Abstract
Active oxygen species (AOS) are central components of the defence reactions of plants against pathogens. Plant respiratory burst oxidase homologues (RBOH) of gp91phox, a plasma membrane protein of the neutrophil nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, play a prominent role in AOS production. The role of two RBOH from Nicotiana benthamiana, NbrbohA and NbrbohB that encode plant NADPH oxidase in the process of elicitor-induced stomatal closure and hypersensitive cell death is described here. NbrbohA was constitutively expressed at a low level, whereas NbrbohB was induced when protein elicitors exist (such as boehmerin, harpin, or INF1). The virus-induced gene-silencing (VIGS) method was used to produce single-silenced (NbrbohA or NbrbohB) and double-silenced (NbrbohA and NbrbohB) N. benthamiana plants. The hypersensitive response (HR) of cell death and pathogenesis-related (PR) gene expression of these gene-silenced N. benthamiana plants, induced by various elicitors, are examined. The HR cell death and transcript accumulation of genes related to the defence response (PR1) were slightly affected, suggesting that RBOH are not essential for elicitor-induced HR and activation of these genes. Interestingly, gene-silenced plants impaired elicitor-induced stomatal closure and elicitor-promoted nitric oxide (NO) production, but not elicitor-induced cytosolic calcium ion accumulation and elicitor-triggered AOS production in guard cells. These results indicate that RBOH from N. benthamiana function in elicitor-induced stomatal closure, but not in elicitor-induced HR.
Keywords: AOS, elicitor, hypersensitive response, Nicotiana benthamiana, stomatal closure, virus-induced gene silencing
Introduction
Plant cell death during the hypersensitive response (HR) has been well studied and has usually been described in incompatible plant–pathogen interactions (Lamb and Dixon, 1997). Defence responses by incompatible pathogens have been modelled using elicitor treatment. Elicitors can induce oxidative burst, which can limit the spread of invading pathogens by generating active oxygen species (AOS) including the moderately reactive radicals hydrogen peroxide (H2O2), superoxide (O2−), and the highly reactive hydroxyl radical (OH–). Plants have evolved many AOS-scavenging systems, but cell death may still occur when excessive numbers of AOS are produced (Pitzschke and Hirt, 2006).
Although many enzymes such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, cell wall peroxidases, amine oxidase, oxalate oxidase, and flavin-containing oxidase are potential H2O2 sources (Bolwell and Wojtaszek, 1997; Bolwell et al., 2002), the NADPH oxidase complex is considered as one of the most important sources of oxidative burst (Bolwell et al., 1998; Grant M et al., 2000; Torres and Dangl, 2005). Plant respiratory burst oxidase homologues (RBOH) of gp91phox, a plasma membrane protein of the neutrophil NADPH oxidase, are believed to have six transmembrane-spanning domains and two elongation factor (EF) hands in the N-terminal region that may function in Ca2+ regulation (Torres and Dangl, 2005).
The RBOH was first isolated from rice (Oryza sativa) as a homologue of gp91phox (Groom et al., 1996), and then identified in other plant species including Arabidopsis, tomato, tobacco, and potato (Keller et al., 1998; Torres et al., 1998; Amicucci et al., 1999; Yoshioka et al., 2001, 2003; Yoshie et al., 2005). Previous studies have shown that RBOH play a central role in AOS production during biotic and abiotic stress. For example, rboh-silenced Nicotiana tabacum showed reduced disease resistance to Phytophthora infestans (Yoshioka et al., 2003); rboh from Zinnia elegans was involved in xylem differentiation (Barcelo, 2005); rboh knockdowns in tomato resulted in growth anomalies (Sagi et al., 2004); rbohC from Arabidopsis may have regulated cell expansion during root hair formation (Foreman et al., 2003). Stomatal closure was also severely inhibited in Arabidopsis rbohD/F double-mutants after abscisic acid (ABA) treatment (Bright et al., 2006). All of these data suggest that multiple isoforms of RBOH may act in different AOS-dependent functions in different plants.
AOS signalling may also be associated with nitric oxide (NO), a highly reactive nitrogen species produced after pathogen and elicitor recognition (Delledonne et al., 1998; Durner et al., 1998; Lamotte et al., 2004; Zhang et al., 2004; Ji et al., 2005; Asai et al., 2008). NO may work in conjunction with AOS, Ca2+, and protein kinases in plant signalling (Delledonne et al., 2001; Wendehenne et al., 2004; Courtois et al., 2008). Under non-stressed conditions, plants balance the states between AOS and NO. Cytological studies have indicated that AOS and NO determine the fate of the cell, and one signal modulates the accumulation of the other (Tada et al., 2004; Zeier et al., 2004). In addition, both AOS and NO collaborate to mediate ABA-induced stomatal closure in Arabidopsis (Garcia-Mata and Lamattina, 2002; Desikan et al., 2002, 2004; Neill et al., 2002a; Bright et al., 2006).
Elicitors include a variety of compounds, such as proteins, glycoproteins, glycans, lipids, and synthetic molecules. They may be cell components, pathogen secretions, or substances released by hydrolytic enzymes of pathogens and plants (Garcia-Brugger et al., 2006). The recognition of an elicitor by the plant cell is followed by calcium ion influx, AOS, and NO production. After successive signal transduction, it can induce cell death and stomatal closure (Allan and Fluhr, 1997; Lee et al., 1999; Nürnberger et al., 2004).
During incompatible interactions, rboh Arabidopsis mutants with reduced H2O2 production had an opposite response to the bacterial pathogen Pseudomonas syringae pv. tomato DC3000 (avrRpm1) and the oomycete parasite Peronospora parasitica (Torres et al., 2002). In addition, rboh also played a role in ABA signalling for stomatal closure regulation (Bright et al., 2006). However, research on RBOH in elicitor signalling is still lacking. Two NADPH oxidase catalytic subunit genes, NbrbohA and NbrbohB, from Nicotiana benthamiana were chosen to investigate their function in elicitor-induced plant response and stomatal closure. Transient knock-down via virus-induced gene silencing (VIGS) was performed to assess the role of the two genes.
Materials and methods
Plant materials, elicitors, and treatment protocol
The N. benthamiana plants were grown in a controlled growth chamber under a 16/8 h light/dark cycle at 25 °C. Elicitation with the elicitor (50 nM) was conducted on plants by infiltrating an equivalent elicitor solution of 25 μl with a needleless syringe into tiny cuts on the underside of the leaf, thereby flooding the apoplastic space. To prepare Phytophthora infestans INF1 and Phytophthora boehmeriae boehmerin, overnight cultures of E. coli cells, BL21 carrying pET32b with the inf1 (GenBank accession no. AY830094) or boehmerin (GenBank accession no. AY196607) gene, were diluted (1:100) in Luria–Bertani medium containing ampicillin (50 mg ml−1) and incubated at 37 °C. To prepare the E. coli-expressed harpin, overnight cultures of E. coli cells, BL21 carrying pET30a with the hrf1 (GenBank accession no. AY875714) gene, were diluted (1:100) in Luria–Bertani medium containing kanamycin (50 mg ml−1) and incubated at 37 °C. When the OD600 of cultures reached 0.6, boehmerin, INF1, and harpin were induced in the cultured medium by the addition of 0.4 mM isopropyl-β-D-thiogalactopyranoside for 6 h. The deposit was harvested by centrifugation, washed repeatedly, stored in 10 mM PBS (pH 6.5), and then broken up by ultrasonification. Supernatants collected by centrifugation (12 000 g, 15 min, 4 °C) were dialysed successively against 0.8%, 0.6%, 0.4%, 0.2%, and 0.1% SDS at 15 °C. Finally, supernatants were dialysed against 10 mM PBS (pH 6.5) and stored at –20 °C prior to use. Protein concentrations were determined using Bradford reagent (Qutob et al., 2006), and concentrated stock solutions (500 nM) were prepared.
