Abstract
Objective
Bronchogenic Carcinoma Can Mimic Or Be Masked By Pulmonary Tuberculosis (Tb), And The Aim Of This Study Was To Describe The Radiologic Findings And Clinical Significance Of Bronchogenic Carcinoma And Pulmonary Tb Which Coexist In The Same Lobe.
Materials and Methods
The findings of 51 patients (48 males and three females, aged 48-79 years) in whom pulmonary TB and bronchogenic carcinoma coexisted in the same lobe were analyzed. The morphologic characteristics of a tumor, such as its diameter and margin, the presence of calcification or cavitation, and mediastinal lymphadenopathy, as seen at CT, were retrospectively assessed, and the clinical stage of the lung cancer was also determined. Using the serial chest radiographs available for 21 patients, the possible causes of delay in the diagnosis of lung cancer were analyzed.
Results
Lung cancers with coexisting pulmonary TB were located predominantly in the upper lobes (82.4%). The mean diameter of the mass was 5.3 cm, and most tumors (n=42, 82.4%) had a lobulated border. Calcification within the tumor was seen in 20 patients (39.2%), and cavitation in five (9.8%). Forty-two (82.4%) had mediastinal lymphadenopathy, and more than half the tumors (60.8%) were at an advanced stage [IIIB (n=11) or IV (n=20)]. The average delay in diagnosing lung cancer was 11.7 (range, 1-24) months, and the causes of this were failure to observe new nodules masked by coexisting stable TB lesions (n=8), misinterpretation of new lesions as aggravation of TB (n=5), misinterpretation of lung cancer as tuberculoma at initial radiography (n=4), masking of the nodule by an active TB lesion (n=3), and subtleness of the lesion (n=1).
Conclusion
Most cancers concurrent with TB are large, lobulated masses with mediastinal lymphadenopathy, indicating that the morphologic characteristics of lung cancer with coexisting pulmonary TB are similar to those of lung cancer without TB. The diagnosis of lung cancer is delayed mainly because of masking by a tuberculous lesion, and this suggests that in patients in whom a predominant or growing nodule is present and who show little improvement of symptoms despite antituberculous or other medical therapy, coexisting cancer should be suspected.
Keywords: Lung neoplasms; Lung neoplasms, CT; Tuberculosis, pulmonary
Several reports have shown that the incidence of lung cancer is greater in patients with pulmonary tuberculosis (TB) than in the general population (1-3). Although the incidence of bronchogenic carcinoma in patients with active pulmonary TB has been reported as 5-6.4% (1, 4), these data are outdated, and to our knowledge, no report published during the era of CT has focused on this topic. Because the signs, symptoms, and radiologic findings can be masked by preexisting disease, a diagnosis of bronchogenic carcinoma superimposed on pulmonary TB is difficult (5). In most cases, the diagnosis of tumors in such patients is delayed, probably until an advanced stage. It has been reported that in patients with TB, the average delay in the diagnosis of lung cancer is 6-9 months (6), though in this patient group, the early detection of cancer is clearly important. The aim of this study is to describe the radiologic findings of coexisting bronchogenic carcinoma and pulmonary TB in the same lobe. We also discuss the causes of delayed diagnosis of lung cancer in patients with pulmonary TB, as revealed by chest radiography, and the diagnostic clues which facilitate the detection of such lesions at CT.
MATERIALS AND METHODS
Between January 1993 and May 1999, 335 patients with coexisting bronchogenic carcinoma and pulmonary TB were selected on the basis of the ACR (American College of Radiology) code and the computerized disease coding system in use at our institution. Among the 335 cases, CT scans were available in 219. After review of the related medical records and radiologic reports, we enrolled patients who had both histologic proof of lung cancer and coexisting TB in the same lobe as the cancer.
There were 51 such patients (48 males and three females), and their age ranged from 48 to 79 (mean, 64.5) years. The presence and localization of pulmonary TB was determined on the basis of CT findings; three radiologists reached a consensus regarding the location and activity of tuberculous lesions. Lesions which were calcified granulomas or demonstrated fibrotic change were regarded as inactive, while those which were cavitary or consolidated, and showed typical bronchogenic spread, were considered active. Acid-fast bacilli in sputum were demonstrated in ten patients, while 35 had a history of antituberculous chemotherapy. The mean duration of TB prior to the diagnosis of lung cancer was 16.8 (range, 3-40) years.
