Maggie Cruickshank, Graeme Murray, David Parkin, Louise Smart, Eric Walker, Norman Waugh (principal investigator 2004-7) (University of Aberdeen and NHS Grampian, Aberdeen); Mark Avis, Claire Chilvers, Katherine Fielding, Rob Hammond, David Jenkins, Jane Johnson, Keith Neal, Ian Russell, Rashmi Seth, Dave Whynes (University of Nottingham and Nottingham NHS, Nottingham); Ian Duncan, Alistair Robertson (University of Dundee and NHS Tayside, Dundee, Tayside); Julian Little (principal investigator 1999-2004) (University of Ottawa, Ottawa, Canada); Linda Sharp (National Cancer Registry, Cork); Ian Russell (Bangor University, Bangor); Leslie Walker (University of Hull, Hull).
Staff in clinical sites and coordinating centres: Breda Anthony, Sarah Bell, Adrienne Bowie, Katrina Brown, Joe Brown, Kheng Chew, Claire Cochran, Seonaidh Cotton, Jeannie Dean, Kate Dunn, Jane Edwards, David Evans, Julie Fenty, Al Finlayson, Marie Gallagher, Nicola Gray, Maureen Heddle, Alison Innes, Debbie Jobson, Mandy Keillor, Jayne MacGregor, Sheona Mackenzie, Amanda Mackie, Gladys McPherson, Ike Okorocha, Morag Reilly, Joan Rodgers, Alison Thornton, Rachel Yeats (Grampian); Lindyanne Alexander, Lindsey Buchanan, Susan Henderson, Tine Iterbeke, Susanneke Lucas, Gillian Manderson, Sheila Nicol, Gael Reid, Carol Robinson, Trish Sandilands (Tayside); Marg Adrian, Ahmed Al-Sahab, Elaine Bentley, Hazel Brook, Claire Bushby, Rita Cannon, Brenda Cooper, Ruth Dowell, Mark Dunderdale, E Gabrawi, Li Guo, Lisa Heideman, Steve Jones, Salli Lawson, Zoë Philips, Christopher Platt, Shakuntala Prabhakaran, John Rippin, Rose Thompson, Elizabeth Williams, Claire Woolley (Nottingham).
Statistical analysis: Massoud Boroujerdi, Seonaidh Cotton, Kirsten Harrild, John Norrie.
External trial steering committee: Nicholas Day (chair, 1999-2004), Theresa Marteau (chair 2004-), Mahesh Parmar, Julietta Patnick, Ciaran Woodman.
External data monitoring and ethics committee: Doug Altman (chair), Sue Moss, Michael Wells.
Contributors: Julian Little, Linda Sharp, Seonaidh Cotton, Maggie Cruickshank, Ian Duncan, Kirsten Harrild, David Jenkins, Louise Smart, Norman Waugh, Claire Cochran, Nicola Gray, Rob Hammond, Keith Neal, Alison Thornton, and Claire Woolley carried out these analyses and wrote the paper. JL is guarantor.
Funding: This study was supported by the Medical Research Council (grant No G9700808) and the NHS in England and Scotland. The MRC had no role in design, data collection, analysis, interpretation, or the writing of the report.
Competing interests: During the past five years ID has served on British and European Boards advising GlaxoSmithKline regarding the connection between human papillomavirus and cervical neoplasia, for which he has received expenses and fees for professional services. He has participated in a symposium sponsored by GlaxoSmithKline as part of a EUROGIN conference in Paris and was partly sponsored as a result. He has assisted in GlaxoSmithKline’s and MSD Sanofi Pasteur’s education programmes increasing professional awareness of the link between the human papillomavirus and cervical neoplasia, receiving fees for his professional services in creating a set of educational slides and lecturing to doctors and nurses. JL has received fees from GlaxoSmithKline as a member of an independent data and safety monitoring committee for a trial of the efficacy of vaccination against HSV. NG was reimbursed for attending an international clinical advisory board on health related quality of life issues related to cervical cancer in April 2005 by GlaxoSmithKline. From 2003 until September 2007 DJ was director of clinical research into papillomavirus vaccines for GlaxoSmithKline Biologicals and continues to act as a consultant to GSK.
