Abstract
Because the tongue is superficially located and the initial manifestation of most diseases occurring there is mucosal change, lingual lesionscan be easily accessed and diagnosed without imaging analysis. Some lingual neoplasms, however, may manifest as a submucosal bulge and be located in a deep portion of the tongue, such as its base; their true characteristics and extent may be recognized only on cross-sectional images such as those obtained by CT or MRI.
Some uncommon tongue neoplasms may have characteristic radiologic features, thus permitting quite specific radiologic diagnosis. Lipomas typically manifest at both CT and MR imaging as homogeneous nonenhancing lesions. Relative to subcutaneous fat they are isoattenuating on CT images, and all MR sequences show them as isointense. Due to the paramagnetic properties of melanin, metastases from melanotic melanoma usually demonstrate high signal intensity on T1-weighted MR images and low signal intensity on T2-weighted images.
Although the radiologic findings for other submucosal neoplasms are nonspecific, CT and MR imaging can play an important role in the diagnostic work-up of these unusual tumors. Delineation of the extent of the tumor, and recognition and understanding of the spectrum of imaging and the pathologic features of these lesions, often help narrow the differential diagnosis.
Keywords: Tong; Tong, MR; Tong, neoplasms
Although the vast majority of lingual tumors are squamous cell carcinomas, a variety of non-squamous cell neoplasms may affect the tongue. Most squamous cell carcinomas first manifest with mucosal change and can be easily accessed and diagnosed without imaging analysis. Some non-squamous cell neoplasms, however, may manifest as a submucosal bulge and be located in a deep portion of the tongue such as its base. Thus, the characteristics and extent of these lesions may be recognized only on cross-sectional CT or MR images.
In this article, we describe the imaging findings of uncommon non-squamous cell neoplasms of the tongue provide clinical and pathologic-radiologic correlation, and discuss the clinical role of CT and MR imaging in the diagnostic work-up of these lesions.
Lipoma
Although lipoma is the most common of all connective tissue lesions, it is rarely found in the tongue. When it does occur there, it is localized immediately beneath the mucosa and is seen at the organ's lateral edge and in the anterior two-thirds.
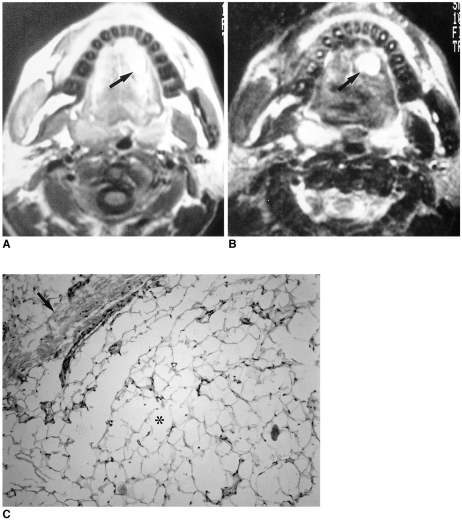
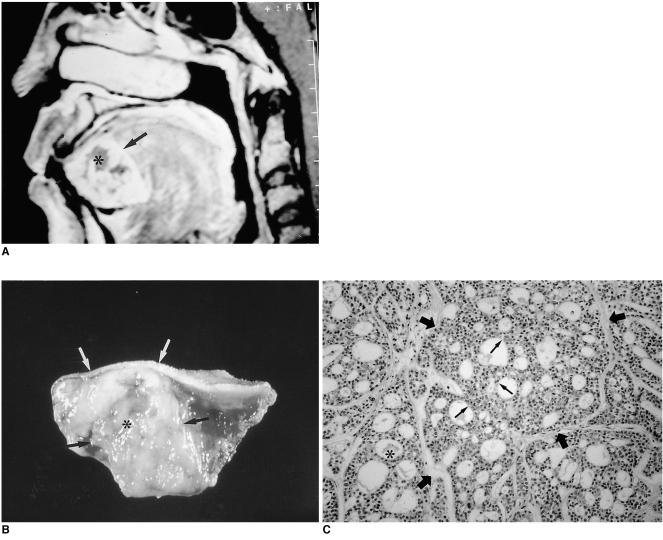
The radiologic diagnosis of lipoma is straightforward: in virtually all cases both CT and MR imaging provide a definitive diagnosis. On CT, lipoma typically manifests as a homogeneous nonenhancing lesion with attenuation values of between -65 HU and -125 HU (1). All MR pulse sequences show that the lesion is isointense relative to subcutaneous fat (i.e. hyperintense on T1-weighted images, moderately intense on T2-weighted images, and hypointense on fat-suppressed T1-weighted images) (Fig. 1).
