Abstract
Crop losses caused by pests and weeds have been estimated at 42% worldwide, with plant pathogens responsible for almost $10 billion worth of damage in the USA in 1994 alone. Elevated carbon dioxide [ECO2] and associated climate change have the potential to accelerate plant pathogen evolution, which may, in turn, affect virulence. Plant–pathogen interactions under increasing CO2 concentrations have the potential to disrupt both agricultural and natural systems severely, yet the lack of experimental data and the subsequent ability to predict future outcomes constitutes a fundamental knowledge gap. Furthermore, nothing is known about the mechanistic bases of increasing pathogen agressiveness. In the absence of information on crop species, it is shown here that plant pathogen (Erysiphe cichoracearum) aggressiveness is increased under ECO2, together with changes in the leaf epidermal characteristics of the model plant Arabidopsis thaliana L. Stomatal density, guard cell length, and trichome numbers on leaves developing post-infection are increased under ECO2 in direct contrast to non-infected responses. As many plant pathogens utilize epidermal features for successful infection, these responses provide a positive feedback mechanism facilitating an enhanced susceptibility of newly developed leaves to further pathogen attack. Furthermore, a screen of resistant and susceptible ecotypes suggest inherent differences in epidermal responses to ECO2.
Keywords: ABA, carbon dioxide, global change, plant–pathogen interactions, stomatal density, trichomes
Introduction
Plant–pathogen interaction and climate change
Investigations into the potential effects of CO2-induced climate change (IPCC, 2007) on plant–pathogen interactions largely consist of epidemic models coupled to general circulation models (GCMs) to predict future outcomes. Results predict increased disease pressure in Northern Italy with each decade, with more severe epidemics as a direct consequence of increasing temperature during specific months (Salinari et al., 2006). In the UK, increased disease severity of Phoma stem canker on oilseed rape is predicted under regional changes in both temperature and rainfall (Evans et al., 2008).
Experimental research into the effects of increasing atmospheric CO2 on plant–pathogen interactions has received little attention and produces conflicting results. Free Air Concentration Enrichment (FACE) facilities allow for an assessment of effects under field conditions. Such studies (increasing CO2 from 365 ppmv to 550 ppmv) found that disease incidence of leaf spot on mature leaves of Solidago rigida was reduced by half under ECO2 concentration (Strengbom and Reich, 2006), whereas in rice (Oryza sativa L.) both rice blast and sheath blight increased under similar FACE conditions in Japan (Kobayashi et al., 2006).
Controlled environment growth facility studies also report conflicting results. Under ambient (350 ppmv) and elevated (700 ppmv) CO2 conditions, the anthracnose pathogen Colletotrichum gloeosporioides increased in aggressiveness over 25 sequential infection cycles on the host Stylosanthes scabra. An increase in both aggressiveness and fecundity of isolates suggested that a favourable microclimate, due to increased canopy size under ECO2 could result in accelerated pathogen evolution (Chakraborty and Datta, 2003; Pangga et al., 2004). The importance of this result is heightened when account is taken of the short generation times and efficient dispersal mechanisms of many plant pathogens (Salinari et al., 2006). An investigation into the systemic responses of tobacco to the plant virus potato virus Y, found increased resistance of both inoculated older and uninfected younger leaves under ECO2 (1000 ppvm) compared to control conditions. The authors point out, however, that due to changes in the biochemical profiles of virus-infected plants under ECO2, this may result in increased susceptibility to bacterial and fungal pathogens (Matros et al., 2006). Recent reviews highlighting the future research needs in this area (Garrett et al., 2006; Berger et al., 2007), point out that the majority of recent work has focused on genomic level advances which allow high throughput analyses of post-infection gene expression. Such results are useful at pin-pointing when expression levels change, however, little attempt is made to translate gene expression changes in terms of whole plant responses (either anatomically or physiologically). Such results must be coupled to the physiology of both plant and pathogen (Berger et al., 2007), at the whole plant level (Bruce and Pickett, 2007) to increase knowledge at the population and global levels (Burdon et al., 2006; Glazebrook and Ton, 2007) before accurate predictions of pathogen interactions under climate change scenarios can be made: an issue that has been identified as a fundamental knowledge gap (Garrett et al., 2006). Furthermore, there is a general lack of information on the physiological or mechanistic bases of reported changes in pathogenesis.
