Abstract
Under apparently similar field conditions individual plants of Cistus creticus turn transiently red during winter, while neighbouring plants remain green. These two phenotypes provide a suitable system for comparing basic photosynthetic parameters and assessing critically two hypotheses, i.e. anthocyanins afford photoprotection and anthocyanins induce shade characteristics on otherwise exposed leaves. With that aim, pigment levels and in vivo chlorophyll fluorescence parameters were monitored in dark-acclimated (JIP-test) and light-acclimated (saturation pulse method) leaves during both the green and the red period of the year. No evidence for actual photoprotection by anthocyanins was obtained. On the contrary, all fluorescence parameters related to yields and probabilities of photochemical energy conversion and electron flow, from initial light trapping to final reduction of ultimate electron acceptors in PSI, declined in the red phenotype after leaf reddening. Moreover, the pool sizes of final electron acceptors of PSII diminished, indicating that both photosystems were negatively affected. Vulnerability to winter stress was also indicated by sustained chlorophyll loss, inability to increase the levels of photoprotective xanthophylls and increased quantum yield of non-regulated energy loss during reddening. However, during the same period, the relative PSII antenna size increased, indicating an apparent shade acclimation after anthocyanin accumulation, while changes in the photosynthetic pigment ratios were also compatible to the shade acclimation hypothesis. All parameters recovered to pre-reddening values upon re-greening. It is concluded that the photosynthetic machinery of the red leaf phenotype has an inherently low capacity for winter stress tolerance, which is not alleviated by anthocyanin accumulation.
Keywords: Cistus creticus, electron transport, epoxidation state, OJIP curves, photoinhibition, photoprotection, pigments, PSI, PSII, shade acclimation
Introduction
In some plants and under some circumstances, leaves may appear red due to accumulation of anthocyanins. These pigments absorb visible radiation without being photosynthetic, hence they compete with chlorophylls for photon capture and, accordingly, their presence entails a photosynthetic cost, equal to the lost photons. Yet, the cost of any conserved trait should be paid-off by a corresponding benefit. The adaptive significance of leaf redness, however, is not well understood. Ecologically oriented hypotheses link red leaf colour with the avoidance of overconsumption by folivorous insects (Archetti, 2000; Hamilton and Brown, 2001; Ougham et al., 2005) and earned support from observations that young or senescing red leaves seem to be less attractive for food or oviposition (Archetti and Leather, 2005; Karageorgou and Manetas, 2006). Physiologically oriented hypotheses consider that anthocyanins are photoprotective, either as passive light screens or through their antioxidant function (Lee and Gould, 2002; Steyn et al., 2002; Ougham et al., 2008). They may act as a last line of defence against photoinhibitory damage when the first lines of defence (i.e. chloroplast and leaf movements, xanthophyll and water-water cycles, photorespiration) are not adequate or collapse. The photoprotective function is reasonable since in many cases the abundance of red leaves or red individuals is higher in exposed than in shaded sites (Kytridis et al., 2008). Moreover, a variety of stresses that increase the photoinhibitory risk (nutrient deficiency, water or salt stress, high light in combination with cold temperatures) do induce the accumulation of anthocyanins in some plants (Manetas, 2006).
The photoprotective hypothesis was checked by examining whether red leaves are more tolerant of an applied photoinhibitory treatment in the laboratory. In most cases, a positive correlation between leaf redness and tolerance of photoinhibition has been reported (Krol et al., 1995; Feild et al., 2001; Manetas et al., 2002; Pietrini et al., 2002; Hughes et al., 2005; Hughes and Smith, 2007), but see Burger and Edwards (1996) for a different result. However, the ultimate test for the sunscreen hypothesis is whether anthocyanin accumulation offers any actual photoprotection in the field. Results, again, are contradictory. Thus, Liakopoulos et al. (2006) reported a slight photoprotective function in young, developing Vitis vinifera leaves. Yet no evidence for an actual photoprotection was found in young leaves of Quercus coccifera (Manetas et al., 2003), in mature leaves of Prunus cerasifera (Kyparissis et al., 2007) and Erythronium dens-canis (Esteban et al., 2008), and in senescing leaves of many woody species (Lee et al., 2003). A by-product of these investigations (Gould et al., 2002; Manetas et al., 2003; Hughes and Smith, 2007; Kyparissis et al., 2007) was that, irrespective of the likely function of the red-leaf trait, the anthocyanic screen imposes some morphological and physiological adjustments, partly compatible with the shade acclimation syndrome.
A slightly different version of the photoprotective hypothesis was recently adopted by Kytridis et al. (2008). These authors took advantage of the intra-species variation in the anthocyanic character displayed by the Mediterranean shrub Cistus creticus, where mature leaves in some individuals turn transiently red during winter, while neighbouring individuals in the same site remain green. In this system, red individuals displayed slightly inferior photosynthetic and photoprotective capabilities and lower levels of leaf nitrogen. Yet, no sign of differential photoinhibitory damage between the two phenotypes was found. Thus, anthocyanin accumulation was assumed to compensate for the photosynthetic and photoprotective inferiority of the red plants through a light screen and/or an antioxidant function (Kytridis et al., 2008).
In the present investigation, the photoprotective hypothesis and the assumed shade acclimation of red leaves were re-examined with the C. creticus system by using a new approach. Previous investigations used only the maximum PSII yield as a measure of photoinhibition, considering just two points in the fluorescence rise kinetics, i.e. the initial (F0) fluorescence when all PSII centres are open and the maximum (FM) fluorescence when all PSII centres close after a saturating light pulse. However, the fluorescence rise kinetics from F0 to FM is polyphasic, with characteristic steps denoting the step-wise flow of energy through PSII (Strasser et al., 1995, 2004; Papageorgiou et al., 2007) and up to the final electron acceptors of PSI (Jiang et al., 2008; Tsimilli-Michael and Strasser, 2008; Yordanov et al., 2008). If they are analysed appropriately by the so-called JIP-test, the original fluorescence data reveal several structural and functional attributes of PSII and PSI, which also encompass their donor and acceptor sides. Thus, additional information linked to photoinhibition and light/shade adjustments was sought by analysing the shape of the fast chlorophyll fluorescence transients.
It was also questioned whether the imposed anthocyanic screen would adjust the xanthophyll cycle pool sizes and the extent of diurnal inter-conversions of its components. Such adjustments accompany shade acclimation (Thayer and Björkman, 1990), and the corresponding information for red leaves is lacking. All parameters were monitored in both phenotypes at frequent intervals, starting just before the onset of reddening in early winter and ending at re-greening in late spring.
