Abstract
Objective
To describe the HRCT findings of cytomegalovirus (CMV) pneumonia in non-AIDS immunocompromised patients.
Materials and Methods
This retrospective study involved the ten all non-AIDS immunocompromised patients with biopsy-proven CMV pneumonia and without other pulmonary infection encountered at our Medical Center between January 1997 and May 1999. HRCT scans were retrospectively analysed by two chest radiologists and decisions regarding the findings were reached by consensus.
Results
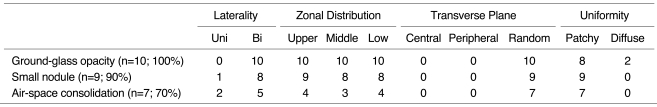
The most frequent CT pattern was ground-glass opacity, seen in all patients, with bilateral patchy (n = 8) and diffuse (n = 2) distribution. Other findings included poorly-defined small nodules (n = 9) and consolidation (n = 7). There was no zonal predominance. The small nodules, bilateral in eight cases and unilateral in one, were all located in the centrilobular region. Consolidation (n = 7), with patchy distribution, was bilateral in five of seven patients (71%). Pleural effusion and bilateral areas of thickened interlobular septa were seen in six patients (60%).
Conclusion
CMV pneumonia in non-AIDS immunocompromised patients appears on HRCT scans as bilateral mixed areas of ground-glass opacity, poorly-defined centrilobular small nodules, and consolidation. Interlobular septal thickening and pleural effusion are frequently associated.
Keywords: Lung, abnormalities; Lung, CT; Lung, infection
Human cytomegalovirus (CMV), which belongs to the group of herpes viruses, is an important cause of morbidity and mortality in immunocompromised patients (1-12).
The radiographic findings of CMV pneumonia are variable and include diffuse reticulonodular densities (2, 3, 7, 8), diffuse haze, and dense areas of consolidation (13-15). McGuinness et al. (9) recently described the CT appearances of CMV pneumonitis in 21 AIDS patients; the findings included ground-glass attenuation, dense consolidation, and bronchial wall thickening. In a recent report, Kang et al. (16) described the CT findings of CMV pneumonia in ten patients who had undergone transplants; their findings differed from those seen in AIDS patients and included micronodules (1-5 mm in diameter), consolidation, and-less frequently-reticulation and ground-glass opacity. In their study, however, only three patients underwent HRCT scanning.
According to our experience, however, findings of CMV infection seen on high-resolution CT scans especially in non-AIDS immunocompromised patients, were somewhat different from those of Kang et al. The aim of the present study is to describe the clinical and CT findings of CMV pneumonia in ten non-AIDS immunocompromised patients.
MATERIALS AND METHODS
This retrospective study involved ten consecutive patients with pathologically proven CMV pneumonia but without other coexisting pulmonary infections encountered between January 1997 and May 1999. All patients (M: 6; F: 4), who ranged in age from 27 to 70 (mean, 46) years, underwent CT examination. In all cases, CMV infection was confirmed by transbronchial lung biopsy using fiberoptic bronchoscopy, and by immunohistochemical staining for CMV monoclonal antibody. All patients were immunocompromised. Seven of the ten had received organ transplants [bone marrow (n = 2), liver (n = 2), kidney (n = 2), and heart (n = 1)]. Two had diabetes and one had acute myelogenous leukemia (Table 1).
Table 1.
Clinical Data of Ten Patients with Cytomeglovirus Pneumonia
Note.-DM: diabetes mellitus, AML: acute myelogenous leukemia, ARDS: acute respiratory distress syndrome, ARF: acute renal failure, AV: atrioventricular, RML: right middle lobe, RLL: right lower lobe, LUL: left upper lobe, LLL: left lower lobe, BMT: bone marrow transplantation
In all patiets, high-resolution CT scanning using a HiSpeed Advantage scanner (GE Medical Systems, Milwaukee, WI) was performed throughout the thorax (1-mm collimation at 10-mm intervals; scans obtained at end inspiration). Both mediastinal (width, 400 H; level, 20 H) and lung (width, 1,500 H; level, -700 H) window images were printed. HRCT scans were analyzed by two chest radiologists and decisions regarding the findings were reached by consensus.