Epidermal morphology: plant–pathogen and elevated CO2 interactions
The exchange of CO2 and water vapour between a leaf and the atmosphere is principally controlled by stomatal density (SD, the number of stomatal pores per unit area) and their mean aperture. The observation that changes to epidermal morphology, in the form of SD on herbarium leaves, had decreased over the last century (Woodward, 1987) represents compelling evidence that plants have responded to anthropogenic increases in CO2 concentration. Furthermore, this contemporary increase in atmospheric CO2 may stimulate the photosynthetic uptake of CO2 (Woodward and Lomas, 2004). Altered epidermal morphology of plants under ECO2 has the potential to affect plant–pathogen interactions as many pathogens utilize the epidermal characteristics of leaves to facilitate infection; stomatal pores afford a natural entry point for the establishment of infection (Underwood et al., 2007), therefore both number and size of stomata may affect plant resistance to fungal attack.
SD of the adaxial leaf surface has been found to correlate positively with the density of bean rust on the common bean (Phaseolus vulgaris) (Shaik, 1985). In a separate study of the same species investigating passive resistance to pathogens in the form of epidermal characteristics (SD), an inverse relationship of susceptibility with altitudinal range (1000 to 2800 m. asl) indicated that stomatal numbers may be affected by decreasing CO2 partial pressure (with increasing altitude), and that such responses may contribute to pathogen resistance in this species (Stenglein et al., 2005).
Trichomes have previously been considered to present a physical barrier to infection (Martin and Glover, 2007) and have been shown to retard the germination of Uromyces on the surface of bean leaves (Mmbaga et al., 1994). Trichome density (TD), as well as SD, were shown to have a positive relationship with total microbial density in several resistant and susceptible rice cultivars under both greenhouse and field conditions (De Costa et al., 2006), whereas a study of the bacterial pathogen Psuedomonas syringae on bean leaves showed the aggregation of pathogen cells predominantly around glandular trichome bases (Monier and Lindow, 2004). Such results suggest that specific pathogens may utilize different anatomical features to promote successful infection. A recent study using Arabidopsis thaliana demonstrated, using glabrous (trichome-less) accessions, that these mutants were less susceptible to infection by Botrytis cinerea than their wild-type counterpart (Landsberg erecta), the authors suggesting that trichomes trap airborne spores allowing a better chance of establishing infection (Calo et al., 2006).
Rationale
The model plant Arabidopsis thaliana has been successfully used in the elucidation of many genes involved in stomatal development and of biochemical events resulting in epidermal responses to environment factors (CO2 and light), recently reviewed by Casson and Gray (2008).
The powdery mildews, including Erysiphe cichoracearum, are obligate biotrophic fungi, and constitute one of the most economically important groups of plant pathogens (Oerke et al., 1994; Agrios, 1997). Because infections are limited to the leaf surface (epidermis) (Koh et al., 2005) and Arabidopsis ecotype Col-0 is classified as susceptible to E. cichoracearum (Adam et al., 1999), this makes an ideal model system for the investigation of plant–pathogen interactions with epidermal morphology under manipulated environmental conditions.
An experimental approach was taken here to examine the effect of ECO2 on both pathogen aggressiveness and plant morphological changes (epidermal features; SD, guard cell length (GCL), and TD) of leaves developing post-infection, and therefore resulting from systemic responses, using the powdery mildew (Erysiphe cichoracearum) and A. thaliana Col-0. Following the results from Col-0, a screen of 17 Arabidopsis ecotypes, classified as resistant or susceptible (Adam et al., 1999) were grown at ambient and ECO2 but not challenged with a pathogen.
The experiment and subsequent analyses tested four hypotheses designed to assess the impact that ECO2 concentration has on pathogen agressiveness, the impact that pathogen challenge has on epidermal characteristics, and the effects of inherent resistance or susceptibility on epidermal features under ECO2. (i) Pathogen infection of mature leaves has no systemic effect on epidermal morphology of newly developed leaves. (ii) Interaction of ECO2 and pathogen challenge has no effect on epidermal morphology of newly developed leaves. (iii) ECO2 concentration has no effect on pathogen aggressiveness. (iv) There is no inherent effect on epidermal morphological changes under ECO2.