Materials and methods
Plant material study site and sampling protocol
Cistus creticus L. (Cistaceae) is an evergreen Mediterranean shrub pioneer on post-fire sites. The study area (38.14°N, 21.44°E, 250 m a.s.l.) was burnt in 1989 and today is covered by a mixture of short shrubs dominated by C. creticus and regenerating evergreen sclerophylls and pine trees. Previous observations (Kytridis and Manetas, 2006; Kytridis et al., 2008) indicated that certain individuals of C. creticus turn red roughly at mid-winter and revert back to green at mid-spring. Hence, during this period, a field of C. creticus is a mosaic of green and red phenotypes. The frequency of red individuals is higher in the most well-lit sites and the anthocyanic trait is stable, i.e. the same shrubs turn red every year. This permits the permanent tagging of red and green plants to be studied both during the ‘green’ and the ‘red’ period of the year. Ten (five red and five green), south-facing shrubs were used throughout this study. Measurements started in early December 2007, i.e. 2 weeks before commencement of colour change, and ended in late summer 2008. As it is shown in Table 1, the mean monthly temperatures during the experimental period were comparable to the corresponding mean values over a period of 10 years in the study area.
Table 1.
Mean monthly temperatures (°C) for the study area during the experimental season and during the previous 10 years (1997–2006)
| Nov | Dec | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | |
| 1997–2006 | 14.7 | 11.1 | 9.5 | 9.9 | 12.0 | 14.9 | 20.3 | 25.1 | 27.3 | 27.8 |
| 2007–2008 | 14.5 | 10.2 | 10.5 | 10.7 | 14.1 | 16.5 | 21.0 | 26.7 | 28.2 | 29.3 |
Temperature data were provided by the Regional Institute of Plant Protection (Patras).
Sampling was performed with a frequency of ∼10 d during the first month and ≥20 d afterwards, always on clear days. On each sampling date the site was visited twice, at mid-day and at pre-dawn of the following day; leaves were removed for pigment analysis and chlorophyll fluorescence measurements. Mature, exposed leaves were used throughout.
Pigment determination
Two leaves from every shrub (i.e. a total of 20 leaves, equally divided between red and green phenotypes), were harvested at midday and sampling was similarly repeated at pre-dawn of the following day. Both were immediately immersed in liquid nitrogen until extraction. They were extracted by grinding in a mortar with pure methanol plus a small amount of CaCO3 under dim (0.3 μmol m−2 s−1) light. After centrifugation at 5000 g for 10 min, the supernatant was further cleared by passing through 0.45 μm filters. Chlorophylls were measured spectrophotometrically in a Shimadzu (UV-160A) double-beam spectrophotometer by using the equations of Lichtenthaler and Wellburn (1983). A portion of the initial extract was acidified with concentrated HCl for spectrophotometric determination of anthocyanins according to Lindoo and Caldwell (1978). Carotenoid separation was performed with a Shimadzu LC-10 AD HPL chromatograph equipped with a calibrated, non-end-capped Zorbax ODS column and a photodiode array detector (Shimadzu, SPD-M10AVP) as previously described (Manetas et al., 2003).
Chlorophyll a fluorescence measurements
Six leaves from each tagged shrub (i.e. a total of 60 leaves, equally divided between red and green phenotypes) were sampled at pre-dawn of each sampling date and kept moistened in the dark for at least 1 h before measurement. Fast chlorophyll a fluorescence transients were induced by an array of six red (peak at 650 nm) LEDs, providing 3000 μmol photons m−2 s−1 for 2 s at leaf level. Fluorescence was captured by a Hansatech (Handy-PEA; Hansatech Instruments Ltd, King's Lynn, Norfolk, UK) analyser, recording with a time scan from 10 μs to 2 s and with a data acquisition rate of 105, 104, 103, 102, and 10 readings s−1 in the time intervals of 10–300 μs, 0.3–3 ms, 3–30 ms, 30–300 ms, and 0.3–2 s, respectively. The cardinal points in the fluorescence versus time curve used for further calculation of biophysical parameters were: maximal fluorescence intensity (FM); fluorescence intensity at 20 μs, considered as the first credible measurement (F0); fluorescence intensity at 300 μs (F300μs) needed for calculation of the initial slope (M0) of the relative variable fluorescence versus time curve; fluorescence intensity at 2 ms (i.e. at the J-step, FJ); fluorescence intensity at 30 ms (i.e. at the I-step, FI). These primary data were used to derive the following parameters, according to the JIP-test (Strasser et al., 2004; Jiang et al., 2008; Tsimilli-Michael and Strasser, 2008; Yordanov et al., 2008):
(i) The photosynthetic efficiencies at the onset of illumination, i.e. the maximum quantum yield of primary photochemistry φPo=TR0/ABS, also known as FV/FM (where TR and ABS denote the trapped and absorbed excitation energy); the efficiency to conserve trapped excitation energy as redox energy (i.e. electron transfer, ET) ψEο=ET0/TR0; the quantum yield of electron transfer to intermediate electron carriers φEο=ET0/ABS = φPο·ψEo; the efficiency of electron transfer between intermediate carriers to final acceptors of PSI, δRo=REo/ET0; and the quantum yield of reduction of final electron acceptors of PSI, φRο=φPο·ψEο·δRο.
(ii) The specific fluxes per active (i.e. QA-reducing) reaction centre (RC) for absorption (ABS/RC), trapping (TR0/RC), electron transport (ET0/RC), and dissipation (DI0/RC).
The formulas used to calculate the above parameters are given in the Appendix.
Chlorophyll fluorescence measurements in the light acclimated state
During the middle of the ‘red’ period, leaves were sampled at pre-dawn, put in air-tight plastic bags and kept for at least 1 h in the dark. All further manipulations were done under dim background laboratory light of <1 μmol m−2 s−1. Leaf discs were cut from both green and red leaves and placed in Petri dishes on moistened filter paper. An IMAGING-PAM (Walz, Effeltrich, Germany), equipped with blue LEDs providing measuring, actinic, and saturation pulse light, and a CCD camera for capturing the fluorescence was used. Before each measurement, the instrument probes the sample reflectance in the red and infra-red band. A built-in equation is then used for the calculation of absorptance (A), to be used later in the calculation of linear electron transport rates. Following a saturation pulse for the measurement of dark-acclimated maximum PSII yield (as FV/FM=(FM–F0)/FM), the sample was illuminated with step-wise increasing actinic irradiances, during which saturation pulses (0.8 s, 2400 μmol m−2 s−1) were given every 60 s to close all PSII reaction centres. Duration of each actinic irradiance step was long enough for full photosynthetic induction, typically not <5 min. Light-acclimated PSII yield, ΦPSII, was computed as ΔF/FM′=(FM′–F)/FM′ and linear electron transport rates as ETR=ΦPSII·PAR·A·0.5, where PAR is the incident photosynthetically active radiation, A the computed sample absorptance, and 0.5 a correction factor assuming equal light absorption by the two photosystems (Genty et al., 1989). Both abaxial and adaxial leaf sides were probed. However, calculation of ETR is only applicable in the abaxial side which does not contain anthocyanins (Kytridis and Manetas, 2006). Anthocyanin presence in the palisade mesophyll introduces an uncertainty as to the actual PAR reaching the probed chloroplasts, since anthocyanins also absorb in the blue band. The quantum yield of regulated non-photochemical energy loss, ΦNPQ, was calculated as (F/FM′)–(F/FM) and the quantum yield of non-regulated energy loss, ΦNO, as F/FM according to Hendrickson et al. (2004). Apparently, the quantum yields of photochemichal energy conversion and combined energy losses would sum to unity, i.e. ΦPSII+ΦNPQ+ΦNO=1.