As seen on HRCT scans, patterns of parenchymal abnormalities were subdivided into areas of ground-glass opacity, consolidation, and small nodules (< 7 mm in diameter). The presence of septal thickening, crazy paving, air trapping, and pleural effusion was also recorded, and zonal distribution (upper/middle/lower) and laterality (unilateral vs. bilateral) of the lesions were evaluated. Consolidation on CT indicated the area of opacity with obscuration of underlying vessels. Ground-glass opacity was defined as a hazy increase in lung attenuation without obscuration of underlying vessels. Lesions were considered to be centrally distributed if located within one third of the lung from the mediastinum in transverse plane, and peripheral if located within the inner one third of the lung from the chest wall. If they did not fit either of these categories, they were regarded as random. If scattered widely they were considered to be diffuse, and if inconsistent and variable, to be patchy. Additional findings including the presence of mediastinal nodal enlargement (positive when short axis was greater than 10 mm in diameter), were also recorded.
Transbronchial lung biopsy specimens were reviewed by an experienced lung pathologist. Using a limited number of these, the findings of CT and of the pathologic findings obtained at preoperatively determined biopsy sites were correlated.
RESULTS
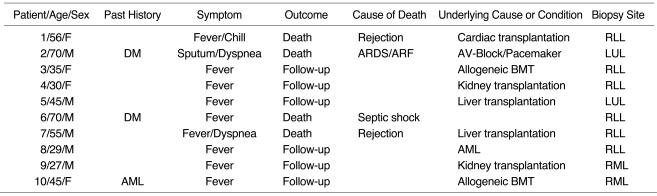
The most frequent CT appearance was ground-glass opacity, seen in all patients (Fig. 1). It was mixed with other patterns, mainly areas of small nodules (n = 9) and consolidation (n = 7). Areas of ground-glass opacity were distributed bilaterally in all patients, and were patchy in eight and diffuse in two. There was no zonal predominance (Table 2).
Fig. 1.
Cytomegalovirus pneumonia in a 27-year-old man who underwent kidney transplantation. High-resolution (1.0-mm collimation) CT scan obtained at level of distal trachea 32 days after transplantation shows bilateral patchy areas of ground-glass opacity with crazy paving appearance. The presence of a few poorly-defined centrilobular nodules (arrows) and bilateral pleural effusion should also be noted.
Table 2.
High-Resolution CT Findings of Cytomeglovirus Pneumonia
Note.-Uni: unilateral, Bi: bilateral
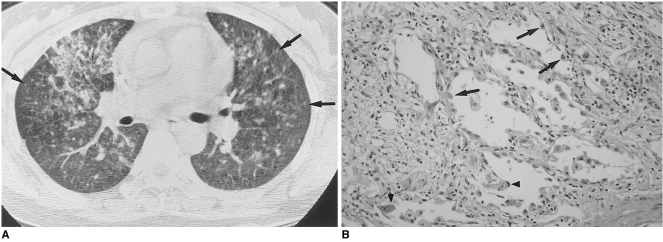
Small nodules were seen in nine patients (Figs. 2, 3), being associated with ground-glass opacity in all and with consolidation in six. They were distributed bilaterally in eight patients and unilaterally in one, and in all nine were located in the centrilobular region of the secondary pulmonary lobule.
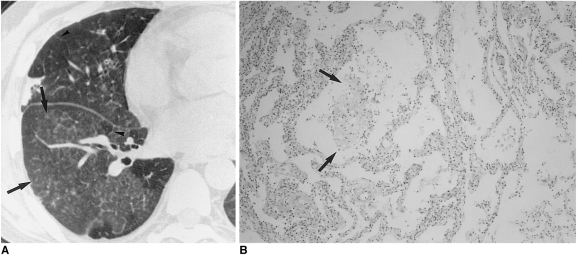
Fig. 2.
Cytomegalovirus pneumonia in a 35-year-old woman who underwent bone marrow transplantation 27 days earlier.
A. High-resolution (1.0-mm collimation) CT scan obtained at level of bronchus intermedius shows numerous small nodules predominantly in centrilobular regions (arrows). There are associated focal patchy areas of ground-glass opacity.
B. Photomicrograph of transbronchial lung biopsy specimen obtained from superior segment of right lower lobe shows diffuse alveolar damage consisting of interstitial fibroblastic proliferation and type-2 pneumocyte hyperplasia (arrows), in addition to characteristic cells (arrowheads) showing intra-nuclear inclusions (H & E, ×100).
Fig. 3.
Cytomegalovirus pneumonia in a 30-year-old woman who presented with fever two months after kidney transplantation.
A. High-resolution (1.0-mm collimation) CT scan obtained at level of right inferior pulmonary vein shows mixed areas of ground-glass opacity and small poorly-defined centrilobular nodules (arrows). There is associated interlobular septal thickening (arrowheads).