DNA constructs and seedling infection for virus-induced gene silencing
Silencing of NbrbohA and NbrbohB genes in N. benthamiana by Potato virus X (PVX) VIGS was performed as described by Sharma et al. (2003). The NbrbohA (GenBank accession no. AB079498) and NbrbohB (GenBank accession no. AB079499) inserts were 235 bp and 217 bp and showed 12% and 10% nucleotide identity to the corresponding regions of NbrbohB and NbrbohA, respectively. The inserts of NbrbohA and NbrbohB were both derived from the 3′ terminus of the respective open reading frame (ORF), and inserted into the PVX vector separately or simultaneously in the antisense direction to generate PVX.NbrbohA, PVX.NbrbohB, and PVX.NbrbohA/B. The constructs containing the inserts were transformed into Agrobacterium tumefaciens strain GV3101. Bacterial suspensions were applied to the undersides of N. benthamiana leaves using a 1 ml needleless syringe. Plants exhibited mild mosaic symptoms 3 weeks after inoculation. The third or fourth leaf above the inoculated one, where silencing was most consistently established, was used for further analysis.
DAB staining
Following the methods of Samuel et al. (2005), leaves collected 6 h after elicitor treatment were incubated in diaminobenzidine (DAB) solution for 8 h at 25 °C in light. The leaves were then boiled in 96% ethanol for 10 min to remove the dye. After 4 h of further incubation in ethanol, brown precipitates were observed, indicating H2O2 burst. Quantitative scoring of H2O2 staining in leaves was analysed using the software Quantity One (Bio-Rad, Milan, Italy).
RNA isolation and RT-PCR analysis
Total RNA was extracted following the Trizol extraction protocol (Invitrogen, Carlsbad, CA) and treated with RNAse-free DNAse I (TaKaRa, Dalian, China). First-strand cDNA was synthesized using Superscript II reverse transcriptase (Invitrogen) following the manufacturer's directions. PCR was performed in 50 μl reactions using 1 μl cDNA template, 1 μM of each gene-specific primer, 2 units of Taq polymerase, and the buffer provided by the manufacturer (containing 1.5 mM MgCl2). To ensure that similar amounts of cDNA were used for silenced and non-silenced plants, parallel reactions were run with elongation factor 1α (EF1α) primers as controls (29 cycles). Each PCR cycle included denaturation at 94 °C for 30 s, annealing at 54 °C for 30 s, and elongation at 72 °C for 30 s, as described in Zhang et al. (2004). The PCR products were analysed on a 1.2% agarose gel stained with ethidium bromide. RT-PCR -specific primers for NbrbohA, NbrbohB, and EF1α are: NbrbohA forward primer: 5′-CgTgCTTgATAAAgAAACACTgA-3′, NbrbohA reverse primer: 5′-CCCACCCAACCAAAATACgC-3′; NbrbohB forward primer: 5′-CgggTgATgCTCgTTCTgCTC-3′, NbrbohB reverse primer: 5′-CCAggCgTgTTgTCTTAgTTCTT-3′; and EF1α forward primer: 5′-AGACCACCAAGTACTACTGCAC-3′, EF1α reverse primer: 5′-CCACCAATCTTGTACACATCC-3′. The RT-PCR primers of defence-related genes are described below. Primer sequences are as follows: PR-1a, PR-1b, and PR-1c forward primer, 5′-ATGCCCATAACAGCTCG-3′; PR-1a reverse primer, 5′-GAGGATCATAGTTGCAAGAG-3′; PR-1b reverse primer, 5′-GTATGGACTTTGGCCATGAC-3′; and PR-1c reverse primer, 5′-GGATCATAGTTGCAAGAGAC-3′.
Stomatal aperture measurements
Stomatal apertures were measured as described by Chen et al. (2004) in 5 mM KCl, 50 mM CaCl2, and 10 mM MES-Tris (pH 6.15).
NO measurement in guard cells
NO accumulation was determined using fluorophore 4, 5′-diaminofluorescein diacetate (DAF-2DA, Sigma-Aldrich) according to Ali et al. (2007). Epidermal strips were prepared from control and gene-silenced plants, respectively; the strips were then incubated in 5 mM KCl and 10 mM MES-Tris (pH 6.15) in light for 2 h, followed by incubation in 20 μM DAF-2DA for 1 h in the dark at 25 °C, and finally rinsed three times with 10 mM Tris-HCl (pH 7.4) to wash off excessive fluorophore. Guard cell images were taken 3 h after elicitor treatment, by fluorescence microscopy at 470 nm excitation using a 515 nm emission filter. Fluorescence emission of guard cells was analysed using the software Quantity One.
AOS measurement in guard cells
Dihydrorhodamine 123 (DHR, Merck, Whitehouse Station, NJ) was used to analyse elicitor-induced AOS production in guard cells. The epidermal strips were incubated in 20 μM DHR for 2 h in the dark at 37 °C and then rinsed three times with PBS (pH 7.4) to wash off excessive fluorophore. Subsequently, 3 h after elicitor treatment, guard cell images were taken by using Adobe Photoshop 5.5 (Mountain View, CA) during a 2 s short UV exposure (one UV exposure per sample) under a fluorescence microscope equipped with a digital camera. Fluorescence emission of the guard cells was analysed using the software Quantity One.
Measurement of Ca2+ in guard cells
Following Chen et al. (2004), the epidermal strips were peeled gently and incubated in 10 mM 1-[2-amino-5-(2,7-dichloro-6-hydroxy-3-oxo-9-xanthenyl) phenoxy]-2-(2-amino-5-methylphenoxy) ethane-N,N,N′,N′-tetra-acetic acid and penta-acetoxymethyl ester (fluo-3 AM, Merck, Whitehouse Station, NJ) loading buffer (10 mM MES-Tris, pH 6.15) at 4 °C for 2 h in darkness. Because the activities of esterases at 4 °C were low, fluo-3 AM permeated through the membranes without being hydrolysed by esterases in the cell walls. After washing the strips three times with MES buffer, they were kept at room temperature for 1 h. During this period, fluo-3 AM inside the cell was hydrolysed by intracellular esterases, and the hydrolysed form of fluo-3 AM bound to free Ca2+, indicating dynamic changes in Ca2+ in guard cells. Three hours after elicitor treatment, guard cell images were taken with confocal laser scanning microscopy and analysed with the software Quantity One.
Results
NbrbohA and NbrbohB participate in elicitor-induced H2O2 generation
It has been reported that NbrbohA is expressed constitutively at a low level, whereas the accumulation of NbrbohB protein is induced by cell wall elicitors (Yoshioka et al., 2003). To investigate whether agro-infiltrated N. benthamiana exhibited lower rboh transcription, all inoculated antisense N. benthamiana were subjected to semi-quantitative reverse transcriptase (RT)-PCR analysis specific to each gene, using EF1α transcript as an expression level control (Fig. 1A). The transcript of NbrbohA decreased 75% in both NbrbohA-silenced and NbrbohA/B-silenced plants compared to the control, while the transcript of NbrbohB decreased 67% in NbrbohB-silenced plants and 75% in NbrbohA/B-silenced plants compared to the control (Fig. 1B, C). Therefore, it could be concluded that both genes were silenced in all three agro-infiltrated N. benthamiana lines.