Materials and methods
Plant material and growth conditions
Arabidopsis thaliana L. ecotype Columbia (Col-0) was grown in 8 cm pots of multi-purpose potting compost (Arthur Bowers, UK) in controlled environment chambers (Fitotron SGC2352/FM/HFL, Sanyo Gallenkamp) under an 8/16 h day/night cycle with a temperature regime of 22/18±0.5 °C, 150 μmol m−2 s−1 irradiance, following 4 d stratification at 4 °C in the dark. CO2 concentrations were 400 ppmv (ambient) or 800 ppmv (elevated).
Pathogen challenge
Actively growing cultures of Erysiphe cichoracearum were maintained on marrow (Cucurbita L. cv. Long Green Bush). Inoculum was used at the 7–10 d stage.
Col-0 was at the 4 week stage (10 rosette leaves present). Leaf insertions 6 to 10 were painted with inoculum at a spore concentration of 1×105 ml−1 in a suspension of Fluorinert (Sigma-aldrich, UK); control plants were painted with Fluorinert only. Plants were enclosed in propagators to isolate the inoculated from the control plants held within the same controlled environment chamber and to raise humidity to 80–85% for effective infection. This resulted in a decrease in light level of 30% (to 100 μmol m−2 s−1); thus changes in humidity and light level were experienced by both infected plants and the non-infected controls. Seven days later, infection status (see below) of inoculated mature leaves was confirmed by staining, whilst leaf insertions 14 to 17 (newly developed leaves) were harvested before they became infected, halved longitudinally, and analysed for both staining (non-infection check) and epidermal analyses.
Staining pathogen mycelium
Mature (infected) and half laminas of newly developed (non-infected) leaves were stained to check for infection. Leaves were placed abaxial surface down on ethanol:acetic acid (3:1 v/v) for 2 h on cotton pads. This process was repeated and left overnight. Leaf tissue was then relaxed on water-soaked pads for 2 h, then on pads moistened with lactic acid:glycerol:water (1:1:1 by vol.) overnight. Mycelia were stained with 0.6% Brilliant Blue R (Sigma-Aldrich, UK) for 10 s, rinsed with water, and slide mounted in 50% glycerol. Infection was determined using light microscopy (Leitz Laborlux S, Leitz, Germany).
Infection status
Following staining, the infection of mature infected and non-infected (control) rosette leaves was checked. The number of conidiophores (specialized hyphae on which conidia, the external asexual spores, are produced) and established colonies (network of mycelia) on mature leaves were counted across the entire leaf surface using light microscopy (Leitz Laborlux S, Leitz, Germany). Newly developed leaves were halved longitudinally and checked for lack of infection.
Ecotype screening
Arabidopsis thaliana L. resistant (score 0–1, Adam et al., 1999) ecotypes Zu-0, Edi-0, Can-0 Ll-0, Su-0, C24, and Kas-1 and susceptible (score 2–3, Adam et al., 1999) ecotypes Columbia (Col-0), Sf-2, Wu-0, Ler, Ba-1, Mc-0, Lc-0, Pla-0, Ws and Rld-1, were grown in 8 cm pots of multi-purpose potting compost (Arthur Bowers, Lincoln,UK) in controlled environment chambers (Fitotron SGC2352/FM/HFL, Sanyo Gallenkamp) under an 8/16 h day/night cycle with a temperature regime of 22/18±0.5 °C, 150 μmol m−2 s−1 irradiance, following 4 d stratification at 4 °C in the dark. CO2 concentrations were 400 ppmv (ambient) or 800 ppmv (elevated).
Epidermal analysis
Negative impressions of both abaxial and adaxial leaf surfaces are taken with high precision polyvinylsiloxane (Coltene-Whaledent Ltd, UK) either side of the midrib, mid lamina. Positive impressions are then made from the silicone rubber impressions using clear cellulose varnish, which were then mounted onto microscope slides. Stomatal density (mm−2), index [(no. of stomata/no. of stomata + no. of epidermal cells)×100], and guard cell length was determined by light microscopy using an image analysis software package (Quantimet 500, Leitz, Germany) coupled to a light microscope (Leitz Laborlux S, Leitz, Germany).
Trichome density on the adaxial surface was determined using light microscopy (Leitz Laborlux S, Leitz, Germany).
Statistics
Two-way ANOVA was performed with variables as CO2 environment and pathogen infection using MINITAB v. 12. (Minitab Inc.)
