Abstract
Objective
The purpose of this study was to describe the CT findings of hepatic hypereosinophilic syndrome in which hepatic lobes or segments were involved.
Materials and Methods
Seven patients with hypereosinophilic syndrome with hepatic lobar or segmental involvement were included in our study. In all seven, diagnosis was based on liver biopsy and the results of corticosteroid treatment. CT findings were retrospectively reviewed by three radiologists, who reached a consensus. Biopsy specimens were examined, with special reference to portal and periportal inflammation.
Results
CT demonstrated well-defined, homogeneous or heterogeneous low attenuation with a straight margin limited to a hepatic lobe (n = 2), segments (n = 3), or subsegments (n = 2), particularly during the portal phase. Where there was subsegmental involvement, lesions were multiple, ovoid or wedge-shaped, and showed low attenuation. In two patients with lobar or segmental involvement, segmental portal vein narrowing was observed. Histopathologic examination disclosed eosinophilic infiltration in the periportal area, sinusoids and central veins, as well as portal phlebitis.
Conclusion
Hypereosinophilic syndrome may involve the presence of hepatic lobar, segmental, or subsegmental low-attenuated lesions, as seen on CT images. Their presence may be related to damage of the liver parenchyma and to portal phlebitis.
Keywords: Liver, CT; Liver, abscess; Liver, disease
Hypereosinophilic syndrome, first described by Hardy and Anderson (1), is a spectrum of diseases characterized by severe peripheral eosinophilic leukocytosis and eosinophilic infiltration of organs such as the heart, nervous system, skin and mucous membranes, the hematopoietic system, liver, spleen, gastrointestinal tract, lung and urinary system. Clinical manifestation depends upon the organ involved and the severity of infiltration, as well as upon thromboembolic phenomena (2); symptoms and signs are, therefore, rather nonspecific. Diagnosis is usually based on persistent peripheral eosinophilia of 1500 eosinophils/mm3 for more than six months, the absence of parasitic, allergic, or other causes of eosinophilia, and evidence of organ involvement (3).
Several reports have described the sonographic, CT, and scintigraphic findings of the liver in patients with hepatic involvement (4-10), though such studies have focused only upon small, focal hepatic lesions. Over the last five years, several cases have come to light in which CT has revealed hepatic involvement over a wider area: subsegmental, segmental or lobar, for example. To our knowledge, no report has described hepatic lobar and segmental involvement in patients with hypereosinophilic syndrome. In this paper, we describe the CT findings of hypereosinophilic syndrome with hepatic lobar, segmental, or subsegmental involvement. In order to demonstrate the correlation between the CT and histopathologic findings, these latter are also reviewed.
MATERIALS AND METHODS
Medical record searches at three institutions over the last five years (1995-1999) disclosed seven patients with hypereosinophilic syndrome with hepatic lobar, segmental or subsegmental involvement, as seen on CT scans. Six were male and one was female, and their ages ranged from 17 to 50 (mean, 32) years. All patients were referred for abdominal CT scanning because of abdominal pain, fever, swelling of the upper and lower extremities, and abnormal liver function tests. In all patients, the history of any allergic disease was obtained, and stool and skin tests for parasites were performed. All results were negative. The Elisa test was not performed. In all patients, diagnosis was based on findings of hypereosinophilia in peripheral blood (eosinophil count of 3410-42, 390/mm3; 28-94%) in conjunction with biopsy, and on the response to corticosteroid therapy. Biopsy was performed under ultrasonographic guidance using a 19.5-gauge automated biopsy gun (Angiomed; Aktiengeselshaft, Karlsruhe, Germany). Three patients underwent triphasic dynamic helical CT scanning (HiSpeed Advantage; General Electric Medical Systems, MilwanRee, WI) with a bolus injection of 120 ml of nonionic contrast media (Iopamiro 300; Bracco, Milano, Italy) via the anticubital vein at a rate of 3 mL/sec by power injector, with images obtained 30, 60, and 180 sec after the start of contrast injection during the hepatic arterial, portal venous, and equilibrium phase, respectively. The scans were obtained through the liver in a craniocaudal direction with 7 mm collimation, 7-mm/sec table speed (pitch, 1.0) during a single breath-hold helical acquisition of 25-30 sec, and 7-mm reconstruction interval. The other four patients underwent conventional CT scanning (Hilight; GE, Mil-waukee, Wis., U.S.A., and Somatom DR, Siemens, Erlagen, Germany) with bolus injection of 100~120 mL ionic or nonionic contrast medium. Scans of 1.0-cm thickness were obtained during the portal and delayed phases. In one patient, follow-up helical CT scanning was performed one month after the resolution of clinical findings and laboratory abnormalities following corticosteroid treatment.
