Abstract
Objective
To compare the efficacy of suprapapillary and transpapillary methods of transhepatic biliary metallic stent placement in malignant biliary strictures and to specify the indications of each method applied.
Materials and Methods
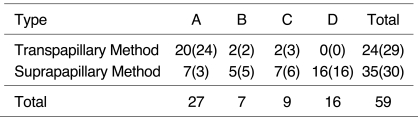
Stents were placed in 59 patients. Strictures were categorized as type A (within 3 cm of the ampulla, n = 27), type B (over 3 cm from ampulla, n = 7), type C (within 3 cm of the bending portion, n = 9), or type D (over 3 cm above the bending portion, n=16). The stenting method was suprapapillary in 34 cases and transpapillary in 25. The rates of initial and long-term patency and of early recurrence were compared.
Results
Initial patency rates for the suprapapillary and transpapillary methods were 1/7 (14.3%) and 20/20 (100%) respectively for type A (p < 0.0001), 4/5 (80.0%) and 2/2 for type B, 3/7 (42.9%) and 2/2 for type C, and 15/16 (93.8%) and 0/0 for type D. Early recurrence rates were 7/30 (23.3%) using the suprapapillary method and 4/29 (13.8%) using the transpapillary method (p = 0.51). The long-term patency rate did not differ significantly according to either type (p = 0.37) or method (p = 0.62).
Conclusion
For good initial patency, the transpapillary method is recommended for strictures of the distal extrahepatic duct near the ampulla and just above the bending portion. Long-term patency is not influenced by the stenting method employed.
Keywords: Bile ducts, stenosis or obstruction; Bile ducts, interventional procedure; Bile ducts, stents and prostheses
Metallic stents have been successfully applied for the palliation of malignant biliary strictures due to various causes (1-7), and from a technical viewpoint, the placement technique in extrahepatic strictures is relatively straightforward. There is a consensus among interventional radiologists that to prevent tumor overgrowth, a safety margin of at least 2 cm should be left at each end of the upper and lower margins of the stricture (over-stenting) (4-6, 8). A high proportion of patients with extrahepatic duct (EHD) strictures can be palliated without crossing the ampulla of Vater (suprapapillary method).
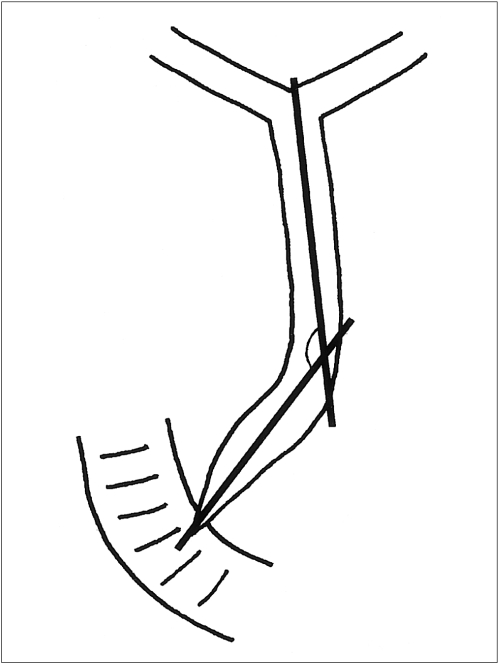
Not infrequently, however, this basic rule regarding the level of strictures and anatomic characteristics of the EHD must be modified, and the stent crossing is thus placed at the ampulla of Vater (transpapillary method) (2). In addition, we have often been faced with a cholangiogram showing various degrees of angulation formed by the distal and proximal portion of the EHD, especially at its distal one third portion, and this can be exaggerated after stent placement (Fig. 1) (9, 10). When the suprapapillary method is used for stent placement, this kind of anatomic feature may play a negative role in stent patency (10).
Fig. 1.
Anatomically, the extrahepatic duct is not straight for its entire course. Varying degrees of angulation between the proximal and distal axes of the extrahepatic duct can be noted.
In spite of concerns over the disadvantages of the transpapillary method, only a few case reports have lent weight to such assertations (11-15). The purpose of this study is to compare the efficacy of the suprapapillary and transpapillary methods of percutaneous transhepatic biliary metallic stent placement in the palliation of malignant EHD strictures and to specify the indications of each method. We focused on the cholangiographic features of EHD strictures: location of the stricture within the bile duct and the relationship between the strictures and angulation of the EHD.