Bronchogenic carcinomas were pathologically confirmed by percutaneous needle biopsy (n=21), bronchoscopic biopsy (n=16), sputum cytology (n=4), open lung biopsy (n=1), or biopsy of metastatic lesions (supraclavicular lymph node, n=6; pleura, n=1; adrenal gland, n=1; jejunum, n=1). Histological types were squamous cell carcinoma (n=32), adenocarcinoma (n=15), and small cell lung cancer (n=4). Forty-six patients (90.2%) were smokers, and the mean pack-year figure was 42.7 (more than 40 pack-year, n=23; between 40 and 20 pack-year, n=21; less than 20 pack-year, n=2).
All images were reviewed by three radiologists, decisions being reached by consensus. The location of the tumor, its diameter and margin, the presence of calcification and cavitation, and of mediastinal lymphadenopathy, were assessed retrospectively on CT scans and the stage of the cancer was also determined. If the short diameter of a lymph node was greater than 1 cm, this was taken to indicate metastatic lymphadenopathy.
Twenty-one of the 51 patients underwent serial chest radiography prior to the diagnosis of lung cancer, and the duration and possible causes of diagnostic delay were investigated. When chest radiography or CT in the 21 patients suggested lung cancer, the three radiologists retrospectively reviewed previous serial chest radiographs. If they determined by consensus that a lesion had been missed at earlier chest radiography, prior to the diagnosis of lung cancer, the duration of the delay was calculated. The causes of this were categorized as failure to observe new nodules masked by coexisting stable TB lesions, the misinterpretation of new lesions as aggravation of TB, the misinterpretation of lung cancer as tuberculoma at initial radiography, masking of the nodule by an active TB lesion, or subtleness of the lesion.
In patients who underwent curative surgery, the pathologic findings were reviewed to determine whether scar cancer was present. This was defined as a tumor which - according to the histologic and clinical evidence - arose from a previously documented tuberculous lesion. The following criteria were required for a diagnosis of scar cancer: macroscopic evidence of scarring, a central nidus of fibrous tissue suggestive of an old tuberculous granuloma, and anthracotic pigmentation (7).
RESULTS
The location of tuberculous lesions was as follows: both upper lobes (n=36), the right upper lobe (n=8), the left upper lobe (n=3), the right lower lobe (n=1), both upper lobes and the left lower lobe (n=1), the right lung (n=1), all lobes (n=1). Thus, the predominant location was the upper lobes (n=42, 82.4%), followed by the lower lobes (n=6, 11.7%), and the right middle lobe (n=3, 5.9%). The mean diameter was 5.3 (1.5-12) cm, and the tumor margin was lobulated (n=43, 84.3%) or spiculated (n=7, 13.7%). In one case of adenocarcinoma with bronchioloalveolar carcinoma, the form of the tumor was consolidative. Calcification within the tumor was seen in 20 patients (39.2%), and was located eccentrically in 15 cases and centrally in five. Cavitation within the tumor was observed in five patients (9.8%), and mediastinal lymphadenopathy in 42 (82.4%). The stage of non-small-cell lung cancer was I in ten patients, II in two, IIIA in four, IIIB in 11, and IV in 20. At the time of diagnosis, all small-cell lung cancers (n=4) were extended.
Serial chest radiography showed that in 21 patients, the average delay in diagnosing lung cancer was 11.7 (range, 1-24) months and the causes of this were failure to observe new nodules masked by coexisting stable TB lesions (n=8) (Fig.1), misinterpretation of new lesions as aggravation of TB (n=5) (Fig. 2), misinterpretation of lung cancer as tuberculoma at initial radiography (n=4) (Fig. 3), masking of the nodule by an active TB lesion (n=3) (Fig. 4), and subtleness of the lesion (n=1).