All CT images were reviewed retrospectively by three experienced abdominal radiologists with regard to the shape and distribution of hepatic abnormalities. Biopsy specimens were reviewed by one hepatobiliary pathologist at each hospital, with special reference to portal and periportal abnormalities.
RESULTS
There were two cases of hepatic lobar involvement, three of segmental involvement, and two of subsegmental involvement. The clinical, pathologic and CT findings in seven patients are summarized in Table 1. In two patients, CT scans involving the right lobe of the liver (cases 1 and 2), revealed moderate hepatomegaly and low-attenuated lobar lesions involving the right hepatic lobe. In one patient (case 1), helical dynamic CT revealed heterogeneous low attenuation with a straight border limited to the right hepatic lobe (Fig. 1). There was a small, subsegmental low-attenuated lesion in the medial segment of the left lobe, but the remainder of the left lobe was normal. These findings were prominent during the portal venous phase of CT scanning, while scans taken during the hepatic arterial and equilibrium phases showed only a small, faint, subsegmental lesion. The posterior branch of the right portal vein was considerably narrowed, the diameter being 50% or less of normal. A follow-up CT scan one month after corticosteroid therapy revealed a small peripheral wedge-shaped attenuated lesion only during the portal venous phase of CT scanning. The posterior branch of the right portal vein returned to normal. In the other patient with lobar involvement (case 2), CT scans demonstrated irregular, confluent, low-attenuated lesions around the periphery of the right hepatic lobe; these findings suggest the confluence of multiple subsegmental lesions (Fig. 2).
Table 1.
Clinical, Pathologic, and CT Findings in Seven Patients with Hypereosinophilia
Note.-WBC = white blood cell count in peripheral blood, infilt = infiltration; c/w = consistent with
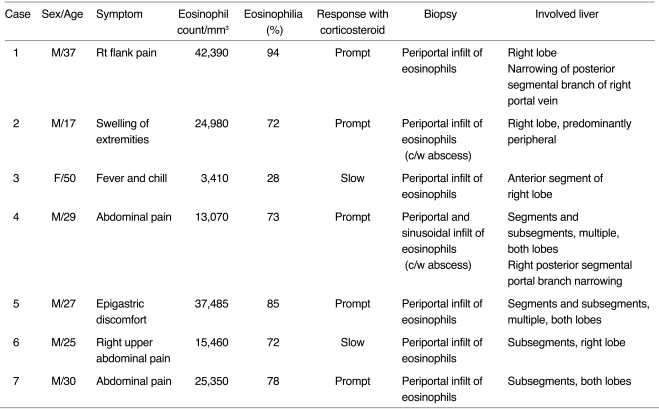
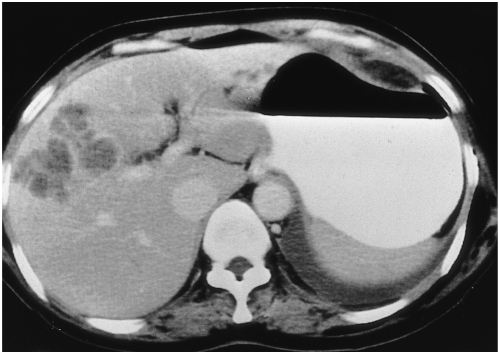
Fig. 1.
