Abstract
Objective
To document the imaging findings of hepatic cavernous hemangioma detected in cirrhotic liver.
Materials and Methods
The imaging findings of 14 hepatic cavernous hemangiomas in ten patients with liver cirrhosis were retrospectively analyzed. A diagnosis of hepatic cavernous hemangioma was based on the findings of two or more of the following imaging studies: MR, including contrast-enhanced dynamic imaging (n = 10), dynamic CT (n = 4), hepatic arteriography (n = 9), and US (n = 10).
Results
The mean size of the 14 hepatic hemangiomas was 0.9 (range, 0.5-1.5) cm in the longest dimension. In 11 of these (79%), contrast-enhanced dynamic CT and MR imaging showed rapid contrast enhancement of the entire lesion during the early phase, and hepatic arteriography revealed globular enhancement and rapid filling-in. On contrast-enhanced MR images, three lesions (21%) showed partial enhancement until the 5-min delayed phases. US indicated that while three slowly enhancing lesions were homogeneously hyperechoic, 9 (82%) of 11 showing rapid enhancement were not delineated.
Conclusion
The majority of hepatic cavernous hemangiomas detected in cirrhotic liver are small in size, and in many, hepatic arteriography and/or contrast-enhanced dynamic CT and MR imaging demonstrates rapid enhancement. US, however, fails to distinguish a lesion of this kind from its cirrhotic background.
Keywords: Liver, cirrhosis; Liver neoplasms, angiography; Liver neoplasms, MR; Liver neoplasms, US
Cavernous hemangioma is the most common benign hepatic tumor. Various imaging techniques all show that the condition usually presents with fairly distinctive findings, but there can also be signs of a lesion with atypical findings that must be distinguished from one that is malignant (1-6). Most hemangiomas do not change in size or appearance during follow-up studies (7-9), though some investigators have suggested that cavernous hemangiomas degenerate and become smaller or vanish during the process of liver cirrhosis (9-11). To our knowledge, no report has described the imaging findings in cavernous hemangiomas detected in cirrhotic liver. Because of the difficulty of distinguishing between hepatocellular carcinomas and hepatic cavernous hemangiomas in this circumstance, a description of the imaging findings of hepatic cavernous hemangioma during the changes occurring in liver cirrhosis is needed. In this report, we describe the imaging findings of hepatic cavernous hemangiomas obtained during various imaging studies of cirrhotic liver.
MATERIALS AND METHODS
A search of dictation records at our institution, including those relating to CT and MR imaging, revealed that during the five-year period 1995-1999, 822 cases of liver cirrhosis had been recorded. In addition, the available hard copies of various imaging studies disclosed 21 cases of hepatic cavernous hemangioma in 15 cirrhotic livers. In the process of case selection, five patients with seven hemangiomas were excluded because only one imaging finding was available, with no additional evidence from further radiologic investigation or histologic proof. The remaining ten patients, in whom 14 cavernous hemangiomas were diagnosed as a result of two or more imaging procedures, were included in this study.
The lesions depicted on heavily T2-weighted static MR images (n = 10) showed bright high signal intensities, as well as contrast-enhancement on delayed contrast-enhanced images. On contrast-enhanced dynamic CT (n = 4) or MR imaging (n = 10), peripherally discontinuous nodular enhancement on early-phase images, with progressive and persistent enhancement on delayed images, was considered diagnostic. On angiograms (n = 9), a pooling of contrast media within a lesion that had a characteristic 'cotton wool' appearance, together with the retention of contrast media well beyond the venous phase, was considered a typical finding. Because of the variety of findings, as well as the limitation of lesion-to-lesion correlation in each case, US (n = 10) was not regarded as a confirmative study.
Seven of the ten patients were male and three were female, and their age ranged from 45 to 72 (mean, 57.5) years. Liver cirrhosis was diagnosed pathologically (n = 2) or on the basis of a patient's laboratory data and radiologic findings. The causes of cirrhosis were chronic B-viral hepatitis (n = 6), chronic C-viral hepatitis (n = 1), alcoholic cirrhosis (n = 2), or primary biliary cirrhosis (n = 1). At the time that hemangiomas were detected, four patients also had hepatocellular carcinoma.