Fig. 1.
Evaluation of NbrbohA and NbrbohB silencing in leaves of N. benthamiana infected with PVX, PVX.NbrbohA, PVX.NbrbohB, or PVX.NbrbohA/B. RT-PCR was performed with first-strand cDNA obtained from total RNA derived from various plants silenced for NbrbohA, NbrbohB, NbrbohA/B, and for controls. After a 3-week inoculation, leaf samples were harvested from the third and fourth leaves above the inoculation site, and total RNA was isolated and used for RT-PCR. A 7 μl aliquot was removed from each reaction after three-cycle increments starting after 20 cycles. The aliquots were separated on an agarose gel and stained with ethidium bromide. Equal input of cDNA template for PCR was demonstrated by amplification of the constitutively expressed EF1α gene (29 cycles); Lane M shows the DL2000 DNA ladder (TaKaRa, Dalian, China). (A1) The control PVX N. benthamiana, (A2) NbrbohA-silenced N. benthamiana (left) and NbrbohB-silenced N. benthamiana (right), (A3) both NbrbohA- and NbrbohB-silenced N. benthamiana, and (A4) EF1α control. The RT-PCR analysis was repeated for three sets of independently silenced plants in each experiment and in three independent experiments. (B) Relative amount of the transcript accumulation of NbrbohA (B) and NbrbohB (C) to EF1α using software Quantity One, as shown in (A). Values are the mean ±SD from three independent experiments.
Repression of RBOH polypeptide expression may imply a reduction in the constitutive level of AOS. RBOH produce superoxide radicals (Sagi and Fluhr, 2001), and staining for H2O2 produced by endogenous superoxide dismutation of superoxide radicals has been used to quantify RBOH activity (Yoshioka et al., 2003). Elicitor-induced H2O2 production measurements were performed using DAB staining, which indicated H2O2 accumulation by the formation of a brown precipitate. Figure 2A shows the development of the DAB-H2O2 reaction product in leaves of the control and Nbrboh-silenced plants 6 h after elicitor treatment. Brown precipitate in control leaves triggered by boehmerin was highest among the three elicitors. However, the brown precipitate decreased, with lighter colouring and lower distribution, in Nbrboh-silenced leaves after various elicitor treatments (Fig. 2A). Results of further quantitative analysis, using the software Quantity One, revealed that Nbrboh-silencing attenuated elicitor-induced H2O2 production (Fig. 2B). The results suggest that NADPH oxidases mediate elicitor-induced AOS generation in N. benthamiana, and that NbrbohA and NbrbohB may be the major catalytic subunits in this response.
Fig. 2.
In situ detection of hydrogen peroxide using DAB staining on PVX, PVX.NbrbohA-, PVX.NbrbohB-, and PVX.NbrbohA/B-infected N. benthamiana leaves. (A) Photographs of representative leaves ethanol-bleached from control and Nbrboh-silenced plants 6 h after boehmerin (50 nM), harpin (50 nM), or INF1 (50 nM) treatment; elicitation with the elicitor was conducted on plants by infiltrating an equivalent elicitor solution of 25 μl; (B) quantitative scoring of staining in leaves of the control and Nbrboh-silenced plants with elicitor treatment with the software Quantity One. The analysis was repeated for three sets of independently silenced plants in each experiment; the values shown are the means ±SD of duplicate assays.
RBOH are not involved in elicitor-triggered HR
Gene-silenced plants were selected for further evaluation of elicitor-triggered HR. Photographs of representative (control and Nbrboh-silenced plants) leaves infiltrated with the elicitor are shown in figure 3. After inoculation, all three elicitors rapidly induced a water-soaked appearance of the leaves (12 h), followed by brown-pigmented necrosis characteristic of HR (12–24 h). Necrosis was restricted to the inoculated area of the leaf, and the lesion became fully desiccated 2–3 d after inoculation. Inoculation of serially diluted elicitor solutions indicated that minimal threshold concentrations of 1–10× were necessary for HR induction by boehmerin, harpin, and INF1. No obvious difference was observed among the elicitors in the specificity and severity of HR induction on the controls or the Nbrboh-silenced N. benthamiana. These results indicate that NbrbohA and NbrbohB may not be the key contributors to HR caused by these elicitors.
Fig. 3.
Local induction of hypersensitivity responses with boehmerin (50 nM), harpin (50 nM), and INF1 (50 nM). A leaf (representative of three replicate treatments) was infiltrated with the three elicitors simultaneously. (A) Control PVX N. benthamiana, (B) NbrbohA/B dual-silenced N. benthamiana, (C) NbrbohA-silenced N. benthamiana, (D) NbrbohB-silenced N. benthamiana. Leaves were removed from plants after 3 d of treatment and bleached in ethanol.
Elicitor-induced PR gene expression is regulated in an RBOH-independent manner
Incompatible interactions can induce not only HR cell death but also systemic acquired resistance (SAR), which requires both local and systemic salicylic acid (SA) accumulation, and the induction of a subset of pathogenesis-related (PR) genes (Grant and Lamb, 2006). To investigate whether Nbrboh deficiency affects transcript accumulation of the PR gene, semi-quantitative RT-PCR was performed to monitor transcript accumulation of defence genes including PR1a, PR1b, and PR1c after treatment with boehmerin, harpin, and INF1. In control plants, boehmerin induced rapid transcript accumulation of PR1a, PR1b, and PR1c 6 h after inoculation (Fig. 4A). This result is consistent with other reports that transcripts of PR-1 genes began to accumulate after 6 h during N gene-mediated HR (Seo et al., 2000; Hatsugai et al., 2004). There is no obvious increased transcript accumulation of PR1a, PR1b, and PR1c in both gene-silenced and control plants after PBS treatment (Fig. 4B, E). RT-PCR was performed to analyse transcript accumulation of PR1a, PR1b, and PR1c in N. benthamiana plants infected with PVX, PVX.NbrbohA, PVX.NbrbohB, or PVX.NbrbohA/B at certain time points (0 h, 6 h) after various elicitor treatments (Fig. 4C, D). The results show increased transcript accumulation of PR1a, PR1b, and PR1c 6 h after elicitor treatment, but no obvious difference among the Nbrboh single-silenced, dual-silenced, or control plants (Fig. 4F, G). The experimental data suggest that Nbrboh may have a slight effect on the transcription of PR genes upon elicitor induction, which is consistent with HR cell death.
Fig. 4.