Fig. 1.
Lipoma in a 40-year-old man with a non-tender, soft mass at the left dorsum of the tongue.
A. T1-weighted axial image shows an ovoid mass, its high signal intensity similar to that of subcutaneous fat, at the left anterior aspect of the tongue (arrow).
B. T2-weighted axial image also shows that the high signal intensity of this mass is similar to that of subcutaneous fat (arrow).
C. Photomicrograph (original magnification ×100; H & E staining) shows that the lipoma is composed of mature adipocytes (*). The mass is well demarcated by a fibrous pseudocapsule (arrow) and separated from lingual muscle (not shown). An easily detachable yellow-to-white soft tissue mass was excised.
Schwannoma
Schwannoma is a solitary tumor of the Schwann sheath of the cranial, sympathetic or peripheral nerves. About 25% of all extracranial schwannomas are located in the head and neck, the lateral cervical region and the mouth being the most common sites. Schwannomas arising from the base of the tongue are very rare (2). Tumors are generally well encapsulated and their growth is slow. As they become larger they tend to outgrow their blood supply and undergo cystic degeneration in some areas. Histologically, schwannoma of the tongue is characterized by regimented pallisading of cells.
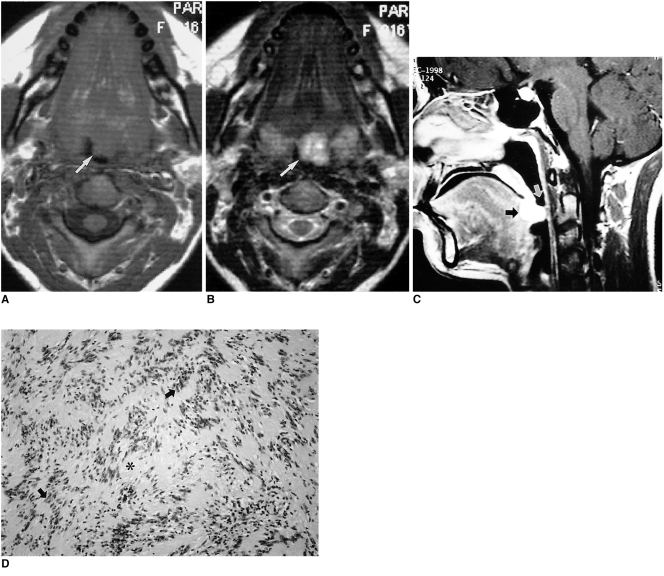
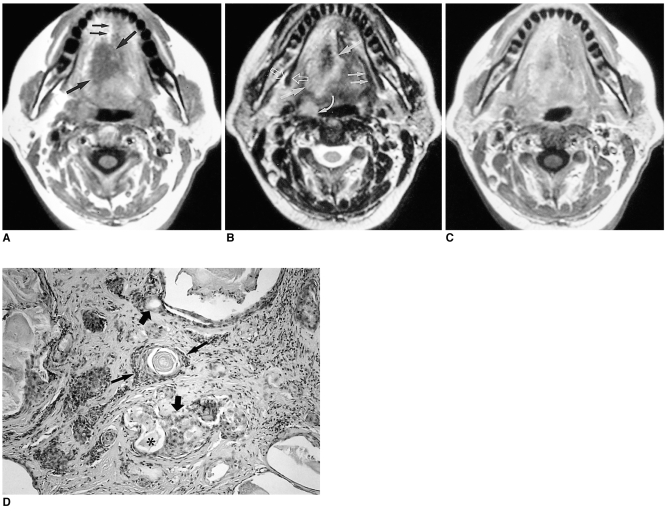
Radiologically, most schwannomas are shown by non-enhanced CT to be well-circumscribed, dense, homogeneous soft-tissue masses which exhibit contrast enhancement. Calcification or hemorrhage is uncommon, but cystic or fatty degeneration is frequent. Most schwannomas appear hypointense or isointense relative to muscle on T1-weighted images, hyperintense on T2-weighted images, and show strong enhancement after contrast administration (Fig. 2). In our case, because the tumor occurred in the region of the foramen cecum, the possibility of ectopic thyroid was considered (Fig. 2).
