Abstract
Central diabetes insipidus (DI) can be the outcome of a number of diseases that affect the hypothalamic-neurohypophyseal axis. The causes of the condition can be classified as traumatic, inflammatory, or neoplastic. Traumatic causes include postoperative sella or transection of the pituitary stalk, while infectious or inflammatory causes include meningitis, lymphocytic hypophysitis, and granulomatous inflammations such as sarcoidosis and Wegener's granulomatosis. Various neoplastic conditions such as germinoma, Langerhans cell histiocytosis, metastasis, leukemic infiltration, lymphoma, teratoma, pituitary adenoma, craniopharyngioma, Rathke cleft cyst, hypothalamic glioma, and meningioma are also causes of central DI. In affected patients, careful analysis of these MR imaging features and correlation with the clinical manifestations can allow a more specific diagnosis, which is essential for treatment.
Keywords: Diabetes insipidus, Magnetic resonance (MR)
Diabetes insipidus (DI) is a clinical syndrome characterized by the excretion of copious volumes of dilute urine combined with the persistent intake of abnormally large quantities of fluid. There are two general forms of DI: central (vasopressin-deficient) and nephrogenic (vasopressin-resistant). DI of central origin most often results from lesions in the hypothalamic-neurohypophyseal axis. The differential diagnosis of central DI should include the pathologic processes (e.g. traumatic, infectious or inflammatory, and neoplastic) that involve the structures normally found in the pituitary gland or contiguous structures. However, idiopathic central DI is diagnosed when central DI occurs in the absence of any alteration that is known to be responsible for DI (1).
MR imaging provides high-resolution images of the pituitary gland, pituitary stalk, and adjacent structures. The modality's multiplanar capability allows the diagnosis of various diseases that cause central DI, and thus helps guide its treatment.
In this article, we review the pathophysiology of central DI and the MR features of neurohypophysis, and demonstrate the MR imaging findings of various pathologic conditions that cause central DI.
The Pathophysiology of Diabetes Inspidus and the MR Features of Neurohypophysis
The neurohypophysis is divided into three parts: the posterior lobe, the pituitary stalk (infundibulum), and the median eminence. These structures derive from the downward outgrowth of diencephalic neuroectoderm and come into contact with the anterior lobe, which develops upward from stomodeal ectoderm. Antidiuretic hormone (ADH) and oxytocin are synthesized in the supraoptic and periventricular nucleiof the hypothalamus, are transported down along the pituitary stalk, and released by exocytosis into the posterior lobe. The functional state of the neural lobe is thus highly dependent upon the integrity of the pituitary stalk and the hypothalamus. Probably the most common causative defect in central DI is failure of ADH release in response to normal physiologic stimuli, caused by lesions involving the hypothalamic-neurohypophyseal axis.
In most healthy individuals, a normal posterior lobe appears hyperintense on T1-weighted MR images, due to phospholipid or secretory granules contained in pituicytes (Fig. 1) (2). In longstanding DI, this high signal intensity of the posterior lobe is absent as a result of failure to synthesize, transport or store neurosecretory granules. After the administration of contrast material, the pituitary gland and pituitary stalk enhance homogeneously.
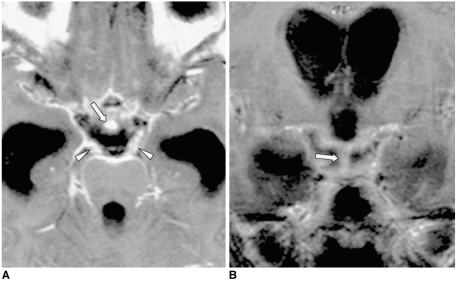
Fig. 1.