Response of PR1a, PR1b, and PR1c genes in elicitor-treated N. benthamiana leaves using semi-quantitative RT-PCR. RT-PCR was performed with RNA isolated from N. benthamiana plants with primers specific for PR1a, PR1b, PR1c, and EF1α of N. benthamiana after PBS (10 mM), boehmerin (50 nM), harpin (50 nM), and INF1 (50 nM) treatment, respectively. PCR conditions, ranging from 20 to 40 amplification cycles were tested in both cases. Presented here are 29 cycles corresponding to the log-linear phase of amplified PCR product in N. benthamiana. (1) Control PVX N. benthamiana, (2) NbrbohA-silenced N. benthamiana, (3) NbrbohB-silenced N. benthamiana, (4) NbrbohA/B-dual-silenced N. benthamiana. The RT-PCR analysis was repeated for three independent control plants in each experiment and in three independent experiments. (A) Time-course accumulation of transcripts of PR1a, PR1b, and PR1c genes in boehmerin-treated leaves of PVX-infected N. benthamiana. The elicitor-treated leaves were removed at the indicated time point. (B) RT-PCR analysis to examine transcript levels of defence-related genes in N. benthamiana leaves after PBS treatment. (C) RT-PCR analysis to examine transcript levels of defence-related genes in N. benthamiana leaves 0 h after elicitor treatment. (D) RT-PCR analysis to examine transcript levels of defence-related genes in N. benthamiana leaves 6 h after elicitor treatment. (E) Relative transcript accumulation of PR1a, PR1b, and PR1c to EF1α according to the software Quantity One as shown in (B). Values are the mean ±SD from three independent experiments. (F) Relative transcript accumulation of PR1a, PR1b, and PR1c to EF1α with the software Quantity One as shown in (C). Values are the mean ±SD from three independent experiments. (G) Relative transcript accumulation of PR1a, PR1b, and PR1c to EF1α, calculated by the software Quantity One as shown in (D). Values are the mean ±SD from three independent experiments.
Elicitor-induced stomatal closure is impaired in Nbrboh-silenced N. benthamiana
Stomata are specialized epidermal structures formed by two guard cells surrounding a pore, through which carbon dioxide (CO2) for photosynthesis is absorbed and water evaporates. The stomatal pores open in light and close in response to water stress through the action of ABA (Pei et al., 2000). Because elicitor PB90 from P. boehmeriae induces stomatal closure (Zhang et al., 2007) and rbohD/F from Arabidopsis has an effect on inhibiting ABA-induced stomatal closure, guard cells display a classic innate immune response to both pathogen-associated molecular pattern (PAMP) compounds and pathogens (Lee et al., 1999; Wright et al., 2000). Elicitor-induced stomatal closure analysis was performed with Nbrboh single- and dual-silenced N. benthamiana. As shown in figure 5, harpin induced stomatal closure of control leaves, which was inhibited in the NbrbohA/B dual-silenced N. benthamiana. Nbrboh dual-silenced N. benthamiana significantly inhibited boehmerin-induced stomatal closure compared to the Nbrboh single-silenced N. benthamiana and controls (Table 1A; P=0.01). The results of harpin treatment were the same as those with boehmerin (Table 1B; P=0.01). However, INF1 treatment led to significantly different results between the gene-silenced plants and controls, but not between the dual-silenced and single-silenced N. benthamiana (Table 1C, P=0.01). These results suggest that NbrbohA and NbrbohB function in elicitor-induced stomatal closure. The impact of Nbrboh dual-silencing and single-silencing on stomatal closure induced by boehmerin, harpin, and INF1 is somewhat different.
Fig. 5.
Elicitor-induced stomatal closure is impaired in the NbrbohA/B dual-silenced plants after harpin treatment. Leaf epidermal peels prepared from control (top panels) and NbrbohA/B dual-silenced plants (bottom panels) were incubated in harpin (50 nM).
Table 1.
Stomatal aperture measurements show that elicitor-induced stomatal closure is partially reduced in Nbrboh single-silenced and dual-silenced N. benthamiana
| Nb | Stomatal aperture (μm) P=0.01 | |
| (A) | PVX Nb | 0.41±0.08 c |
| NbrbohA-silenced Nb | 1.37±0.40 bc | |
| NbrbohB-silenced Nb | 3.01±0.86 bc | |
| NbrbohA/B-silenced Nb | 3.56±1.03 a | |
| (B) | PVX Nb | 0.40±0.24 b |
| NbrbohA-silenced Nb | 1.20±0.94 b | |
| NbrbohB-silenced Nb | 1.31±0.77 b | |
| NbrbohA/B-silenced Nb | 3.65±0.40 b | |
| (C) | PVX Nb | 0.35±0.42 b |
| NbrbohA-silenced Nb | 0.88±0.64 ab | |
| NbrbohB-silenced Nb | 2.21±1.10 ab | |
| NbrbohA/B-silenced Nb | 2.70±1.11 a |
Stomatal aperture was measured 3 h after incubation in boehmerin (50 nM) (A), harpin (50 nM) (B), and INF1 (50 nM) (C). Data were compared by using the DPS at the 95% significance level.
NO is associated with elicitor-induced stomatal closure
NO co-ordinates HR and plant innate immunity, serving as a cellular signalling molecule in a wide range of organisms including plants, especially in stomatal guard cells (Dangl, 1998; Ali et al., 2007). NO is involved in ABA-induced stomatal closure (Neill et al. 2002a). To determine whether NO plays a role in the inhibition of elicitor-induced stomatal closure, NO generation was compared in guard cells isolated from controls and Nbrboh-silenced N. benthamiana 3 h after treatment with boehmerin, harpin, and INF1. As shown in figure 6A, PBS-treated guard cells showed almost no fluorescence in guard cells of the control and gene-silenced plants. Elicitor treatment evoked NO generation in guard cells of the control plants, and this response was inhibited in both single- and dual-silenced N. benthamiana. Results of further quantitative analysis of Nbrboh-silencing effects on elicitor-induced NO production, with the software Quantity One, are shown in figure 6B. NO production in the dual-silenced plants decreased severely after elicitor treatment compared to the controls, suggesting that NO is associated with elicitor-induced stomatal closure.
Fig. 6.
Elicitor activation of NO is reduced in guard cells of Nbrboh-silenced plants. (A) In all cases, NO-sensitive dye DAF-2DA was loaded into cells of the epidermal peels, and fluorescence was measured after addition of PBS (10 mM), boehmerin (50 nM), harpin (50 nM), and INF1 (50 nM). For each treatment, fluorescence and bright-field images are shown. Results from several experiments are compiled in this figure. Experiments were repeated at least three times, and representative images are shown. (B) Quantitative analysis of in vivo NO generation monitored using DAF-2DA fluorescence as shown in (A). Results are presented as the mean (n ≥3) fluorescence intensity per pixel.
The increase of cytosolic calcium induced by elicitors is independent or acts upstream of Nbrboh
Cytosolic Ca2+ quickly increases upon pathogen infection (Garcia-Brugger et al., 2006), and Ca2+ influx is necessary for AOS production after elicitation (Blume et al., 2000; Grant M et al., 2000). The interplay between Ca2+ influx through channels and Ca2+ efflux from pumps and carriers will determine the form of a Ca2+ spike that is potentially specific to relevant sensors (Sanders et al., 2002). To evaluate the relative contributions of NADPH oxidases and whether elicitor-induced AOS production has an effect on [Ca2+]cyt elevation, calcium fluorescence imaging analysis of guard cells from intact epidermal strips was conducted. In control PBS-treated guard cells of gene-silenced and control plants, there was almost no fluorescence. Elicitors were applied to guard cells that showed obvious Ca2+ fluorescence. Cytosolic Ca2+ fluorescence in guard cells from both the controls and Nbrboh-silenced plants was altered slightly after elicitor treatment for 3 h (Fig. 7A). Further quantification using the software Quantity One revealed that Nbrboh-silencing caused little alterations of Ca2+ fluorescence intensity (Fig. 7B). This result indicates that the elicitor-induced calcium spike is independent of oxidative burst or acts upstream of the oxidative burst induced by Nbrboh. This finding is consistent with reports that a cytosolic Ca2+ spike precedes NADPH oxidase (NOX) activation as part of the elicitor-induced defence response (Nürnberger and Scheel, 2001; Zhao et al., 2005).