Ethical approval: This study was approved by the joint research ethics committee of NHS Grampian and the University of Aberdeen, the Tayside committee on medical research ethics, and the Nottingham research ethics committee. All participants provided informed consent.
Cite this as: BMJ 2009;339:b2546
References
- 1.Vizcaino AP, Moreno V, Bosch FX, Munoz N, Barros- Dios XM, Borras J, et al. International trends in incidence of cervical cancer: II. Squamous-cell carcinoma. Int J Cancer 2000;86:429-35. [DOI] [PubMed] [Google Scholar]
- 2.Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet 2004;364:249-56. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Semenciw R, Probert A, Mao Y. Cervical cancer in Canada: changing patterns in incidence and mortality. Int J Gynecol Cancer 2001;11:24-31. [DOI] [PubMed] [Google Scholar]
- 4.Taylor RJ, Morrell SL, Mamoon HA, Wain GV. Effects of screening on cervical cancer incidence and mortality in New South Wales implied by influences of period of diagnosis and birth cohort. J Epidemiol Community Health 2001;55:782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray F, Loos AH, McCarron P, Weiderpass E, Arbyn M, Moller H, et al. Trends in cervical squamous cell carcinoma incidence in 13 European countries: changing risk and the effects of screening. Cancer Epidemiol Biomarkers Prev 2005;14:677-86. [DOI] [PubMed] [Google Scholar]
- 6.Raffle AE, Gray JAM. Screening: evidence and practice. Oxford: Oxford University Press, 2007.
- 7.Bentley E, Cotton SC, Cruickshank ME, Duncan I, Gray NM, Jenkins D, et al. Refining the management of low-grade cervical abnormalities in the UK National Health Service and defining the potential for human papillomavirus testing: a commentary on emerging evidence. J Low Genit Tract Dis 2006;10:26-38. [DOI] [PubMed] [Google Scholar]
- 8.Raffle AE, Alden B, Quinn M, Babb PJ, Brett MT. Outcomes of screening to prevent cancer: analysis of cumulative incidence of cervical abnormality and modelling of cases and deaths prevented. BMJ 2003;326:901-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin-Hirsch P, Rash B, Martin A, Standaert B. Management of women with abnormal cervical cytology: treatment patterns and associated costs in England and Wales. BJOG 2007;114:408-15. [DOI] [PubMed] [Google Scholar]
- 10.Rana DN, Marshall J, Desai M, Kitchener HC, Perera DM, El Teraifi H, et al. Five-year follow-up of women with borderline and mildly dyskaryotic cervical smears. Cytopathology 2004;15:263-70. [DOI] [PubMed] [Google Scholar]
- 11.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Kehoe S, Flannelly G, Mitrou S, et al. Management of minor cervical cytological abnormalities: A systematic review and a meta-analysis of the literature. Cancer Treat Rev 2007;33:514-20. [DOI] [PubMed] [Google Scholar]
- 12.Johnson N, Sutton J, Thornton JG, Lilford RJ, Johnson VA, Peel KR. Decision analysis for best management of mildly dyskaryotic smear. Lancet 1993;342:91-6. [DOI] [PubMed] [Google Scholar]
- 13.Etherington IJ, Luesley DM, Shafi MI, Dunn J, Hiller L, Jordan JA. Observer variability among colposcopists from the West Midlands region. Br J Obstet Gynaecol 1997;104:1380-4. [DOI] [PubMed] [Google Scholar]
- 14.Cardenas-Turanzas M, Follen M, Benedet JL, Cantor SB. See-and-treat strategy for diagnosis and management of cervical squamous intraepithelial lesions. Lancet Oncol 2005;6:43-50. [DOI] [PubMed] [Google Scholar]
- 15.Dunn TS, Killoran K, Wolf D. Complications of outpatient LLETZ procedures. J Reprod Med Obstet Gynecol 2004;49:76-8. [PubMed] [Google Scholar]
- 16.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet 2006;367:489-98. [DOI] [PubMed] [Google Scholar]
- 17.Marteau TM. Psychology and screening—narrowing the gap between efficacy and effectiveness. Br J Clin Psychol 1994;33:1-10. [DOI] [PubMed] [Google Scholar]
- 18.Jones MH, Singer A, Jenkins D. The mildly abnormal cervical smear: patient anxiety and choice of management. J R Soc Med 1996;89:257-60. [PMC free article] [PubMed] [Google Scholar]
- 19.Eggington S, Hadwin R, Brennan A, Walker P. Modelling the impact of referral guideline changes for mild dyskaryosis on colposcopy services in England. Sheffield: NHS Cancer Screening Programmes, 2006. (NHSCSP Publication No 24.)
