Abstract
Wortmannin, a specific inhibitor of phosphatidyl-inositol 3-kinase, is a useful tool for studying protein trafficking and identifying organelles in the plant secretory and endocytic pathways. It has recently been demonstrated that wortmannin at 16.5 μM or 33 μM caused the prevacuolar compartments (PVCs), identified as multivesicular bodies (MVBs) by their enrichment in vacuolar sorting receptor (VSRs) proteins and the BP-80 reporter, to form small vacuoles rapidly. However, the source(s) of the membrane needed for the rapid enlargement of PVCs/MVBs has been unclear. Using both confocal immunofluorescence and immunogold EM with high pressure freeze substitution of plant samples, it has been demonstrated here that wortmannin induces homotypic fusions of PVCs/MVBs thus providing an explanation for the demand for extra membrane. In addition, possible wortmannin-induced fusions between the trans-Golgi network (TGN) and PVC, as well as between the small internal vesicles and PVC membrane, were also observed and they may also contribute to the membranes needed for PVC enlargement. In contrast to mammalian cells and yeast, wortmannin-induced fusion of PVCs appears to be unique to plants.
Keywords: Homotypic fusion, multivesicular body, prevacuolar compartment, trans-Golgi network, wortmannin
Introduction
Multivesicular bodies (MVBs) are membrane-bound compartments containing multiple small vesicles. Recently, MVBs have been identified to function as the prevacuolar compartment (PVC) (Bethke and Jones, 2000) and are characteristically enriched in vacuolar sorting receptors (VSRs), which mediate protein trafficking between the Golgi apparatus and the vacuole (Li et al., 2002; Tse et al., 2004; Miao et al., 2006, 2008; Wang et al., 2007). MVBs also lie on the endocytic pathway to the vacuole and appear to be equivalent to the late endosome of mammalian cells (Lam et al., 2007a, b, c, 2008; Robinson et al., 2008).
The drug wortmannin is often used to perturb receptor-mediated traffic to the lytic compartment of the cell, a feature well-documented for yeast (Schu et al., 1993; Wurmser et al., 1999), mammalian (Davidson, 1995; Arighi et al., 2004), and plant (Matsuoka et al., 1995; da Silva et al., 2005) cells. This effect of wortmannin is achieved through inhibition of phosphatidylinositol 3-kinase (Vps34p in yeast), an enzyme responsible for the production of PI-3P, a lipid characteristic of endosomal membranes. The proteins constituting the small subunit of retromer in yeast (Vps5p, Vps17p) as well as their functional equivalents in mammalian cells, the sorting nexins (SNX1, SNX2) interact with PI-3P through their characteristic Phox domains (Yu and Lemmon, 2001; Cozier et al., 2002). Thus, wortmannin interferes with the transport of proteins to the lysosome/vacuole by preventing retromer-mediated receptor recycling to the trans-Golgi network (TGN) (Kundra and Kornfeld, 1998; Seaman, 2005).
Morphologically, wortmannin is known to cause a dilation/swelling of multivesicular endosomes in both mammalian (Reaves et al., 1996; Shpetner et al., 1996; Bright et al., 2001) and plant cells (Tse et al., 2004; Jaillais et al., 2006; Miao et al., 2006, 2008; Lam et al., 2007a, b; Silady et al., 2008; Tse et al., 2009). This is often attributed to the prevention of retrograde transport from these organelles (Bright et al., 2001; Poupon et al., 2003), but since PI-3 kinase is also required for the formation of the internal vesicles in endosomes (Fernandez-Borja et al., 1999; Futter et al., 2001), the dilation may be the net sum of both inhibitory events.
Wortmannin-induced swelling of plant PVCs can be readily observed in the confocal microscope by using fluorescently-labelled PVC markers, for example, various GFP–VSR fusion constructs in transgenic tobacco BY-2 cells (Tse et al., 2004; Miao et al., 2006, 2008), or GFP–ARA7 in root cells of transgenic Arabidopsis plants (Robinson et al., 2008; Silady et al., 2008). With these membrane-bound reporters, it is relatively easy to follow the development of doughnut-like structures from punctate signals that are distinguishable from Golgi stacks. In a recent review (Lam et al., 2007b) it was speculated about the possibility of a further mechanism for the wortmannin-induced vacuolation of plant PVCs: fusion of PVCs and/or fusion of the TGN with the PVC as well as fusion betweeen the small internal vesicles and MVB membrane. In this paper, evidence for these scenarios is now presented.
Materials and methods
Cell and plant materials
The construction of the recombinant plasmid GFP–BP-80 and the characterization of a transgenic tobacco BY-2 cell line stably expressing GFP–BP-80 have been described previously (Tse et al., 2006). The transient expression of GFP–BP-80 in protoplasts of suspension-cultured Arabidopsis cells was performed as described previously (Miao and Jiang, 2007; Miao et al., 2008). Mung bean was grown in pots in the greenhouse of the Department of Biology, the Chinese University of Hong Kong.
Antibodies
The production and characterization of the polyclonal antibody VSRAt-1 specifically recognizing VSR proteins has been described previously (Tse et al., 2004). The generation and characterization of the polyclonal antibody SCAMP1a, raised by a peptide of rice OsSCAMP1 in rabbit, were described by Lam et al. (2007a).
Drug treatment
For the treatment of suspension-cultured tobacco BY-2 cells or Arabidopsis protoplasts with wortmannin, aliquots of wortmannin solution (stock solution at 1 mM in DMSO) were added to liquid culture to a final concentration of 16.5 μM or 33 μM, followed by incubation for 1 h at room temperature prior to chemical fixation or confocal imaging.
For the treatment of developing mung bean seeds with wortmannin, mung bean seeds at 6 d after flowering (DAF) were collected from the greenhouse and cut across into two halves with a sharp blade. The surfaces of half the seeds were immersed in MS medium containing wortmannin at 16.5 μM or 33 μM, followed by incubation for 1 h at room temperature prior to chemical fixation or high pressure freezing.