MATERIALS AND METHODS
Patients
We retrospectively reviewed the cholangiographic findings and hospital records of 67 patients who between January 1997 and May 1999 had undergone metallic biliary stent placement for the palliation of malignant extrahepatic strictures including Klatskin tumors and peripheral cholangiocarcinoma invading the confluence of the intrahepatic ducts. Patients with recurrence of biliary obstruction after bilioenteric anastomosis (n = 4) or inadequate follow-up for the evaluation of stent patency (n = 4) were excluded, and a total of 59 patients were thus enrolled in this study. Thirty-six were male and 23 were female, and their mean age was 63.6 (range, 25-82) years. The causes of extrahepatic strictures were as follows: common duct cancer (n = 5), Klatskin tumor (n = 5), peripheral cholangiocarcinoma invading the hepatic confluence (n = 5), gall bladder cancer (n = 5), cancer of the pancreas (n = 16), and metastasis (n = 13) (12 gastric cancer and one adenocarcinoma of unknown origin). Malignancy was confirmed by surgery in 18 cases, by percutaneous needle biopsy in 19, by bile cytology with and without brushing in eight, and on the basis of the imaging findings of cholangiography and CT in 14. In all 59 patients, surgical resection was deemed impossible.
Lesion characteristics
When reviewing the cholangiograms obtained during percutaneous transhepatic biliary drainage (PTBD) and before stent placement, we focused on the angulation formed by the distal and proximal portion of the EHD (Fig. 1) and the location of strictures within it (Fig. 2). All cases except one showed a varying degree of angulation (80-170°) between the proximal and distal portions of the EHD; in the exceptional case, the course of the EHD from the biliary confluence to the ampulla of Vater was straight. The area of angulation within the EHD was termed 'bending portion'.
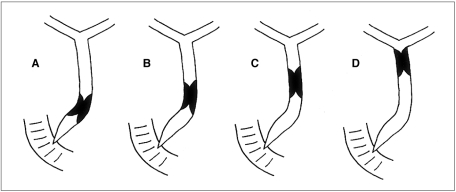
Fig. 2.
Cholangiographic categorization of extrahepatic duct strictures according to location of distal margin of stricture within the bile duct and relationship with the angulation of the extrahepatic duct. (A) a stricture located within 3 cm of the ampulla (type A); (B) a stricture located more than 3 cm above the ampulla and at or below the bending portion (Type B); (C) a stricture located above the bending portion but within 3 cm of the bending portion (type C); (D) a stricture located over 3 cm from the bending portion (type D)
To facilitate analysis, the classical method of describing EHD strictures (16) was abandoned. According to the location of the distal margin of the stricture relative to the ampulla of Vater and the bending portion of the EHD, lesions were categorized as one of four types. Type A represented a lesion whose distal margin was located within 3 cm of the ampulla of Vater, regardless of the bending portion (Fig. 2A); a type-B lesion was one whose distal margin was located more than 3 cm above the ampulla of Vater and at or below the bending portion (Fig. 2B); type C represented a lesion whose distal margin was located not more than 3 cm above the bending portion (Fig. 2C), while a lesion whose distal margin was located over 3 cm from the bending portion was categorized as Type D (Fig. 2D).
The number of type A, B, C and D cases was 27, 7, 9, and 16, respectively (Table 1). A case in which a straight extrahepatic duct without angulation was noted was categorized as type A because the stricture was located within 3 cm of the ampulla.
Table 1.
Placement Technique Used for Each Type
The number in parentheses denotes the number of cases after secondary intervention.
Procedure
Fifty-seven patients had undergone PTBD before stent placement, and the remaining two underwent placement as a single step. The mean time lag between PTBD and stent placement was 9.5 (range, 0-25) days. Informed consent was obtained from all patients.
Using self-expandable stainless stents (Hanaro stent; MI Tech, Seoul, Korea) in 48 patients and Nitinol stents (StenTech, Seoul, Korea) in 11, placement was effected with and without balloon dilatation of the stricture. The stainless stent was made of 0.25-mm stainless steel wire bent in a zigzag pattern, with alternating different leg lengths forming a spiral cylindric configuration, and for the Nitinol stent, 0.18 or 0.20-mm nitinol wire bent in a mesh was used.