A 37-year-old man (case 1) presented with right flank pain. Peripheral white blood cell count was 45,100/mm3, with 94% eosinophilia.
A. Arterial phase CT scan shows a small, wedge-shaped low-attenuated lesion in the peripheral part of the posterior segment of the right hepatic lobe (white arrow). Attenuation of the right hepatic lobe is slightly lower than that of the left lobe. The diameter of the posterior branch of the portal vein (arrowheads) is less than 50% of normal. Note the right hepatic artery (black arrow). The liver is moderately enlarged.
B. CT scan at 3.5 cm cephalad to A in the portal venous phase shows low attenuation at the right hepatic lobe, with slight heterogeneity. Note the sharp border between the lesion and normal parenchyma (open arrow).
C. CT scan at the same level as A during the portal phase shows heterogeneous low attenuation in the right hepatic lobe, with a sharp straight border (open arrow). A small subsegmental low attenuated lesion was also seen in the medial segment of the left hepatic lobe (arrow). Note abrupt narrowing of the posterior branch of the right portal vein.
D. CT scan during the equilibrium phase shows a small, wedge-shaped low attenuated lesion in the right hepatic lobe (the same area as in A, open arrow) and another area of faint low attenuation (arrow). Note the significantly narrow posterior branch of the right portal vein (arrowheads).
E. Follow-up CT scan one month after corticosteroid treatment shows a subsegmental, faint zone of low-attenuation zone (open arrow) and a tiny subcapsular low attenuated lesion (arrow). Note the presence of normal portal venous branches in the posterior segment of the right hepatic lobe (arrowheads).
F. Photomicrograph of needle biopsy specimen reveals severe infiltration of the periportal area by eosinophils and mononuclear cells, resulting in widening of this area and destruction of the hepatic cell cord architecture. Note infiltration of the wall of the portal venule by eosinophils (arrows) and mononuclear cells (arrowheads), resulting in thickening of the wall as well as distortion and narrowing of the lumen (H & E, × 400).
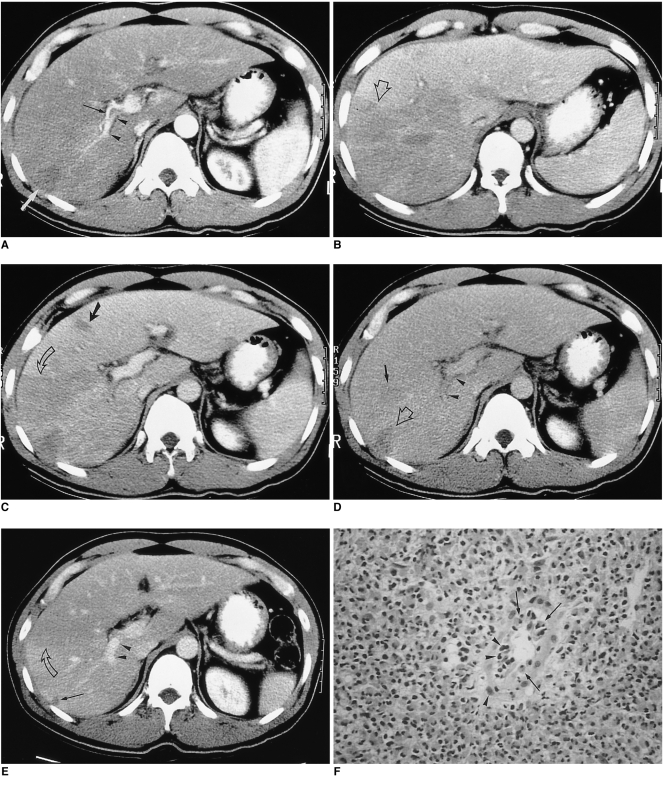
Fig. 2.
A 17-year-old man (case 2) presented with swelling of the upper and lower extremities. White blood cell count was 34,700/mm3, with 72% eosinophilia.