Confocal immunofluorescence studies
Fixation and preparation of tobacco BY-2 cells and developing mung bean seeds and their labelling and analysis by confocal immunofluorescence have been described previously (Jiang and Rogers, 1998; Jiang et al., 2000; Li et al., 2002; Tse et al., 2004). Here, thin sections from the surface of the half mung bean seeds immersed in medium containing wortmannin were used in confocal immunofluorescence studies. The settings for collecting confocal images within the linear range were as described previously (Jiang and Rogers, 1998; Tse et al., 2004). For single immunolabelling, polyclonal antibodies were incubated at 4 °C overnight for both VSRAt-1 and SCAMP1a antibodies. All confocal immunofluorescence images were collected using the Bio-Rad Radiance 2100 system. Images were processed using Adobe Photoshop software as described previously by Jiang and Rogers (1998).
Structural and immunogold EM study
Immunogold EM on ultra-thin sections prepared from high-pressure freeze-substitution of developing mung bean cotyledons was performed essentially as previously described (Tse et al., 2004; Wang et al., 2007, 2009). Briefly, thin sections of developing mung bean cotyledons immersed in medium containing wortmannin were frozen in a high-pressure freezing apparatus (EMP2, Leica, Bensheim, Germany). Substitution was performed in dry acetone containing 0.1% uranyl acetate at –85 °C for 5 d in an AFS freeze-substitution unit (Leica, Wetzlar, Germany). Samples were stepwise infiltrated with HM20, embedded, and UV polymerized at –35 °C. Immunogold labelling on ultra-thin sections with anti-VSRAt-1 antibody at 40 μg ml−1 was performed using standard procedures (Tse et al., 2004). The ultra-thin sections, post-stained by aqueous uranyl acetate/lead citrate, were examined using a Hitachi H-7650 transmission electron microscope with a CCD camera (Hitachi High-Technologies Corporation, Japan) operating at 80 kV (Tse et al., 2004; Lam et al., 2007a).
Results
Wortmannin induces PVC fusions in transgenic tobacco BY-2 cells
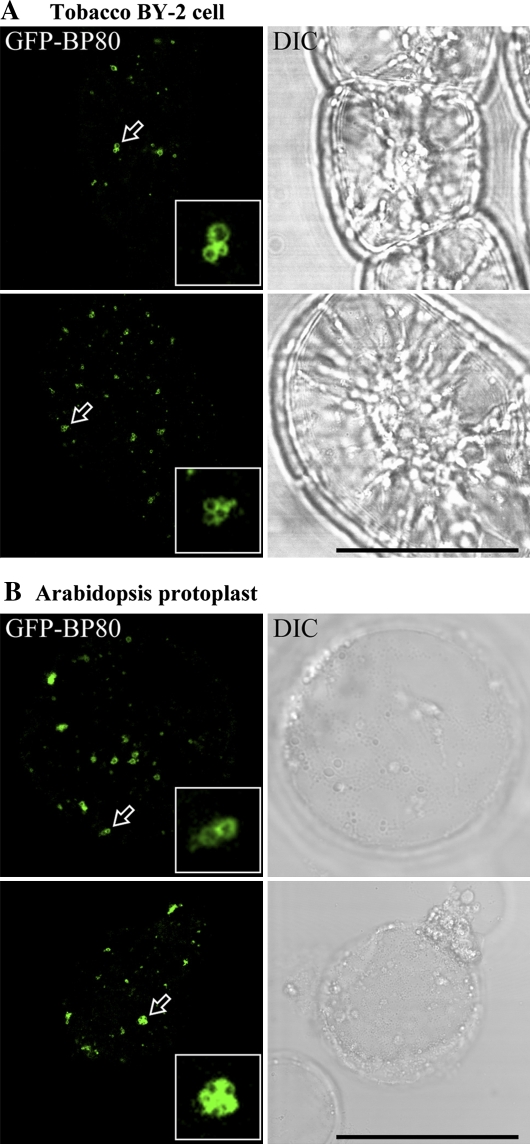
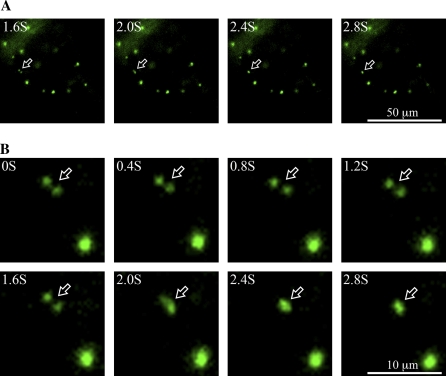
As a first step to test if a homotypic fusion of PVCs might occur upon wortmannin treatment, transgenic tobacco BY-2 cells expressing the PVC marker GFP–BP-80 (Tse et al., 2004) were treated with wortmannin at 16.5 μM, and observations were made using the confocal microscope after 30 min. As shown in Fig. 1A, clusters of enlarged PVCs were readily observed. Similar aggregates of dilated PVCs were also visualized in Arabidopsis protoplasts transiently expressing the same PVC marker GFP–BP-80 after 30 min of identical wortmannin application (Fig. 1B). The punctate PVC numbers were reduced by more than 70% within 2 h after wortmannin treatment, and those remaining were enlarged (Table 1). Since these results are very suggestive of a wortmannin-induced homotypic fusion between PVCs in both BY-2 and Arabidopsis cells, real-time observations were next performed in living transgenic BY-2 cells expressing the PVC marker GFP–BP-80 using a fluorescence microscope equipped with a CCD camera for image collection. As shown in Fig. 2 and Supplementary movies 1 and 2 (see Supplementary data at JXB online), fusion between two punctate GFP–BP-80 labelled PVCs was observed over a period of 3 s in BY-2 cells after 15 min of 16.5 μM wortmannin treatment, The fusion signals did not subsequently separate (data not shown). These results thus provide direct evidence for a wortmannin-induced homotypic fusion of PVC in tobacco BY-2 cells, explaining at least in part the enlargement of the PVC and giving a clue to the sources of membrane required for this rapid dilation.