The diameter of the stent was 8 or 10 mm. The length of stent was determined by the length of the stricture. In order to leave sufficient safety margin at each end of the stent within the bile duct, we generally selected a stent at least 4 cm longer than the stricture. If a stent was placed across the ampulla of Vater (transpapillary placement), a longer one, which would cover the distal duct, was chosen. Mean stent length was 6.2 (range, 3-8) cm for the suprapapillary method, and 7.3 (range, 5-9) cm for the transpapillary. The routes of stent insertion were right transhepatic in 25 patients, left transhepatic in 24, and bilateral trans-hepatic in ten.
To simplify analysis, methods were classified only from the viewpoint of location of the distal end of the stent: whether or not it crossed the ampulla of Vater. The term 'suprapapillary method' was used to denote a stent placement technique which did not cross the ampulla of Vater regardless of the location of the distal end of the stent within the EHD. The term 'transpapillary method' denoted a placement technique that crossed the ampulla, the distal end of the stent thus being located in the duodenal lumen. In this way the function of the ampulla of Vater was sacrificed. The stent was placed using the suprapapillary method in 35 cases and the transpapillary method in 24 (Table 1).
To permit bile drainage until the following morning, an 8.5-Fr drainage catheter (Simp-Loc; Cook, Bloomington, Ind.) was positioned after stent placement, and the tube was then clamped for one day. When no problem was evident after clamping the drainage tube and the cholangiogram indicated good passage of contrast media into the duodenum, the drainage tube was removed. When the remaining waist of the stent hindered the passage of contrast media, as occurred in a limited number of cases, a balloon Catheter (Olbert; Boston Scientific, Watertown, Mass, U.S.A.) with a diameter of 8 or 10 mm was used to relieve the waist. If it was found that a previously inserted stent inadequately decompressed the biliary system, a further stent was placed.
Many type-A lesions were managed using the transpapillary method, whereas for all type-D lesions, the suprapapillary method was used (Table 1). In the 35 cases in which the superapapillary method was employed initially, another stent was used by way of secondary intervention in five lesions because the initial stent failed to maintain patency. Finally they were classified into the suprapapillary method. There were four such type-A lesions and one type C. Their numbers are shown in parentheses in the table.
Analysis
Percutaneous cholangiography was used to assess initial stent patency immediately after placement. If passage of contrast media through the stent was poor, the procedure was regarded as inadequate. Using the Fisher exact test, initial patency rates for both the suprapapillary and transpapillary method were compared for each of the four types. The causes of inadequate procedures were analyzed using the cholangiograms obtained before, during, and after stent placement.
Regardless of the cause of reobstruction, recurrence of symptoms within two months of initial stent placement was defined as 'early recurrence'. Using Fisher's exact test, early recurrence rates were also compared for each of the four types.
Long-term patency rates were also calculated. The duration of stent patency was defined as the interval between initial placement and recurrence of symptoms because of obstruction. Patient survival and duration of stent patency were calculated with regard to type of lesion and the method of stent placement. Using the Kaplan-Meier method, differences in long-term patency were compared.
RESULTS
Initial patency
All 24 cases involving the transpapillary method and 23 of 35 (65.7%) in which the suprapapillary method was used showed good initial patency. The difference in initial patency rate between the two methods was statistically significant (p < .001).
In type A, only one (14.3%) involving the seven cases of the suprapapillary method showed good patency, whereas all 20 in which the transpapillary method was employed showed good passage through the stent (Fig. 3). The difference was statistically significant (p <.0001). The causes of failure of initial patency were unpredicted stricture of the distal extrahepatic duct near the ampulla of Vater in three cases (Fig. 4), aggravation of distal angulation after stenting in two, and misplacement of the stent resulting in inadequate coverage of the stricture at the distal portion of the stent in one.
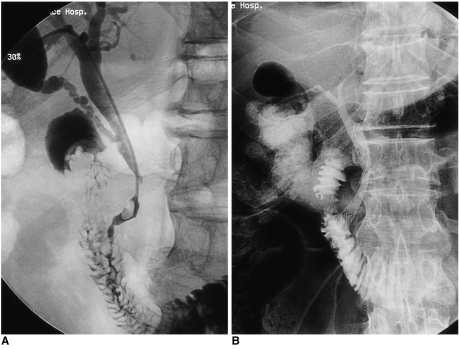
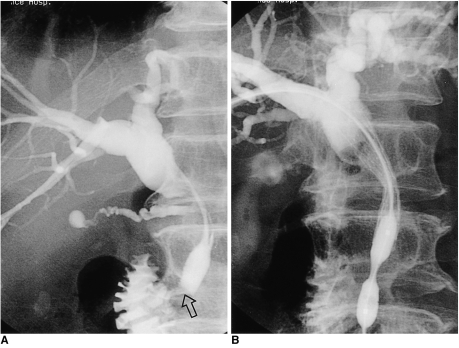
Fig. 3.