Results and discussion
CO2 response of stomatal characteristics
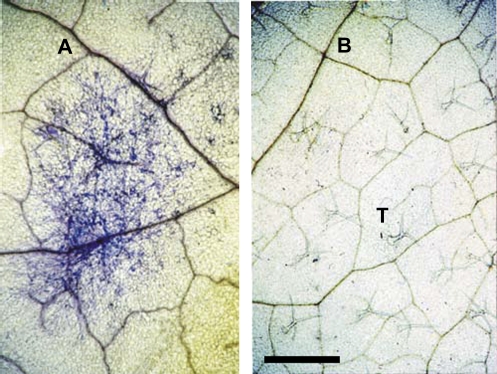
Figure 1 shows the successful establishment of a pathogen colony on the adaxial surface of a mature rosette leaf (Fig. 1A, blue stain) and a lack of infection on a non-infected control leaf (Fig. 1B) (leaf insertions 6–10).
Fig. 1.
Infection status. Mature leaves of Col-0 showing (A) blue stained E. cichoracearum infection or (B) non-infected (control) adaxial leaf surface 7 d following application of innoculum or Fluorinert control. T, trichome. Scale bar=2 mm.
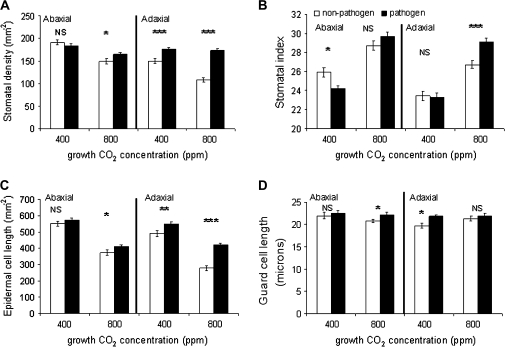
The effects of both CO2 concentration and pathogen challenge on the stomatal density (SD) and index (SI) of leaves that developed post-infection (leaf insertions 14–17) are shown in Fig. 2A and B. Significant reductions in both SD (22%; P <0.0001) and SI (10%: P <0.005) on the abaxial surface, and SD (27%; P <0.0001) and SI (8%; P <0.005) on the adaxial surface of non-infected controls occur when comparing ambient and ECO2 concentrations (Table 1) commensurate with previous findings (Lake et al., 2001, 2002).
Fig. 2.
Stomatal characteristics. (A) Stomatal density, (B) stomatal index, (C) epidermal cell density, and (D) guard cell length of newly developed leaves following pathogen challenge of mature leaves under ambient (Amb–400 ppm) and elevated (Elev–800 ppm) CO2 concentrations, with non-pathogen challenged controls. Both abaxial and adaxial leaf surfaces are shown. ***P <0.0001, **P <0.001, *P <0.05, NS, not significant between pathogen and non-pathogen challenge. Two-way ANOVA, Table 1, n=70, bar=SEmean.
Table 1.
Results of two-way analysis of variance for leaf morphological traits in Arabidopsis thaliana Col-0
| Pathogen |
CO2 |
Pathogen×CO2 |
||||
| F | P | F | P | F | P | |
| Abaxial surface | ||||||
| Stomatal density | 0.07 | 0.79 | 32.1 | <0.001 | 0.87 | 0.35 |
| Stomatal index | 4.5 | <0.05 | 14.8 | <0.0001 | 0.19 | 0.66 |
| Epidermal cell density | 5.23 | <0.05 | 7.3 | <0.005 | 0.4 | 0.52 |
| Guard cell size | 5.21 | <0.05 | 4.7 | <0.05 | 1.1 | 0.3 |
| Adaxial surface | ||||||
| Stomatal density | 43.7 | <0.001 | 12.19 | <0.005 | 8.06 | <0.005 |
| Stomatal index | 32.5 | <0.0001 | 23.8 | <0.0001 | 25.2 | <0.0001 |
| Epidermal cell density | 27.2 | <0.0001 | 6.6 | <0.05 | 6.2 | <0.05 |
| Guard cell size | 4.2 | <0.05 | 0.74 | 0.39 | 0.46 | 0.51 |
| Trichome density | 32.8 | <0.0001 | 29.1 | <0.001 | 0.76 | 0.38 |
The two-way component tested effects of pathogen challenge and CO2 concentration (grown at ambient 400 ppm or elevated 800 ppm). F=F value, P=probability value.