Statistics
When appropriate, Student's t-test was used to assess significance of differences in the measured parameters between green and red leaves at each sampling date (SPSS 15.0 statistical package). In the case of pigment measurements, Student's t-test was performed separately for the ‘green’ and the ‘red’ period in order to assess significance of differences between green and red leaves.
Results
Pigments
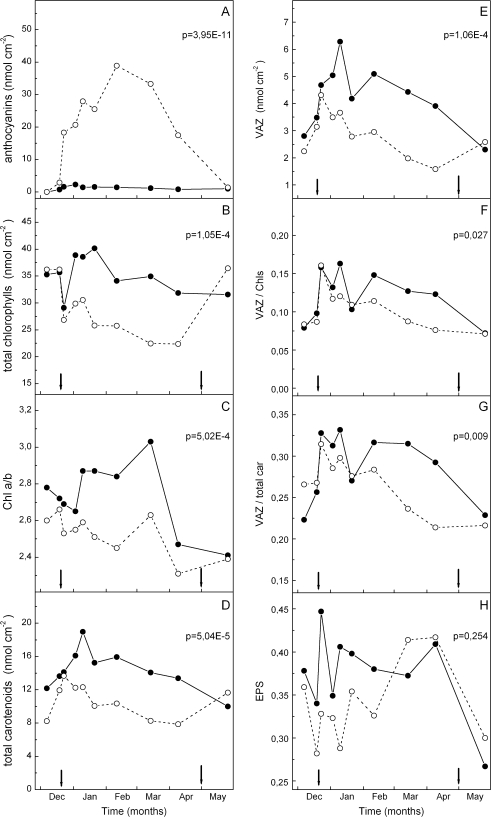
Leaves of the red phenotype started turning red at late December. Anthocyanins displayed a steep initial increase to half-saturated levels within 4 d, followed by a more gradual increase up to a plateau reached in mid-February, and kept at high levels up to leaf senescence in mid-April (Fig. 1A). New leaves burst in mid-April and were green in both phenotypes. Chlorophyll (a + b) levels in the green period were similar in the two phenotypes and displayed an equal drop synchronized with the first appearance of anthocyanins in the red phenotype (Fig. 1B). Thereafter, greens recovered to previous levels, yet the reds remained at ∼20–25% lower levels compared with the pre-reddening period. The chlorophyll a/b ratio was slightly higher in the green phenotype at almost all sampling dates, yet a lowering trend was evident in the reds during their ‘red’ period, resulting in a gradual increase in the percentage difference of greens over reds, as shown in Fig. 1C. Total carotenoids and the pools of xanthophyll cycle components (violaxanthin+antheraxanthin+zeaxanthin, VAZ) were consistently higher in the green phenotype during most of the sampling period, if expressed on a leaf area basis (D and E, respectively, in Fig. 1). The differences were more pronounced during the middle of the ‘red’ period. This was due to a stronger trend in the green phenotype to increase the photoprotective carotenoids during winter (Fig. 1E). When total carotenoids were expressed on a chlorophyll (Chl) basis no differences were observed between the two phenotypes (data not shown). When the VAZ/Chl and VAZ/total carotenoids ratios were computed (F and G, respectively, in Fig. 1), there was a slight, yet statistically significant, difference between the two phenotypes, with red leaves displaying lower ratios in the ‘red’ period. Finally, although the xanthophyll cycle pool size in the red leaves was lower, a trend for a lower mid-day epoxidation state was observed during the first half of the sampling period (Fig. 1H), i.e. the cycle seems to be more actively engaged at midday on clear days in the reds.
Fig. 1.
Pigment levels and their ratios in green (closed circles) and red (open circles) phenotypes of C. creticus sampled on the dates indicated. The arrows denote the start and the end of the ‘red’ period. Leaves senesce and fall from mid-April to mid-May in parallel with new leaf emergence. During April, when old and new leaves co-occur, only old leaves (green and red, respectively) were sampled. The May sampling concerns new green leaves for both phenotypes. Values are means from two independent extractions per sampling date. Differences in each parameter between the two phenotypes are not significant during the ‘green’ period, but they are during the ‘red’ period, with the exception of the epoxidation state (EPS). P-values for the red period are inserted. VAZ=violaxanthin+antheraxanthin+zeaxanthin; EPS=(V+0.5A)/VAZ.