A 65-year-old man with pancreatic head cancer.
A. Because it was located within 3 cm of the ampulla of Vater, the stricture was categorized as type A.
B. Using the transpapillary method of stent placement, the stricture was successfully palliated.
Fig. 4.
A 52-year-old man with pancreatic head cancer.
A. Because it was located within 3 cm of the ampulla of Vater, the stricture was categorized as type A. The passage of contrast medium injected via a catheter below the main stricture was good, and we therefore initially neglected a focal narrowing (open arrow) near the ampulla of Vater.
B. The passage of contrast medium was poor after the suprapapillary method of stent placement was applied. The stricture was dilated using an angioplasty balloon catheter.
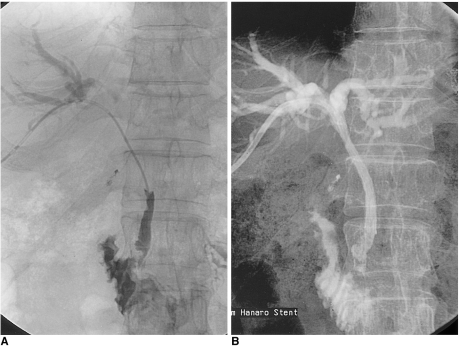
With regard to type-B strictures, four of the five cases involving the suprapapillary method (80.0%) showed good patency (Fig. 5); the cause of failure of initial patency in one case was aggravation of angulation after stent placement. In two cases the transpapillary method was used, and both were also patent.
Fig. 5.
A 62-year-old man presented with obstructed jaundice due to metastatic lymphadenopathy arising from advanced gastric cancer.
A. Because it was located over 3 cm from the ampulla of Vater at the level of the bending portion of the extrahepatic duct, the stricture was categorized as type B.
B. By means of the suprapapillary placement method, the stricture was successfully palliated.
For type C, three of the seven cases employing the suprapapillary method showed good patency. In the other four, the cause of failure of initial patency was aggravation of the preexisting angulation of the duct due to longitudinal rigidity of the distal portion of the stent (Fig. 6). Two cases involved the transpapillary method, and both were also patent.
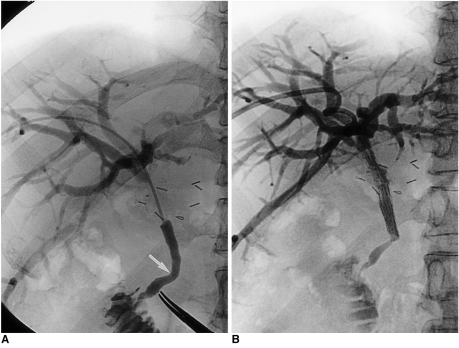
Fig. 6.
A 56-year-old man with common hepatic duct cancer.
A. Because it was located not more than 3 cm above the bending portion of the extrahepatic duct, the stricture was categorized as type C. Note the bending portion below the stricture (arrow).
B. The stricture was palliated by means of the suprapapillary placement method. A cholangiogram obtained the following day demonstrated, however, that the angulation below the stricture was aggravated by the stretching effect of the stent. Note the presence of a significantly dilated intrahepatic duct, indicating poor passage of contrast medium through the extrahepatic duct.
For type D, stricture management relied in all cases on the suprapapillary method, and 15 of 16 (93.8%) showed good patency. The cause of failure of initial patency in one case was aggravation of angulation, which can be prevented if the stent length chosen is appropriate. Because of the small sample size, statistical significance for types B, C, and D could not be calculated.
Secondary intervention
Of the six type-A cases which showed poor patency after initial stent placement, four underwent additional placement across the ampulla of Vater, and in two balloon dilatation of the unpredicted stricture of the distal EHD near the ampulla of Vater was performed (Fig. 4B). In one type-B case of poor patency due to aggravation of angulation at the distal portion of the stent, further intervention was not attempted. Among the four type-C cases with poor patency, one underwent transpapillary placement of an additional stent immediately after initial placement, but in the other three there was no further intervention. In one type-D case of poor patency, no further intervention took place.