B. Photomicrograph of transbronchial lung biopsy specimen obtained from superior segment of right lower lobe shows intra-alveolar collection of macrophages, fibrin and red blood cells (arrows) that corresponds to the centrilobular nodules seen on CT scans. There is associated alveolar wall thickening, with interstitial inflammatory cell infiltration (H & E, ×200).
The area of consolidation, seen in seven patients, was bilateral in five (71%). The lesion was patchy and random in distribution, and was associated with ground-glass opacity in all patients and with small nodules in six of seven.
Bilateral areas of thickened interlobular septa were present in six patients (60%) (Fig. 3). Pleural effusion was seen in six, in three of whom it was bilateral (Fig. 1); in four of the six, the amount of was small. Additional findings were crazy paving (2/10; 20%) (Fig. 1), a small amount of pericardial effusion (2/10; 20%), air trapping in the right upper and lower lobes (1/10; 10%), and cystic lesions in both upper lobes (1/10; 10%).
Limited correlation between the CT findings and the pathologic findings of transbronchial lung biopsy revealed that areas of ground-glass attenuation or consolidation seen on CT scans corresponded to areas of the exudative or proliferative phase of diffuse alveolar damage consisting of interstitial fibroblastic proliferation and lymphocytic infiltration (Fig. 2B). Type-2 pneumocyte hyperplasia, hyaline membrane formation, and intra-alveolar exudate with or without organization were associated. Histopathologically, the poorly-defined centrilobular nodules seen on HRCT represented intra-alveolar collections of macrophages, red blood cells and fibrin (Fig. 3B). CMV-infected cells showing intra-nuclear inclusions were seen in all pathologic specimens.
DISCUSSION
Due to its physical characteristics, CMV is considered a member of the Herpetoviridae family. One of the major biological characteristics of CMV (as with other herpes viruses) is its ability to become latent in the human host, with the potential for reactivation (6). It is evident that in immunocompromised patients, CMV may cause severe symptomatic pulmonary disease (13, 14, 17-22).
It has been suggested that CMV pneumonia in allogeneic transplant recipients is caused by immune mechanisms mediated by a T-cell response to virally-induced antigens expressed in the lungs. Severe necrotizing pneumonia may occur in spite of the suppression of virus replication during antiviral therapy. AIDS patients with more profound immune deficiency than transplant recipients may be unable to initiate the immune response necessary to cause CMV pneumonia by this mechanism. In AIDS patients, lung damage may be directly due to the cytopathogenic effects of CMV, related to the extent of active virus replication (23). These differences in the pathogenic mechanisms of pulmonary CMV infection may result in different histopathologic features between AIDS patients and transplant recipients. In transplant recipients with CMV pneumonia, necrotizing inflammation is dominant, and relatively few CMV-infected cells are revealed by histopathologic examination. In AIDS patients, there is a high density of CMV inclusion bodies, and this leads to severe symptomatic pneumonia (23). In AIDS patients, the cytopathogenic effect of CMV may well cause diffuse alveolar damage more frequently than in non-AIDS patients.
The CT findings of CMV pneumonia are diverse and have been described without distinction between AIDS and non-AIDS patients (9, 10). Common findings are mixed alveolar-interstitial infiltrates such as ground-glass opacity, consolidation, bronchiectasis and interstitial reticulation. McGuinness et al. (9) noted that masses or mass-like infiltrates were more frequent in AIDS patients with CMV pneumonia (12/21) than in non-AIDS patients. Kang et al. (16) reported the CT findings of CMV pneumonia in ten transplant patients in whom diagnosis was confirmed by open lung biopsy in nine and autopsy in one. In their study, parenchymal abnormalities were revealed by CT in nine of ten patients, while in one, CT scans were normal. In those nine patients, the findings were as follows: small nodules (n = 6), consolidation (n = 4), ground-glass opacity (n = 4), and irregular linear opacity (n = 1). The nodules were bilaterally and symmetrically distributed and involved all lung zones. Consolidation was prominent mostly in the lower zones, while ground-glass opacity was combined only with nodules and/or consolidation. The distribution of ground-glass opacity was not specifically mentioned.