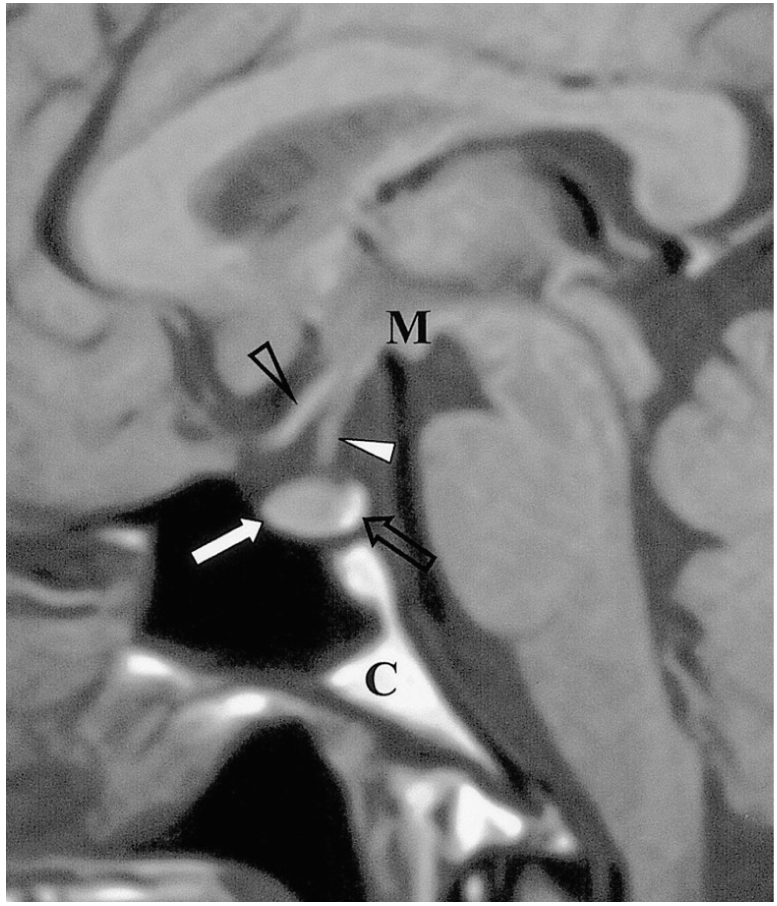
Sagittal T1-weighted MR image shows the normal anatomy of the sella turcica and juxtasellar region. The anterior lobe (thick arrow), posterior lobe (open arrow), pituitary stalk (thick arrowhead), optic chiasm (open arrowhead), mamillary body (M), and clivus (C) are indicated.
Traumatic Lesions
Transection of the Infundibular Stalk
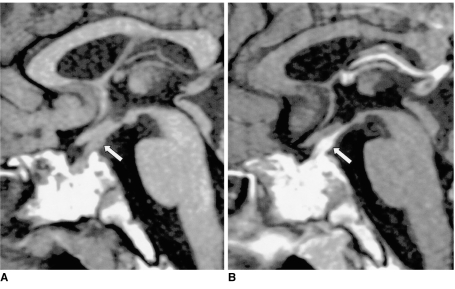
Post-traumatic central DI can be caused by any type of head trauma, in particular by motor vehicle accidents. Clinically, severe head trauma may cause anterior pituitary insufficiency and/or DI, and when this occurs, it is frequently associated with skull fractures and other neurological abnormalities. While the immediate onset of DI is believed to result from direct injury to the posterior lobe, its delayed onset is caused by transection of the stalk, since ADH stored in the posterior lobe is able to maintain its function over a period of time (3). In most cases, DI develops a few days after stalk transection and is commonly transient (Fig. 2). In addition to the absence of the high signal intensity normally seen in the posterior lobe, an ectopic bright spot in the proximal stump of the transected, retracted proximal stalk or hypothalamus can occasionally be identified.
Fig. 2.
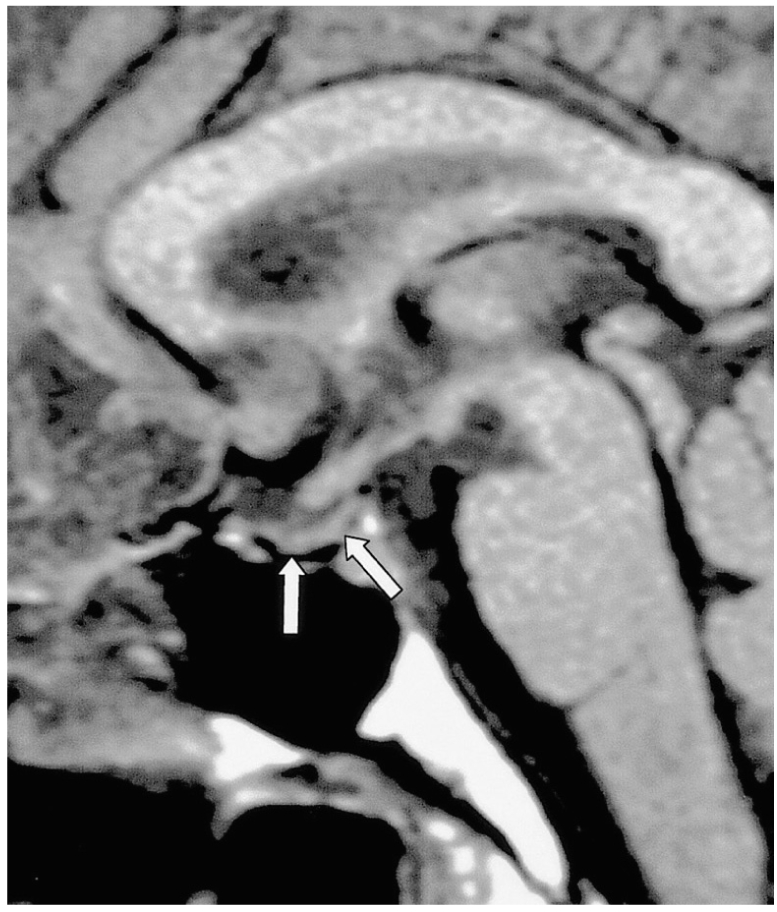
Transection of the pituitary stalk caused by a fall in a 17-year-old male. The signs and symptoms of DI developed two months after trauma. Sagittal T1-weighted MR image (600/15) reveals diffuse atrophy of the pituitary gland (arrows). The high signal intensity normally seen in the posterior lobe is absent, and the normal pituitary stalk is not seen. Two foci of high signal intensity anterior and posterior to the pituitary fossa represent the fat marrow of the sella turcica.