Fig. 7.
Elicitor activation of Ca2+ generation in guard cells of control and Nbrboh-silenced plants. (A) Leaf epidermal peels prepared from the control (top panels) or Nbrboh-silenced plants were loaded with the Ca2+ dye fluo-3 AM prior to incubation in PBS (10 mM), boehmerin (50 nM), harpin (50 nM), and INF1 (50 nM). In each case, corresponding fluorescence and bright-field images are shown. The areas of the peel subjected to analysis are greater than those shown in the figure. This experiment was repeated three times. Representative cells from one of three experiments are shown. In each experiment, a minimum of three epidermal peels were used as treatment replicates. (B) Quantitative analysis of in vivo Ca2+ generation monitored using fluo-3 AM fluorescence as shown in (A). Results are presented as the mean (n ≥3) fluorescence intensity per pixel.
AOS, apart from H2O2 and NO, are not involved in elicitor-induced stomatal closure but are related to elicitor-induced HR
Reduced DAB-H2O2 production was observed in leaves and decreased NO fluorescence in guard cells of Nbrboh-silenced N. benthamiana. However, the accumulation of AOS is characteristic of the HR in plant tissues and functions as a second signal mediating plant HR (Lamb and Dixon, 1997; Gechev and Hille, 2005; Li et al., 2006; Gan et al., 2009). To evaluate whether other AOS were involved in elicitor-induced stomatal closure, AOS such as peroxide and peroxynitrite were analysed by incubation with DHR, which is oxidized to the fluorochrome rhodamine 123 in the presence of AOS (Schulz et al., 1996). All epidermal peels (controls, single-silenced, and dual-silenced N. benthamiana) showed similar, bright fluorescence after elicitor treatments. By contrast, the control guard cells showed almost no fluorescence (Fig. 8A, B), indicating that Nbrboh-silencing has little effect on AOS production (except H2O2 and NO) in guard cells. Another enzyme might account for H2O2 production apart from superoxide and hydroxyl radical production. The observed AOS production is consistent with elicitor-induced HR, and Nbrboh was not sufficient for elicitor-induced HR. A growing body of evidence indicates that a balance between H2O2 and NO is key , and that the redox state determines the fate of cell death (Zeier et al., 2004; Frank and Dat, 2006). Moreover, AOS may serve as a secondary message, contributing to the establishment of defence (Torres et al., 2006).
Fig. 8.
Elicitor-induced AOS increase in guard cells of the control and Nbrboh-silenced plants. (A) In all cases, AOS dye DHR was loaded into cells of epidermal peels, and fluorescence was detected after incubation in PBS (10 mM), boehmerin (50 nM), harpin (50 nM), and INF1 (50 nM). For each treatment, fluorescence and bright-field images are shown. Results from several experiments are compiled in this figure. Experiments were repeated at least three times, and representative images are shown. (B) Quantitative analysis of in vivo AOS generation monitored using DHR fluorescence as shown in (A). Results are presented as mean (n ≥3) fluorescence intensity per pixel.
Discussion
Our results indicate that AOS production is required for elicitor signal transduction in guard cells. NbrbohA and NbrbohB are the main genes that mediate elicitor-induced H2O2 production in leaves and affect the aperture of guard cells of N. benthamiana upon elicitor treatment. The inhibition of elicitor-induced stomatal closure was accompanied by less NO generation, and cytosolic calcium induced by the elicitor increased to the same level in both controls and the Nbrboh-silenced plants. These results suggest that H2O2 and NO are signalling molecules for elicitor-activated signal transduction in guard cells.
RBOH are the main contributors to elicitor-induced AOS production
NbrbohA-, NbrbohB-, and NbrbohA/B-silenced N. benthamiana showed impaired elicitor-induced H2O2 production in leaves. Upon elicitor treatment, the production of H2O2 was obviously decreased, but some brown precipitate remained in the silenced leaves. This result indicates that RBOH are the major H2O2 source. Extracellular AOS production by NbrbohA and NbrbohB is required for elicitor-induced AOS production. Our data do not exclude the possibility that other Nbrboh or AOS-producing/scavenging genes contribute to the elicitor response. Other proteins (peroxidases, amine oxidase, oxalate oxidase, and flavin-containing oxidase) may account for reactive oxygen burst (Bolwell et al., 2002), and other cellular mechanisms can generate AOS in guard cells. AOS are associated with photosynthesis, chloroplasts, and cell wall peroxisomes (Grant JJ et al., 2000; Bolwell et al., 2002; Karpinski et al., 2003; Apel and Hirt, 2004). Distinct sources of elicitor-induced oxidative bursts may differentiate according to catalase sensitivity (Allan and Fluhr, 1997). On the other hand, various AOS-scavenging systems, including ascorbate peroxidases, glutathione, superoxide dismutases, and catalases, maintain AOS homeostasis in different compartments of the plant cell (Mittler et al., 2004) and may also be regulated by elicitors.
RBOH may not be a critical factor in elicitor-induced HR
H2O2 and NO function as stress signals in plants, mediating a range of responses to environmental stress (Neill et al., 2002b). Nbrboh-silenced plants showed less H2O2 production in leaves and less NO fluorescence in guard cells, but normal HR cell death upon treatment with elicitors. This result indicates that Nbrboh is not necessary for elicitor-triggered HR, but PR gene expression is accompanied by HR cell death. Our findings are consistent with those of Dorey et al. (1999), who reported that H2O2 was neither necessary nor sufficient for HR cell death, PAL activation, or SA accumulation in cultured tobacco cells. Although both H2O2 and NO play a role in the HR of plants infected by bacteria and viruses (Delledonne et al., 1998; Durner et al., 1998), a critical balance between AOS and NO determines the fate of the cell. NO is generated at the same time as H2O2 in response to pathogen attack and mediates defence responses similar to observations following H2O2 generation. Planchet et al. (2006) argued that the role of NO in HR should be reconsidered. In addition to AOS- and NO-scavenging systems, Rho-family GTPase (rac) isoforms may also regulate a cell response. A combination of rac isoforms with specific RBOH isoforms may mediate differential regulatory outcomes, which could explain the different functions of NADPH oxidases in regulating cell death. In rice (O. sativa) rac1 is a positive regulator of AOS production and cell death (Ono et al., 2001), whereas in tobacco (N. tabacum) rac5 acts as a negative regulator of AOS production via rbohD (Morel et al., 2004).
Ca2+ acts upstream of Nbrboh
All elicitors in this study induced bright fluorescence, which is consistent with the early elicitor-induced Ca2+ spike reported by Garcia-Brugger et al. (2006). There was little visible difference in Ca2+ fluorescence between the controls and the rboh-silenced plants 3 h after elicitor treatment. Apoplastic Ca2+ influx is important to the oxidative burst, and Ca2+ can activate RBOH proteins in vitro in tobacco and tomato (Miura et al., 1995, 1999; Sagi and Fluhr, 2001). Overexpression of TPC1 from O. sativa, a putative voltage-gated Ca2+-permeable channel, enhances elicitor-induced oxidative burst (Kurusu et al., 2005). Ectopic expression of Arabidopsis CDPK (calcium-dependent protein kinase) in tomato protoplasts elevates plasma membrane-associated NADPH oxidase activity (Xing et al., 2001). Transient expression of the constitutive active form of CDPK2 in N. benthamiana leads to oxidative burst-mediated cell death against hypo-osmotic stress (Ludwig et al., 2005). In potato, CDPK5 activates rbohB by phosphorylation of the N-terminal region and regulates oxidative burst (Kobayashi, 2007). Therefore, plant NADPH oxidases may be regulated by Ca2+ signalling.