Pathogen challenge alone affects the epidermal characteristics of newly developed leaves
The effect of pathogen challenge to newly developed leaves, under ambient CO2, conditions suggests that pathogen challenge alone has the capacity to alter the adaxial stomatal characteristics of newly emergent leaves and that these changes are enhanced (SD) or reversed (SI) under ECO2.
There is no effect of pathogen challenge on SD of the abaxial surface under ambient CO2 concentration, however, under ECO2 concentration, a significant increase in SD of 15% is recorded. The adaxial surface shows a significant 15% increase in stomatal numbers following infection of mature leaves under ambient CO2 which increases to 38% under CO2 enrichment (Fig. 2A).
A slight but significant post-infection reduction of SI (P <0.05) is recorded on the abaxial surface at ambient CO2, with no significant difference at ECO2, however, again the adaxial surface shows a significant increase under ECO2 concentration (P <0.0005) (Fig. 2B). There is significant interaction between CO2 concentration and pathogen challenge on the adaxial surface only (Table 1). The adaxial surface of a non-infected leaf is likely to be the initial point of contact for incoming wind-blown pathogen spores.
Epidermal cell density of the adaxial surface shows a significant increase at ambient CO2 and both surfaces increase under ECO2 conditions (Fig. 2C; Table 1) suggesting that the infection of mature leaves causes a reduction in expansion of leaves developing post-infection and may be related to the metabolic costs of defence responses, despite the greater availability of CO2 for assimilation, and/or alterations in plant water status.
Stomatal guard cell length (GCL) reveals a non-pathogen induced response under ambient versus ECO2 as a decrease in guard cell size on the abaxial surface of new leaves (Table 1). Pathogen challenge results in an increase in adaxial GCL under ambient CO2 and abaxial GCL under ECO2 (Fig. 2D). Guard cell dynamics are now suggested as playing an active role in part of the plant's innate immune system (Schultz-Lefert and Robatzek, 2006). It is known that stomatal closure of leaves may be elicited in response to the application of several pathogen-derived compounds (Underwood et al., 2007); fusicoccin (a fungal toxin), oligogalacturonic acid (OGA; a product of plant cell wall degradation), and chitosan (a component of the fungal cell wall). These compounds are able to antagonize abscisic acid (ABA)-induced stomatal closure and disrupt normal stomatal movement (Schultz-Lefert and Robatzek, 2006). Effects on assimilation and water relations are not known as recovery to normal stomatal movement may occur within 3 h (Schultz-Lefert and Robatzek, 2006). This apparent contradictory response of compound-induced closure and antagonistic overriding of ABA-induced closure may be the result of unknown biochemical interactions, possibly involving the initiation of defence responses which up-regulate ABA, as it appears to be a short-term effect. Such interactions require further investigation.
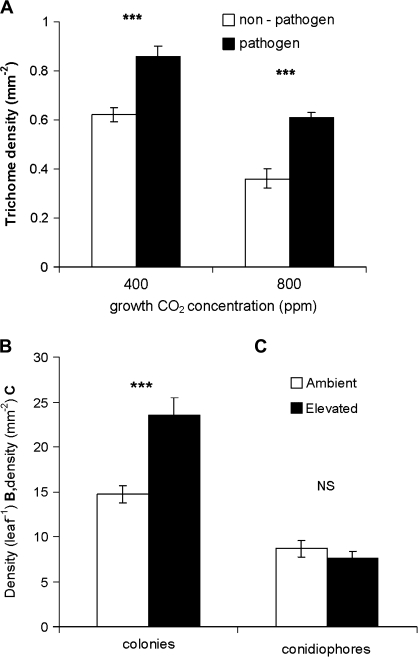
It is demonstrated here that trichome density (TD) of the adaxial surface of newly developed leaves increased significantly following pathogen challenge to mature leaves (Fig. 3A; Table 1), with the effect of CO2 concentration reducing the number of trichomes in both infected and non-infected controls. An increase in TD of the new leaves in response to the infection of mature leaves under both ambient and ECO2 appears to be a pathogen-induced response. Susceptibility of pathogen attack positively correlates with the possession of trichomes in some accessions of A. thaliana (Calo et al., 2006), an increase in TD may again facilitate further pathogen establishment.