Fig. 1.
Wortmannin-induced PVC fusion in transgenic tobacco BY-2 and Arabidopsis suspension-cultured cells. Tobacco BY-2 cells stably expressing the PVC marker GFP–BP-80 (A) or Arabidopsis protoplasts transiently expressing the PVC marker GFP–BP-80 (B) were treated with wortmannin at 16.5 μM for 30 min before samples were collected for confocal imaging. Arrows indicate examples of possible PVC fusions where inserted images are the enlarged examples. DIC, differential interference contrast. Scale bar=50 μm. (This figure is available in colour at JXB online.)
Table 1.
Numbers of punctate PVCs in untreated cells versus number of enlarged PVCs at the end of a 2 h wortmannin treatment in transgenic BY-2 cells expressing the PVC reporter GFP–BP-80
| Drug treatment | Total no. of PVCs or enlarged PVCs | No. of cells counted | Average no. of PVC or enlarged PVCs per cell |
| Before treatment | 1079 | 30 | 36 |
| After treatment | 315 | 30 | 10.5 |
The numbers of punctate or enlarged PVCs before or after wortmannin treatment were calculated from observation of confocal images.
Fig. 2.
Dynamics of wortmannin-induced PVC fusion in transgenic tobacco BY-2 cells. Tobacco BY-2 cells stably expressing the PVC marker GFP–BP-80 were treated with wortmannin at 16.5 μM for 15 min, followed by time-lapse image collection using an epifluorescent microscope equipped with a CCD camera. Shown are a series of images collected within 3 s at the time intervals indicated. Arrows point to examples of PVC fusion between the two GFP–BP-80-marked PVCs. (A) An overview of the cell under investigation. (B) The enlarged areas with the two fusing PVCs. Scale bars are 50 μm for (A) and 10 μm in (B), respectively. (This figure is available in colour at JXB online.)
In order to demonstrate wortmannin-induced PVC fusion at the ultra-structural level, ultra-thin sections, prepared from high-pressure freeze-substituted wortmannin-treated and untreated BY-2 cell samples, were also cut for both structural and immunogold electron microscopy (EM) studies. However, despite repeated attempts no success was achieved from these samples, which is perhaps due to the relatively poor freezing quality of highly vacuolated suspension-cultured cells.
Wortmannin also induces PVC fusions in developing mung bean seeds
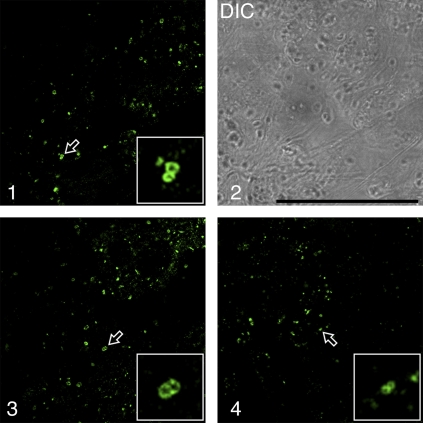
To overcome the freeze-fixation difficulties experienced with BY-2 cells, the parenchymatous cells in developing mung bean cotyledons, with a better ratio of cytoplasm to vacuole, were investigated instead. In addition, it had previously been shown by immunogold EM with VSRAt-1 antibodies that MVBs were also enriched in VSRs in mung bean cotyledons (Wang et al., 2007). Therefore, the cotyledons from developing mung bean seeds were first treated with wortmannin (16.5 μM) for 30 min before the cells were chemically fixed and immunolabelled with VSRAt-1 antibodies to detect multivesicular PVCs. As shown in Fig. 3, images of two closely adpressed VSR-marked ring structures (enlarged PVCs) were frequently observed (as indicated by arrows in panels 1–4). Only punctate PVCs were detected by VSRAt-1 antibodies in control cells without wortmannin treatment (data not shown). These clusters were similar to those seen in transgenic BY-2 cells and Arabidopsis protoplasts described before.
Fig. 3.
Wortmannin induced vacuolation and PVC fusion in developing mung bean cotyledons. Developing (6 DAF) mung bean cotyledons were treated with wortmannin at 16.5 μM for 30 min, followed by chemical fixation and immunolabelling with VSRat-1 antibodies to detect PVCs/MVBs. Arrows with enlarged inserted images indicate examples of possible PVC fusions. DIC, differential interference contrast. Scale bar=50 μm. (This figure is available in colour at JXB online.)