Ten patients underwent MR imaging on a 1.5-T imager (Magnetom Vision; Siemens, Erlangen, Germany) including a T1-weighted spoiled gradient-echo sequence (TR [msec] TE [msec], 113-161/4.1 for single-echo sequence or 140/2.7 and 5.3 for double-echo sequence) and a T2-weighted turbo spin-echo sequence (TR range/effective TE, 3540-4060/138; echo train length, 29) for pre-contrast imaging. For these same patients, gadolinium-enhanced multiphase dynamic imaging (including the arterial, parenchymal and 5-min delayed equilibrium phase) employed a T1-weighted spoiled gradient-echo sequence with the same imaging parameters as in unenhanced imaging. Four patients underwent unenhanced and contrast-enhanced two-phase (arterial and parenchymal or delayed) dynamic CT scanning (HiSpeed Advantage; General Electric Medical Systems, Milwaukee, WI). US, for all ten, involved the use of an Acuson 128 scanner (Acuson, Mountain View, CA) or an HDI-3000 scanner (Advanced Technology Laboratories, Bothell, WA) fitted with a 3.5-MHz convex or 5-MHz linear transducer, or both.
For the nine patients who underwent hepatic arteriography, a Multistar T.O.P (Siemens, Erlangen, Germany) capable of digital subtraction angiography was used.
All images were reviewed retrospectively by three experienced abdominal radiologists, and a consensus was reached: size, speed of enhancement (percent enhancement during the portal or parenchymal phase of dynamic CT or MR imaging), the enhancement pattern seen during hepatic arteriography, and echogenicity during sonography were the factors considered. For lesion-to-lesion analysis and in order to investigate the imaging findings of hemangioma detected in cirrhotic liver, all images for each patient, obtained during various imaging studies, were directly compared.
RESULTS
The mean size of the 14 hepatic cavernous hemangiomas was 0.9 (range, 0.5-1.5) cm in the longest dimension, measured on T2-weighted MR images in which all lesions showed very bright signal intensity (Fig. 1). Nine (64%) of 14 lesions showed low signal intensity on T1-weighted MR images (Fig. 1), but five could not be distinguished from surrounding liver parenchyma.
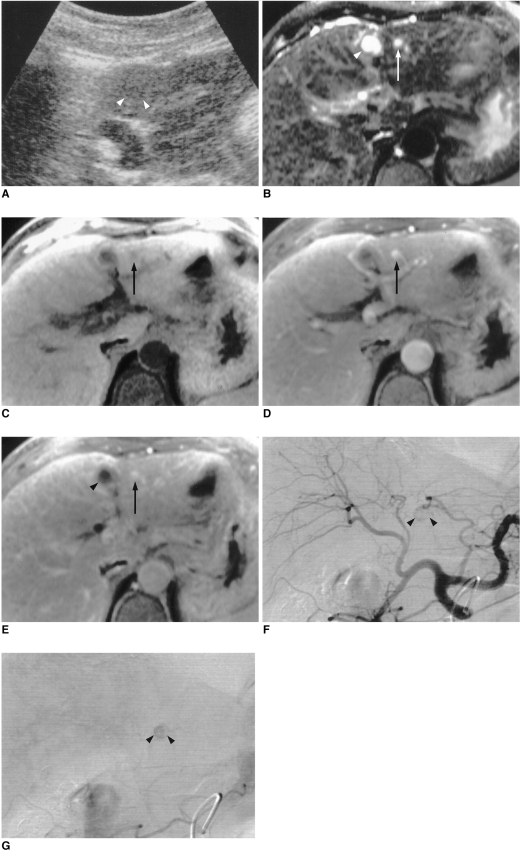
Fig. 1.
A 62-year-old woman with hypoechoic, rapidly enhancing small hemangioma detected in a cirrhotic liver caused by chronic B-viral hepatitis.
A. US depicts a 0.8-cm hypoechoic tumor (arrowheads) in the left lobe of the liver.
B. T2-weighted turbo spin-echo MR image (TR/TE, 4060/138) shows a tumor (arrow) with bright signal intensity. Another high signal intensity tumor is seen (arrowhead), and this is a simple cyst.
C. Unenhanced T1-weighted spoiled gradient-echo MR image with fat suppression (160.8/4.1) shows a low signal intensity tumor (arrow).
D. Arterial dominant phase spoiled gradient-echo image (160.8/4.1) reveals strong enhancement of the tumor (arrow) seen in E.
E. Delayed phase spoiled gradient-echo image (160.8/4.1) obtained 5 min after the start of contrast injection demonstrates persistent contrast enhancement of the tumor (arrow). Simple cyst (arrowhead) is not enhanced after contrast injection.
F. Arterial phase of hepatic arteriography shows small C-shaped contrast puddling (arrowheads).
G. Capillary phase of hepatic arteriography depicts more diffuse contrast accumulation in the lesion (arrowheads) seen in F.