Postoperative Sella
An association between transient DI and postoperative sella is not uncommon. The reported incidence of this is up to 60%, and it appears to represent selective damage to the posterior lobe without concurrent damage to the hypothalamic neurosecretory nuclei (4). Permanent DI after transsphenoidal hypophysectomy has been reported to occur in 0.4-3% of cases (5), and is usually caused by pituitary stalk injury. Fortunately, permanent DI is not a particularly disabling deficit because hormone replacement is a satisfactory treatment. MR imaging shows marked structural distortion and an area of high signal intensity in the pituitary fossa, with an associated mass effect on T1-weighted images within two weeks of surgery. This most likely represents methemoglobin accumulation due to hemorrhage in the surgical bed, a fairly typical feature of early postoperative sella (4).
Infectious or Inflammatory Lesions
Tuberculous Meningitis
Tuberculosis is one of the common infective causes of central DI. The infiltrative nature of tuberculous meningitis can result in damage to the hypothalamic-neurohypophyseal axis leading to central DI (4). MR imaging reveals a uniformly thickened pituitary stalk, as seen in other granulomatous diseases, and diffuse meningeal enhancement, especially in the basal cistern (Fig. 3).
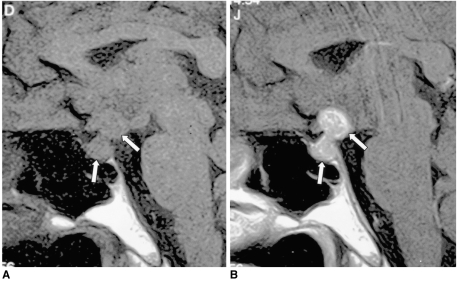
Fig. 3.
Tuberculous meningitis in a 10-year-old boy with multiple cranial nerve palsy.
A, B. Contrast-enhanced axial (A) and coronal (B) T1-weighted MR images (600/20) depict enhancement of the uniformly thickened pituitary stalk (arrows) and diffuse leptomeningeal enhancement in the basal cistern. Strong enhancement of the bilateral thickened third nerves (arrowheads in A) and severe communicating hydrocephalus are noted. Tuberculous meningitis was confirmed by cerebrospinal fluid analysis.
Epidemic Hemorrhagic Fever
Korean hemorrhagic fever, more commonly known in the Western world as epidemic hemorrhagic fever, is an acute disease caused by viruses belonging to the genus Hantavirus (6). It is characterized clinically by fever, circulatory collapse, hemorrhage, and renal failure. In fatal cases, necrosis of the anterior lobe often develops, and there may be occasional hemorrhage at this location. The eventual result is atrophic change in the pituitary gland. Depending on the location and stage of the hemorrhage and the severity of the disease, the signal intensity of the hemorrhagic area varies (6). DI is a very uncommon symptom in patients with epidemic hemorrhagic fever, though it can result when atrophic change extends into the pituitary stalk (Fig. 4).
Fig. 4.