In vivo fast transients of chlorophyll fluorescence in pre-darkened leaves
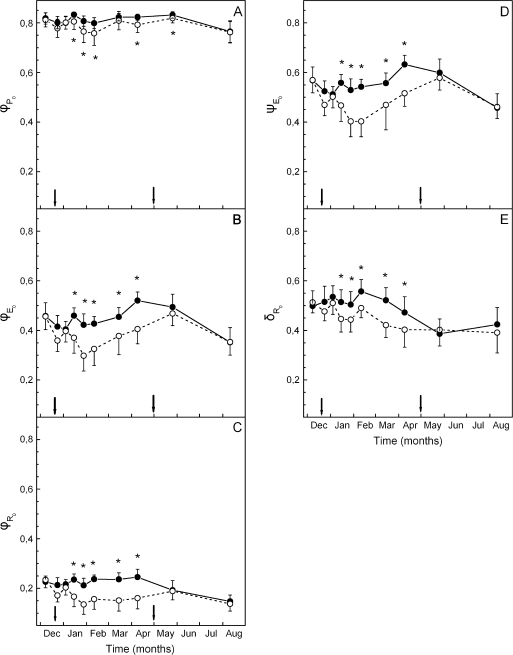
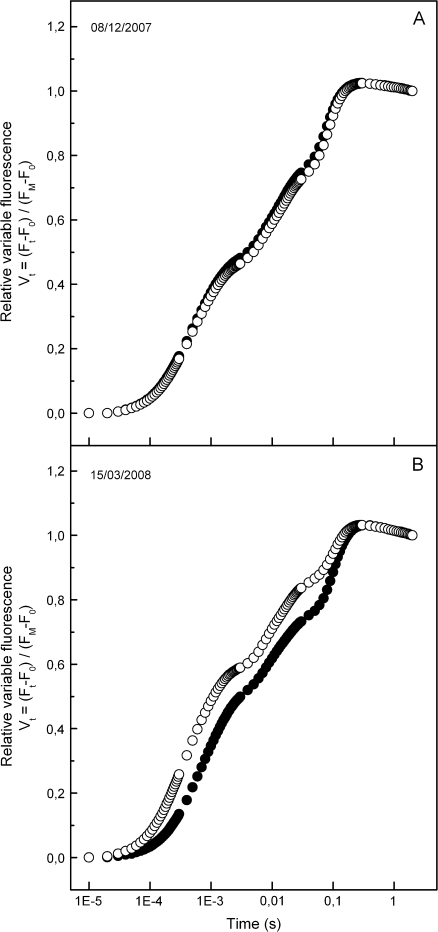
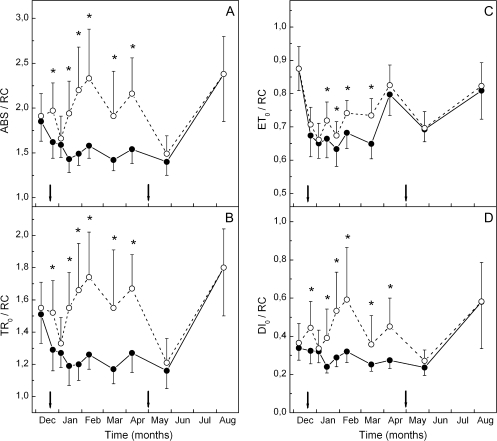
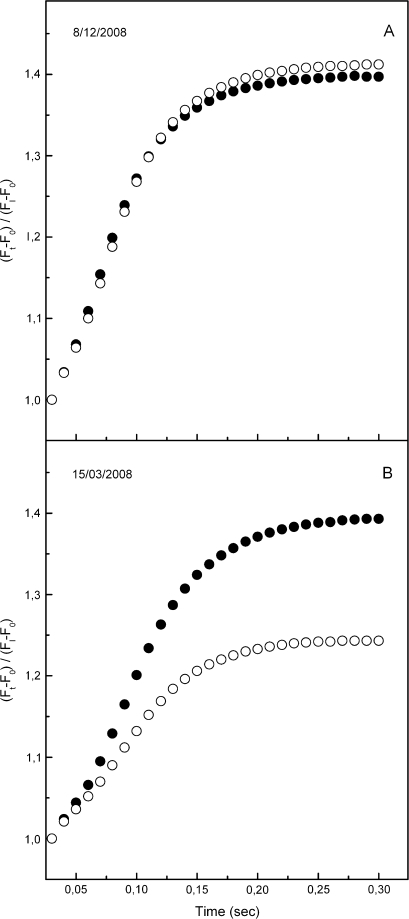
The maximum (pre-dawn) efficiency of PSII photochemistry (φPο or FV/FM) of the green phenotype was high and constant at ∼0.8 during the whole sampling period. In the red phenotype, a slight, yet significant trend for lower FV/FM values was observed ∼2–3 weeks after the onset of reddening and kept up to the end of the red period in mid-spring (Fig. 2A). Much more pronounced differences between the two phenotypes were displayed in the parameters derived after further analysis of the fluorescence rise curves according to the JIP-test. Plotted on a logarithmic time scale, fluorescence rise curves were similar in the two phenotypes during the green and early red period (Fig. 3A), but quite different in the middle of the red period (Fig. 3B). Concomitantly, the derived parameters of photosystem structure and function were similar for the two leaf kinds during the green period and up to 2 weeks after the first signs of reddening (Figs 2, 3). After that date, parameters related to quantum yields or flux ratios (φEο, φRο, ψEo, and δRo; see Materials and methods for definitions) were considerably reduced in the red phenotype (Fig. 2). Concerning those parameters related to specific energy fluxes per active (QA-reducing) PSII reaction centre, these were either reduced (i.e. absorption, ABS/RC; trapped, TR/RC; electron transport, ET0/RC) or remained constant (dissipation, DI0/RC) in the green phenotype (Fig. 4). However, in the red phenotype, the parameters either increased (ABS/RC, TR/RC, DI0/RC) or decreased to a lesser extent (ET0/RC) during the red period. In Fig. 5, the relative amplitude of the I–P phase is given after normalization of the curves at the I-step. This amplitude gives a relative measure of the pool size of the final electron acceptors on the reducing side of PSI (Jiang et al., 2008; Tsimilli-Michael and Strasser, 2008). As shown, the pool size is similar in both phenotypes during the green period (Fig. 5A), remains constant in the green phenotype during the red period but it declines considerably in the red phenotype after reddening (Fig. 5B). All differences in the chlorophyll fluorescence parameters were abolished after leaf re-greening at mid-spring. An additional measurement was performed in late winter to examine possible differences in chlorophyll fluorescence parameters obtained from the abaxial (shaded) leaf side. Results were qualitatively similar to those obtained from the adaxial (exposed) leaf side. Characteristic parameters are shown in Table 2.
Fig. 2.
Quantum yields of energy capture and electron flow as well as probabilities for trapped excitation energy to move electrons along the linear electron transport in green (closed circles) and red (open circles) phenotypes of C. creticus sampled on the dates indicated; φP0 (equivalent to FV/FM), maximum quantum yield of primary photochemistry; φE0, quantum yield of electron transfer to intermediate carriers; φR0, quantum yield of reduction of electron acceptors of PSI; ψE0, efficiency to conserve trapped excitation energy to electron transfer; δR0, efficiency of electron transfer between reduced intermediate carriers to final electron acceptors of PSI. The arrows denote the start and the end of the ‘red’ period. Values are means ±standard deviation from five plants (six leaves per plant). The asterisks denote statistically significant (P <0.05) differences between the two phenotypes in the parameter indicated for each sampling date.
Fig. 3.
Fast chlorophyll fluorescence transients of green (closed circles) and red (open circles) phenotypes sampled before (08/12/2007) and after (15/03/2008) leaf reddening of the red phenotype. Note the logarithmic time scale. On the vertical axis the relative variable fluorescence is given as Vt=(Ft–F0)/(FM–F0), i.e. after double normalization at F0 and FM. F0 is the fluorescence yield at 20 μs. Values are means from five plants (six leaves per plant).
Fig. 4.