- 20.Raffle AE. Cervical Screening. BMJ 2004;328:1272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheungraber C, Kleekamp N, Schneider A. Management of low-grade squamous intraepithelial lesions of the uterine cervix. Br J Cancer 2004;90:975-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luesley D, Leeson S. Colposcopy and programme management. Guidelines for the NHS Cervical Screening Programme. Sheffield: NHS Cervical Screening Programmes, 2004. (NHSCSP Publication No 20.)
- 23.Duncan ID. Guidelines for clinical practice and programme management. 2nd ed. Sheffield: NHS Cervical Screening Programme, 1997. (NHSCSP Publication No 8.)
- 24.Cotton SC, Sharp L, Little J, Duncan I, Alexander L, Cruickshank ME, et al. The TOMBOLA Group. Trial of management of borderline and other low grade abnormal smears (TOMBOLA): trial design. Cont Clin Trials 2006;27:449-71. [DOI] [PubMed] [Google Scholar]
- 25.ALTS Group. A randomized trial on the management of low-grade squamous intraepithelial lesion cytology interpretations. Am J Obstet Gynecol 2003;188:1393-400. [DOI] [PubMed] [Google Scholar]
- 26.ALTS Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol 2003;188:1383-92. [DOI] [PubMed] [Google Scholar]
- 27.Flannelly G, Anderson D, Kitchener HC, Mann EMF, Campbell M, Fisher P, et al. Management of women with mild and moderate cervical dyskaryosis. BMJ 1994;308:1399-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shafi MI, Luesley DM, Jordan JA, Dunn JA, Rollason TP, Yates M. Randomised trial of immediate versus deferred treatment strategies for the management of minor cervical cytological abnormalities. Br J Obstet Gynaecol 1997;104:590-4. [DOI] [PubMed] [Google Scholar]
- 29.Jones MH, Jenkins D, Cuzick J, Wolfendale MR, Jones JJ, Balogun-Lynch C, et al. Mild cervical dyskaryosis: safety of cytological surveillance. Lancet 1992;339:1440-3. [DOI] [PubMed] [Google Scholar]
- 30.Kitchener HC, Burns S, Nelson L, Myers AJ, Fletcher I, Desai M, et al. A randomised controlled trial of cytological surveillance versus patient choice between surveillance and colposcopy in managing mildly abnormal cervical smears. BJOG 2004;111:63-70. [DOI] [PubMed] [Google Scholar]
- 31.Ferris DG, Kriegel D, Cote L, Litaker M, Woodward L. Women’s triage and management preferences for cervical cytologic reports demonstrating atypical squamous cells of undetermined significance and low-grade squamous intraepithelial lesions. Arch Fam Med 1997;6:348-53. [DOI] [PubMed] [Google Scholar]
- 32.Melnikow J, Kuppermann M, Birch S, Chan BKS, Nuovo J. Management of the low-grade abnormal pap smear: what are women’s preferences? J Fam Pract 2002;51:849-55. [PubMed] [Google Scholar]
- 33.Jacobs MV, Snijders PJ, van den Brule AJ. A general primer GP5+/GP6(+)-mediated PCR-enzyme immunoassay method for rapid detection of 14 high-risk and 6 low-risk human papillomavirus genotypes in cervical scrapings. J Clin Microbiol 1997;35:791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.TOMBOLA Group. Options for managing low-grade cervical abnormalities detected at screening cost effectiveness study. BMJ 2009;339: b2549. [DOI] [PMC free article] [PubMed]
- 35.TOMBOLA Group. Biopsy and selective recall compared with immediate large loop excision in management of women with low grade cervical cytology referred for colposcopy: multicentre randomised controlled trial. BMJ 2009;339:b2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gage JC, Hanson VW, Abbey K, Dippery S, Gardner S, Kubota J, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol 2006;108:264-72. [DOI] [PubMed] [Google Scholar]
- 37.Byrom J, Douce G, Jones PW, Tucker H, Millinship J, Dhar K, et al. Should punch biopsies be used when high-grade disease is suspected at initial colposcopic assessment? A prospective study. Int J Gynecol Cancer 2006;16:253-6. [DOI] [PubMed] [Google Scholar]
- 38.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. [DOI] [PubMed] [Google Scholar]
- 39.Fayers P, Machin D. Quality of life. Assessment, analysis and interpretation. Chichester: John Wiley, 2000.