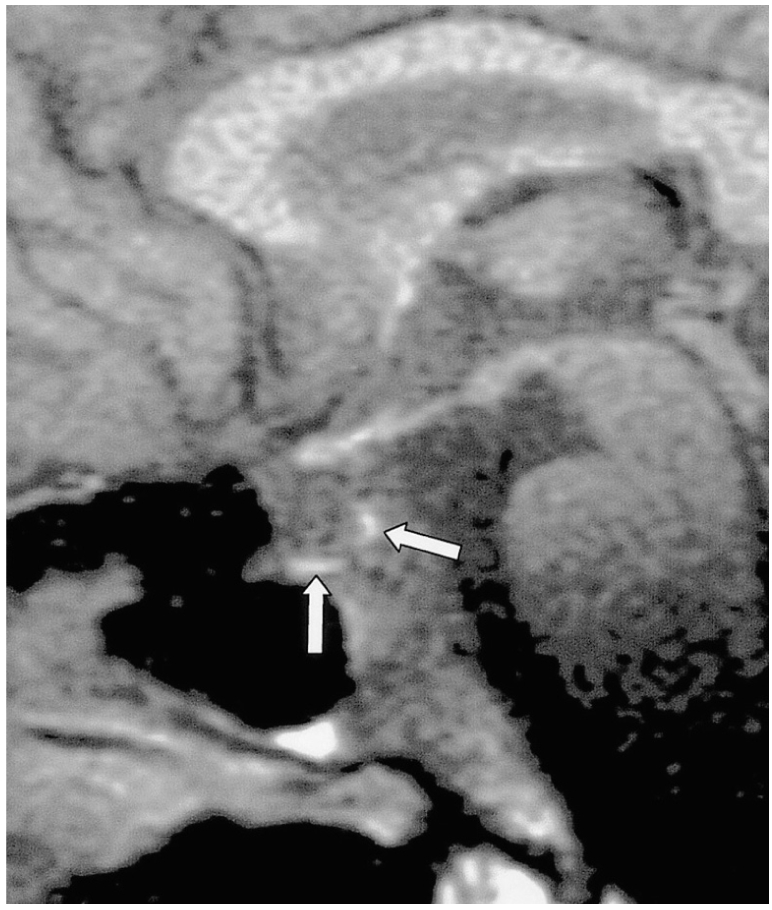
Epidemic hemorrhagic fever in a 52-year-old man. Sagittal fat-suppressed T1-weighted MR image (600/20) shows diffuse atrophy of the pituitary gland. The focal high signal intensities observed (arrows) suggest hemorrhage in the pituitary gland, which may occur in the course of this disease.
Lymphocytic Hypophysitis
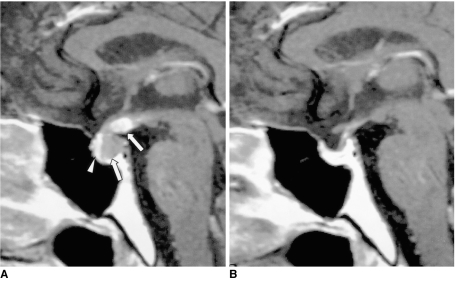
In the last few years, a broad spectrum of presentation of the condition known as lymphocytic hypophysitis has been established. The entity is not confined to the anterior lobe but can involve the posterior lobe and pituitary stalk (7). Furthermore, both men and women may be affected, and the condition is thus not necessarily related to pregnancy. According to the anatomical site and severity of the inflammatory process, lymphocytic hypophysitis may be subclassified as lymphocytic adenohypophysitis, lymphocytic infundibuloneurohypophysitis, or necrotizing infundibulohypophysitis (8). The first and second of these are distinctly different entities, and are probably caused by different autoimmune processes (9). It has been reported that in lymphocytic infundibuloneurohypophysitis, inflammation is localized in the nerurohypophyseal system and forms a mass lesion in the posterior lobe and/or pituitary stalk, whereas MR imaging and histologic studies have shown that the anterior lobe is spared (9). However, simultaneous involvement of the anterior adenophysis has been reported (10), and in this situation, the term 'lymphocytic infundibulohypophysitis' is more appropriate than 'lymphocytic infundibuloneurohypophysitis' (Fig. 5) (10).
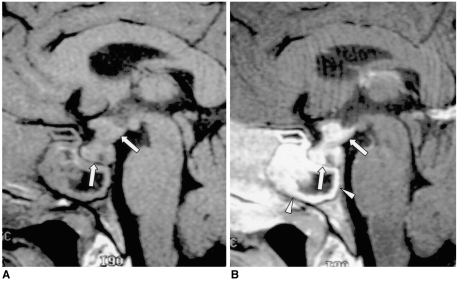
Fig. 5.
Lymphocytic infundibulohypophysitis in a 43-year-old man.