Specific energy fluxes per active (i.e. QA-reducing) PSII reaction centre (RC) in green (closed circles) and red (open circles) phenotypes sampled on the dates indicated. The arrows denote the start and the end of the ‘red’ period. ABS, TR0, ET0, and DI0 stand for absorbed energy, trapped energy, electron transport, and dissipated energy, respectively. Values are means ±standard deviation from five plants (six leaves per plant). The asterisks denote statistically significant (P <0.05) differences between the two phenotypes in the indicated parameter for each sampling date.
Fig. 5.
Fluorescence rise kinetics of the I–P phase in green (closed circles) and red (open circles) phenotypes before (08/12/2007) and after (15/03/2008) leaf reddening of the red phenotype. On the vertical axis fluorescence is given as (Ft–F0)/(FI–F0), after normalization at FI. F0 and FI stand for fluorescence yield at 20 μs and at 30 ms, respectively. Values are means from five plants (six leaves per plant).
Table 2.
Chlorophyll fluorescence parameters obtained from the abaxial (lower) leaf side of leaves of the green and red phenotype in late winter
| Fluorescence parameter | Green leaves | Red leaves |
| φPo | 0.838±0.011 | 0.822±0.024 |
| φEo | 0.507±0.044 | 0.392±0.063 |
| φRo | 0.201±0.028 | 0.132±0.026 |
| ψEο | 0.605±0.048 | 0.476±0.064 |
| δRo | 0.396±0.049 | 0.337±0.035 |
| ABS/RC | 1.947±0.158 | 2.385±0.446 |
Data are means ±standard deviation from 10 leaves (two leaves/shrub). Differences in each parameter between the two phenotypes are statistically significant (P <0.05), with the exception of φPo.
Light dependence of PSII yield and development of the non-photochemical energy losses
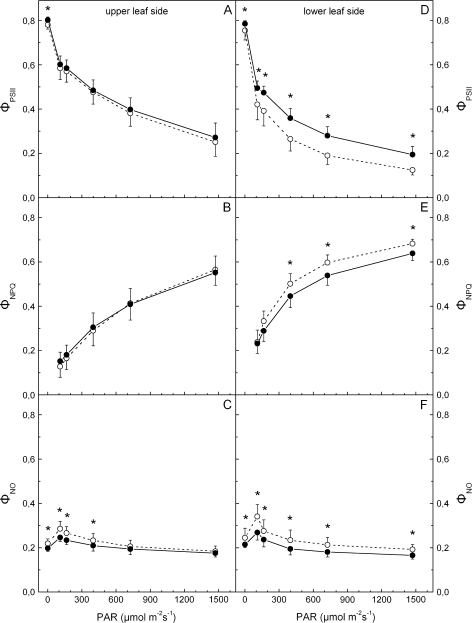
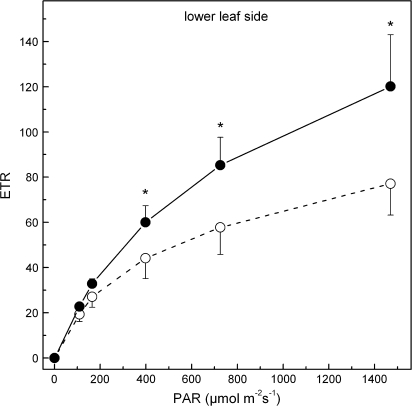
Figure 6A indicates that light-acclimated PSII yield, ΦPSII, obtained from the adaxial leaf side, is similar in the two leaf types, and the same is true for the light-dependent development of regulated, ΦNPQ, and non-regulated, ΦNO, non-photochemical energy quenching (B and C, respectively, in Fig. 6). Corresponding measurements obtained from the lower leaf side (which does not contain anthocyanins) indicate that ΦPSII of red leaves is considerably lower at all PAR levels tested (Fig. 6D). However, abaxial ΦNPQ was higher in the red leaves at high PAR, while ΦNO was higher at all PAR levels tested (E and F, respectively, in Fig. 6). In the case of the abaxial side which is devoid of anthocyanins, the accurate measurement of absorbed PAR (see Material and methods) permits the calculation of linear ETRs along PSII. As shown in Fig. 7, the values of ETR in red leaves at high PAR are lower.
Fig. 6.
Changes in light-acclimated PSII effective yield, ΦPSII, and non-photochemical energy quenching (either regulated, ΦNPQ, or non-regulated, ΦNO), versus incident light level, in green (closed circles) or red (open circles) leaves. The experiment was performed during the ‘red’ period (5 March 2008) and the left and right panels refer to values obtained from the upper and lower leaf side, respectively. The leaves remained under the indicated PAR until stable values for each parameter were obtained. Values are means ±standard deviation from five plants (four leaves per plant). The asterisks denote statistically significant (P <0.05) differences between the two phenotypes in the parameter indicated for each PAR level.
Fig. 7.
Linear electron transport rates (ETR) along PSII in the lower leaf side of green (closed circles) and red (open circles) phenotypes. The experiment was performed during the ‘red’ period (5 March 2008). The leaves remained under the indicated PAR until stable values for each parameter were obtained. Values are means ±standard deviation from five plants (four leaves per plant). The asterisks denote statistically significant (P <0.05) differences between the two phenotypes in the parameter indicated for each PAR level.
Discussion
The photoprotective hypothesis for leaf anthocyanins entails a lower photoinhibitory risk in the red leaves during the stressful period of the year. Mediterranean plants (García-Plazaola et al., 1999), including C. creticus (Karavatas and Manetas, 1999), may suffer from sustained photoinhibition under cold winter temperatures combined with high light. However, assessing the mean photosynthetic behaviour in a population may hinder identification of intra-species differences between strong and weak phenotypes. In this investigation, it was shown that the green phenotype of C. creticus is more tolerant of winter photoinhibition while the red phenotype is quite sensitive. Traditionally, sustained photoihibition is assessed through measurements of maximum quantum yield of PSII photochemistry in dark-acclimated leaves. It has been argued, however, that this parameter (usually given as FV/FM or φPο) is not a sensitive indication of stress vulnerability (Strasser et al., 2004). A high efficiency of exciton trapping indicates that primary photochemistry is not limiting, yet limitations in further steps of the excitation energy processing and its transformation to redox energy and electron transport may be hidden behind a high φPo. In the case of C. creticus, the green phenotype maintained the yield-related parameters (i.e. φPo, φEo, φRo, ψEo, δRo) almost constant during the whole sampling period (Fig. 2). Unexpectedly though, the corresponding parameters in the red phenotype dropped to lower values during late winter and early spring, in spite of anthocyanin accumulation (Fig. 2). The decline in φPo was minor (∼7% difference between green and red leaves at most), confirming that primary photochemistry was not, in fact, limiting in the red phenotype. Corresponding differences in the other parameters of quantum yields depended on sampling date and ranged between ∼20% for efficiency of electron flow from QA– to intersystem electron carriers (φEo) and ∼30% for the rest of partial quantum yields. Hence, it can be concluded that the red-leaf phenotype suffers from a loss of photosynthetic activity during winter. Since the green/red differences in partial quantum yields are increasing along the linear electron flow from PSII to PSI (Fig. 2), an enhanced transformation of active PSII centres to non-QA- and/or non-Q-reducing centres can be inferred for the red phenotype. In addition, PSI is also negatively affected, as judged by the considerable reduction in the efficiency of electron transfer from intermediate carriers to final acceptors of PSI and the decline in the pools of these final acceptors (Figs 2C, E, 5). It can also be noted that the loss of photosynthetic activity in red leaves does not recover even after the full anthocyanin complement is realized in February. We consider that the above results weaken the photoprotective hypothesis, unless it is assumed that the target for protection is not related to photosynthesis. Alternatively, some photoprotection could be assumed, yet not enough to fully compensate for the higher vulnerability of the red phenotype to winter stress.
