Abstract
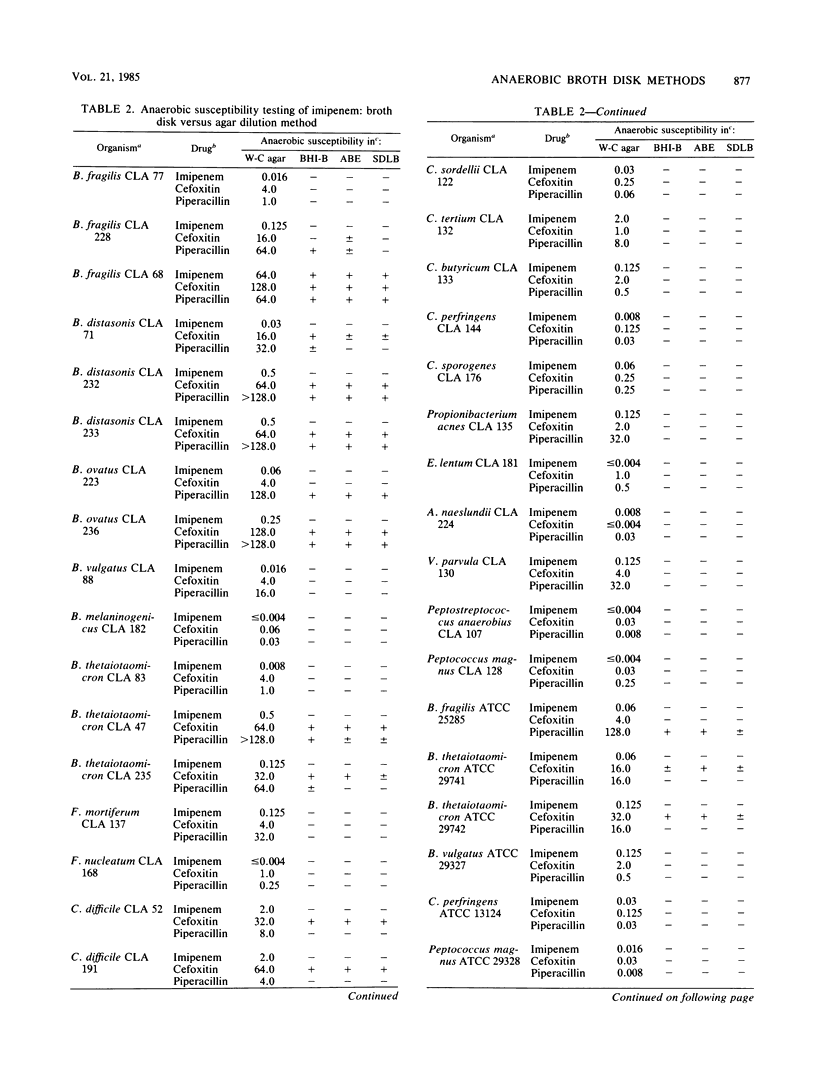
Imipenem is a member of a new class of highly potent beta-lactam antibiotics, carbapenems, with an antibacterial spectrum that includes nearly all currently known aerobic and anaerobic bacterial species of clinical significance. Although relatively stable in most standard laboratory media used for antimicrobial susceptibility testing, imipenem undergoes rapid inactivation in thioglycolate broth, a recommended medium for susceptibility testing of anaerobic bacteria by the broth disk method. In the current study, a panel of 36 anaerobic bacteria consisting of 28 clinical isolates and eight quality control strains was used to determine the suitability and accuracy of the broth disk methods with brain heart infusion, Schaedler, and anaerobic broths, in comparison to the reference agar dilution method, for the anaerobic susceptibility testing of imipenem. To achieve single test concentrations of approximately 8, 16, and 64 micrograms/ml for imipenem, cefoxitin, and piperacillin, respectively, which correspond to the MIC breakpoints of the test drugs, four 10-microgram imipenem disks, three 30-microgram cefoxitin disks, and three 100-microgram piperacillin disks were used in 5 ml of broth. The correlation between the reference agar dilution method and each of the three broth disk elution procedures evaluated was excellent, for imipenem (100% agreement) and somewhat less so for cefoxitin and piperacillin. Therefore, brain heart infusion, Schaedler, and anaerobic broths, but not thioglycolate broth, are suitable for anaerobic susceptibility testing of imipenem by the disk elution method.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge K. E., Sanders C. V., Janney A., Faro S., Marier R. L. Comparison of the activities of penicillin G and new beta-lactam antibiotics against clinical isolates of Bacteroides species. Antimicrob Agents Chemother. 1984 Sep;26(3):410–413. doi: 10.1128/aac.26.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge K. E., Sanders C. V., Lewis A. C., Marier R. L. Susceptibility of anaerobic bacteria to beta-lactam antibiotics and beta-lactamase production. J Med Microbiol. 1983 Feb;16(1):75–82. doi: 10.1099/00222615-16-1-75. [DOI] [PubMed] [Google Scholar]
- Kahan F. M., Kropp H., Sundelof J. G., Birnbaum J. Thienamycin: development of imipenen-cilastatin. J Antimicrob Chemother. 1983 Dec;12 (Suppl 500):1–35. doi: 10.1093/jac/12.suppl_d.1. [DOI] [PubMed] [Google Scholar]