Fig. 2.
Schwannoma in a 16-year-old girl with swallowing difficulty.
A. T1-weighted axial image shows a well-defined, homogeneous mass (arrow), isointense to surrounding muscle, at the base of the tongue and encroaching on the airway.
B. T2-weighted axial image shows a mass with heterogeneous high signal intensity (arrow).
C. Enhanced T1-weighted sagittal image shows that the mass is located at the posterior one-third of the tongue, which corresponds to the region of the foramen cecum (black arrow). This mass significantly encroaches on the airway (white arrow).
D. Photomicrograph (original magnification ×40; H & E staining) shows spindle cells with some whirling and pallisading of their nuclei (arrows). The cells resemble Verocay bodies and enclose a space nearly devoid of nuclei (*). There is no evidence of malignancy.
Myoepithelioma
Myoepithelioma is a rare salivary gland tumor composed entirely of myoepithelial cells. Although these are an important element in many salivary gland tumors, pure myoepithelioma is rare, accounting for less than 1% of all such tumors (3). Malignant myoepithelioma is even rarer. The parotid gland is by far the most frequently affected site; myoepithelioma arising from the base of the tongue has not been reported.
Myoepithelioma is biologically benign in the majority of cases, but occasionally infiltrates locally and metastasizes to other organs including the inguinal lymph node.
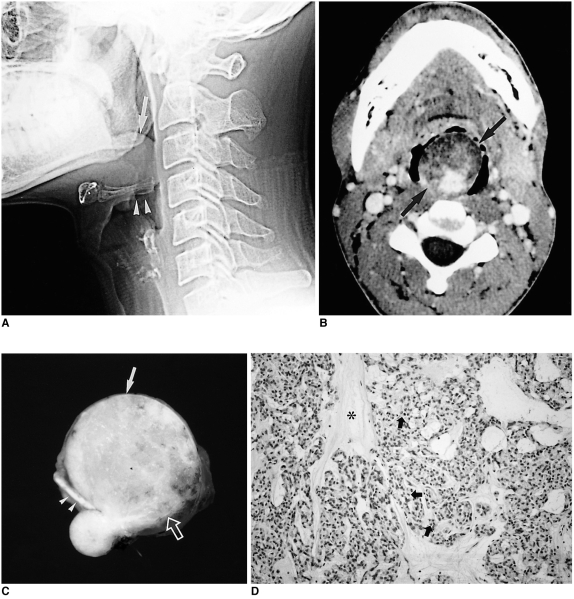
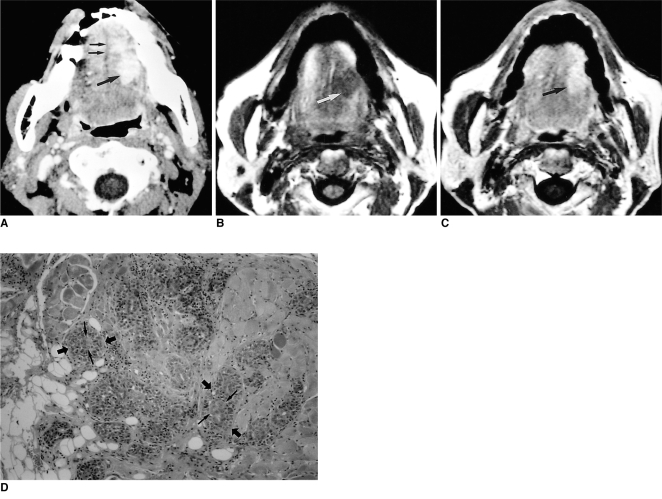
The imaging features of this tumor strongly suggest a benign mass (Fig. 3), but using current imaging modalities it cannot be differentiated from other benign lesions unless a biopsy is performed.