In 11 (79%) of the 14 lesions, contrast-enhanced dynamic CT (n = 4) or MR imaging (n = 10) showed rapid contrast enhancement of the entire lesion during the arterial and/or parenchymal phase and prolonged enhancement during the delayed phase (Fig. 1). In four (36%) of these 11, perilesional parenchymal enhancement was noted during the arterial phase (Fig. 2). Three others (21%) enhanced slowly, and until the 5-min delayed phase showed partial enhancement (Fig. 3).
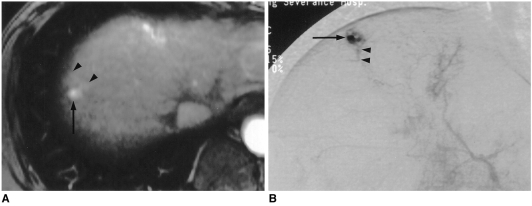
Fig. 2.
A 45-year-old man with small rapidly enhancing hemangioma in a cirrhotic liver resulting from chronic B-viral hepatitis.
A. Arterial dominant phase contrast-enhanced spoiled gradient-echo MR image (140/2.7) shows strong enhancement of a 0.9-cm tumor (arrow) with wedge-shaped temporal peritumoral enhancement (arrowheads). On unenhanced T1-and T2-weighted, and delayed contrast-enhanced MR images (not shown), the area of wedge-shaped enhancement could not be distinguished from surrounding hepatic parenchyma.
B. Capillary phase of hepatic arteriography shows near complete filling-in of the tumor by contrast agent (arrow), with opacification of a small, proximal portal vein branch (arrowheads), suggesting a transtumoral shunt or drainage of the hyperdynamic tumor by this branch.
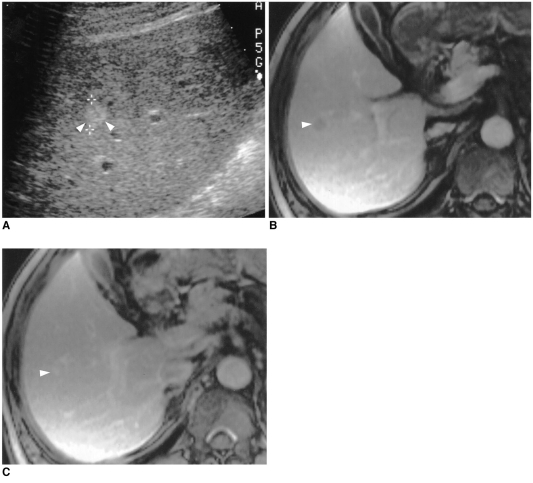
Fig. 3.
A 52-year-old man with hyperechoic, slowly-enhancing small hemangima detected in a cirrhotic liver caused by chronic B-viral hepatitis.
A. US shows 1.1-cm hyperechoic tumor (arrowheads) in the right lobe of the liver.
B. Parenchymal phase contrast-enhanced spoiled gradient-echo MR image (140/2.7) demonstrates minimal tumoral enhancement (arrowhead).
C. Delayed phase contrast-enhanced spoiled gradient-echo MR image (140/2.7) obtained 5 mins after the administration of contrast agent depicts more diffuse, but still partial, contrast enhancement of the tumor (arrowhead) seen in B. Hepatic arteriogram (not shown) demonstrated no tumoral contrast enhancement.
During hepatic arteriography, 11 (85%) of 13 hemangiomas, distributed among nine patients, showed punctate or nodular contrast accumulation during the arterial phase and complete filling-in during the capillary phase (Figs. 1, 2). Two (18%) of the 11 showed early contrast-filling of perilesional small portal vein branches during the capillary phase (Fig. 2). Two hemangiomas that enhanced slowly during dynamic MR imaging were not delineated on hepatic arteriograms.
Regarding the detection rate of US, and the echogenicity demonstrated, only five lesions (36%) were distinguished from surrounding hepatic parenchyma, either with (n = 2) or without (n = 3) knowledge of the presence of the lesions. Three were homogeneously hyperechoic, and two were hypoechoic (Fig. 1). The three sonographically hyperechoic lesions corresponded with the slowly enhancing lesions depicted by dynamic MR imaging (Fig. 3), while the two hypoechoic lesions showed rapid enhancement during dynamic imaging (Fig. 1). US failed to demonstrate the remaining nine rapidly enhancing lesions.