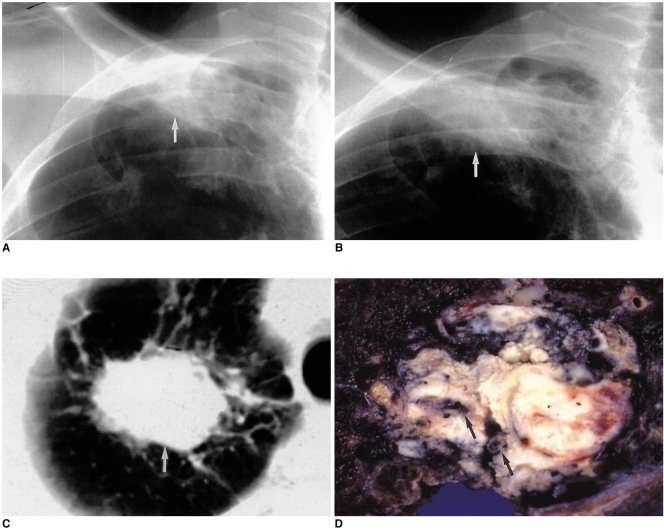
Fig. 1.
60-year-old male with a two-year history of pulmonary TB.
A. Chest radiograph shows ill-defined patchy opacity (arrow) at the right apex. Because acid-fast bacilli were present in sputum, anti-tuberculous medication was administered. Staining for acid-fast bacilli then proved negative.
B. Chest radiograph obtained two years after A demonstrates increased opacity (arrow), which was disregarded by both the radiologist and the patient's clinician.
C. Follow-up CT scan obtained 10 months after B shows a 3.5 cm-sized mass (arrow) at the right apex.
D. Photograph of a cut section of the resected specimen shows a hard yellowish mass which proved to be squamous cell carcinoma. Dark pigmentations (arrows) within the tumor were composed of tuberculous granulomas.
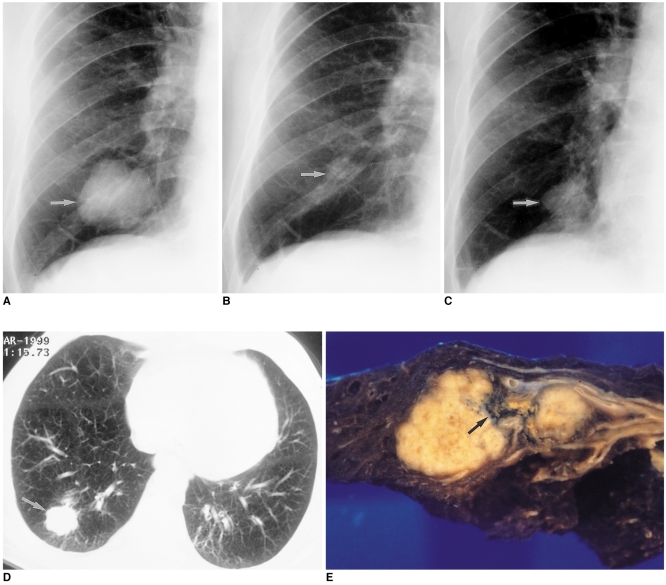
Fig. 2.
64-year-old male who presented with sputum.
A. Initial chest radiograph reveals the presence of a large lobulated mass (arrow), proven by percutaneous needle biopsy to be an active tuberculous lesion, in the right lower lung zone.
B. The patient received anti-tuberculous medication, and a follow-up plain radiograph obtained six months after the initial study showed that the lesion (arrow) was very much smaller.
C, D. Follow-up chest radiograph (C) and CT scan (D) obtained seven months after B show an enlarged mass (arrows) in spite of anti-tuberculous medication.
E. Photograph of a cut section of the resected specimen shows a dumbbell-shaped mass in the right lower lobe. Histopathologic examination showed that squamous cell carcinoma surrounded the scar tissue (arrow).
(From Lee KS, Im JG, Kang DS. Notes from the 1999 annual meeting of the Korean Society of Thoracic Radiology. J Thorac Imaging 2000; 15:30-35, with permission.)