A. Sagittal T1-weighted MR image (500/25) depicts an isointense mass comprising the hypothalamus, the pituitary stalk, and the pituitary gland (arrows). The posterior lobe no longer shows high signal intensity.
B. Contrast-enhanced sagittal T1-weighted MR image (500/25) shows strong enhancement of the mass (arrows). Lymphocytic infundibulohypophysitis was pathologically proven.
Granulomatous Inflammation
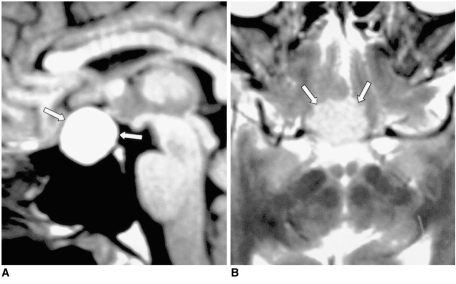
Granulomatous diseases such as sarcoidosis, Wegener's granulomatosis and Churg-Strauss syndrome can involve the hypothalamic-neurohypophyseal axis, and result in central DI. MR imaging reveals a uniformly thickened pituitary stalk, with occasional involvement of the adjacent hypothalamus or pituitary gland. The development of neurosarcoidosis is primarily leptomeningeal and vascular in nature and most commonly involves the meninges, cranial nerves, hypothalamus, infundibular stalk and pituitary gland. Wegener's granulomatosis is a disease characterized by necrotizing vasculitis and granulomatous inflammation of the upper and lower respiratory tracts, together with glomerulonephritis. DI is a very rare complication, the presumed mechanism of which is thought to be either hypothalamic vasculitis or direct granulomatous involvement, or both. Churg-Strauss syndrome, known as allergic granulomatosis and angiitis, is characterized by systemic vasculitis, extravascular granulomas, and eosinophilia, which occur in patients with bronchial asthma and allergy (11). MR imaging reveals diffuse swelling of the pituitary stalk, adjacent hypothalamus, and pituitary gland, which enhances strongly after the injection of contrast material (Fig. 6), a finding similar to that of other granulomatous diseases.
Fig. 6.
Churg-Strauss syndrome in a 38-year-old man.
A. Sagittal T1-weighted MR image (600/15) reveals an isointense mass involving the hypothalamus, pituitary stalk and pituitary gland (arrows). The posterior lobe no longer shows high signal intensity. Prominent mucosal thickening of the sphenoid sinus is apparent.
B. Contrast-enhanced sagittal T1-weighted MR image (600/15) demonstrates strong enhancement of the mass (arrows), and there is a non-enhancing portion. The thickened mucosa of the sphenoid sinus is intensely enhanced, representing sphenoid sinusitis (arrowheads). Churg-Strauss syndrome was pathologically proven.
Neoplastic Lesions
Germinoma
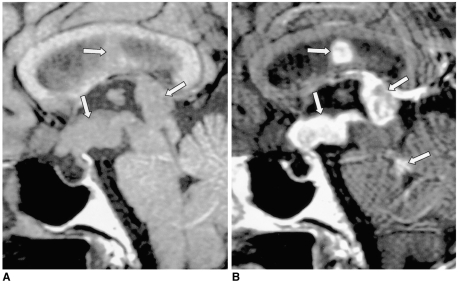
In Asian countries, germinoma is the most common intracranial tumor accompanied by central DI, especially in children. Since the hypothalamic-neurohypophyseal axis is one of the most common sites from which a germinoma originates, DI occurs frequently. Because a hypothalamic-pituitary area lesion is too subtle to be detected during the early stages of suprasellar germinoma, idiopathic central DI is sometimes diagnosed. In such cases, but where a germinoma is still suspected, follow-up MR imaging is therefore necessary, especially in teenage boys. In typical cases, MR imaging clearly demonstrates a thickened pituitary stalk with or without a lobulated pineal mass (Fig. 7). In patients with germinoma, the response to chemotherapy and radiotherapy is usually dramatic.
Fig. 7.
Germinoma in a 29-year-old man.
A. Initial contrast-enhanced sagittal T1-weighted MR image (600/15) depicts mild thickening and homogeneous enhancement of the pituitary stalk. In addition, small nodular enhancing lesions are seen in the pineal gland outlet of the fourth ventricle (arrows).
B. Contrast-enhanced sagittal T1-weighted MR image (480/14) obtained one year later, without treatment, shows a marked increase in the size and inhomogeneous enhancement of those lesions, and also of the pituitary gland (arrows). The focal enhancing lesion seen within the fourth ventricle appears to be cerebrospinal seeding. Germinoma was pathologically proven.