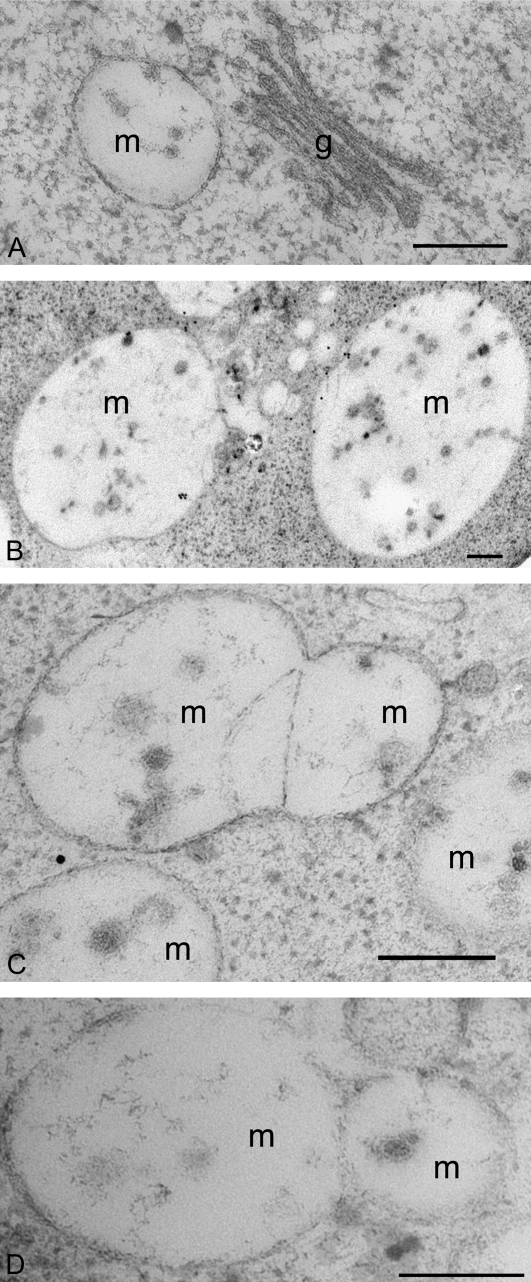
Next wortmannin-treated and control-untreated developing mung bean cotyledons were subjected to high-pressure freeze substitution. Ultra-thin sections were prepared for both structural and immunogold studies. As shown in Fig. 4A, untreated cells revealed typical Golgi stacks and MVBs with a diameter of about 200–400 nm. However, in wortmannin-treated cells, enlarged VSR-positive MVBs (larger than 1 μm in diameter) were observed (Fig. 4B). In addition, images of potential fusion profiles of MVBs were obtained (Fig. 4C, D). These results are good evidence that a wortmannin-induced homotypic fusion of PVC/MVBs also occurs in this tissue. In addition, the average numbers of internal vesicles μm−2 in sections of the enlarged MVBs in wortmannin-treated cells were at least six times less than those within the MVBs of untreated cells (Table 2).
Fig. 4.
Ultra-structural studies of wortmannin-induced PVC vacuolation and PVC fusion in developing mung bean cotyledons. Developing (6 DAF) mung bean cotyledons were first treated with wortmannin at 16.5 μM for 30 min, and then subjected to high-pressure freeze-substitution, followed by preparation of ultra-thin sections for structural (C, D) and immunogold EM studies with VSRAt-1 antibodies (B). Sections prepared from untreated samples were used as control (A). (A) Normal PVC/MVB and Golgi apparatus in cells without wortmannin treatment; (B) examples of the wortmannin-induced enlarged VSR-positive PVCs/MVBs; (C, D) examples of the fusing PVC/MVB in wortmannin-treated cells. Scale bar, 200 nm. g, Golgi; m, multivesicular body.
Table 2.
Wortmannin treatment of developing mung bean seeds reduces the number of internal vesicles inside MVBs
| Drug treatment | No. of MVB counted | Total no. of internal vesicles | Average no. of internal vesicles per MVB | Average diameter of MVB (nm) | Average no. of internal vesicles μm−2 inside MVBa |
| Untreated | 24 | 197 | 8.2 | 300 | 121.4** |
| Treated | 20 | 166 | 8.3 | 826 | 19.15** |
Significant difference between wortmannin-treated and untreated MVBs was analysed using two-sided paired t test (** P <0.01). Data were collected and analysed from four independent labelling experiments.
Evidence for wortmannin-induced TGN–PVC fusions
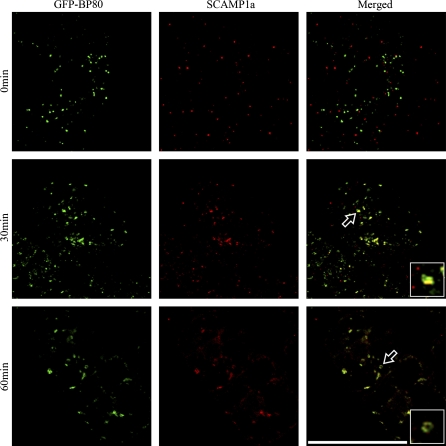
To test if wortmannin also induces fusion between the trans-Golgi network (TGN) and PVC, BY-2 cells expressing GFP–BP-80 were treated with wortmannin (16.5 μM) and samples were collected at various times (0, 15, 30, and 60 min) before subjecting them to chemical fixation. The fixed cells were then labelled with SCAMP1a antibodies, a recognized endocytic marker for the TGN (Lam et al., 2007a). As shown in Fig. 5, before wortmannin treatment, the GFP–BP-80-marked PVCs (green, first panel) were separate from the SCAMP-labelled TGN (red, first panel). Within 15 min of wortmannin treatment a closer relationship between these organelles was observed (data not shown), and at 30 min after the beginning of the drug treatment, colocalization between these two signals became more obvious. After 60 min, the organelles marked by SCAMPs were largely colocalized with the enlarged PVCs (Fig. 5, third panel). These confocal observations were fruther confirmed by immunogold EM studies where possible fusion between the SCAMP-labelled TGN (arrows) and the enlarged MVB in wortmannin-treated developing mung bean were observed (Fig. 6B, C) while no such fusion was detected in control untreatd cells (Fig. 6A). These results strongly suggest that wortmannin causes the redistribution of the SCAMPs from TGN into the enlarged PVCs, and that this may be due to the physical fusion between these two compartments. In addition, in wortmannin-treated developing mung bean cells, possible fusion between the small internal vesicles of MVB and the limited membrane of enlarged MVB was also detected (Fig. 7A, B), possibly accounting for the reduced internal vesicles observed for the enlarged MVBs in wortmannin-treated cells (Lam et al., 2007b).
Fig. 5.