The conclusion for a lower photosynthetic activity in the red leaf phenotype is further strengthened by measurements of light-acclimated PSII yields of the upper leaf surface at various incident light levels (Fig. 6A). Although values of PSII effective yield, ΦPSII, are only slightly (and non-significantly) lower in the red leaves, it has to be noted that PAR levels actually penetrating to red leaf mesophyll are in fact lower compared with their green counterparts, due to anthocyanin absorption of the blue actinic light used in these measurements. Accordingly, for the same incident PAR, higher effective PSII yields would be expected in reds than greens. Instead of that, lower PSII yields were obtained. This is shown more clearly if the PSII effective yield and ETR are considered versus incident PAR obtained from the lower leaf surface when this surface is given actinic light (Figs 6D, 7). Under the conditions of this experiment, spongy mesophyll chloroplasts on both leaf types enjoy similar actual PAR (for a given incident PAR), since anthocyanins accumulate only in the palisade mesophyll (Kytridis and Manetas, 2006) and the abaxial leaf surface is always green. Figures 6D and 7 show that the lower leaf side of adaxially red leaves displays considerably lower ΦPSII and ETR, especially at high PAR. Although lower effective PSII yield and ETR could be the result of a shade-acclimation syndrome of red leaves (see below), as would be expected for the lower leaf side due to the overlying anthocyanic screen, we point out that such an acclimation should not affect the maximum quantum yield of PSII photochemistry nor the quantum yields of partial electron flow reactions (see Table 2). These findings also reject the possibility that the alleged photoprotective function of leaf anthocyanins is targeted preferentially to the lower leaf surface.
Concomitant to the above may be the finding that the yield of regulated non-photochemical quenching, ΦNPQ, of the adaxial surface is similar in the two leaf types, although one should expect a lower ΦNPQ in red leaves due to the actually lower PAR reaching the palisade mesophyll (Fig. 6B). Corresponding values of ΦNPQ obtained from the abaxial surface are significantly higher in red leaves at the higher PAR levels (Fig. 6E). This may be consistent with the lower midday epoxidation state of the xanthophyll cycle components in the red leaves during early winter (Fig. 1H), indicating a need for more active engagement of the cycle, in spite of the anthocyanic screen. Concerning the quantum yield of non-regulated energy loss, ΦNO, this was significantly higher in the red leaves at all PAR levels (Fig. 6F). Since a high value of ΦNO indicates vulnerability to photoinhibition (Hendrickson et al., 2004), the assumption of an actual photoprotective function for leaf anthocyanins is further weakened.
Apart from the lower photosynthetic activity of red leaves suggested from the above results, a reduced photoprotective capacity is also evident if pigment levels and their changes are considered during the sampling period. Total carotenoids and the pool size of xanthophyll cycle components, expressed on a leaf area basis, were considerably lower in the red leaf phenotype throughout the sampling period and the differences became more pronounced during the red period (Fig. 1D, E). This is due to the strong increasing trend in both parameters during winter in the green leaves, while the capacity for such an increase in the reds is less evident. One may argue that a better way of expressing photoprotective carotenoid levels would be on a chlorophyll basis. Indeed the total carotenoids/Chl ratio was similar in the two leaf types throughout the sampling period, while the VAZ/Chl ratio became marginally lower in red leaves only during winter (Fig. 1F). This is due to the considerable decline in chlorophyll levels of the red leaves (Fig. 1B). We argue, however, that the crucial parameter in photoprotection by carotenoids may not be the concentration of the target molecules (chlorophylls) but their absorptance. Leaf absorptance is a weak function of chlorophyll levels, especially when these levels are normal. Thus, a 50% decrease in Chl results in a ∼10% decrease in leaf absorptance (Dima et al., 2006) due to the sieve effect observed in heterogeneous, chlorophyll-containing tissues (Vogelmann, 1993). It can therefore be concluded that the photoprotective capacity of the red-leaf phenotype is smaller. This photoprotective inefficiency, together with the report that the nitrogen content in the red phenotype of C. creticus is also lower (Kytridis et al., 2008) may underlie the observed decline of photosynthetic activity during winter. According to the above, we could suggest that Chl loss, combined with anthocyanin accumulation, may have the adaptive significance of reducing (albeit slightly) the excitation pressure in the chloroplasts of the red leaf phenotype. Nevertheless, their additive effect may not be enough to alleviate photosynthetic inactivation fully. Increases in xanthophyll cycle pool sizes and chlorophyll loss have been reported as part of an adaptive repertoire of Mediterranean plants during adverse environmental conditions (Kyparissis et al., 1995, 2000). Red leaves of C. creticus display a capacity for Chl loss, but not for an increase in carotenoids. Thus, even if anthocyanins exert a photoprotective function, their effect is slight.
It has been repeatedly argued that an anthocyanic screen would lead to a shade acclimation of photosynthesis (Gould et al., 2002; Manetas et al., 2003; Hughes and Smith, 2007; Kyparissis et al., 2007). Ample evidence for an apparent shade acclimation was also obtained in this investigation. Some of the evidence, however, could be partly linked to the higher sensitivity of the red phenotype to the superimposed winter stress. Hence, the lower ratios of VAZ/total carotenoids in the red leaves during winter could be interpreted either as part of the shade acclimation syndrome (Thayer and Björkman, 1990) or as an inherent inability of the red phenotype to up-regulate the xanthophyll pool size (Fig. 1E, G). Moreover, the enhanced mean antenna size (as judged by the specific flux of light absorption per reaction centre, ABS/RC) is an indication of shade acclimation (Anderson, 1986) and it was shown in this study to increase considerably after anthocyanin accumulation (Fig. 4A). Yet, according to the assumptions of the JIP-test (Strasser et al., 2004), the antenna size is expressed per active (i.e. QA-reducing) PSII centre. Hence, the increase in this parameter could be the combined effect of an increase in the antenna size and a decrease in active PSII centres. However, the diminishing Chl a/b ratios (Fig. 1C) can be better accommodated within an assumed shade acclimation in red leaves, than interpreted on the basis of their higher winter stress sensitivity (Anderson, 1986).