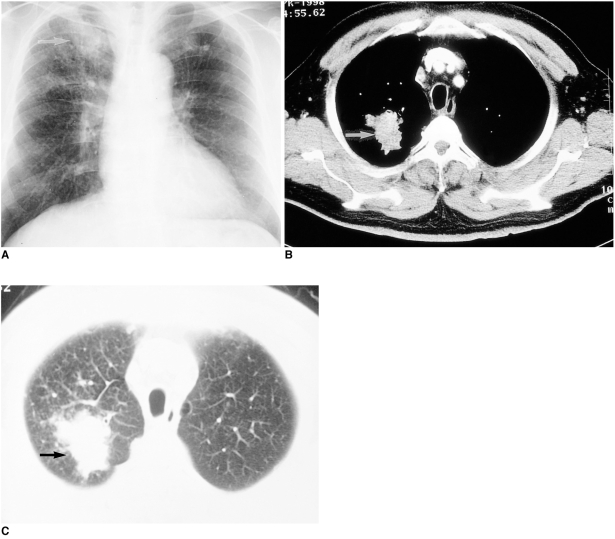
Fig. 3.
69-year-old male who presented with cough and dyspnea, and had been treated with antituberculous medication at the age of 39.
A. Chest radiograph shows reticulonodular opacities in both upper lung zones, suggestive of TB. A large lobulated mass (arrow) in the right upper lobe was regarded as tuberculous granuloma rather than lung cancer.
B, C. Antituberculous medication offered no improvement, however, and the CT scan obtained ten months after A reveals a 3.0-cm sized, irregularly marginated mass (arrows) at the right apex. Sputum cytology showed that an adenocarcinoma was present.
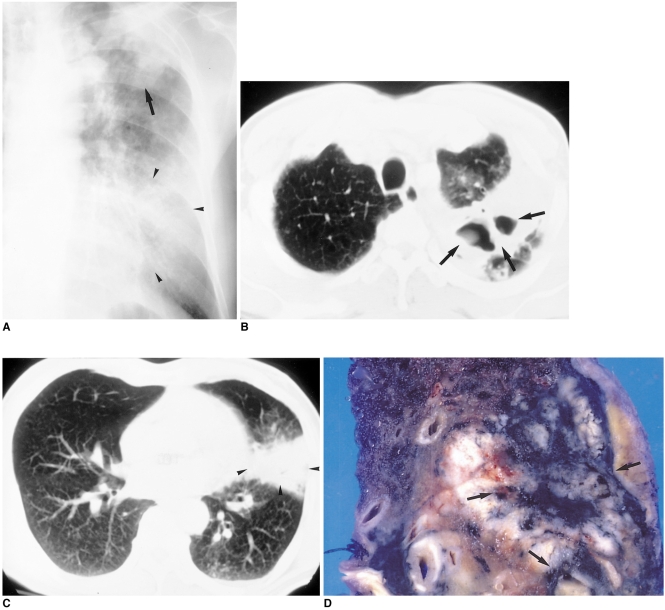
Fig. 4.
60-year-old male who presented with hoarseness.
A. Initial chest radiograph shows consolidation (arrow) in the left upper lung zone and ill-defined ground-glass opacity (arrowheads) in the left lower lung zone. Because acid-fast bacilli were present in sputum, the patient underwent anti-tuberculous chemotherapy.
B, C. CT scans obtained two months after A, due to persistent symptoms, show cavitary lesions (arrows) in the apicoposterior segment and segmental consolidation (arrowheads) in the lingular division of the left upper lobe.
D. Bronchoscopy demonstrated adenocarcinoma in the lingular division. In the pathologic specimen, a pinkish tumor, which proved to be tuberculous granuloma, engulfed the pigmented area (arrows).
Mainly because of poor pulmonary function and the advanced stage of the tumor, only ten patients (19.6%) underwent curative surgery. Of these, five (50%) had scar cancer, the histological types of which were adenocarcinoma in three cases, and squamous cell carcinoma in two (Figs. 1 and 2). Three patients had old tuberculous granulomas and lung cancers in the same lobe, but pathologic examination showed that the lesions were completely separated. In the remaining two patients, lung cancer and active TB were concurrent, and some portion of the cancer was in close contact with the TB lesions. However, because macroscopic evidence of scarring or anthracotic pigmentation was not detected pathologically, the criteria for scar cancer were not met.