Wortmannin induced fusion between SCAMP-marked TGN/EE and enlarged PVCs in transgenic tobacco BY-2 cell. Tobacco BY-2 cells expressing the PVC marker GFP–BP-80 were first treated with wortmannin at 16.5 μM, followed by sample collection and chemical fixation at indicated times (0, 30, and 60 min). The fixed samples were then subjected to immunolabelling using SCAMP1a antibodies (red). Arrows with inserted enlarged images indicated examples of colocalization between SCAMPs and GFP–BP-80 probably due to the fusion between the SCAMP-marked TGN and GFP–BP-80-marked PVCs. Scale bar=50 μm.
Fig. 6.
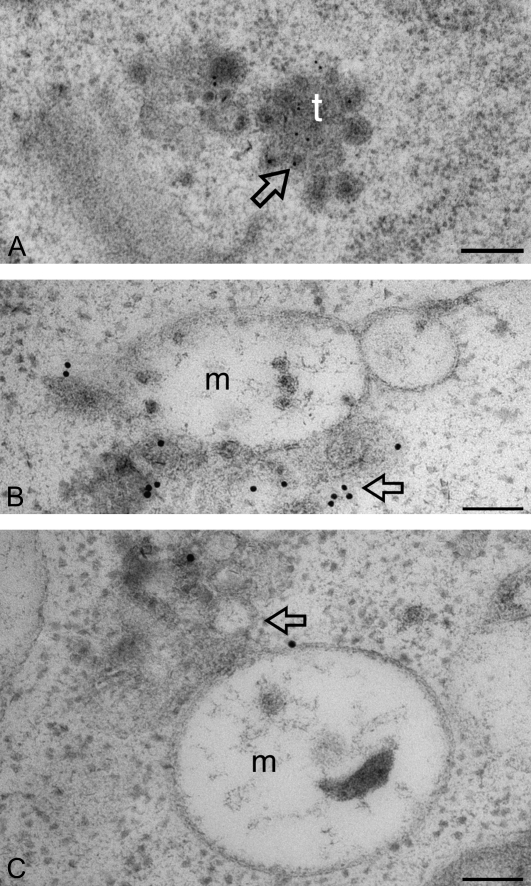
Ultra-structural studies of wortmannin-induced TGN–PVC fusion in developing mung bean cotyledons. Developing (6 DAF) mung bean cotyledons were first treated with wortmannin at 16.5 μM for 30 min (B, C), and then subjected to high-pressure freeze-substitution, followed by preparation of ultra-thin sections for structural and immunogold EM studies with SCAMP1a antibodies. Sections prepared from untreated samples were used as control (A). (A) Normal SCAMP-positive TGN in cells without wortmannin treatment; (B, C) examples of possible wortmannin-induced fusions between SCAMP-positive TGN (arrow) and the enlarged MVB in wortmannin-treated cells. m, multivesicular body; t, trans-Golgi network. Scale bar=200 nm.
Fig. 7.
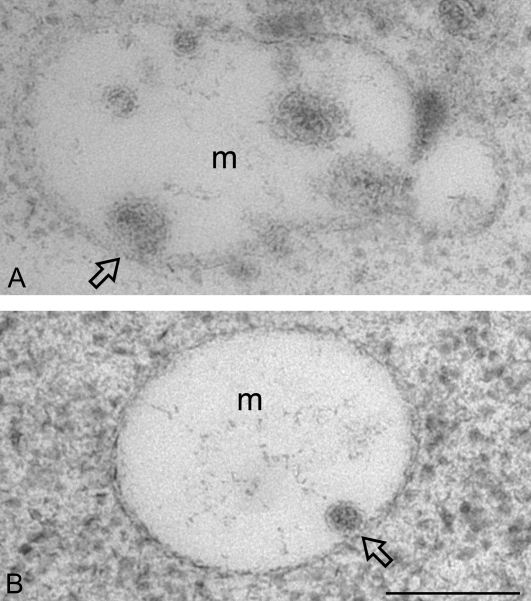
Ultra-structural studies of wortmannin-induced fusion between internal vesicles and MVB in developing mung bean cotyledons. Developing (6 DAF) mung bean cotyledons were first treated with wortmannin at 16.5 μM for 30 min, and then subjected to high-pressure freeze-substitution, followed by preparation of ultra-thin sections for structural and immunogold EM studies with SCAMP1 antibodies. (A, B) Examples (as indicated by arrow) of possible fusions between the internal vesicle and the limited membrane of enlarged MVB in wortmannin-treated cells. m, multivesicular body. Scale bar=200 nm.
Discussion
Wortmannin has been shown to function as an inhibitor of protein trafficking in the secretory and endocytic pathways. In mammalian cells, wortmannin prevents the recycling of the mannose 6-phosphate/insulin-like factor II receptor and its ligand from the endosome to the TGN (Kundra and Kornfeld, 1998). Similarly, in plant cells, protein recycling from PVC/MVB to the TGN or Golgi apparatus also appears to be blocked by wortmannin (daSilva et al., 2005, 2006), since wortmannin significantly increased the secretion of a transiently expressed vacuolar α-amylase construct in tobacco protoplasts. This observation indicates that, while protein secretion still operates through the Golgi apparatus in the presence of wortmannin, treatment with this drug prevents receptor-mediated vacuolar traffic out of the Golgi apparatus or TGN (daSilva et al., 2005, 2006). As in mammalian cells, this effect can be explained through the lack of vacuolar protein receptors at the TGN, a consequence of the inhibition of retromer-based receptor recycling from the PVC (Oliviusson et al., 2006; Yamazaki et al., 2008). However, in addition to targeting the PVC, wortmannin also seems to exert an effect upstream in the endocytic pathway, since it also prevents the uptake of the fluorescent endocytosis marker FM4-64 (Emans et al., 2002; Dettmer et al., 2006). Exactly how and where wortmannin exerts this additional inhibitory effect remains to be determined.