In conclusion, this investigation demonstrated that the red leaf phenotype of the evergreen C. creticus is inherently more sensitive to winter stress, displaying a reduction in electron flow in both photosystems and a difficulty in improving the photoprotective potential based on the xanthophyll cycle components, in spite of the higher need for non-photochemical quenching. Anthocyanin accumulation and the concomitant chlorophyll loss may afford some protection by reducing excitation pressure and increasing the anti-oxidative potential, yet not to the extent of a full photosynthetic competence with the green phenotype.
Acknowledgments
We thank the Research Committee of the University of Patras (project K Karatheodori) for funding this work.
Appendix
Cardinal points in the kinetics of fast chlorophyll fluorescence rise and the formulae used for the calculation of biophysical parameters, according to the JIP-test.
| F0 | F20μs, fluorescence intensity at 20 μs, considered as the fluorescence intensity when all reaction centres (RC) are open |
| FM | Maximal fluorescence intensity, considered as the fluorescence intensity when all RCs are closed |
| FV | Variable fluorescence, FM–F0 |
| FJ or FI | Fluorescence intensity at 2 ms or 30 ms, respectively |
| M0 or (dV/dt)0 | 4(F300μs–F0)/(FM–F0), initial slope of the fluorescence transient |
| VJ | (F2ms–F0)/(FM–F0), relative variable fluorescence at 2 ms |
| VI | (F30ms–F0)/(FM–F0), relative variable fluorescence at 30 ms |
| Quantum yields or flux ratios | |
| φPo or TR0/ABS | FV/FM=1–F0/FM |
| φEo or ET0/ABS | φPο·ψEo=1–FJ/FM |
| φRo | φPο·ψE·δRο=1–FI/FM |
| ψEο or ET0/TR0 | 1–VJ=(FM–FJ)/(FM–F0) |
| δRo | (1–VI)/(1–VJ) = (FM–FI)/(FM–FJ) |
| Specific energy fluxes per RC | |
| ABS/RC | (M0/VJ)·FM/(FM–F0) |
| TR0/RC | M0/VJ |
| ET0/RC | (M0/VJ)·(1–VJ) |
| DI0/RC | (M0/VJ)·(F0/FV) |
References
- Anderson JM. Photoregulation of the composition, function, and structure of thylakoid membranes. Annual Review of Plant Physiology and Plant Molecular Biology. 1986;37:93–136. [Google Scholar]
- Archetti M. The origin of autumn colours. Journal of Theoretical Biology. 2000;205:625–630. doi: 10.1006/jtbi.2000.2089. [DOI] [PubMed] [Google Scholar]
- Archetti M, Leather SR. A test of the coevolution theory of autumn colours: colour preference of Rhopalosiphum padi on Prunus padus. Oikos. 2005;110:339–343. [Google Scholar]
- Burger J, Edwards GE. Photosynthetic efficiency, and photodamage by UV and visible radiation, in red versus green leaf in Coleus varieties. Plant and Cell Physiology. 1996;37:395–399. [Google Scholar]
- Esteban R, Fernández-Marín B, Becerril JM, García-Plazaola JI. Photoprotective implications of leaf variegation in E. dens-canis L. and P. officinalis L. Journal of Plant Physiology. 2008;165:1255–1263. doi: 10.1016/j.jplph.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Dima E, Manetas Y, Psaras GK. Chlorophyll distribution pattern in inner stem tissues: evidence from epifluorescence microscopy and reflectance measurements in 20 woody species. Trees. 2006;20:515–521. [Google Scholar]
- Feild TS, Lee DW, Holbrook M. Why leaves turn red in autumn: the role of anthocyanins in senescing leaves of red-osier dogwood. Plant Physiology. 2001;127:566–574. [PMC free article] [PubMed] [Google Scholar]
- García-Plazaola JI, Artetxe U, Dunabeitia MK, Becceril JM. Role of photoprotective systems of holm-oak (Quercus ilex) in the adaptation to winter conditions. Journal of Plant Physiology. 1999;155:625–630. [Google Scholar]
- Genty B, Briantais J-M, Baker NR. The relationship between quantum yield of photosynthetic electron transport rate and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Gould KS, Vogelmann TC, Han T, Clearwater MJ. Profiles of photosynthesis within red and green leaves of Quintinia serrata A. Cunn. Physiologia Plantarum. 2002;116:127–133. doi: 10.1034/j.1399-3054.2002.1160116.x. [DOI] [PubMed] [Google Scholar]
- Hamilton WD, Brown SP. Autumn tree colours as a handicap signal. Proceedings of the Royal Society of London B: Biological Sciences. 2001;268:1489–1493. doi: 10.1098/rspb.2001.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson L, Furbank RT, Chow WS. A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynthesis Research. 2004;82:73–81. doi: 10.1023/B:PRES.0000040446.87305.f4. [DOI] [PubMed] [Google Scholar]
- Hughes NM, Neufeld HS, Burkey KO. Functional role of anthocyanins in high-light winter leaves of the evergreen herb Galax urceolata. New Phytologist. 2005;168:575–587. doi: 10.1111/j.1469-8137.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Hughes NM, Smith WK. Attenuation of incident light in Galax urceolata (Diapensiaceae): concerted influence of adaxial and abaxial anthocyanic layers on photoprotection. American Journal of Botany. 2007;94:784–790. doi: 10.3732/ajb.94.5.784. [DOI] [PubMed] [Google Scholar]
- Jiang HX, Chen LS, Zheng JG, Han S, Tang N, Smith BR. Aluminum-induced effects on Photosystem II photochemistry in Citrus leaves assessed by the chlorophyll a fluorescence transient. Tree Physiology. 2008;28:1863–1871. doi: 10.1093/treephys/28.12.1863. [DOI] [PubMed] [Google Scholar]
- Karageorgou P, Manetas Y. The importance of being red when young: anthocyanins and the protection of young leaves of Quercus coccifera from insect herbivory and excess light. Tree Physiology. 2006;26:613–621. doi: 10.1093/treephys/26.5.613. [DOI] [PubMed] [Google Scholar]
- Karavatas S, Manetas Y. Seasonal patterns of photosystem 2 photochemical efficiency in evergreen sclerophylls and drought semi-deciduous shrubs under Mediterranean field conditions. Photosynthetica. 1999;36:41–49. [Google Scholar]
- Krol M, Gray GR, Hurry VM, Oquist G, Malker L, Huner NPA. Low-temperature stress and photoperiod affect an increased tolerance to phoinhibition in Pinus banksiana seedlings. Canadian Journal of Botany. 1995;73:1119–1127. [Google Scholar]