Fig. 3.
(A) Trichome density on the adaxial surface of newly developed leaves, following infection of mature leaves, (B) number of established E. cichoracearum colonies, and (C) number of conidiophores of mature leaves following pathogen challenge under ambient (400 ppm) and elevated (800 ppm) CO2 concentrations. Student t tests between infected and non-infected (A) and ambient and elevated CO2 (B, C), n=25 (trichome density), n=40 (colonies), n=60 (conidiophores), ***P <0.0001, NS, not significant, bar=SE mean.
Elevated CO2 facilitates pathogen infection
The number of established colonies (networks of mycelia) on mature leaves increased significantly (P <0.001) under ECO2 conditions, with 40% more colonies successfully established (Fig. 3B), however, the number of conidiophores (specialized hyphae on which conidia, the external asexual spores are formed) remained unchanged (Fig. 3C) over the establishment period of 7 d. There is not, therefore, an increase in pathogen fecundity over this time scale, but a facilitated establishment.
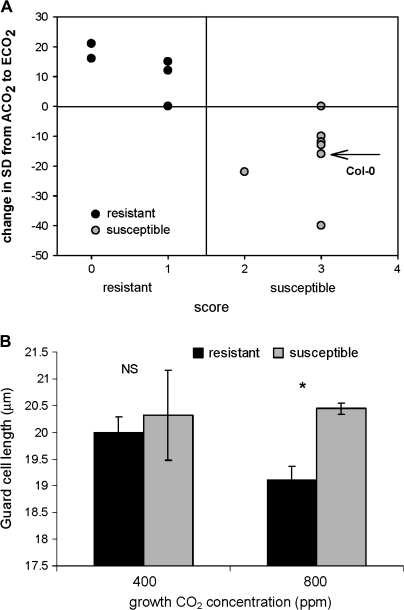
Inherent traits associated with morphological responses to ECO2
Figure 4A shows the results of an initial screening of 17 resistant and susceptible (Adam et al., 1999) ecotypes for changes in SD of when grown under ambient and ECO2 but not challenged with a pathogen. A general trend of increased SD for resistant and decreased SD for susceptible plants when grown under ECO2 is recorded, suggesting that inherent factors associated with resistance or susceptibility to E. cichoracearum impinge on stomatal responses to CO2. Mean GCL of resistant ecotypes decreases under ECO2, whilst susceptible ecotypes remain larger (Fig. 4B). These results suggest that resistant ecotypes under current CO2 levels may become more susceptible in the future in terms of increased stomatal numbers under ECO2, which may be aggravated by pathogen challenge as demonstrated in Col-0 (Fig. 2A). Susceptible ecotypes may become more susceptible following pathogen attack at ECO2 in terms of both increased SD (Col-0, Fig. 2A, B) and larger GCL (Col-0, Fig. 2D; susceptible ecotypes, Fig. 3C).
Fig. 4.
Ecotype screen for change in stomatal density (A) and mean guard cell length (B) of 17 ecotypes of A. thaliana (7 resistant and 10 susceptible to E. cichoracearum) grown under ambient (400 ppm) and elevated (800 ppm) CO2 concentrations: (A) arrow indicates Col-0 SD response to elevated CO2, n=70. (B) Student's t test of mean guard cell length, n=50, *P <0.05, NS, not significant between ambient and elevated CO2 concentration, bar, SEmean.
Trichome densities of the 17 accessions show either a decrease or no change under ECO2 across the range of resistant and susceptible ecotypes (data not shown), which corroborates with Col-0 when grown at ECO2 without pathogen challenge (Fig. 3A, white bars) and suggests that increases seen in Col-0 (Fig. 3A) are solely the result of pathogen challenge.