In addition to acting as an inhibitor of vacuolar protein transport, wortmannin causes fluorescently-tagged PVCs to dilate, resulting in fluorescent ring-like structures (Tse et al., 2004; Miao et al., 2006, 2008). This swelling has also been confirmed in the electron microscope which revealed enlarged MVBs, sized from 500 nm to a few μm in diameter (Tse et al., 2004, 2009; Miao et al., 2006; Mo et al., 2006). The wortmannin effect is rapid since the formation of small-dilated PVCs can already be seen 15 min of application of the drug. It seems that the wortmannin-induced vacuolation of PVCs is a general response involving a similar mechanism in plants, since it has been observed in a variety of plant cell types including suspension-cultured cells of tobacco BY-2, Arabidopsis, and rice (Tse et al., 2004; Miao et al., 2006, 2008; Delhaize et al., 2007; Miao and Jiang, 2007; and this study), root cells of Arabidopsis, tobacco, pea, and mung bean (Jaillais et al., 2006, 2008; Miao et al., 2006; Robinson, et al., 2008), germinating mung bean seeds (Wang et al., 2007), and developing mung bean seeds (this study).
In addition to a swelling of the PVC, the number of internal vesicles decreases considerably (Table 2). This is in agreement with the observation that the expression of a mutant AAA ATPase (ATPase Associated with diverse cellular Activities) (a component of the terminal ESCRT complex responsible for the invagination event in multivesicular endosomes; Williams and Urbe, 2007), also leads to a reduction in internal vesicles (Haas et al., 2007). It also indicates that, in addition to interfering with retromer–receptor recognition (Oliviusson et al., 2006), wortmannin also prevents invagination of the boundary membrane of the MVB/PVC. Although these effects are similar to those observed in mammalian cells (see Introduction), our data on homotypic PVC fusion is somewhat surprising, considering that a standard in vitro assay exists for studying the homotypic fusion of mammalian endosomes, and wortmannin inhibits this event (Jones and Clague, 1995; Jones et al., 1998). On the other hand, wortmannin does not prevent the fusion between late endosomes and lysosomes in mammalian cells (Bright et al., 1997; Poupon et al., 2003), nor does it inhibit the homotypic fusion of yeast vacuoles (Mayer et al., 2000).
TGN fusion with the PVC/MVB may also contribute to the enlargement of PVC/MVB as a result of wortmannin treatment (Lam et al., 2007b). The TGN has been identified as the early endosome in plant cells (see Robinson et al., 2008, for a recent review). Moreover, as in mammalian cells, wortmannin does not appear to prevent endocytic traffic from early to late endosomes (Bright et al., 2001). Thus, the merge of fluorescent signals relating to endocytic molecules (SCAMPs) and the PVC marker GFP–BP-80 after wortmannin treatment is in agreement with continued endocytic traffic into the MVB/PVC during wortmannin treatment. In addition, since the numbers of the internal small vesicles of PVCs/MVBs in wortmannin-treated cells are much less than those of the untreated cells (Table 2; Fig. 6), it is thus likely that the fusion between the internal vesicles and the limiting PVC membrane also contribute the membranes needed for rapid PVC enlargement. However, the possibility cannot be excluded that both newly synthesized membranes in the secretory pathway and internalized molecules via endocytosis also contribute the membranes required for the wortmannin-induced PVC vacuolation. Indeed, our recent data indicate that wortmannin only prevents the uptake of FM4-64 from the exterior to the PM, but this drug does not prevent further internalization of FM4-64 from the PM to the endosomal compartments in both BY-2 and Arabidopsis cells (Y Miao, L Jiang, unpublished results).
In conclusion, wortmannin-induced vacuolation can now be seen to be a useful tool for the identification of PVCs (Tse et al., 2004; Miao et al., 2006, 2008; Lam et al., 2007b). In this study, we have shown for the first time that wortmannin induces the homotypic fusion of PVC/MVBs and this appears to be the principal source of membrane required for the enlargement. It also seems that a fusion between the TGN and the PVC, as well as the fusion between the small internal vesicles and the outer membrane of PVC/MVB, may also contribute, in part, to the membrane required for PVC vacuolation or enlargement. These observations are unique to plant cells and may be of use in future studies on organelle dynamics and protein trafficking in the secretory and endocytic pathways.
Supplementary data
Supplementary data are available at JXB online.
Supplementary movies 1 and 2. Time-lapse detection of homotypic fusion of the fluorescently-tagged PVCs in transgenic tobacco BY-2 cells expressing the PVC reporter GFP–BP-80. Movie 1, ×1; movie 2, ×5.
Acknowledgments
This work was supported by grants from the Research Grants Council of Hong Kong (CUHK4580/05M, CUHK488707, CUHK465708, and HKUST6/CRF/08), UGC-AoE, CUHK Schemes B/C, and the National 863 Program of China (2007AA02Z102) to L Jiang. We thank Drs David Robinson and Stefan Hillmer (University of Heidelberg, Germany) for their continuous support of our EM study.