Fig. 3.
Myoepithelioma in a 35-year-old man with severe dyspnea and difficulty in swallowing.
A. Lateral view of plain cervical spine shows a lobulating, well-defined soft tissue density mass (arrow) at the base of the tongue, resting against the epiglottis (arrowheads) and encroaching on the airway.
B. Contrast-enhanced axial CT scan shows a well-defined mass with heterogeneous attenuation (arrows) at the base of the tongue. Since the anterior portion of this mass has low attenuation similar to that of subcutaneous fat, initial differential diagnosis included benign mature teratoma and benign minor salivary gland tumor such as pleomorphic adenoma.
C. Photograph of a cut section of gross specimen shows a well-demarcated yellowish mass (arrow) arising from the base of the tongue (open arrow). A lower small daughter nodule is covered by intact lingual mucosa. Note the intervening epiglottis (arrowheads) between the main mass and the nodule.
D. Photomicrograph (original magnification ×100; H & E staining) shows plasmacytoid-type myoepithelioma composed of small polygonal cells (arrows) with centrally located nuclei and abundant cytoplasm in a myxoid ground substance (*).
Hemangioma
Hemangioma is the most common tumor of infancy. On the basis of the cellular features described by Mulliken and Glowacki in 1982, vascular lesions may be classified as either hemangioma or vascular malformation (4). They proposed that the term 'hemangioma' should be limited to those lesions that show increased mitotic activity. Most hemangiomas are present at birth, but some do not manifest clinically until early childhood. They may regress spontaneously due to internal bleeding, thrombosis, or organization. The most common sites of oral occurrence are the lip, buccal mucosa, tongue, and palate.
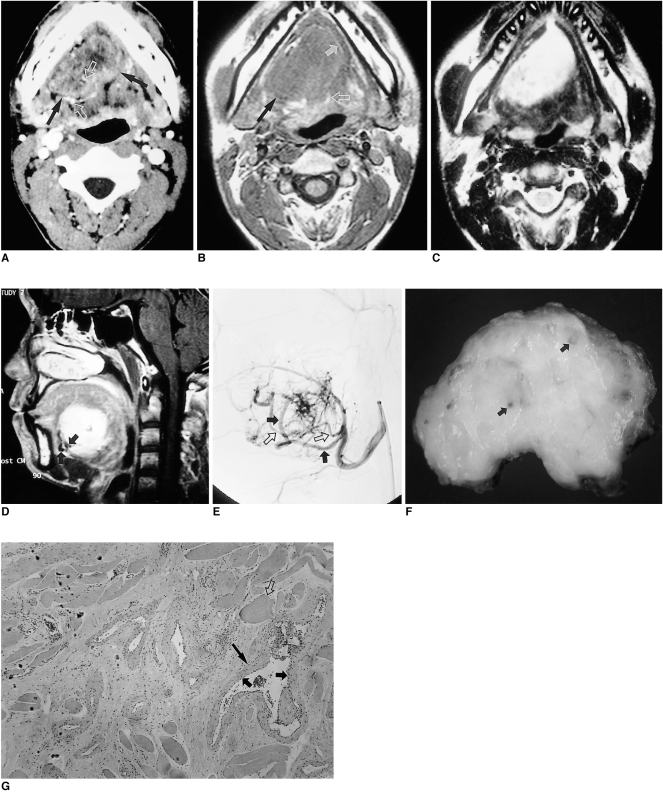
Angiographically, a hemangioma appears as a well-circumscribed mass characterized by the presence of a lobular pattern of intense, persistent tissue staining (5) (Fig. 4). Arteriovenous shunting and high flow are frequent, but do not permit differentiation from vascular malformations.
Fig. 4.
Hemangioma in a 20-year-old man with lingual bulging.