A, B. CT scan obtained conventionally, during the portal venous phase, shows peripheral low attenuation with an irregular, ragged margin along the subcapsular area of the right hepatic lobe. Note the two small splenic lesions (B) (arrows). The hepatic parenchyma adjacent to the low attenuated lesion is slightly higher in attenuation. The liver is moderately enlarged. Liver biopsy revealed severe infiltration of the periportal area and central veins by eosinophils, as well as hepatic parenchymal infarction (not shown).
Five patients with segmental and/or subsegmental involvement (case 3-7) were found to have a few or multiple, medium-sized or small, oval or wedge-shaped low-attenuated lesions, varying in size from 1 to 4 cm, in both lobes of the liver (Fig. 3). Lesion margins were either distinct or indistinct, and some were confluent. In two patients (cases 4 and 7) who underwent three-phase helical CT scanning, segmental and subsegmental low-attenuated lesions were prominent during portal venous phase CT, but these were not prominent or were totally invisible on both arterial and delayed phase CT images. In one patient (case 4), the posterior segmental branch of the right portal vein was significantly narrowed. One patient with subsegmental involvement (case 3) exhibited clustered, round or oval low-attenuated lesions, with enhanced rims in the anterior segment of the right hepatic lobe (Fig. 4).
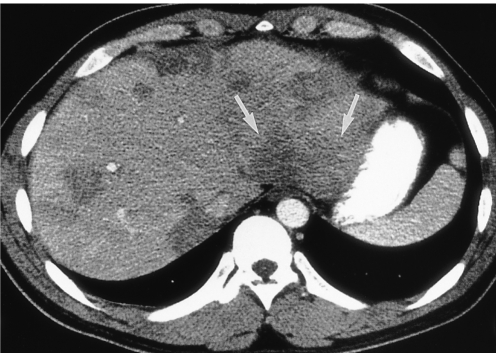
Fig. 3.
A 27-year-old man (case 5) presented with epigastric discomfort. White blood cell count was 44,100/mm3, with 85% eosinophilia. CT scan obtained using a conventional technique shows multiple, well- or ill-defined, round, oval or wedge-shaped low-attenuated lesions of both lobes of the liver. Note the presence of a segmental lesion in the posterior part of the lateral segment of the left hepatic lobe (arrows). Liver biopsy revealed portal and periportal eosinophilic infiltration (not shown).
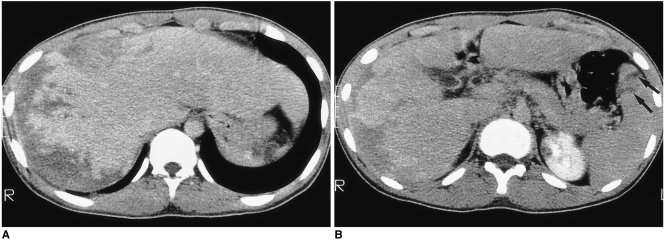
Fig. 4.
A 50-year-old woman (case 3) presented with fever and chill. White blood cell count was 12,180/mm3, with 28% eosinophilia. CT scan obtained using a conventional technique shows multiseptated, lobulated, low-attenuated lesions with peripheral enhancement in the anterior segment of the right hepatic lobe. Biopsy disclosed periportal eosinophilic infiltration and eosinophilic abscess (not shown).
Histopathologic examination of liver biopsy specimens disclosed severe infiltration of periportal spaces, the sinusoids and the central veins by eosinophils and mononuclear cells (Fig. 1F). Small portal venules demonstrated severe inflammation, and the lumens were obliterated. In two patients (cases 3 and 4), an eosinophilic abscess was also noted.
DISCUSSION
Hepatic involvement is known to occur in 40-90% of patients with hypereosinophilic syndrome presenting with hepatomegaly and abnormal liver function tests (1, 2, 6). Several reports have described the varied radiological appearance of hypereosinophilia with hepatic involvement (4-10), characterizing the hepatic lesions as small and focal. CT images demonstrated that these hepatic lesions were small, oval or round, showed low attenuation, and were scattered throughout the liver, especially in areas adjacent to the portal veins. Their average diameter was 2 (range, 1-4) cm. These foci were either well- or ill-defined, and were hypoattenuated (6-9); they were reported to be most conspicuous during the portal venous phase, gradually becoming obscured during the equilibrium phase (7, 8). The number of foci was closely correlated with the percentage of eosinophils in peripheral blood, and decreased as the level of eosinophils decreased (7, 9). Ultrasound revealed that the foci were mostly hypo- (7, 10) but sometimes hyperechoic (10).