RBOH-silencing with decreased H2O2 and NO production affects elicitor-induced stomatal closure, but not HR
It was found that Nbrboh-silenced plants decreased H2O2 production and that inhibition of elicitor-induced stomatal closure was associated with less NO fluorescence in guard cells of Nbrboh-silenced N. benthamiana, which is consistent with other reports. Elicitor-induced H2O2 production leads to stomatal closure (McAinsh et al., 1996; Lee et al., 1999), while inhibition of H2O2 production compromises ABA-induced stomatal closure (Shintaro et al., 2007). AOS and NO collaborate to mediate ABA-induced stomatal closure (Desikan et al., 2004). NO synthesis and stomatal closure in response to ABA are severely reduced in Arabidopsis NADPH oxidase (rbohD/F) double mutants, suggesting that endogenous H2O2 production elicited by ABA is required for NO synthesis (Bright et al., 2006). ABA, which induces stomatal closure in a cADPR and cGMP-dependent manner, stimulates NO synthesis in guard cells, indicating that NO is an even earlier secondary messenger in this response pathway.
Nbrboh-silencing did not affect elicitor-induced HR. Similar and bright fluorescence was observed in Nbrboh-silenced and control plants stained with DHR. The results suggest that Nbrboh is not the key contributor to elicitor-induced HR. AOS (other than H2O2 and NO) and the balance between AOS and NO participate in elicitor-triggered HR. Other enzymes (such as cell wall peroxidases, amine oxidase, oxalate oxidase, and flavin-containing oxidase) may account for AOS production (Bolwell and Wojtaszek, 1997; Bolwell et al., 2002; Tada et al., 2004; Zeier et al., 2004).
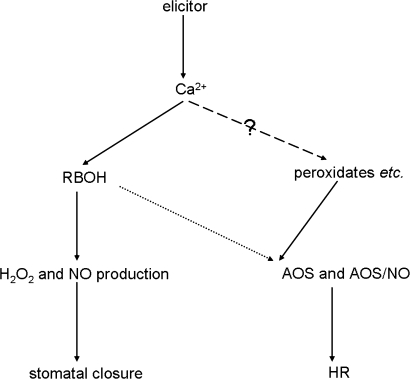
Based on these results, a simple model of elicitor signalling is presented (Fig. 9). This model considers how RBOH, AOS, and NO production (including the balance between AOS and NO), and Ca2+ are associated with elicitor-induced stomatal closure and HR cell death. Elicitors may trigger a Ca2+ spike activating upstream of RBOH and induce NO-associated stomatal closure. On the other hand, AOS of another origin and the balance between AOS and NO may be associated with elicitor-triggered HR cell death. These results are in agreement with previous evidence that AOS are activated in response to elicitors, and that H2O2 is crucial to the regulation of stomatal movements, thus shedding further light on this complex and important topic.
Fig. 9.
A simple model of the elicitor signalling of stomatal closure and HR. Elicitor-induced Ca2+ generation, the branch point of stomatal closure signalling, and HR signalling. rboh-silencing, which disrupts elicitor-induced H2O2 and NO production affected elicitor-induced stomatal closure, but not HR (‘..…>’ shows that RBOH are not the key contributors to elicitor-induced HR because rboh-silencing did not effect elicitor-triggered HR). In plants, plant cell wall peroxidases, amine oxidase, oxalate oxidase, and flavin-containing oxidase, which may act downsteam of Ca2+ generation, account for AOS production. RBOH-dependent AOS production is not the key contributor to elicitor-induced HR. AOS of other origin and the balance between both AOS and NO determine the fate of the cell.
Acknowledgments
This research was supported in part by the National Natural Science Foundation of China (Grant nos 30871605 and 30471123, ZG Zhang and no. 30571206, XB Zheng), the National 863 project of China (No. 2008AA10Z410, ZG Zhang), Commonweal Specialized Research Fund of China Agriculture (3-20), and the New Century Excellent Scholar Project of the Ministry of Education of China (NCET-07-0442 to ZG Zhang). We thank David Baulcombe (Sainsbury Laboratory, John Innes Centre, Norwich, UK) for the gift of the PVX vector and Agrobacterium strains, Sophien Kamoun (Department of Plant Pathology, Ohio State University, Ohio Agricultural Research and Development Centre, Wooster, OH, USA) for the gift of the inf1 gene, Gongyou Chen (Department of Plant Pathology, Nanjing Agricultural University, Nanjing, China) for the gift of the hrf1 gene, Xueping Zhou (College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, China) for the gift of Nicotiana benthamiana seeds. We also thank several anonymous reviewers for important suggestions on a previous version of the manuscript.
References
- Ali R, Ma W, Lemtiri-Chlieh F, Tsaltas D, Leng Q, von Bodman S, Berkowitz GA. Death don't have no mercy and neither does calcium: Arabidopsis cyclic nucleotide gated channel2 and innate immunity. The Plant Cell. 2007;19:1081–1095. doi: 10.1105/tpc.106.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan AC, Fluhr R. Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. The Plant Cell. 1997;9:1559–1572. doi: 10.1105/tpc.9.9.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amicucci E, Gaschler K, Ward JM. NADPH oxidase genes from tomato (Lycopersicon esculentum) and curly-leaf pondweed (Potamogeton crispus) Plant Biology. 1999;1:524–528. [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Asai S, Ohta K, Yoshioka H. MAPK signalling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. The Plant Cell. 2008;20:1390–1406. doi: 10.1105/tpc.107.055855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelo AR. Xylem parenchyma cells deliver the H2O2 necessary for lignification in differentiating xylem vessels. Planta. 2005;220:747–756. doi: 10.1007/s00425-004-1394-3. [DOI] [PubMed] [Google Scholar]
- Blume B, Nürnberger T, Nass N, Scheel D. Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defence in parsley. The Plant Cell. 2000;12:1425–1440. doi: 10.1105/tpc.12.8.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F. The apoplastic oxidative burst in response to biotic stress in plants: a three component system. Journal of Experimental Botany. 2002;53:1367–1376. [PubMed] [Google Scholar]
- Bolwell GP, Davies DR, Gerrish C, Auh CK, Murphy TM. Comparative biochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanisms. Plant Physiology. 1998;116:1379–1385. doi: 10.1104/pp.116.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GT, Wojtaszek P. Mechanisms for the generation of reactive oxygen species in plant defence: a broad perspective. Physiological and Molecular Plant Pathology. 1997;51:347–366. [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. The Plant Journal. 2006;45:113–122. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- Chen YL, Huang RF, Xiao YM, Lu P, Chen J, Wang XC. Extracellular calmodulin-induced stomatal closure is mediated by heterotrimeric G protein and H2O2. Plant physiology. 2004;136:4096–4103. doi: 10.1104/pp.104.047837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois C, Besson A, Dahan J, Bourque S, Dobrowolska G, Pugin A, Wendehenne D. Nitric oxide signalling in plants: interplays with Ca2+ and protein kinases. Journal of Experimental Botany. 2008;59:155–163. doi: 10.1093/jxb/erm197. [DOI] [PubMed] [Google Scholar]
- Dangl JL. Innate immunity: plants just say NO to pathogens. Nature. 1998;394:525–527. doi: 10.1038/28958. [DOI] [PubMed] [Google Scholar]