A. Postcontrast CT scan shows a well enhancing, clearly demarcated soft tissue mass with heterogeneous attenuation at the right lateral aspect of the tongue (arrows). Note the multiple enhancing linear or curvilinear structures suggesting hypervascularity (open arrows).
B. T1-weighted axial MR image shows a homogeneous iso-intense mass (black arrow) extending across the midline. The peripheral high signal intensity rim (white arrow) increases the conspicuity of the mass. The lingual septum is seen only posteriorly, still displaced to the left (open arrow).
C. T2-weighted axial image shows heterogeneously high signal intensity of the lesion.
D. Enhanced T1-weighted sagittal image shows strong enhancement of the lesion and increased conspicuity of its extent. This mass is truly located in the submucosa with intact overlying mucosa. Note the large serpentine vascular structures with signal void due to rapid flow at the anterior aspect of the mass (arrows).
E. Selective lingual arteriography performed for selective embolization of the lesion shows tumor staining arising mainly from hypertrophied sublingual (arrows) and deep lingual (open arrows) arteries. After embolization, the patient underwent total excision of the mass.
F. Photograph of a cut section of gross specimen shows a yellowish mass with multiple vascular channels (arrows).
G. Photomicrograph (original magnification ×100; H & E staining) demonstrates dilated vessels lined by flattened endothelium (short arrows), and a thick smooth muscle wall (long arrow). Note the presence of intervening skeletal muscles of the tongue (open arrow).
On CT, hemangioma usually appears as a well demarcated enhancing mass that often contains calcified phleboliths. MR imaging shows the lesion as a solid mass with iso- or slightly high signal intensity to muscle on T1-weighted images and heterogeneous signal intensity on T2-weighted images. Postcontrast T1-weighted imaging commonly demonstrates prominent enhancement (Fig. 4). Due to the presence of multiple low signal intensity vessels with rapidly flowing blood, some hemangiomas have a typical serpentine appearance (Fig. 4). The conspicuity of these signal void areas increases with tumor size.
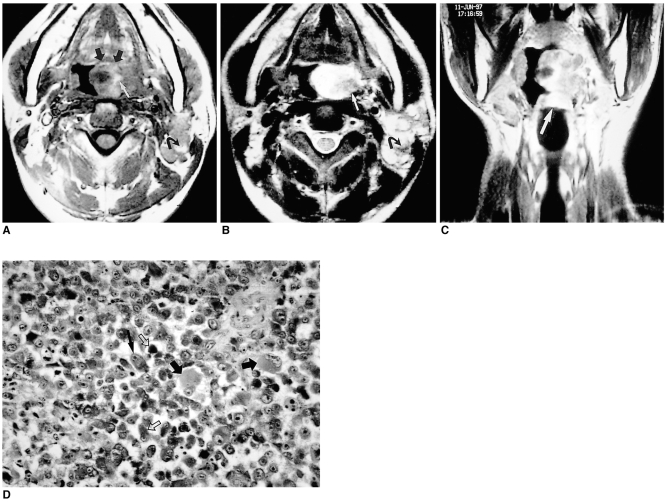
Adenoid cystic carcinoma
Adenoid cystic carcinoma is the most common malignant tumor of the minor salivary glands and accounts for 10~51% of all malignant lingual tumors. It is well known for its propensity to spread through perineural spaces. Dysphagia, a tongue mass and pain are the most common presenting symptoms. A history of pain, suggesting neural invasion by the tumor, is associated with poor prognosis. The tumor is classified histologically as one of three types: cribriform, tubular, or solid or basaloid.
At the time of diagnosis, both CT and MR imaging usually depict extensive submucosal spread. The signal intensity demonstrated by MRI and the pattern of contrast enhancement revealed by CT and MRI are nonspecific (Fig. 5), and differentiation from other types of tumor is therefore difficult. Nevertheless, low signal intensity on T2-weighted MR images appears to correspond to highly cellular tumors (solid type) with poor prognosis, whereas high signal intensity appears to correspond to less cellular tumors (cribriform or tubular type) with better prognosis (6) (Fig. 5).
Fig. 5.
Adenoid cystic carcinoma in a 63-year-old woman with growing lingual mass.