- Kahan J. S., Kahan F. M., Goegelman R., Currie S. A., Jackson M., Stapley E. O., Miller T. W., Miller A. K., Hendlin D., Mochales S. Thienamycin, a new beta-lactam antibiotic. I. Discovery, taxonomy, isolation and physical properties. J Antibiot (Tokyo) 1979 Jan;32(1):1–12. doi: 10.7164/antibiotics.32.1. [DOI] [PubMed] [Google Scholar]
- Kurzynski T. A., Yrios J. W., Helstad A. G., Field C. R. Aerobically incubated thioglycolate broth disk method for antibiotic susceptibility testing of anaerobes. Antimicrob Agents Chemother. 1976 Oct;10(4):727–732. doi: 10.1128/aac.10.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. A., Sanders C. V., Marier R. L. N-formimidoyl thienamycin (MK0787): in vitro activity against anaerobic bacteria. Antimicrob Agents Chemother. 1982 Jan;21(1):168–169. doi: 10.1128/aac.21.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. R., Niles A. C. Inactivation of penicillins by Thiol broth. J Clin Microbiol. 1982 Nov;16(5):982–984. doi: 10.1128/jcm.16.5.982-984.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. Comparative in vitro activity of N-formimidoyl thienamycin against gram-positive and gram-negative aerobic and anaerobic species and its beta-lactamase stability. Antimicrob Agents Chemother. 1982 Jan;21(1):180–187. doi: 10.1128/aac.21.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Cuchural G. J., Jacobus N. V., Gorbach S. L., Aldridge K. E., Cleary T. J., Finegold S. M., Hill G. B., Iannini P. B., McCloskey R. V. Susceptibility of the Bacteroides fragilis group in the United States in 1981. Antimicrob Agents Chemother. 1983 Apr;23(4):536–540. doi: 10.1128/aac.23.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E., Shungu D. L., Gadebusch H. H. Effectiveness of the antimicrobial removal device, BACTEC 16B medium, and thiol broth in neutralizing antibacterial activities of imipenem, norfloxacin, and related agents. J Clin Microbiol. 1984 Feb;19(2):207–209. doi: 10.1128/jcm.19.2.207-209.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. E., Wilkins T. D. Vaspar broth-disk procedure for antibiotic susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1980 Feb;17(2):288–291. doi: 10.1128/aac.17.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins T. D., Thiel T. Modified broth-disk method for testing the antibiotic susceptibility of anaerobic bacteria. Antimicrob Agents Chemother. 1973 Mar;3(3):350–356. doi: 10.1128/aac.3.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]