In our study, the CT findings of CMV pneumonia in immunocompromised non-AIDS patients were somewhat different from those described by Kang et al. (16). Bilateral patchy areas of ground-glass opacity were the commonest pattern, seen in all patients. These differences may be due to the fact that in our study, HRCT images were available, while Kang et al. used the conventional technique in seven and the HRCT technique in only three. In our study, poorly-defined centrilobular small nodules (90%) and areas of consolidation (70%) were also common. Kim et al. (24) recently reported the HRCT findings of CMV pneumonia in eleven immunocompromised patients. Their findings were very similar to ours and included ground-glass opacity (n = 11), consolidation (n = 7), reticular opacity (n = 10), multiple small nodules or mass (n = 6), and bronchiectasis or bronchial wall thickening (n = 5).
In cases of CMV pneumonia, pathologic examination reveals cytomegalic cells within areas of alveolar damage, the alveolar-filling process being caused by hemorrhage and fibrinous exudate. These areas may correspond to the areas of ground-glass opacity and/or consolidation seen on HRCT scans. Kang et al. (16) reported small nodules, revealed by CT seen in six of ten patients, which corresponded histopathologically to areas of inflammatory or hemorrhagic nodules or organizing pneumonia. In our study, the poorly-defined centrilobular nodules seen on HRCT scans represented histopathologically areas of intra-alveolar collection of macrophages, red blood cells, and fibrin (hemorrhagic and inflammatory nodule).
In their study of 21 AIDS patients with CMV pneumonia, McGuinness et al. (9) suggested that patterns of disease, revealed by CT closely correspond to the pathologic changes seen in CMV pneumonia. Diffuse alveolar damage, which is probably caused by the cytopathogenic effect of CMV, involves pneumocyte injury and reactive proliferation, as well as neutrophilic and fibrinous exudates, and hyaline membrane formation and hemorrhage fill the alveolar spaces. Interstitial infiltrates are comprised mainly of lymphocytes and result in alveolar wall or interlobular septal thickening. Interspersed throughout these changes there may be areas of hemorrhage, fibrin deposition, and -rarely-necrosis. These pathologic changes, depending on their severity, distribution, and relative proportions of each disease process, may result in ground-glass opacity, dense consolidation, mass-like infiltrates and irregular linear opacity on CT scans (9).
Our investigation suffers from two limitations. First, it was retrospective. Because true CMV infection is not easily diagnosed, we might not have included all patients with pulmonary CMV infection. It may be that only patients diagnosed by means of transbronchial lung biopsy, and in whom, therefore, apparent abnormalities were revealed by CT scanning, were included. Second, because we had limited pathologic specimens, obtained only by means of transbronchial lung biopsy, pathological correlation was incomplete.
In conclusion, the most common HRCT findings of CMV pneumonia in non-AIDS immunocompromised patients are bilateral mixed areas of ground-glass opacity, poorly-defined centrilobular small nodules, and consolidation. Interlobular septal thickening and pleural effusion are frequently associated.
References
- 1.Rubin RH, Cosimi AB, Tolkoff-Rubin NE, Russel PS, Hirsch MS. Infectious disease syndromes attributable to cytomegalovirus and their significance among renal transplantation recipients. Transplantation. 1977;24:458–464. doi: 10.1097/00007890-197712000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Abdallah PS, Mark JBD, Merigan TC. Diagnosis of cytomegalovirus pneumonia in compromised hosts. Am J Med. 1976;61:326–332. doi: 10.1016/0002-9343(76)90368-5. [DOI] [PubMed] [Google Scholar]
- 3.Ravin CE, Smith GW, Ahern MJ, et al. Cytomegaloviral infection presenting as a solitary pulmonary nodule. Chest. 1977;71:220–222. doi: 10.1378/chest.71.2.220. [DOI] [PubMed] [Google Scholar]
- 4.Krowka MJ, Rosenow EC, Hoagland HC. Pulmonary complications of bone marrow transplantation. Chest. 1985;87:237–246. doi: 10.1378/chest.87.2.237. [DOI] [PubMed] [Google Scholar]