After reintervention there were changes in the numbers of cases in which the suprapapillary/transpapillary method was employed. The new totals are shown in parentheses (see Table 1).
Early recurrence
Among the 30 cases in which the suprapapillary method was used, there were seven in which early recurrence occurred, including five of poor patency at initial stent placement but without additional placement. There were just two cases of early recurrence in the remaining patients, all of whom showed good patency of the biliary tree after stent placement. In four of 29 cases involving the transpapillary method (13.8%), early recurrence was noted, though the difference was statistically insignificant (p = .51).
Long-term patency
The overall median patency interval was 317 days. The six-month and one-year patency rates were 58.9% and 39.4%, respectively, while the median patency intervals for type A, B, and C were 201, 317, and 50 days, respectively. We were unable to calculate the median patency interval for type D. All differences were statistically insignificant (p = .39). The median patency intervals for cases in which the suprapapillary and transpapillary methods were used were 317 and 253 days, respectively, with no significant statistical difference (p = .57).
DISCUSSION
For the effective palliation of malignant EHD strictures, it is essential to consider the anatomic characteristics of the EHD. These include the length of the bile duct and the location of cystic duct insertion, as well as the size and anatomic level of the obstructing tumor itself within the EHD (4, 8). For the prevention of tumor overgrowth it is very important to cover the bile duct for as great a disttance as possible (over-stenting), leaving safety margins below and above the tumorous stricture (3, 8). When stenting the strictures located adjacent to the ampulla of Vater, however, it is sometimes impossible to leave adequate distal safety margins. In our series, when the stent was placed by means of the suprapapillary method in such lesions (type A), we were often faced with poor patency just after stent placement; this was due to unexpected narrowing distal to the stent or angulation of the bile duct below the stent. Though we cannot adequately explain the presence of an unexpected stricture, it may have been caused by edematous change or spasm of the ampulla of Vater associated with the procedure, by extrinsic compression of metastatic lymphadenopathy, or by infiltration of the tumor. We believe that in such cases the transpapillary method should be used.
In addition, we emphasize the importance of natural bending (angulation) of the EHD for successful palliation of the obstruction. The course of the extrahepatic duct from the confluence of the hepatic duct to the ampulla of Vater is, in most cases, not straight. Anatomically, the supraduodenal and retroduodenal segments of the extrahepatic duct slant obliquely from the right to the left, and below them the pancreatic segment curves rather sharply to the right to enter the descending duodenum via its posteromedial surface (18). The overall course of the extrahepatic duct results in convexity or angulation to the left, the degree of angulation varying according to the patient. By reviewing the cholangiographic findings of 270 patients, Liu et al. (9) measured the degree of angulation, the mean value of which was 140-150°. A tumor such as pancreatic cancer around the duct sometimes aggravates the degree of angulation (9).
We often encountered cases showing aggravation by the distal portion of the stent itself of preexisting angulation of the EHD after stent placement. Sometimes the angulation, which hindered effective bile flow, progressed on follow-up due to the longitudinal stretching force (straightening) of the metallic stent. In such cases another stent was required for distal angulation, and the function of ampulla of Vater had to be sacrificed.
Lameris et al. (4) reported an interesting case of stent malfunction caused by exaggerated angulation at the proximal portion of a stent placed for the palliation of distal EHD stricture. Straightening of the short stent caused acute angulation 'above' the stricture. They pointed out certain disadvantages of angulation, such as ineffective drainage and increased likelihood of damage to the bile duct epithelium by sharp wires (4). Stoker and Lameris (10) encountered two cases of proximal occlusion caused by straightening of the stent, a phenomenon also observed by Huibergtse (19), using a Wallstent. They found that the straightened proximal tip of a stent embedded in the bile duct wall hindered bile flow, and this facilitated sludge formation. Stoker and Lameris (10) suggested that in selected cases, a longer stent or one with a smaller diameter and less expansive force should be used. Self-expanding metallic stents will eventually straighten with time, to a certain extent, although the longitudinal flexibility of a Wallstent is slightly less than that of the stents we used (20, 21). This implies that if the proximal or distal ends of the stent are located near the bending portion of the bile duct when the rule of simple over-stenting is applied, it is important to overcome the problem of angulation. It is therefore wise to choose a stent which is long enough to successfully traverse the bending portion of the bile duct (4). When this is located below the stricture, the transpapillary method should be applied.