Langerhans Cell Histiocytosis
Although isolated Langerhans cell histiocytosis of the central nervous system is rare, central nervous system involvement as part of the systemic process is common. This usually takes the form of neuroendocrine disturbances involving the hypothalamic-neurohypophyseal axis. The absence of normal high signal intensity of the posterior lobe, associated with a thickened pituitary stalk, although non-specific for Langerhans cell histiocytosis, should, because of frequent systemic involvement, prompt further studies such as chest radiography, bone scanning, and temporal bone CT (Fig. 8) (12).
Fig. 8.
Langerhans cell histiocytosis in a 15-year-old boy.
A. Sagittal T1-weighted MR image (600/15) demonstrates a uniformly thickened pituitary stalk (arrow). The posterior lobe no longer shows high signal intensity.
B. Contrast-enhanced sagittal T1-weighted MR image (600/15) shows strong enhancement of the lesion (arrow). The high signal intensity in the sphenoid sinus presumably represents fat marrow associated with poor pneumatization. Langerhans cell histiocytosis was pathologically proven.
Metastasis
Metastases to the pituitary-hypothalamic axis account for 1% of sellar masses. Breast cancer metastases are most common, followed by those from gastrointestinal carcinoma (4). About 20% of these metastases to the pituitary-hypothalamic axis are diagnosed clinically, with DI as the main presenting symptom (13). DI of a transient nature may represent metastases to the posterior lobe of the pituitary, without significant encroachment on the supraoptic and periventricular nuclei of the hypothalamus. At MR, a destructive and inhomogeneously enhancing intrasellar and suprasellar lesion, with involvement of the adjacent structure, can be observed (Fig. 9).
Fig. 9.
Metastasis from lung cancer in a 41-year-old man.
A. Contrast-enhanced sagittal T1-weighted MR image (600/16) depicts the markedly enhancing thickened median eminence and pituitary stalk, and less enhancing posterior lobe (arrows). The posterior lobe no longer shows high signal intensity, and the normally enhanced anterior lobe (arrowhead) is compressed and displaced anteriorly by the enlarged posterior lobe.
B. Contrast-enhanced sagittal T1-weighted MR image (600/23) obtained two months after radiotherapy shows near disappearance of the lesion. The clinical symptoms of DI were also much improved.
Leukemia
DI is a rare complication of acute leukemia, despite the fact that perihypophyseal leukemic infiltrates are found at autopsy in 46% of such patients. In describing the pathogenesis of DI in patients with leukemia, two main histologic findings have been mentioned: diffuse leukemic infiltrates of the neurohypophysis, and thrombosis of the small vessels in the hypothalamic nuclei and neurohypophysis (14). MR may reveal thickening of the pituitary stalk, with or without adjacent hypothalamic enlargement (14).
Lymphoma
Primary lymphoma of the central nervous system is seen either in immunocompetent patients, or in those with human immunodeficiency virus (HIV) infection or in other immunocompromised states. It can cause DI by directly destroying hypothalamic ADH-producing neurons or seeding the third ventricle with lymphomatous cells (15). At MR, an enhancing mass can be seen in the pituitary stalk, hypothalamus, thalamus, basal ganglia, or periventricular white matter (Fig. 10). Lymphomatous spread to the cerebrospinal fluid is, however, rare.
Fig. 10.
Primary intraventricular lymphoma (non-Hodgkin's lymphoma) in a 77-year-old woman.
A. Sagittal T1-weighted MR image (600/15) depicts multiple masses in the hypothalamus, floor and posterior wall of the third ventricle and septum pellucidum (arrows). The posterior lobe no longer shows high signal intensity.
B. Contrast-enhanced sagittal T1-weighted MR image (600/15) shows strong enhancement of the above mentioned lesions and of the superior portion of the fourth ventricle (arrows), indicating lymphomatous involvement. Lymphoma was pathologically proven.
Teratoma
Intracranial teratomas are defined as benign tumors which contain well-differentiated elements derived from the three germinal layers and have a predilection for the midline, that is, the pineal region, the third ventricle, and the infundibulochiasmal region. On rare occasions, intracranial suprasellar teratomas extend into the pituitary fossa, with enlargement of the sella turcica, thereby resulting in visual disturbance, DI, and hypopituitarism. At T1- and T2-weighted MR imaging, high signal intensities representing fat components can be seen in sellar and suprasellar areas.