- 5.Schulman LL. Cytomegalovirus pneumonitis and lobar consolidation. Chest. 1987;91:558–561. doi: 10.1378/chest.91.4.558. [DOI] [PubMed] [Google Scholar]
- 6.Klotman ME, Hamilton JD. Cytomegalovirus pneumonia. Semin Respir Infect. 1987;2:95–103. [PubMed] [Google Scholar]
- 7.Wallace JM, Hannah J. Cytomegalovirus pneumonitis in patients with AIDS. Findings in an autopsy series. Chest. 1987;92:198–203. doi: 10.1378/chest.92.2.198. [DOI] [PubMed] [Google Scholar]
- 8.Johnson PC, Hogg KM, Sarosi GA. The rapid diagnosis of pulmonary infections in solid organ transplant recipients. Semin Respir Infect. 1990;5:2–9. [PubMed] [Google Scholar]
- 9.McGuinness G, Scholes JV, Garay SM, Leitman BS, McCauley DI, Naidich DP. Cytomegalovirus pneumonitis: spectrum of parenchymal CT findings with pathologic correlation in 21 AIDS patients. Radiology. 1994;192:451–459. doi: 10.1148/radiology.192.2.8029414. [DOI] [PubMed] [Google Scholar]
- 10.Aafedt BC, Halvorsen RA, Tylen U, Hertz M. Cytomegalovirus pneumonia: computed tomography findings. J Can Assoc Radiol. 1990;41:276–280. [PubMed] [Google Scholar]
- 11.Northfelt DW, Sollitto RA, Miller TR, Hollander H. Cytomegalovirus pneumonitis. An unusual cause of pulmonary nodule in a patient with AIDS. Chest. 1993;103:1918–1920. doi: 10.1378/chest.103.6.1918. [DOI] [PubMed] [Google Scholar]
- 12.Berger LA. Imaging in the diagnosis of infections in immunocompromised patients. Curr Opin Infect Dis. 1998;11:431–436. doi: 10.1097/00001432-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Austin JH, Schulman LL, Mastrobattista JD. Pulmonary infection after cardiac transplantation: clinical and radiologic correlations. Radiology. 1998;72:259–265. doi: 10.1148/radiology.172.1.2544923. [DOI] [PubMed] [Google Scholar]
- 14.Shreeniwas R, Schulman LL, Berkmen YM, McGregor CC, Austin JHM. Opportunistic bronchopulmonary infections after lung transplantation: clinical and radiographic findings. Radiology. 1996;200:349–356. doi: 10.1148/radiology.200.2.8685324. [DOI] [PubMed] [Google Scholar]
- 15.Afessa B, Gay PC, Plevak DJ, et al. Pulmonary complications of orthotopic liver transplantation. Mayo Clin Proc. 1993;68:427–434. doi: 10.1016/s0025-6196(12)60187-6. [DOI] [PubMed] [Google Scholar]
- 16.Kang EY, Patz EF, Müller NL. Cytomegalovirus pneumonia in transplant patients: CT findings. J Comput Assist Tomogr. 1996;20:295–299. doi: 10.1097/00004728-199603000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Zantel J, Leij L, Prop J, Harmsen MC. The Human cytomegalovirus: a viral complication in transplantation. Clin Transplant. 1998;12:145–158. [PubMed] [Google Scholar]
- 18.Moore EH, Webb WR, Amend WJC. Pulmonary infections in renal transplantation patients treated with cyclosporine. Radiology. 1988;167:97–103. doi: 10.1148/radiology.167.1.2831565. [DOI] [PubMed] [Google Scholar]
- 19.Leung AN, Grosselin MV, Napper CH, et al. Pulmonary infections after bone marrow transplantation: clinical and radiographic findings. Radiology. 1999;210:699–710. doi: 10.1148/radiology.210.3.r99mr39699. [DOI] [PubMed] [Google Scholar]
- 20.Husni RN, Gordon SM, Longworth DL, et al. Cytomegalovirus infection is a risk factor for invasive aspergillosis in lung transplant recipients. Clin Infect Dis. 1998;26:753–755. doi: 10.1086/514599. [DOI] [PubMed] [Google Scholar]
- 21.Chien J, Chan CK, Chamberlain D, et al. Cytomegalovirus pneumonia in allogeneic bone marrow transplantation. An immunopathologic process? Chest. 1990;98:1034–1307. doi: 10.1378/chest.98.4.1034. [DOI] [PubMed] [Google Scholar]
- 22.Worthy SA, Flint JD, Müller NL. Pulmonary complications after bone marrow transplantation: high-resolution CT and pathologic findings. RadioGraphics. 1997;17:1359–1371. doi: 10.1148/radiographics.17.6.9397451. [DOI] [PubMed] [Google Scholar]
- 23.Aukrust P, Farstad IN, Froland SS, Holter E. Cytomegalovirus (CMV) pneumonitis in AIDS patients: the result of intensive CMV replication? Eur Respir J. 1992;5:362–364. [PubMed] [Google Scholar]
- 24.KIM HS, LEE JS. Cytomegalovirus pneumonia in immunocompromised patients: HRCT findings. J Korean Radiol Soc. 1999;41:1133–1138. [Google Scholar]