We recommend the transpapillary method for the palliation of type-A or C lesions in which the stricture is close to the ampulla of Vater or the bile duct is angulated below the stricture. For type-B or D lesions in which the stricture is far from the ampulla of Vater or bile duct angulation occurs apart from the stricture so that the stent can be placed without aggravation of preexisting angulation, a conventional suprapapillary method can easily be applied. If the suggested indication is applied, the number of cases amenable to the transpapillary method increases very considerably. Rieber et al. (22) placed transpapillary stents in 22 of their 65 patients, even though the method was applied in restricted cases. When they were sure that the papilla was not involved, suprapapillary placement was attempted. In our series, where our indication was applied, the transpapillary method was indicated in 38 of 59 patients (64%).
Although some authors have suggested that duodenal ulceration and subsequent bleeding or perforation of the duodenum, as well as both reflux of duodenal content into the bile duct (once believed to be a cause of early stent dysfunction) and pancreatitis are complications of the transpapillary method of stent placement, the cases they encountered were limited (12-15). In spite of concerns over these complications, most reports describing the long-term results of metallic stent insertion have demonstrated that their incidence is very low (2, 12). Interestingly, most reports of bleeding involved cases in which the prototype Wallstent was used (15). The sharp struts of both ends of the stent might cause mucosal injury. Nowadays, Wallstents and other commercially available stents have relatively smooth edges and this problem does not, therefore, exist. Gordon et al. (2) reported that they did not encounter such complications, though Stoker and Lameris (10) described two cases of duodenal pressure ulcer among 135 patients who had undergone transpapillary Wallstent placement. Though we were unable to analyze the complications arising from the methods employed in this retrospective study, the transpapillary method gave rise to no serious complication. There were, however, relatively frequent incidences of epigastric discomfort after placement, and these might be caused by inadvertent dilatation of the ampulla of Vater by the stent. Within two or three days, however, they subsided spontaneously.
Other than a brief comment by Rieber et al. (22), no radiologic report has compared the efficiency with which the suprapapillary and transpapillary methods of stent placement palliate malignant biliary obstruction. Rieber et al. (22) found that the occlusion rate of a stent was not influenced by its position. A further report on the same subject (23) dealt with endoscopically-placed plastic stents, concluding that there was no significant difference in patency and complication rates. We observed similar results: there were no significant differences in early recurrence and long-term patency rates between the two methods.
In conclusion, a careful review of cholangiograms with regard to stricture location and EHD angulation, as well as proper application of stent placement methods, are mandatory procedures if malignant biliary strictures are to be successfully palliated. For strictures involving distal EHD and associated with angulation of the bile duct below the stricture, the transpapillary method of stent placement should be considered.
References
- 1.Wilson MW. The application of metallic stents in malignant biliary obstruction. Tech in vasc Intervent Radiol. 1999;2:39–52. [Google Scholar]
- 2.Gordon RL, Ring EJ, LaBerge JM, Doherty MM. Malignant biliary obstruction: treatment with expandable metallic stents-follow up of 50 consecutive patients. Radiology. 1992;182:697–701. doi: 10.1148/radiology.182.3.1371362. [DOI] [PubMed] [Google Scholar]
- 3.Lee BH, Choe DH, Lee JH, Kim KH, Chin SY. Metallic stents in malignant biliary obstruction: prospective long-term clinical results. AJR. 1997;168:741–745. doi: 10.2214/ajr.168.3.9057527. [DOI] [PubMed] [Google Scholar]