References
- Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. Journal of Cell Biology. 2004;165:123–133. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethke PC, Jones RL. Vacuoles and prevacuolar compartments. Current Opinion in Plant Biology. 2000;3:469–475. doi: 10.1016/s1369-5266(00)00115-1. [DOI] [PubMed] [Google Scholar]
- Bright NA, Lindsay MR, Stewart A, Luzio JP. The relationship between lumenal and limiting membranes in swollen late endocytic compartments formed after wortmannin treatment or sucrose accumulation. Traffic. 2001;2:631–642. doi: 10.1034/j.1600-0854.2001.20906.x. [DOI] [PubMed] [Google Scholar]
- Bright NA, Reaves BJ, Mullock BM, Luzio JP. Dense core lysosomes can fuse with late endosomes and are re-formed from the resultant hybrid organelles. Journal of Cell Science. 1997;110:2027–2040. doi: 10.1242/jcs.110.17.2027. [DOI] [PubMed] [Google Scholar]
- Cozier GE, Carlton J, McGregor AH, Gleeson PA, Teasdale RD, Mellor H, Cullen PJ. The phox homology (PX) domain-dependent, 3-phosphoinositide-mediated association of sorting nexin-1 with an early sorting endosomal compartment is required for its ability to regulate epidermal growth factor receptor degradation. Journal of Biological Chemistry. 2002;277:48730–48736. doi: 10.1074/jbc.M206986200. [DOI] [PubMed] [Google Scholar]
- DaSilva LL, Foresti O, Denecke J. Targeting of the plant vacuolar sorting receptor BP80 is dependent on multiple sorting signals in the cytosolic tail. The Plant Cell. 2006;18:1477–1497. doi: 10.1105/tpc.105.040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva LL, Taylor JP, Hadlington JL, Hanton SL, Snowden CJ, Fox SJ, Foresti O, Brandizzi F, Denecke J. Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. The Plant Cell. 2005;17:132–148. doi: 10.1105/tpc.104.026351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson HW. Wortmannin causes mistargeting of procathepsin D. evidence for the involvement of a phosphatidylinositol 3-kinase in vesicular transport to lysosomes. Journal of Cell Biology. 1995;130:797–805. doi: 10.1083/jcb.130.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Gruber BD, Pittman JK, White RG, Leung H, Miao Y, Jiang L, Ryan PR, Richardson AE. A role for the AtMTP11 gene of Arabidopsis in manganese transport and tolerance. The Plant Journal. 2007;51:198–210. doi: 10.1111/j.1365-313X.2007.03138.x. [DOI] [PubMed] [Google Scholar]
- Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. The Plant Cell. 2006;18:715–730. doi: 10.1105/tpc.105.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emans N, Zimmermann S, Fischer R. Uptake of a fluorescent marker in plant cells is sensitive to brefeldin A and wortmannin. The Plant Cell. 2002;14:71–86. doi: 10.1105/tpc.010339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Borja M, Wubbolts R, Calafat J, Janssen H, Divecha N, Dusseljee S, Neefjes J. Multivesicular body morphogenesis requires phosphatidyl-inositol 3-kinase activity. Current Biology. 1999;9:55–58. doi: 10.1016/s0960-9822(99)80048-7. [DOI] [PubMed] [Google Scholar]
- Futter CE, Collinson LM, Backer JM, Hopkins CR. Human VPS34 is required for internal vesicle formation within multivesicular endosomes. Journal of Cell Biology. 2001;155:1251–1264. doi: 10.1083/jcb.200108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas TJ, Sliwinski MK, Martinez DE, Preuss M, Ebine K, Ueda T, Nielsen E, Odorizzi G, Otegui MS. The Arabidopsis AAA ATPase SKD1 is involved in multivesicular endosome function and interacts with its positive regulator LYST-INTERACTING PROTEIN5. The Plant Cell. 2007;19:1295–1312. doi: 10.1105/tpc.106.049346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y, Fobis-Loisy I, Miege C, Gaude T. Evidence for a sorting endosome in Arabidopsis root cells. The Plant Journal. 2008;53:237–247. doi: 10.1111/j.1365-313X.2007.03338.x. [DOI] [PubMed] [Google Scholar]
- Jaillais Y, Fobis-Loisy I, Miege C, Rollin C, Gaude T. AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature. 2006;443:106–109. doi: 10.1038/nature05046. [DOI] [PubMed] [Google Scholar]
- Jiang L, Phillips TE, Rogers SW, Rogers JC. Biogenesis of the protein storage vacuole crystalloid. Journal of Cell Biology. 2000;150:755–770. doi: 10.1083/jcb.150.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Rogers JC. Integral membrane protein sorting to vacuoles in plant cells: evidence for two pathways. Journal of Cell Biology. 1998;143:1183–1199. doi: 10.1083/jcb.143.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AT, Clague MJ. Phosphatidylinositol 3-kinase activity is required for early endosome fusion. Biochemical Journal. 1995;311:31–34. doi: 10.1042/bj3110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AT, Mills IG, Scheidig AJ, Alexandrov K, Clague MJ. Inhibition of endosome fusion by wortmannin persists in the presence of activated Rab5. Molecular Biology of the Cell. 1998;9:323–332. doi: 10.1091/mbc.9.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundra R, Kornfeld S. Wortmannin retards the movement of the mannose 6-phosphate/insulin-like growth factor II receptor and its ligand out of endosomes. Journal of Biological Chemistry. 1998;273:3848–3853. doi: 10.1074/jbc.273.7.3848. [DOI] [PubMed] [Google Scholar]
- Lam SK, Siu CL, Hillmer S, Jang S, An G, Robinson DG, Jiang L. Rice SCAMP1 defines clathrin-coated, trans-Golgi-located tubular-vesicular structures as an early endosome in tobacco BY-2 cells. The Plant Cell. 2007a;19:296–319. doi: 10.1105/tpc.106.045708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SK, Tse YC, Robinson DG, Jiang L. Tracking down the elusive early endosome. Trends in Plant Science. 2007b;12:497–505. doi: 10.1016/j.tplants.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Lam SK, Tse YC, Miao Y, Li HY, Wang J, Lo SW, Jiang L. Molecular characterization of plant prevacuolar and endosomal compartments. Journal of Integrative Plant Biology. 2007c;49:1119–1128. [Google Scholar]