DISCUSSION
The coexistence of pulmonary TB and bronchogenic carcinoma was first reported by Bayle in 1810 (8). The simultaneous development of unsuspected primary cancer in close vicinity to an active pulmonary tuberculous process can seriously complicate diagnosis, and in most reported cases, a long interval had elapsed before carcinoma was suspected (6). Patients with TB or post-tuberculous pulmonary lesions require more intense attention than those with oncological diseases alone, and the diagnosis of TB in patients with bronchogenic carcinoma requires pathological confirmation based on the findings of biopsy or microbiologic studies. When lung cancer has developed insidiously in a known case of pulmonary TB, the diagnosis of dual disease is more difficult than when a diagnosed case of bronchogenic carcinoma is complicated by the presence in sputum of acid-fast bacilli. The time required for the diagnosis of cancer with inactive TB is somewhat shorter when the disease processes are located in different areas. When cancer is associated with active TB, however, and the two conditions coexist in the same lobe, the time required for the diagnosis of cancer is often considerable. Outstanding progress in imaging techniques has, though, led to increasing accuracy in the diagnosis of lung cancer. In addition, patients in whom active TB is suspected require more attention, and are more likely to be evaluated with CT or HRCT than those with inactive TB.
The relationship between pulmonary TB and bronchogenic carcinoma has been viewed in the following ways: (A) As one of cause and effect (scar cancer). Many researchers believe that scar tissue plays an important causative role in the development of lung cancer (9, 10); (B) As the reactivation of TB by carcinoma. It has also been reported that the development of lung cancer in areas of inactive TB stimulates the reactivation of tubercle bacilli (5, 11). In addition, the association between bronchogenic carcinoma and pulmonary TB may be related to increased susceptibility to opportunistic infections, which can lead to the reactivation of TB in cancer patients (12); (C) As coincidental. Because both pulmonary TB and bronchogenic carcinoma are common in Korean communities, they sometimes co-occur by chance. In our study, lung cancer (proven to be scar cancer) was present in the area of a tuberculotic scar in five of the ten patients who underwent curative surgery. The central focus of lamellated hyaline fibrous tissue in our cases was entirely consistent with old TB. The role of scarring in the pathogenesis of lung cancer is, however, controversial. It was originally postulated that a proportion of such tumors arise at the edge of pre-existing scars, and that parenchymal scarring can stimulate atypical epithelial cell proliferation and metaplasia involving the terminal air-space (13). Others, though, are of the opinion that the scar represents a desmoplastic reaction and is the result, rather than the cause, of tumor growth (14).
Previous studies have suggested that the earliest sign of the coeistence of bronchogenic carcinoma and TB is an atypical course of the latter, as seen on chest radiographs. (15). They insisted that the sudden appearance of new lesions, segmental or lobar atelectasis, unilateral hilar enlargement, thick-walled cavities, and a localized pneumonic process are all suggestive of carcinoma. Ting et al. (4) proposed several plain radiographic features which, if present, increase the suspicion of coexisting lung carcinoma in patients with pulmonary TB. Specifically, these were (A) the progression of pulmonary infiltrate while the patient is on anti-tuberculous drugs; (B) infiltration or atelectasis in the basilar segments of the lower lobes or the anterior segments of the upper lobes; (C) homogeneous infiltration with no air bronchogram rather than a mottled appearance with linear streaking; (D) asymmetrical pleural density at the apex or costophrenic angle while the patient is receiving anti-tuberculous medication; (E) unilateral hilar prominency; (F) a single pulmonary nodule with a diameter greater than 3 cm, and an irregular nodule wall and contour; (G) the impression that a mass is present in a displaced lobar fissure.
In some cases, a CT scan does not clearly distinguish lung cancer from a tuberculoma. In our study, however, CT revealed that masses with the morphologic features previously documented in lung cancer cases were present in most patients. Analysis of the findings of 21 patients who underwent serial chest radiography suggested that common causes of the delayed diagnosis of lung cancer were failure to observe new nodules masked by coexisting stable TB lesions (38.1%), and misinterpretation of new lesions as aggravation of TB (23.8%). CT scanning can reduce image overlap, thus permitting - to an extent which is greater than with chest radiographs - the recognition of tumors masked by tuberculous lesions. We therefore recommend that in patients in whom chest radiographs and CT scans suggest the possible presence of a new tumorous lesion, biopsy is performed. Low-dose CT has recently become popular for screening for lung cancer, and we believe that it may also be used for follow-up study in pulmonary TB patients.