- Delledonne M, Zeier J, Marocco A, Lamb CJ. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proceedings of the National Academy of Sciences, USA. 2001;98:13454–13459. doi: 10.1073/pnas.231178298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delledonne M, Xia Y, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant desease resistance. Nature. 1998;394:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ. ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. Journal of Experimental Botany. 2004;55:205–212. doi: 10.1093/jxb/erh033. [DOI] [PubMed] [Google Scholar]
- Desikan R, Griffiths R, Hancock J, Neill S. A new role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA. 2002;99:16314–16318. doi: 10.1073/pnas.252461999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey S, Kopp M, Geoffroy P, Fritig B, Kauffmann S. Hydrogen peroxide from the oxidative burst is neither necessary nor sufficient for hypersensitive cell death induction, phenylalanine ammonia lyase stimulation, salicylic acid accumulation, or scopoletin consumption in cultured tobacco cells treated with elicitin. Plant Physiology. 1999;121:163–171. doi: 10.1104/pp.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durner J, Wendehenne D, Klessig DF. Defence gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP ribose. Proceedings of the National Academy of Sciences, USA. 1998;95:10328–10333. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- Frank VB, Dat JF. Reactive oxygen species in plant cell death. Plant Physiology. 2006;141:384–390. doi: 10.1104/pp.106.078295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan YZ, Zhang LS, Zhang ZG, Dong SM, Li J, Wang YC, Zheng XB. The LCB2 subunit of the sphingolip biosynthesis enzyme serine palmitoyltransferase can function as an attenuator of the hypersensitive response and Bax-induced cell death. New Phytologist. 2009;181:127–146. doi: 10.1111/j.1469-8137.2008.02642.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Brugger A, Lamotte O, Vandelle E, Bourque S, David L, Benoit P, Lecourieux D, Poinssot B, Wendehenne D, Pugin A. Early signalling events induced by elicitors of plant defences. Molecular Plant–Microbe Interactions. 2006;19:711–724. doi: 10.1094/MPMI-19-0711. [DOI] [PubMed] [Google Scholar]
- Garcia-Mata C, Lamattina L. Nitric oxide and abscisic acid cross talk in guard cells. Plant Physiology. 2002;128:790–792. doi: 10.1104/pp.011020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gechev TS, Hille J. Hydrogen peroxide as a signal controlling plant programmed cell death. Journal of Cell Biology. 2005;168:17–20. doi: 10.1083/jcb.200409170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M, Brown I, Adams S, Knight M, Ainslie A, Mansfield J. The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. The Plant Journal. 2000;23:441–450. doi: 10.1046/j.1365-313x.2000.00804.x. [DOI] [PubMed] [Google Scholar]
- Grant M, Lamb C. Systemic immunity. Current Opinion in Plant Biology. 2006;9:414–420. doi: 10.1016/j.pbi.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Grant JJ, Yun BW, Loake GJ. Oxidative burst and cognate redox signalling reported by luciferase imaging: identification of a signal network that functions independently of ethylene, SA and Me-JA but is dependent on MAPKK activity. The Plant Journal. 2000;24:569–582. doi: 10.1046/j.1365-313x.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- Groom QJ, Torres MA, Fordham-Skelton AP, Hammond-Kosack KE, Robinson NJ, Jones JD. rbohA, a rice homologue of the mammalian gp91phox respiratory burst oxidase gene. The Plant Journal. 1996;10:515–522. doi: 10.1046/j.1365-313x.1996.10030515.x. [DOI] [PubMed] [Google Scholar]
- Hatsugai N, Kuroyanagi M, Yamada K, Meshi T, Tsuda S, Kondo M, Nishimura M, Hara-Nishimura I. A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science. 2004;305:855–858. doi: 10.1126/science.1099859. [DOI] [PubMed] [Google Scholar]
- Ji R, Zhang ZG, Wang XB, Zheng XB. Phytophthora elicitor PB90 induced apoptosis in suspension cultures of tabacco. Chinese Science Bulletin. 2005;50:435–439. [Google Scholar]
- Karpinski S, Gabrys H, Mateo A, Karpinska B, Mullineaux PM. Light perception in plant disease defence signalling. Current Opinion in Plant Biology. 2003;6:390–396. doi: 10.1016/s1369-5266(03)00061-x. [DOI] [PubMed] [Google Scholar]
- Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C. A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. The Plant Cell. 1998;10:255–266. doi: 10.1105/tpc.10.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, Doke N, Yoshioka H. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. The Plant Cell. 2007;19:1065–1080. doi: 10.1105/tpc.106.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu T, Yagala T, Miyao A, Hirochika H, Kuchitsu K. Identification of a putative voltage-gated Ca2+ channel as a key regulator of elicitor-induced hypersensitive cell death and mitogen-activated protein kinase activation in rice. The Plant Journal. 2005;42:798–809. doi: 10.1111/j.1365-313X.2005.02415.x. [DOI] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Lamotte O, Gould K, Lecourieux D, Sequeira-Legrand A, Lebrun-Garcia A, Durner J, Pugin A, Wendehenne D. Analysis of nitric oxide signalling functions in tobacco cells challenged by the elicitor cryptogein. Plant Physiology. 2004;135:516–529. doi: 10.1104/pp.104.038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Choi H, Suh S, Doo IS, Oh KY, Choi EJ, Taylor ATS, Low PS, Lee Y. Oligogalaturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen specied from guard cells of tomato and Commelina communis. Plant Physiology. 1999;121:147–152. doi: 10.1104/pp.121.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhang ZG, Ji R, Wang YC, Zheng XB. Hydrogen peroxide regulates elicitor PB90-induced cell death and defence in nonheading Chinese cabbage. Physiological and Molecular Plant Pathology. 2006;67:220–230. [Google Scholar]
- Ludwig AA, Saitoh H, Felix G, Freymark G, Miersch O, Wasternack C, Boller T, Jones JDG, Romeis T. Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signalling controls stress responses in plants. Proceedings of the National Academy of Sciences, USA. 2005;102:10736–10741. doi: 10.1073/pnas.0502954102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Clayon H, Mansfield TA, Hetherington AM. Changes in stomatal behaviour and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiology. 1996;111:1031–1042. doi: 10.1104/pp.111.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends in Plant Science. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Miura Y, Yoshioka H, Doke N. An autophotographic determination of the active oxygen generation in potato tuber discs during hypersensitive response to fungal infection or elicitor. Plant Science. 1995;105:45–52. [Google Scholar]
- Miura Y, Yoshioka H, Park HJ, Kawakita K, Doke N. Plasma membrane perturbation in association with calcium ion movement followed by fungal elicitor-stimulated oxidative burst and defence gene activation in potato tuber. Annals of the Phytopathological Society of Japan. 1999;65:447–453. [Google Scholar]
- Morel J, Fromentin J, Blein JP, Simon-Plas F, Elmayan T. Rac regulation of NtrbohD, the oxidase responsible for the oxidative burst in elicited tobacco cell. The Plant Journal. 2004;37:282–293. doi: 10.1046/j.1365-313x.2003.01957.x. [DOI] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Clarke A, Hancock JT. Nitric oxide is a novel component of abscisic acid signalling in stomatal guard cells. Plant Physiology. 2002a;128:13–16. [PMC free article] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT. Hydrogen peroxide and nitric oxide as signalling molecules in plants. Journal of Experimental Botany. 2002b;53:1237–1247. [PubMed] [Google Scholar]