- Lam SK, Cai Y, Hillmer S, Robinson DG, Jiang L. SCAMPs highlight the developing cell plate during cytokinesis in tobacco BY-2 cells. Plant Physiology. 2008;147:1637–1645. doi: 10.1104/pp.108.119925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YB, Rogers SW, Tse YC, Lo SW, Sun SS, Jauh GY, Jiang L. BP-80 and homologs are concentrated on post-Golgi, probable lytic prevacuolar compartments. Plant and Cell Physiology. 2002;43:726–742. doi: 10.1093/pcp/pcf085. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Bassham DC, Raikhel NV, Nakamura K. Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. Journal of Cell Biology. 1995;130:1307–1318. doi: 10.1083/jcb.130.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Scheglmann D, Dove S, Glatz A, Wickner W, Haas A. Phosphatidylinositol 4,5-bisphosphate regulates two steps of homotypic vacuole fusion. Molecular Biology of the Cell. 2000;11:807–817. doi: 10.1091/mbc.11.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Jiang L. Transient expression of fluorescent fusion proteins in protoplasts of suspension cultured cells. Nature Protocols. 2007;2:2348–2353. doi: 10.1038/nprot.2007.360. [DOI] [PubMed] [Google Scholar]
- Miao Y, Li KY, Li HY, Yao XQ, Jiang L. Vacuolar transport of aleurain-GFP and 2S albumin-GFP fusions is mediated by the same prevacuolar compartments in tobacco BY-2 and Arabidopsis suspension cultured cells. The Plant Journal. 2008;56:824–839. doi: 10.1111/j.1365-313X.2008.03645.x. [DOI] [PubMed] [Google Scholar]
- Miao Y, Yan PK, Kim H, Hwang I, Jiang L. Localization of green fluorescent protein fusions with the seven Arabidopsis vacuolar sorting receptors to prevacuolar compartments in tobacco BY-2 cells. Plant Physiology. 2006;142:945–962. doi: 10.1104/pp.106.083618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo B, Tse YC, Jiang L. Plant prevacuolar/endosomal compartments. International Review of Cytology. 2006;253:95–129. doi: 10.1016/S0074-7696(06)53003-7. [DOI] [PubMed] [Google Scholar]
- Oliviusson P, Heinzerling O, Hillmer S, Hinz G, Tse YC, Jiang L, Robinson DG. Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. The Plant Cell. 2006;18:1239–1252. doi: 10.1105/tpc.105.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poupon V, Stewart A, Gray SR, Piper RC, Luzio JP. The role of mVps18p in clustering, fusion, and intracellular localization of late endocytic organelles. Molecular Biology of the Cell. 2003;14:4015–4027. doi: 10.1091/mbc.E03-01-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves BJ, Bright NA, Mullock BM, Luzio JP. The effect of wortmannin on the localisation of lysosomal type I integral membrane glycoproteins suggests a role for phosphoinositide 3-kinase activity in regulating membrane traffic late in the endocytic pathway. Journal of Cell Science. 1996;109:749–762. doi: 10.1242/jcs.109.4.749. [DOI] [PubMed] [Google Scholar]
- Robinson DG, Jiang L, Schumacher K. The endosomal system of plants: charting new and familiar territories. Plant Physiology. 2008;147:1482–1492. doi: 10.1104/pp.108.120105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993;260:88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- Seaman MN. Recycle your receptors with retromer. Trends in Cell Biology. 2005;15:68–75. doi: 10.1016/j.tcb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Shpetner H, Joly M, Hartley D, Corvera S. Potential sites of PI-3 kinase function in the endocytic pathway revealed by the PI-3 kinase inhibitor, wortmannin. Journal of Cell Biology. 1996;132:595–605. doi: 10.1083/jcb.132.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silady RA, Ehrhardt DW, Jackson K, Faulkner C, Oparka K, Somerville CR. The GRV2/RME-8 protein of Arabidopsis functions in the late endocytic pathway and is required for vacuolar membrane flow. The Plant Journal. 2008;53:29–41. doi: 10.1111/j.1365-313X.2007.03314.x. [DOI] [PubMed] [Google Scholar]
- Tse YC, Lam SK, Jiang L. Organelle identification and characterization in plant cells: using a combinational approach of confocal immunofluorescence and electron microscope. Journal of Plant Biology. 2009;52:1–9. [Google Scholar]
- Tse YC, Lo SW, Hillmer S, Dupree P, Jiang L. Dynamic response of prevacuolar compartments to Brefeldin A in plant cells. Plant Physiology. 2006;142:1442–1459. doi: 10.1104/pp.106.090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG, Jiang L. Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. The Plant Cell. 2004;16:672–693. doi: 10.1105/tpc.019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li Y, Lo SW, Hillmer S, Sun SS, Robinson DG, Jiang L. Protein mobilization in germinating mung bean seeds involves vacuolar sorting receptors and multivesicular bodies. Plant Physiology. 2007;143:1628–1639. doi: 10.1104/pp.107.096263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Suen PK, Xu ZF, Jiang L. A 64 kDa sucrose binding protein is membrane-associated and tonoplast-localized in developing mung bean seeds. Journal of Experimental Botany. 2009;60:629–639. doi: 10.1093/jxb/ern308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RL, Urbe S. The emerging shape of the ESCRT machinery. Nature Reviews Molecular Cell Biology. 2007;8:355–368. doi: 10.1038/nrm2162. [DOI] [PubMed] [Google Scholar]
- Wurmser AE, Gary JD, Emr SD. Phosphoinositide 3-kinases and their FYVE domain-containing effectors as regulators of vacuolar/lysosomal membrane trafficking pathways. Journal of Biological Chemistry. 1999;274:9129–9132. doi: 10.1074/jbc.274.14.9129. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Shimada T, Takahashi H, Tamura K, Kondo M, Nishimura M, Hara-Nishimura I. Arabidopsis VPS35, a retromer component, is required for vacuolar protein sorting and involved in plant growth and leaf senescence. Plant and Cell Physiology. 2008;49:142–256. doi: 10.1093/pcp/pcn006. [DOI] [PubMed] [Google Scholar]
- Yu JW, Lemmon MA. All phox homology (PX) domains from Saccharomyces cerevisiae specifically recognize phosphatidylinositol 3-phosphate. Journal of Biological Chemistry. 2001;276:44179–44184. doi: 10.1074/jbc.M108811200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.