In conclusion, we suggest that lung cancer is one of the most important complications easily missed in patients with active or inactive TB, which commonly delays the diagnosis of lung cancer due to masking. In TB patients with a predominant or growing nodule, coexisting cancer should be suspected regardless of their activities, and early diagnosis of lung cancer by careful follow-up is essential in the care of patients whose symptoms show little improvement despite antituberculous or other medical therapy. When chest radiographs appear to indicate the concurrence of lung cancer and TB, it is strongly recommended that CT should be performed, and followed by biopsy for pathologic confirmation.
References
- 1.Neeussle WF. Association of bronchogenic carcinoma and active pulmonary TB: Report of 4 cases. Dis Chest. 1953;23:207–216. doi: 10.1378/chest.23.2.207. [DOI] [PubMed] [Google Scholar]
- 2.Steinitz R. Pulmonary TB and carcinoma of the lung: A survey from two population-based disease registers. Am Rev Respir Dis. 1965;92:758–766. doi: 10.1164/arrd.1965.92.5.758. [DOI] [PubMed] [Google Scholar]
- 3.Tunell WP, Koh Y-C, Adkins PC. The dilemma of coincident active pulmonary TB and carcinoma of the lung. J Thorac Cardiovasc Surg. 1971;62:563–567. [PubMed] [Google Scholar]
- 4.Ting YM, Chirch WM, Ravikrishnan KP. Lung carcinoma superimposed on pulmonary TB. Radiology. 1976;119:307–312. doi: 10.1148/119.2.307. [DOI] [PubMed] [Google Scholar]
- 5.Fontenelle LJ, Campbell D. Coexisting bronchogenic carcinoma and pulmonary TB. Ann Thorac Surg. 1970;9:431–435. doi: 10.1016/s0003-4975(10)65535-x. [DOI] [PubMed] [Google Scholar]
- 6.Mok CK, Nandi P, Ong GB. Coexistent bronchogenic carcinoma and active pulmonary TB. J Thorac Cardiovasc Surg. 1978;76:469–472. [PubMed] [Google Scholar]
- 7.Limas C, Japaze H, Gracia-Bunuel R. Scar carcinoma of the lung. Chest. 1971;59:219–222. doi: 10.1378/chest.59.2.219. [DOI] [PubMed] [Google Scholar]
- 8.Bayle CH. Recherches sur la phitisue pulmonaire. Paris, France: Galon; 1810. [Google Scholar]
- 9.Raeburn G, Spencer H. Lung scar cancer. Br J Tuberc Dis Chest. 1957;51:237–245. doi: 10.1016/s0366-0869(57)80080-x. [DOI] [PubMed] [Google Scholar]
- 10.Ripstein CB, Spain DM, Bluth I. Scar cancer of the lung. J Thorac Cardiovasc Surg. 1968;56:362–370. [PubMed] [Google Scholar]
- 11.Gopalakrishnan P, Miller JR, Mclaughlin JS. Pulmonary TB and coexisting carcinoma. Am Surg. 1975;41:405–408. [PubMed] [Google Scholar]
- 12.Ben M'Rad S, Azzabi S, Baccar MA, Aouina H, Bouacha H, Nacef T. Broncho-pulmonary cancer associated with pulmonary TB: Report of 4 cases. Rev Pneumol Clin. 1998 Feb;54:23–25. [PubMed] [Google Scholar]
- 13.Edwards C, Carlile A. Scar adenocarcinoma of the lung: a light and electron microscopic study. J Clin Pathol. 1986;39:423–427. doi: 10.1136/jcp.39.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimosato Y, Hashimoto T, Kodama T, et al. Prognostic implications of a fibrotic focus (scar) in small peripheral lung cancers. Am J Surg Pathol. 1980;4:625–631. doi: 10.1097/00000478-198008000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Berroya Renato B., Polk John W., Raju Radma, Bailey Alan H. Concurrent pulmonary TB and primary carcinoma. Thorax. 1971;26:384–387. doi: 10.1136/thx.26.4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]