- Nürnberger T, Brunner F, Kemmerling B, Piater L. Innate immunity in plants and animals: striking similarities and obvious differences. Immunological Reviews. 2004;198:249–266. doi: 10.1111/j.0105-2896.2004.0119.x. [DOI] [PubMed] [Google Scholar]
- Nürnberger T, Scheel D. Signal transmission in the plant immune response. Trends in Plant Science. 2001;6:372–379. doi: 10.1016/s1360-1385(01)02019-2. [DOI] [PubMed] [Google Scholar]
- Ono E, Wong HL, Kawasaki T, Hasegawa M, Kodama O, Shimamoto K. Essential role of the small GTPase Rac in disease resistance of rice. Proceedings of the National Academy of Sciences, USA. 2001;98:759–764. doi: 10.1073/pnas.021273498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- Pitzschke A, Hirt H. Mitogen-activated protein kinases and reactive oxygen species signalling in plants. Plant Physiology. 2006;141:351–356. doi: 10.1104/pp.106.079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planchet E, Sonada M, Zeier J, Kaiser WM. Nitric oxide (NO) as an intermediate in the cryptogein-induced hypersensitive response: a critical re-evaluation. Plant. Cell and Environment. 2006;29:59–69. doi: 10.1111/j.1365-3040.2005.01400.x. [DOI] [PubMed] [Google Scholar]
- Qutob D, Kemmerling B, Brunner F, et al. Phytotoxicity and innate immune responses induced by Nep1-like proteins. The Plant Cell. 2006;18:3721–3744. doi: 10.1105/tpc.106.044180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, Fluhr R. Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. The Plant Cell. 2004;16:616–628. doi: 10.1105/tpc.019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Fluhr R. Superoxide production by plant homologues of the gp91(phox) NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiology. 2001;126:1281–1290. doi: 10.1104/pp.126.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Hall H, Krzymowska M, Drzewiecka K, Hennig J, Ellis BE. SIPK signalling controls multiple components of harpin-induced cell death in tobacco. The Plant Journal. 2005;42:406–416. doi: 10.1111/j.1365-313X.2005.02382.x. [DOI] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signalling. The Plant Cell. 2002;14:S401–S417. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz JB, Weller M, Klochgeter T. Potassium deprivation-induced apoptosis of cerebellar granule neurons: a sequential requirement for new mRNA and protein synthesis, ICE-like prorease activity, and reactive oxygen species. Journal of Neuroscience. 1996;16:4696–4706. doi: 10.1523/JNEUROSCI.16-15-04696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Okamoto M, Iwai T, Iwano M, Fukui K, Isogai A, Nakajima N, Ohashi Y. Reduced levels of chloroplast FtsH protein in tobacco mosaic virus-infected tobacco leaves accelerate the hypersensitive reaction. The Plant Cell. 2000;12:917–932. doi: 10.1105/tpc.12.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma PC, Ito A, Shimizu T, Terauchi R, Kamoun S, Saitoh H. Virus-induced silencing of WIPK and SIPK genes reduces resistance to a bacterial pathogen, but has no effect on the INF1-induced hypersensitive response (HR) in Nicotiana benthamiana. Molecular Genetics and Genomics. 2003;269:583–591. doi: 10.1007/s00438-003-0872-9. [DOI] [PubMed] [Google Scholar]
- Shintaro M, Kenji O, Megumi WS, Yoshimasa N,Yasuaki S, Yoshiyuki M. The coronatine-insensitive 1 mutation reveals the hormonal signalling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production. Plant Physiology. 2007;143:1398–1407. doi: 10.1104/pp.106.091298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada Y, Mori T, Shinogi T, et al. Nitric oxide and reactive oxygen species do not elicit hypersensitive cell death but induce apoptosis in the adjacent cells during the defence response of oat. Molecular Plant–Microbe Interactions. 2004;17:245–253. doi: 10.1094/MPMI.2004.17.3.245. [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Current Opinion in Plant Biology. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defence response. Proceedings of the National Academy of Sciences, USA. 2002;99:517–522. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Jones JDG, Dangl JL. Reactive oxygen species signalling in response to pathogens. Plant Physiology. 2006;141:373–378. doi: 10.1104/pp.106.079467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Onouchi H, Hamada S, Machida C, Hammond-Kosack KE, Jones JDG. Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox) The Plant Journal. 1998;14:365–370. doi: 10.1046/j.1365-313x.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- Wendehenne D, Durner J, Klessig DF. Nitric oxide: a new player in plant signalling and defence responses. Current Opinion in Plant Biology. 2004;7:449–455. doi: 10.1016/j.pbi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Wright KM, Duncan GH, Pradel KS, Carr F, Wood S, Oparka KJ, Santa Cruz S. Analysis of the N gene hypersensitive response induced by a fluorescently tagged tobacco mosaic virus. Plant Physiology. 2000;123:1375–1385. doi: 10.1104/pp.123.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T, Wang XJ, Malik K, Miki BL. Ectopic expression of an Arabidopsis calmodulin-like domain protein kinase-enhanced NADPH oxidase activity and oxidative burst in tomato protoplasts. Molecular Plant–Microbe Interactions. 2001;14:1261–1264. doi: 10.1094/MPMI.2001.14.10.1261. [DOI] [PubMed] [Google Scholar]
- Yoshie Y, Goto K, Takai R, Iwano M, Takayama S, Isogai A, Che FS. Function of the rice gp91phox homologs OsrbohA and OsrbohE genes in ROS-dependent plant immune responses. Plant Biotechnology. 2005;22:127–135. [Google Scholar]
- Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JD, Doke N. Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. The Plant Cell. 2003;15:706–718. doi: 10.1105/tpc.008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H, Sugie K, Park HJ, Maeda H, Tsuda N, Kawakita K, Doke N. Induction of plant gp91phox homolog by fungal cell wall, arachidonic acid, and salicylic acid in potato. Molecular Plant–Microbe Interactions. 2001;14:725–736. doi: 10.1094/MPMI.2001.14.6.725. [DOI] [PubMed] [Google Scholar]
- Zeier J, Delledonne M, Mishina T, Severi E, Sonoda M, Lamb CJ. Genetic elucidation of nitric oxide signalling in incompatible plant pathogen interactions. Plant Physiology. 2004;136:2875–2886. doi: 10.1104/pp.104.042499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Wang YC, Cai BJ, Zheng XB. Hydrogen peroxide, nitro oxide and calcium participated in stomatal closure induced by elicitor PB90 from Phytophthora boehmeriae. Acta Phytopathologica Sinica. 2007;37:62–68. [Google Scholar]
- Zhang ZG, Wang YC, Li J, Ji R, Shen G, Wang SC, Zhou X, Zheng XB. The role of SA in hypersensitive response and systemic acquired resistance induced by the elicitor PB90 of Phytophthora boehmeriae. Physiological and Molecular Plant Pathology. 2004;65:31–38. [Google Scholar]
- Zhao J, Davis LC, Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnology Advances. 2005;23:283–333. doi: 10.1016/j.biotechadv.2005.01.003. [DOI] [PubMed] [Google Scholar]