CT scans of our seven patients demonstrated hepatic lobar, segmental, and subsegmental low-attenuated lesions. In patients with lobar involvement, the margin was sharp or fuzzy and the border was straight or shaggy. Attenuation within lobar lesions was heterogeneous, and the peripheral zone was more predominantly involved. In patients with segmental or subsegmental involvement, the lesions were located predominantly along the peripheral zone of the liver, and were oval or wedge-shaped, with low attenuation. The margins were usually sharp, but some were ill-defined. Some subsegmental lesions were fused, thus creating a segmental, low-attenuated zone at the periphery of the liver.
As this is the first description of lobar or segmental involvement in patients with hypereosinophilic syndrome, the cause of lobar or segmental distribution of eosinophilic infiltration has not been discussed. We speculate that this may be related to periportal infiltration of eosinophils and portal venous thrombophlebitis, thereby resulting in decreased portal venous blood flow. It is well known that eosinophilic infiltration occurs predominantly in the periportal area (11, 12). Fauci et al. (2) hypothesized that eosinophil cationic protein might be partially responsible for thromboembolic phenomena, particularly through the formation of mural thrombi, leading to embolization. As a result, the diameter of the portal vein branches was reduced. In two of our patients, CT scanning of the right hepatic lobe indicated that the posterior branches of the right portal vein were significantly narrowed and the right hepatic lobe showed low attenuation. In one patient, follow-up CT scans obtained after corticosteroid therapy showed that normal portal vein diameter had been restored, and the right hepatic low-attenuated lesion was nearly normal.
It is well known that a transient attenuation difference seen on CT is related to portal venous obstruction or portal flow cessation caused by diseases such as hepatocellular carcinoma, liver cirrhosis or pylephlebitis (11-17). In such cases, however, the involved parenchyma shows transient hyperattenuation during arterial-phase CT, while the hyperattenuated area becomes isoattenuated during the portal phase. These findings are quite different from the CT findings observed in cases of hypereosinophilic syndrome; attenuation difference is seen mainly during the portal venous phase and the lesions are isoattenuated during the arterial and equilibrium phases. Hepatic parenchymal damage caused by eosinophil infiltration such as eosinophilic abscess, or parenchymal infarction due to vascular damage, may also play a role in decreasing hepatic attenuation during portal venous phase CT. Characteristic hypereosinophilia in peripheral blood is the important clue in establishing a differential diagnosis of lobar or segmental abnormality.
In summary, marked hypereosinophilia causes profound eosinophil infiltration of liver parenchyma and periportal spaces and resultant narrowing of the intrahepatic portal veins. Portal-phase CT scans may thus disclose the presence of lobar or segmental low-attenuated lesions.
Acknowledgment
The authors wish to thank Bonnie Hami, Department of Radiology, University Hospitals of Cleveland, for her editorial assistance, and Young Joo Moon, Department of Radiology, Samsung Medical Center, for her assistance in manuscript preparation.