DISCUSSION
According to a previous report (12), rapidly enhancing hemangiomas tend to be hypoechoic at US, and it may be difficult to distinguish a small hypoechoic lesion from the coarse background of cirrhotic liver. Transient peritumoral enhancement or the early appearance of small portal vein branches adjacent to the lesion during contrast-enhanced dynamic studies has also been described as a characteristic finding of high-flow hemangiomas (13). Such findings suggest that a transtumoral arterioportal shunt is no longer a unique feature of hepatocellular carcinomas.
Regarding the small sizes of cavernous hemangiomas detected in cirrhotic liver, it may be that a larger hemangioma has been reduced in size during the process of cirrhotic change. In a report describing the long-term follow-up of hepatic hemangioma in patients with chronic liver disease (11), one lesion accompanied by chronic hepatitis regressed spontaneously after 48 months. It was suggested that changes in blood flow, and in the intrahepatic environment as a result of hepatic fibrosis, may influence the regression of a hemangioma.
Despite a lack of pathologic proof (3), some investigators have suggested that central fibrotic degeneration, or hemorrhage and thrombosis, might cause decreased echogenicity (7, 9). Central hemorrhage and thrombosis, however, have been observed in larger hemangiomas, particularly giant ones, and should not enhance until the delayed phase of contrast-enhanced studies. For small hemangiomas, decreased echogenicity can not be explained by central thrombosis. Increased echogenicity of surrounding liver parenchyma resulting from diffuse fatty infiltration or fibrotic change would make intrahepatic lesions relatively hypoechoic (14). An actual decrease of echogenicity during the direct compression of liver parenchyma (15) also suggests another mechanism of decreased echogenicity of a hemangioma detected in cirrhotic liver. The surrounding fibrotic changes occurring during cirrhosis would compress the hemangioma, and the collapsed cavernous pool might reduce the echogenic interfaces.
One CT report with pathologic correlation (16) suggested that hemangiomas with smaller vascular spaces would enhance more rapidly during dynamic CT than those with larger vascular spaces. With regard to the high speed of contrast enhancement of cavernous hemangiomas detected in cirrhotic liver, a compressed cavernous pool and the resultant small vascular spaces would be a cause of rapid enhancement in patients involved in the present study. However, a cavernous hemangioma is composed of innumerable cavernous pools, and the arterial perfusional pressure that determines the speed of blood flow in each vascular pool is different. Under such circumstances, the narrowing of vascular pools caused by fibrotic distortion would not be even. In other words, vascular pools with low perfusional pressure would be compressed more easily than those in which pressure is higher. Chronically compressed vascular pools would be obliterated and intermingled with hepatic fibrosis, but those that were hyperdynamic would remain in the cirrhotic liver. The increase in arterial blood and perfusional pressure required to compensate for decreased portal perfusion in cirrhotic liver would partially contribute to the hyperdynamic status of a cavernous hemangioma (17). These speculations should be verified by future research that pathologic correlation.
This study suffers from several limitations. Case selection, for example, depended solely on imaging findings, without pathologic confirmation. On the basis of a statement made by Nelson and Chezmar (18), two or more confirmatory study results were combined and used as a diagnostic gold standard for each lesion. With regard to the US findings which were entirely subjective, the hard copies concerned, with retrospective review, were not useful for lesion-to-lesion analysis in this study. During follow-up however, when it was known that hemangiomas were present, the majority of small ones could not be delineated from the coarse background of cirrhotic liver. Finally, long-term follow-up data for cirrhotic change was not available for the majority of patients, and in no case was cirrhosis-related change in a hepatic cavernous hemangioma clearly demonstrated.
In spite of these limitations, the results of this study suggest that the majority of hepatic cavernous hemangiomas detected in cirrhotic liver are small, and many enhance rapidly at hepatic arteriography and contrast-enhanced dynamic CT or MR imaging. On US, these lesions can not be readily distinguished from a cirrhotic background. In cirrhotic liver, hepatic cavernous hemangiomas are incidentally discovered on dynamic CT or MR imaging during daily practice, and their hyperdynamic nature can be characterized by hepatic arteriography.