- 4.Lameris JS, Stocker J, Nijs HG, et al. Malignant biliary obstruction: percutaneous use of self-expandable stents. Radiology. 1991;179:703–707. doi: 10.1148/radiology.179.3.2027978. [DOI] [PubMed] [Google Scholar]
- 5.Becker CD, Glattli A, Maibach R, Baer HU. Percutaneous palliation of malignant obstructive jaundice with the Wallstent endoprosthesis: follow-up and reintervention in patients with hilar and non-hilar obstruction. J Vasc Intervent Radiol. 1993;4:597–604. doi: 10.1016/s1051-0443(93)71930-2. [DOI] [PubMed] [Google Scholar]
- 6.Lee MJ, Dawson SL, Mueller PR, et al. Percutaneous management of hilar biliary malignancies with metallic endoprostheses: results, technical problems, and causes of failure. RadioGraphics. 1993;13:1249–1263. doi: 10.1148/radiographics.13.6.8290722. [DOI] [PubMed] [Google Scholar]
- 7.Lee BH, Do YS, Byun HS, Kim KH, Jin SY. Metallic stents for management of malignant biliary obstruction. J Korean Radiol Soc. 1992;28:959–967. [Google Scholar]
- 8.Murphy BL, Mueller PR. Metallic biliary stents: technical points on optimizing results. Semin Intervent Radiol. 1996;13:55–67. [Google Scholar]
- 9.Liu Q, Khay G, Cotton B. Feasibility of stent placement above the sphincter of Oddi ("Inside-Stent") for patients with malignant biliary obstruction. Endoscopy. 1998;30:687–690. doi: 10.1055/s-2007-1001389. [DOI] [PubMed] [Google Scholar]
- 10.Stoker J, Lameris JS. Complications of percutaneously inserted biliary Wallstents. J Vasc Intervent Radiol. 1993;4:767–772. doi: 10.1016/s1051-0443(93)71970-3. [DOI] [PubMed] [Google Scholar]
- 11.Dawson SL, Lee MJ, Mueller PR. Metal endoprostheses in malignant biliary obstruction. Semin Intervent Radiol. 1991;8:242–251. [Google Scholar]
- 12.Roebuck DJ, Stanley P, Katz MD, Parry RL, Haight MA. Gastrointestinal hemorrhage due to duodenal erosion by a biliary Wallstent. Cardiovasc Intervent Radiol. 1998;21:63–65. doi: 10.1007/s002709900213. [DOI] [PubMed] [Google Scholar]
- 13.Van Steenbergen W, Van Aken L, Ponette E. Acute pancreatitis complicating the insertion of a self-expandable biliary metal stent. Endoscopy. 1992;24:440–442. doi: 10.1055/s-2007-1010517. [DOI] [PubMed] [Google Scholar]
- 14.Yarze JC, Poulos AM, Fritz HP, Herlihy KJ. Treatment of metallic biliary stent-induced duodenal ulceration using endoscopic laser therapy. Dig Dis Sci. 1997;42:6–9. doi: 10.1023/a:1018812416496. [DOI] [PubMed] [Google Scholar]
- 15.Ee H, Laurence BH. Haemorrhage due to erosion of a metal biliary stent through the duodenal wall. Endoscopy. 1992;24:431–432. doi: 10.1055/s-2007-1010514. [DOI] [PubMed] [Google Scholar]
- 16.Bismuth H. Postoperative strictures of the bile duct. In: Blumgast LH, editor. The biliary tract. Edinburgh: Churchill Livingstone; 1982. pp. 207–218. [Google Scholar]
- 17.Lee BH, Do YS, Lee JH, Kim KH, Chin SY. New self-expandable spiral metallic stent: preliminary clinical evaluation in malignant biliary obstruction. J Vasc Intervent Radiol. 1995;6:635–640. doi: 10.1016/s1051-0443(95)71151-4. [DOI] [PubMed] [Google Scholar]
- 18.Lindner HH. Clinical anatomy. Connecticut: Appleton & Lange; 1989. pp. 418–422. [Google Scholar]
- 19.Huibregtse K. The Wallstent for malignant biliary obstruction. Gastrointest Endosc Clin North Am. 1999;9:491–501. [PubMed] [Google Scholar]
- 20.Lee BH, Kim KH, Chin SY. Mechanical characteristics of self-expandable metallic stents: in vitro study with three types of stresses. J Korean Radiol Soc. 1998;39:497–502. [Google Scholar]
- 21.Roh HG, Kang SG, Cho YK, et al. Physical property and MR imaging of self-expandable metallic stents. J Korean Radiol Soc. 1998;39:503–509. [Google Scholar]
- 22.Rieber A, Brambs H-J. Metallic stents in malignant biliary obstruction. Cardiovasc Intervent Radiol. 1997;20:43–49. doi: 10.1007/s002709900107. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen FM, Lassen AT, Schaffalitzky de Muckadell OB. Randomized trial of stent placed above and across the sphincter of Oddi in malignant bile duct obstruction. Gastrointest Endosc. 1998;48:574–579. doi: 10.1016/s0016-5107(98)70038-0. [DOI] [PubMed] [Google Scholar]