- Kyparissis A, Drilias P, Manetas Y. Seasonal fluctuations in photoprotective (xanthophyll cycle) and photoselective (chlorophylls) capacity in eight Mediterranean plant species belonging to two different growth forms. Australian Journal of Plant Physiology. 2000;27:265–272. [Google Scholar]
- Kyparissis A, Grammatikopoulos G, Manetas Y. Leaf morphological and physiological adjustments to the spectrally selective shade imposed by anthocyanins in Prunus cerasifera. Tree Physiology. 2007;27:849–857. doi: 10.1093/treephys/27.6.849. [DOI] [PubMed] [Google Scholar]
- Kyparissis A, Petropoulou Y, Manetas Y. Summer survival of leaves in a soft-leaved shrub (Phlomis fruticosa L., Labiatae) under Mediterranean field conditions: avoidance of photoinhibitory damage through decreased chlorophyll contents. Journal of Experimental Botany. 1995;46:1825–1831. [Google Scholar]
- Kytridis V-P, Karageorgou P, Levizou E, Manetas Y. Intra-species variation in transient accumulation of leaf anthocyanins in Cistus creticus during winter: evidence that anthocyanins may compensate for an inherent photosynthetic and photoprotective inferiority of the red-leaf phenotype. Journal of Plant Physiology. 2008;165:952–959. doi: 10.1016/j.jplph.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Kytridis V-P, Manetas Y. Mesophyll versus epidermal anthocyanins as potential in vivo antioxidants: evidence linking the putative antioxidant role to the proximity of oxy-radical source. Journal of Experimental Botany. 2006;57:2203–2210. doi: 10.1093/jxb/erj185. [DOI] [PubMed] [Google Scholar]
- Lee DW, Gould KS. Anthocyanins in leaves and other vegetative organs: an introduction. Advances in Botanical Research. 2002;37:1–16. [Google Scholar]
- Lee DW, O'Keefe J, Holbrook NM, Feild TS. Pigment dynamics and autumn leaf senescence in a New England deciduous forest, eastern USA. Ecological Research. 2003;18:677–694. [Google Scholar]
- Liakopoulos G, Nikolopoulos D, Klouvatou A, Vekkos K-A, Manetas Y, Karabourniotis G The photoprotective role of epidermal anthocyanins and surface pubescence in young leaves of grapevine (Vitis vinifera) Annals of Botany. 2006;98:257–265. doi: 10.1093/aob/mcl097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licthenthaler HK, Wellburn AR. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemical Society Transactions. 1983;11:591–592. [Google Scholar]
- Lindoo SJ, Caldwell MM. Ultraviolet-B radiation-induced inhibition of leaf expansion and promotion of anthocyanin production – lack of involvement of low irradiance phytochrome system. Plant Physiology. 1978;61:278–282. doi: 10.1104/pp.61.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetas Y. Why some leaves are anthocyanic and why most anthocyanic leaves are red? Flora. 2006;201:163–177. [Google Scholar]
- Manetas Y, Drinia A, Petropoulou Y. High contents of anthocyanins in young leaves are correlated with low pools of xanthophyll cycle components and low risk of photoinhibition. Photosynthetica. 2002;40:349–354. [Google Scholar]
- Manetas Y, Petropoulou Y, Psaras GK, Drinia A. Exposed red (anthocyanic) leaves of Quercus coccifera display shade characteristics. Functional Plant Biology. 2003;30:265–270. doi: 10.1071/FP02226. [DOI] [PubMed] [Google Scholar]
- Ougham HJ, Morris P, Thomas H. The colors of autumn leaves as symptoms of cellular recycling and defenses against environmental stresses. Current Topics in Developmental Biology. 2005;66:135–160. doi: 10.1016/S0070-2153(05)66004-8. [DOI] [PubMed] [Google Scholar]
- Ougham HJ, Thomas H, Archetti M. The adaptive value of leaf colour: origin and evolution of autumn colours. New Phytologist. 2008;179:9–13. doi: 10.1111/j.1469-8137.2008.02505.x. [DOI] [PubMed] [Google Scholar]
- Papageorgiou GC, Tsimilli-Michael M, Stamatakis K. The fast and slow kinetics of chlorophyll a fluorescence induction in plants, algae and cyanobacteria: a viewpoint. Photosynthesis Research. 2007;94:275–290. doi: 10.1007/s11120-007-9193-x. [DOI] [PubMed] [Google Scholar]
- Pietrini F, Iannelli MA, Massacci A. Anthocyanin accumulation in the illuminated surface of maize leaves enhances protection from photo-inhibitory risks at low temperature, without further limitation to photosynthesis. Plant, Cell & Environment. 2002;25:1251–1259. [Google Scholar]
- Steyn WJ, Wand SJE, Holcroft DM, Jacobs G. Anthocyanins in vegetative tissues: a proposed unified function in photoprotection. New Phytologist. 2002;155:349–361. doi: 10.1046/j.1469-8137.2002.00482.x. [DOI] [PubMed] [Google Scholar]
- Strasser RJ, Srivastava A, Govindjee Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochemistry and Photobiology. 1995;61:32–42. [Google Scholar]
- Strasser RJ, Tsimilli-Michael M, Srivastava A. Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee, editors. Chlorophyll fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration series, Vol. 19. Rotterdam: Kluwer; 2004. pp. 321–362. [Google Scholar]
- Thayer SS, Björkman O. Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynthesis Research. 1990;23:331–343. doi: 10.1007/BF00034864. [DOI] [PubMed] [Google Scholar]
- Tsimilli-Michael M, Strasser RJ. In vivo assessment of plants’ vitality: applications in detecting and evaluating the impact of mycorrhization on host plants. In: Varma A, editor. Mycorrhiza: state of the art, genetics and molecular biology, eco-function, biotechnology, eco-physiology, structure and systematics. 3rd edn. Berlin: Springer; 2008. 679–703. [Google Scholar]
- Vogelmann TC. Plant tissue optics. Annual Review of Plant Physiology and Plant Molecular Biology. 1993;44:231–251. [Google Scholar]
- Yordanov I, Goltsev V, Stefanov D, Chernev P, Zaharieva I, Kirova M, Gecheva V, Strasser RJ. Preservation of photosynthetic electron transport from senescence-induced inactivation in primary leaves after decapitation and defoliation of bean plants. Journal of Plant Physiology. 2008;165:1954–1963. doi: 10.1016/j.jplph.2008.05.003. [DOI] [PubMed] [Google Scholar]