Other Tumors
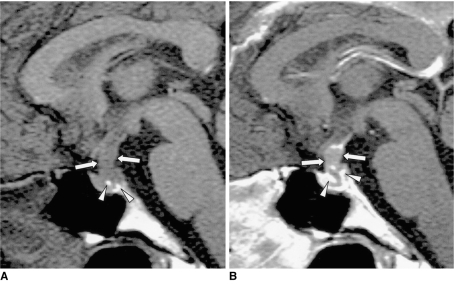
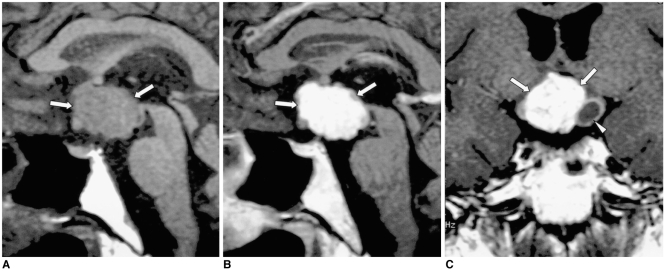
Pituitary adenoma and craniopharyngioma are less common causes of central DI. The former usually shows homogeneous signal intensity, with occasional hemorrhage. Rarely, pituitary adenoma can surround and compress the pituitary stalk, resulting in DI (Fig. 11). Several explanations have been proposed as to how ectopic pituitary tissue arises. Some have suggested that because the anterior lobe originates embryonically from Rathke's pouch, pituitary cells may be deposited along the route of embryologic development of the pituitary gland. Such remnants may become foci of a pituitary adenoma. A craniopharyngioma appears as a rather inhomogeneous mass commonly associated with cyst formation (Fig. 12). A Rathke cleft cyst has non-enhancing, well-defined walls, and the observed MR signal intensity, which depends on the intracystic protein concentration, varies. Most commonly, a Rathke cleft cyst shows a high signal intensity on T1-weighted images and iso- to low signal intensity on T2-weighted images, relative to gray matter, reflecting a high protein concentration in the cystic fluid (Fig. 13). Large hypothalamic/chiasmatic gliomas and meningiomas also can cause DI.
Fig. 11.
Pituitary adenoma in a 42-year-old man.
A. Sagittal T1-weighted MR image (600/15) shows marked thickening of the pituitary stalk (arrows). The posterior lobe, displaced by the lesion, is seen as several foci of high signal intensity (arrowheads).
B. Contrast-enhanced sagittal T1-weighted MR image (600/15) demonstrates homogeneous mild enhancement of the thickened pituitary stalk, which extends into the pituitary fossa and displaces the normal pituitary gland to the left side. Due to strong enhancement, the displaced and compressed pituitary gland is seen as multiple foci of high signal intensity (arrows). Although the mass was found to completely encircle the pituitary stalk, at surgery the two were separated easily. Pituitary adenoma was pathologically proven.
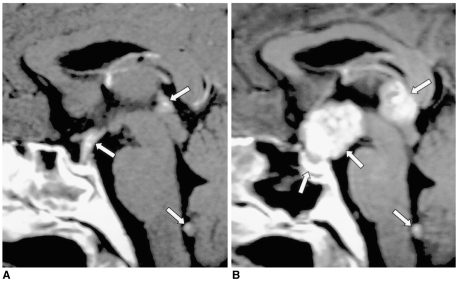
Fig. 12.
Craniopharyngioma in a 34-year-old man.
A. Sagittal T1-weighted MR image (600/15) shows a large lobulated mass (arrows) involving the suprasellar area and hypothalamus. The posterior lobe no longer shows high signal intensity.
B, C. Contrast-enhanced sagittal (B) and coronal (C) T1-weighted MR images (600/15) show strong enhancement of the solid component of the tumor (arrows), which also has a smaller cystic component (arrowhead in C). Craniopharyngioma was pathologically proven.
Fig. 13.
Rathke cleft cyst in a 59-year-old woman.
A. Sagittal T1-weighted MR image (600/15) depicts a well-defined, homogeneous high signal intensity mass (arrows) in the sella turcica and extending into the suprasellar cistern.
B. Axial T2-weighted (3500/99) MR image indicates that the mass is isointense relative to gray matter (arrows), reflecting the high protein concentration of the cystic fluid. A Rathke cleft cyst was pathologically proven.
SUMMARY
The multiplanar capability of MR imaging plays an important role in the assessment of the hypothalamic-pituitary area and in determining the underlying cause of central DI. Although a wide spectrum of disease processes may cause central DI, MR imaging, particularly when coupled with clinical information, can help provide a more specific diagnosis.