References
- 1.Hardy WR, Anderson RE. The hypereosinophilic syndrome. Ann Intern Med. 1968;68:1220–1229. doi: 10.7326/0003-4819-68-6-1220. [DOI] [PubMed] [Google Scholar]
- 2.Fauci AS, Harley JB, Robert WC, Ferrans VJ, Gralnick HR, Bjornson BH. The idiopathic hypereosinophilic syndrome: clinical, pathophysiologic, and therapeutic considerations. Ann Intern Med. 1982;97:78–92. doi: 10.7326/0003-4819-97-1-78. [DOI] [PubMed] [Google Scholar]
- 3.Chusid MJ, Dale DC, West BC, Wolff SM. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine. 1975;54:1–27. [PubMed] [Google Scholar]
- 4.Shiomi S, Kuroki T, Ueda T, Ikeoka N, Kobayashi K, Ochi H. Hypereosinophilic syndrome appearing as a focal defect on liver scan. Ann Nucl Med. 1991;5:171–173. doi: 10.1007/BF03164634. [DOI] [PubMed] [Google Scholar]
- 5.White WL, Wahner HW, Brown ML, James EM. Sequential liver imaging in the hypereosinophilic syndrome: discordant images with scintigraphy, ultrasound, and computed tomography. Clin Nucl Med. 1981;6:75–77. doi: 10.1097/00003072-198102000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Kim GB, Kwon JH, Kang DS. Hypereosinophilic syndrome: imaging findings in patients with hepatic involvement. AJR. 1993;161:577–580. doi: 10.2214/ajr.161.3.8352109. [DOI] [PubMed] [Google Scholar]
- 7.Lee WJ, Lim HK, Lim JH, Kim SH, Choi SH, Lee SJ. Foci of eosinophil-related necrosis in the liver: imaging findings and correlation with eosinophilia. AJR. 1999;172:1255–1261. doi: 10.2214/ajr.172.5.10227499. [DOI] [PubMed] [Google Scholar]
- 8.Cha SH, Park CM, Cha IH, et al. Hepatic involvement in hypereosinophilic syndrome: value of portal venous phase imaging. Abdom Imaging. 1998;23:154–157. doi: 10.1007/s002619900310. [DOI] [PubMed] [Google Scholar]
- 9.Kim KS, Lee M-K, Won YC, et al. Idiopathic hypereosinophilic syndrome involving the liver: CT features vs eosinophilia. J Korean Radiol Soc. 1997;37:673–677. [Google Scholar]
- 10.Nam KJ, Jung WJ, Choi J-C, et al. Hepatic involvement in hypereosinophilia: sonographic findings. J Ultrasound Med. 1999;18:475–479. doi: 10.7863/jum.1999.18.7.475. [DOI] [PubMed] [Google Scholar]
- 11.Foong A, Scholes JV, Gleich GJ, Kephart G, Holt PR. Eosinophil-induced chronic active hepatitis in the idiopathic hypereosinophilic syndrome. Hepatology. 1991;13:1090–1094. [PubMed] [Google Scholar]
- 12.Croffy BC, Kopelman RK, Kaplan M. Hypereosinophilic syndrome: association with chronic active hepatitis. Dig Dis Sci. 1988;33:233–239. doi: 10.1007/BF01535738. [DOI] [PubMed] [Google Scholar]
- 13.Itai Y, Moss AA, Goldberg HI. Transient hepatic attenuation difference of lobar or segmental distribution detected by dynamic computed tomography. Radiology. 1982;144:835–839. doi: 10.1148/radiology.144.4.6287520. [DOI] [PubMed] [Google Scholar]
- 14.Itai Y, Ohtomo K, Kokubo T, et al. Segmental intensity differences of the liver on MR imaging: a sign of intrahepatic portal flow stoppage. Radiology. 1988;167:17–19. doi: 10.1148/radiology.167.1.2831561. [DOI] [PubMed] [Google Scholar]
- 15.Nishikawa J, Itai Y, Tasaka A. Lobar attenuation differences of the liver on computed tomography. Radiology. 1981;141:725–728. doi: 10.1148/radiology.141.3.6272353. [DOI] [PubMed] [Google Scholar]
- 16.Itai Y, Murata S, Kurosaki Y. Straight border sign of the liver: spectrum of CT appearances and causes. RadioGraphics. 1995;15:1089–1102. doi: 10.1148/radiographics.15.5.7501852. [DOI] [PubMed] [Google Scholar]
- 17.Itai Y, Matsui O. Blood flow and liver imaging. Radiology. 1997;202:306–314. doi: 10.1148/radiology.202.2.9015047. [DOI] [PubMed] [Google Scholar]