A. Enhanced T1-weighted sagittal image shows a well demarcated, markedly enhancing mass in the submucosal area of the anterior tongue (arrow), with heterogeneous attenuation suggesting necrosis (*).
B. Photograph of a cut section of gross specimen shows a well-demarcated yellowish mass (black arrows) with a central irregular necrotic area (*) in the anterior region of the tongue. Note the presence of intact overlying lingual mucosa (white arrows).
C. Photomicrograph (original magnification ×100; H & E staining) shows tumor cells composed of uniform, small, angulated cells (thin arrows). Sharply defined cylindrical cores of hyaline material (*) create a cribriform, pseudocystic appearance (thick arrows), characteristic of grade-1 adenoid cystic carcinoma. Due to perineural invasion, the patient underwent hemiglossectomy and adjuvant chemotheraphy.
Mucoepidermoid carcinoma
Mucoepidermoid carcinoma occurs most commonly in the parotid gland; one which arises from the tongue is unusual. This tumor can be classified histologically as low, intermediate or high grade, and grade correlates well with prognosis (7). Metastasis is primarily to subcutaneous tissues, lymph nodes, bone and the lung. Surgery is the treatment of choice, with wide local excision.
The radiologic features of lingual mucoepidermoid carcinoma are nonspecific (Fig. 6), while the CT findings vary with the grade of the tumor. Low-grade lesions are benign in appearance, with apparently well-delineated smooth margins, whereas high-grade lesions have indistinct infiltrating margins. In our case, however, the tumor had a poorly defined margin despite its low-grade histologic features (Fig. 6). Due to the high cellularity of this tumor, both T1- and T2-weighted MR images tend to demonstrate low to intermediate signal intensity (Fig. 6).
Fig. 6.
Mucoepidermoid carcinoma in a 48-year-old female with a painful tongue.
A. T1-weighted axial image shows a soft tissue mass with low signal intensity at the right lateral aspect of the tongue which extends across the midline (thick arrows). The lingual septum is seen only at the anterior half of the tongue (thin arrows). The right lateral margin of the mass is indistinct.
B. T2-weighted axial image shows a mass with increased signal intensity (thick arrows). Anterolateral extension of this mass is more easily recognized than on the T1-weighted image. The right lateral tissue plane and hyoglossus muscle are obliterated, though on the left side are visible (thin arrows). Note the prominent right palatine tonsil (curved arrow), suggesting tonsilar metastasis, and the intact right mylohyoid muscle (open arrows).
C. Enhanced T1-weighted axial image demonstrates heterogeneous, moderate enhancement, making differentiation of the mass from squamous cell carcinoma impossible.
D. Photomicrograph (original magnification ×100; H & E staining) demonstrates a mixture of the glandular component lined by well-differentiated cuboidal to columnar epithelium (thick arrows) containing abundant mucous material (*) and solid nests composed of squamous cells (thin arrows).
Epi-myo-epi carcinoma
Epi-myo-epi carcinoma is an uncommon low-grade tumor occurring mainly in the parotid gland. Tumors are bulky and bosselated, grow slowly, and develop mostly in elderly people. The appellation "epi-myo-epi" is derived from the fact that the tumors form distinctive epithelial tubules or ductules surrounded by neoplastic myoepithelial cells (8) (Fig. 7).
Fig. 7.
Epi-myo-epi carcinoma in a 70-year-old man with tongue pain.
A. Contrast-enhanced axial CT scan shows an irregularly marginated, homogeneously enhancing mass at the left lateral aspect of the tongue (thick arrow). Note the preserved midline fatty septum (thin arrows).
B. T1-weighted axial image shows a mass with low signal intensity (arrow) confined to the mobile part of the tongue.
C. Enhanced T1-weighted axial image shows homogenous dense enhancement of the mass(arrow).
D. Photomicrograph (original magnification ×100; H & E staining) demonstrates distinctive epithelial tubules or ductules (thin arrows) surrounded by neoplastic myoepithelial cells (thick arrows).