- 40.TOMBOLA Group. After-effects reported by women following colposcopy, cervical biopsies and LLETZ: results from the TOMBOLA trial. BJOG (in press). [DOI] [PubMed]
- 41.Gail MH. Eligibility exclusions, losses to follow-up, removal of randomized patients, and uncounted events in cancer clinical trials. Cancer Treat Rep 1985;69:1107-13. [PubMed] [Google Scholar]
- 42.Schiffman M, Rodriguez AC. Heterogeneity in CIN3 diagnosis. Lancet Oncol 2008;9:404-6. [DOI] [PubMed] [Google Scholar]
- 43.Winn CM, Jones H. Outcome of women with index smear showing mild dyskaryosis: effects of age and evidence of HPV infection. Cytopathology 2005;16:281-9. [DOI] [PubMed] [Google Scholar]
- 44.Giannopoulos T, Butler-Manuel S, Tailor A, Demetriou E, Daborn L. Prevalence of high-grade CIN following mild dyskaryotic smears in different age groups. Cytopathology 2005;16:277-80. [DOI] [PubMed] [Google Scholar]
- 45.Lopes A, Beynon G, Robertson G, Daras V, Monaghan JM. Short term morbidity following large loop excision of the cervical transformation zone. J Obstet Gynaecol 1994;14:197-9. [Google Scholar]
- 46.Moss S, Gray A, Legood R, Vessey M, Patnick J, Kitchener H, et al. Effect of testing for human papillomavirus as a triage during screening for cervical cancer: observational before and after study. BMJ 2006;332:83-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lester H, Wilson S. Is default from colposcopy a problem, and if so what can we do? A systematic review of the literature. Br J Gen Pract 1999;49:223-9. [PMC free article] [PubMed] [Google Scholar]
- 48.Olson SH. Reported participation in case-control studies: changes over time. Am J Epidemiol 2001;154:574-81. [DOI] [PubMed] [Google Scholar]
- 49.Morton LM, Cahill J, Hartge P. Reporting participation in epidemiologic studies: a survey of practice. Am J Epidemiol 2006;163:197-203. [DOI] [PubMed] [Google Scholar]
- 50.Recruitment of women to clinical trials. Lancet 2001;358:853. [PubMed] [Google Scholar]
- 51.Sharp L, Cotton SC, Alexander L, Williams E, Gray NM, Reid JM. Reasons for participation and non-participation in a randomized controlled trial: postal questionnaire surveys of women eligible for TOMBOLA (trial of management of borderline and other low-grade abnormal smears). Clin Trials 2006;3:431-42. [DOI] [PubMed] [Google Scholar]
- 52.Castellsague X, Munoz N. Chapter 3: Cofactors in human papillomavirus carcinogenesis—role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr 2003;(31):20-8. [PubMed]
- 53.Parikh S, Brennan P, Boffetta P. Meta-analysis of social inequality and the risk of cervical cancer. Int J Cancer 2003;105:687-91. [DOI] [PubMed] [Google Scholar]
- 54.Bosch FX, Iftner T. The aetiology of cervical cancer. Sheffield: NHS Cancer Screening, 2005. (Report No 22.)
- 55.Smith MC, Keech SE, Perryman K, Soutter WP. A long-term study of women with normal colposcopy after referral with low-grade cytological abnormalities. BJOG 2006;113:1321-8. [DOI] [PubMed] [Google Scholar]