References
- 1.Itai Y, Ohnishi S, Ohtomo K, Kokubo T, Imawari M, Atomi Y. Hepatic cavernous hemangioma in patients at high risk for liver cancer. Acta Radiol. 1987;28:697–701. [PubMed] [Google Scholar]
- 2.Hanafusa K, Ohashi I, Himeno Y, Suzuki S, Shibuya H. Hepatic hemangioma: findings with two-phase CT. Radiology. 1995;196:465–469. doi: 10.1148/radiology.196.2.7617862. [DOI] [PubMed] [Google Scholar]
- 3.Takayasu K, Moriyama N, Shima Y, et al. Atypical radiographic findings in hepatic cavernous hemangioma: correlation with histologic features. AJR. 1986;146:1149–1153. doi: 10.2214/ajr.146.6.1149. [DOI] [PubMed] [Google Scholar]
- 4.Moody AR, Wilson SR. Atypical hepatic hemangioma: a suggestive sonographic morphology. Radiology. 1993;188:413–417. doi: 10.1148/radiology.188.2.8327687. [DOI] [PubMed] [Google Scholar]
- 5.Freeny PC, Vimont TR, Barnett DC. Cavernous hemangioma of the liver: ultrasonography, arteriography, and computed tomography. Radiology. 1979;132:143–148. doi: 10.1148/132.1.143. [DOI] [PubMed] [Google Scholar]
- 6.Jeong MG, Yu JS, Kim KW, Jo BJ, Kim JK. Early homogeneously enhancing hemangioma versus hepatocellular carcinoma : differentiation using quantitative analysis of multiphasic dynamic contrast-enhanced MR imaging. Yonsei Med J. 1999;40:248–255. doi: 10.3349/ymj.1999.40.3.248. [DOI] [PubMed] [Google Scholar]
- 7.Bree RL, Schwab RE, Neiman HL. Solitary echogenic spot in the liver: is it diagnostic of a hemangioma? AJR. 1983;140:41–45. doi: 10.2214/ajr.140.1.41. [DOI] [PubMed] [Google Scholar]
- 8.Gandolfi L, Leo P, Solmi L, Vitelli E, Verros G, Colecchia A. Natural history of hepatic hemangiomas: clinical and ultrasound study. Gut. 1991;32:677–680. doi: 10.1136/gut.32.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibney RG, Hendin AP, Cooperberg PL. Sonographically detected hepatic hemangiomas: absence of change over time. AJR. 1987;149:953–957. doi: 10.2214/ajr.149.5.953. [DOI] [PubMed] [Google Scholar]
- 10.De Caralt TM, Ayuso JR, Ayuso C, Schorlemmer WC. Distortion of subcapsular hepatic hemangioma by hepatic cirrhosis. Can Assoc Radiol. 1999;50:137–138. [PubMed] [Google Scholar]
- 11.Kobayashi T, Kawano M, Tomita Y, et al. Follow-up study of hepatic hemangiomas. Nippon Shokakibyo Gakkai Zasshi. 1995;92:41–46. [in Japanese] [PubMed] [Google Scholar]
- 12.Yu JS, Kim MJ, Kim KW, et al. Hepatic cavernous hemangioma: sonographic patterns and speed of contrast enhancement on multiphase dynamic MR imaging. AJR. 1998;171:1021–1025. doi: 10.2214/ajr.171.4.9762989. [DOI] [PubMed] [Google Scholar]
- 13.Jeong MG, Yu JS, Kim KW. Hepatic cavernous hemangioma: temporal peritumoral enhancement during multiphase dynamic MR imaging. Radiology. 2000;216:692–697. doi: 10.1148/radiology.216.3.r00se08692. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita Y, Ogata I, Urata J, Takahashi M. Cavernous hemangioma of the liver: pathologic correlation with dynamic CT findings. Radiology. 1997;203:121–125. doi: 10.1148/radiology.203.1.9122378. [DOI] [PubMed] [Google Scholar]
- 15.Yu JS, Kim MJ, Kim KW. Intratumoral blood-flow in cavernous hemangioma of the liver: radiologic-pathologic correlation. Radiology. 1998;208:549–550. doi: 10.1148/radiology.208.2.549-b. [DOI] [PubMed] [Google Scholar]
- 16.March JI, Gibney RG, Li DK. Hepatic hemangioma in the presence of fatty infiltration: an atypical sonographic appearance. Gastrointest Radiol. 1989;14:262–264. doi: 10.1007/BF01889211. [DOI] [PubMed] [Google Scholar]
- 17.Choji K, Shinohara M, Nojima T, et al. Significant reduction of the echogenicity of the compressed cavernous hemangioma. Acta Radiol. 1988;29:317–320. [PubMed] [Google Scholar]
- 18.Nelson RC, Chezmar JL. Diagnostic approach to hepatic hemangiomas. Radiology. 1990;176:11–13. doi: 10.1148/radiology.176.1.2191359. [DOI] [PubMed] [Google Scholar]