Lymphoma
Lymphoma is the third most common malignant lesion to occur in the oral region, though because the oral cavity contains only small amounts of lymphoid tissue, extranodal lymphoma in this area is less common (9). The favored intraoral sites of lymphoma are palatal mucosa and bone, and the most common symptoms are local swelling, pain and discomfort in the throat, or an ulcer. Prognosis depends on tumor stage, the aggressiveness of the malignant cell type, and response to treatment (9). Oral lesions seem to be quite sensitive to irradiation (9). Prognosis is worst in cases involving the tongue, and best where the parotid region or tonsils are affected.
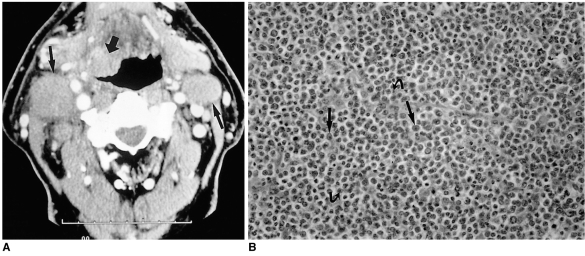
CT demonstrates that lymphoma is isodense to muscle. Although the tumor has no specific imaging characteristics or pathognomonic signs, if more than one extranodal mass is detected, if the mass is huge and there is neither necrosis nor ulceration, or if the mass is associated with large, nonnecrotic nodes, a diagnosis of lymphoma is suggested (Fig. 8). On MR images, lymphoma is isointense to normal muscle on T1-weighted images and hypointense on T2-weighted images. Enhancement is variable.
Fig. 8.
Lymphoma in a 78-year-old-man with palpable neck mass.
A. Contrast-enhanced CT scan obtained at the level of C3 demonstrates a moderately enhancing, poorly-defined mass involving the right-side base of the tongue and right vallecula (thick arrow). Multiple bilateral lymphadenopathies with homogeneous attenuation characteristic of lymphoma (thin arrows) are present.
B. Photomicrograph (original magnification ×200; H & E staining) shows diffuse infiltration of non-cohesive, monotonous neoplastic cells with vesicular nuclei (arrows) and one or two inner nucleoli (curved arrows). The patient underwent chemotheraphy combined with radiation therapy.
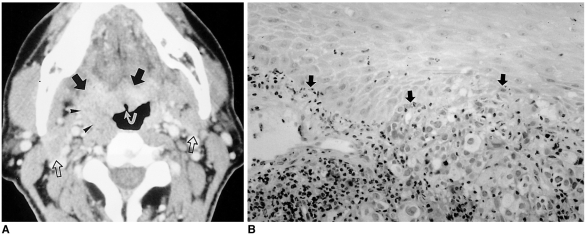
Metastasis
Primary tumors which metastasize to the oral cavity are extremely rare. The gingiva is the most common site, and the tongue the second most common. The two common primary sites from which metastasis to the tongue occurs are the lung and kidney. Possible routes include the systemic, venous, and lymphatic circulation (10). The systemic route is most common. Because of its rich vascular supply and relative immobility compared to other parts of the tongue, the base of this organ is most frequently involved (10). Metastasis to the tongue often occurs at a late stage when there is disseminated disease; prognosis is therefore very poor and only palliative treatment is possible.
In most cases, the radiologic features of lingual metastases are nonspecific: in our case, metastasis mimicked primary carcinoma (Fig. 9). Due to the paramagnetic properties of melanin, however, the typical MR imaging features of metastases from melanotic melanoma are high signal intensity on T1-weighted images and intermediate to low signal intensity on T2-weighted images (Fig. 10). In addition, strong enhancement is noted after the administration of contrast material.
Fig. 9.
Metastasis from carcinoma of the bronchus in a 48-year-old man.
A. Contrast-enhanced axial CT scan shows an ill-defined, well-enhancing mass at the base of the tongue and right vallecula, with exophytic growth to the oropharyngeal cavity (arrows). The superficial portion of the mass is ulcerated (curved arrow). The mass extends laterally, causing prominence of the right palatine tonsil (arrowheads). Multiple bilaterally enlarged lymph nodes can also be seen (open arrows).