Acknowledgements
We are very grateful to Bonnie Hami, M.A., of the Department of Radiology, University Hospitals of Cleveland (U.S.A.), for her editorial assistance.
Footnotes
This paper received a Certificate of Merit award at the 1999 RSNA Scientific Assembly.
References
- 1.Ozata M, Tayfun C, Kurtaran K, et al. Magnetic resonance imaging of posterior pituitary for evaluation of the neurohypophyseal function in idiopathic and autosomal dominant neurohypophyseal diabetes insipidus. Eur Radiol. 1997;7:1098–1102. doi: 10.1007/s003300050261. [DOI] [PubMed] [Google Scholar]
- 2.Kucharczyk W, Lenkinski RE, Kucharczyk J, Henkelman RM. The effect of phospholipid vesicles on the NMR relaxation of water: an explanation for the MR appearance of the neurohypophysis? AJNR. 1990;11:693–700. [PMC free article] [PubMed] [Google Scholar]
- 3.Ma L, Gao Y, Cai Y, Li T, Liang Y. MR evaluation of the brain in central diabetes insipidus. Chin Med J. 1996;109:724–729. [PubMed] [Google Scholar]
- 4.Tien R, Kucharczyk J, Kucharczyk W. MR imaging of the brain in patients with diabetes insipidus. AJNR. 1991;12:533–542. [PMC free article] [PubMed] [Google Scholar]
- 5.Black PM, Zervas NT, Candia GL. Incidence and management of complications of transsphenoidal operation for pituitary adenomas. Neurosurgery. 1987;20:920–924. doi: 10.1227/00006123-198706000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Suh DC, Park JS, Park SK, Lee HK, Chang KH. Pituitary hemorrhage as a complication of hantaviral disease. AJNR. 1995;16:175–178. [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed SR, Aiello DP, Page R, Hopper K, Towfighi J, Santen RJ. Necrotizing infundibulo-hypophysitis: a unique syndrome of diabetes insipidus and hypopituitarism. J Clin Endocrinol Metab. 1993;76:1499–1504. doi: 10.1210/jcem.76.6.8501157. [DOI] [PubMed] [Google Scholar]
- 8.Maghnie M. Lymphocytic hypophysitis and central diabetes insipidus during adolescence: what are the criteria for diagnosis? Eur J Pediatr. 1998;157:693–694. doi: 10.1007/pl00008292. [DOI] [PubMed] [Google Scholar]
- 9.Imura H, Nakao K, Shimatsu A, et al. Lymphocytic infundibuloneurohypophysitis as a cause of central diabetes insipidus. N Engl J Med. 1993;329:683–689. doi: 10.1056/NEJM199309023291002. [DOI] [PubMed] [Google Scholar]
- 10.Maghnie M, Genovese E, Sommaruga MG, et al. Evolution of childhood central diabetes insipidus into panhypopituitarism with a large hypothalamic mass: is lymphocytic 'infundibuloneurohypophysitis' in children a different entity? Eur J Endocrinol. 1998;139:635–640. doi: 10.1530/eje.0.1390635. [DOI] [PubMed] [Google Scholar]
- 11.Vomacka Z, Ehrmann J, Skvarilova M, Krc I. Granulomatous vasculitis of Churg-Strauss type in a patient with diabetes insipidus. Acta Univ Palacki Olomuc Fac Med. 1993;135:17–19. [PubMed] [Google Scholar]
- 12.Tien RD, Newton TH, McDermott MW, Dillon WP, Kucharczyk J. Thickened pituitary stalk on MR images in patients with diabetes insipidus and Langerhans cell histiocytosis. AJNR. 1990;11:703–708. [PMC free article] [PubMed] [Google Scholar]
- 13.Szuwart U, Konig HJ, Bennefeld H, Weritz C, Kleinhans G. Clinical aspects of hypophyseal metastases. Onkologie. 1988;11:66–69. doi: 10.1159/000216489. [DOI] [PubMed] [Google Scholar]
- 14.Ra'anani P, Shpilberg O, Berezin M, Ben-Bassat I. Acute leukemia relapse presenting as central diabetes insipidus. Cancer. 1994;73:2312–2316. doi: 10.1002/1097-0142(19940501)73:9<2312::aid-cncr2820730912>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 15.Balmaceda CM, Fetell MR, Selman JE, Seplowitz AJ. Diabetes insipidus as first manifestation of primary central nervous system lymphoma. Neurology. 1994;44:358–359. doi: 10.1212/wnl.44.2.358. [DOI] [PubMed] [Google Scholar]