B. Photomicrograph (original magnification ×200; H & E staining) demonstrates poorly differentiated adenocarcinoma (arrows) metastasized from bronchogenic carcinoma. Palliative radiotherapy was performed.
Fig. 10.
Metastasis from melanotic melanoma in a 40-year-old man with severe dyspnea and hoarseness.
A. T1-weighted axial image demonstrates a large mass at the left lateral base of the tongue and left lingual tonsil (thick arrows). The mass contains areas of low and high signal intensity (white arrow). In addition, multiple lymphadenopathies with high signal intensity foci (curved arrow) are seen in the left jugulodigastric chain.
B. T2-weighted axial image reveals that the hyperintense areas on the T1-weighted image have become slightly hypointense (arrow). Multiple enlarged lymph nodes show mixed and heterogeneous signal intensity (curved arrow). The signal intensity characteristics of this mass suggest melanotic melanoma of the base of the tongue, with lymph node metastasis.
C. Enhanced T1-weighted coronal image shows moderate enhancement of the tumor. Note extension of the tumor to the epiglottis (arrow).
D. Photomicrograph (original magnification ×200; H & E staining) demonstrates the typical appearance of melanotic melanoma: large, patternlessly distributed round cells with vesicular nuclei and prominent inner nucleoli (thin arrow). A large amount of finely granulated, brown to black melanin (open arrows) is seen in the eosinophilic cytoplasm (thick arrows) of the neoplastic cells. Multiple masses where melanotic melanoma was diagnosed were present on the patient's back.
References
- 1.Som PM, Scherl MP, Rao VM, Biller HF. Rare presentations of ordinary lipomas of the head and neck: a review. AJNR. 1986;7:657–664. [PMC free article] [PubMed] [Google Scholar]
- 2.Dreher A, Gutmann R, Grevers G. Extracranial schwannoma of the ENT region. Review of the literature with a case report of benign schwannoma of the base of the tongue. HNO. 1997;45:468–471. [Article in German] [PubMed] [Google Scholar]
- 3.Sciubba JJ, Brannon RB. Myoepithelioma of salivary glands: report of 23 cases. Cancer. 1982;49:562–572. doi: 10.1002/1097-0142(19820201)49:3<562::aid-cncr2820490328>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69:412–420. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Burrows PE, Mulliken JB, Fellows KE, Strand RD. Childhood hemangiomas and vascular malformations: angiographic differentiation. AJR. 1983;141:483–488. doi: 10.2214/ajr.141.3.483. [DOI] [PubMed] [Google Scholar]
- 6.Sigal R, Monnet O, De Baere T, et al. Adenoid cystic carcinoma of the head and neck: evaluation with MR imaging and clinical-pathologic correlation in 27 patients. Radiology. 1992;184:95–101. doi: 10.1148/radiology.184.1.1319079. [DOI] [PubMed] [Google Scholar]
- 7.Healey WV, Perzin KH, Smith L. Mucoepidermoid carcinoma of salivary gland origin: Classification, clinical-pathologic correlation, and results of treatment. Cancer. 1970;26:368–388. doi: 10.1002/1097-0142(197008)26:2<368::aid-cncr2820260219>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 8.Corio RL. Epithelial-myoepithelial carcinoma. In: Ellis G, Auclair P, Gnepp D, editors. Surgical pathology of the salivary glands. Philadelphia, Pa.: WB Saunders Co.; 1991. pp. 100–109. [Google Scholar]
- 9.Griffin TJ, Hurst PS, Swanson J. Non-Hodgkin's lymphoma: a case involving four third molar extraction sites. Oral Surg Oral Med Oral Pathol. 1988;65:671–674. doi: 10.1016/0030-4220(88)90006-0. [DOI] [PubMed] [Google Scholar]
- 10.Zegarelli DJ, Tsukada Y, Pickren JW, Greene GW., Jr Metastatic tumor to the tongue: Report of twelve cases. Oral Surg Oral Med Oral Pathol. 1973;35:202–211. doi: 10.1016/0030-4220(73)90286-7. [DOI] [PubMed] [Google Scholar]