Increases in pathogen aggressiveness under increasing CO2 concentrations has profound implications for agriculture, both in terms of costs associated with control measures (defined as disease pressure; Salinari et al., 2006) and in geographic distribution of major economic cropping patterns already under pressure from regional climate shifts associated with rising CO2 emissions (IPCC, 2007). An increase in stomatal numbers per se provides an advantage to pathogens for colonization, however, an increase in stomatal density also has the potential to alter source/sink relationships, which under ECO2 conditions, would facilitate higher rates of carbon assimilation (Ainsworth and Rogers, 2007). This may also be of benefit to colonizing pathogens. Source/sink relationships may be altered under pathogen infection alone as demonstrated by the infection of barley leaves with powdery mildew, causing down-regulation of photosynthesis in cells which accumulate phenolic compounds associated with defence responses (Swarbrick et al., 2006). The authors point out that source/sink interactions of both susceptible and resistant, as well as sink or source targeted leaves, are complex. The interaction of such responses with increased carbon supply under ECO2 concentrations is not known.
Chakraborty and Datta (2003) have suggested that changes in leaf canopy microclimate under ECO2 may favour infection. The results presented here provide a physiological explanation of such microclimatic changes; pathogen-induced increases in SD, GCL, and TD which are exacerbated under ECO2 would increase humidity at the leaf surface and, as many pathogens require or prefer higher humidity for successful germination of spores, alteration of epidermal characteristics may afford a more favourable microclimate.
Stomatal development and environmental signalling mechanisms
It has been demonstrated in Arabidopsis that new leaves are influenced by the environment experienced by mature leaves in response to both CO2 concentration and light intensity and that long-distance signalling occurs to effect changes in the epidermal morphology of abaxial and adaxial leaf surfaces independently (Lake et al., 2001; Thomas et al., 2004). Research into the elucidation of the biochemical signalling components involved has progressed over the last several years (Casson and Gray, 2008), however, the full mechanism remains elusive.
It has recently been shown that the responses of SD to CO2 and humidity are positively related to and primarily driven by transpiration rate (E) and ABA concentration: an increase in E and/or ABA concentration in mature leaves produces increased SD in newly developed leaves (Lake and Woodward, 2008). Several studies report a decrease in E following pathogen infection (Fleischmann et al., 2005; Guo et al., 2005), however these decreases occur from 5 d to 5 weeks post-inoculation. Downy mildew infection of cucumber leaves reduced leaf water-content dramatically due to increased E in areas of leaf chlorosis which coincided with increased stomatal aperture during the early stages (2–3 d) of infection (Oerke et al., 2006). A study of Erysiphe graminis on winter wheat at ambient (350 μmol mol−1) and elevated (700 μmol mol−1) CO2 concentrations concluded that the severity of infection was significantly increased when host water content increased (Thompson et al., 1993). These results are not in conflict with the mechanistic relationship between E, ABA, and SD (Lake and Woodward, 2008) if signalling to new leaves occurs in the early stages of infection. ABA was demonstrated to increase within 24 h in Arabidopsis Col-0 following infection with Pseudomonas syringae (Schmelz et al., 2003). Furthermore, water loss from leaves to developing hyphae prior to visible symptoms of disease under ECO2 is not reported in the literature.
Pathogen challenge reverses normal stomatal responses to elevated CO2 in Arabidopsis Col-0
Mitogen-activated protein kinases (MAPKs) play a crucial role in plant growth and development, as well as many biotic and abiotic stress responses and constitute a complex signalling network (Menges et al, 2008). They are known to interact with defence signal molecules such as jasmonic acid (JA), salicylic acid (SA), ethylene, and ABA in the rice host-defence response (Reyna and Yang, 2006), and oligosaccharide elicitors in cultured cells of Lycopersicon peruvianum (Holley et al., 2003). Stomatal development in Arabidopsis is regulated by two sets of MAPKinases; MPK3 and MPK6 are environmentally sensitive, whilst upstream MAPKinase kinases, MKK4 and MKK5 are key regulators of stomatal development (Wang et al., 2007). A loss of function of these MAKKs results in a plethora of stomata and the disruption of the one-cell spacing pattern and, conversely, a gain in function results in a lack of stomatal initiation (Wang et al., 2007). Mechanical wounding (Chassot et al., 2008) and necrotrophic pathogen challenge in response to Botrytis cinerea (Schweighofer et al., 2007) induce MAPK activity in Arabidopsis. MKK4 and MKK5 were found to be activated by the elicitor flg22, with subsequent activation of MPK3 and 6 which act as paired components (Asai et al., 2002). As activation or gain of function of MKK4/5 should result in reduced stomatal initiation (Wang et al., 2007) the increase in SD and SI under pathogen treatment seen here on the adaxial surface, suggests that MAPK signalling as part of the innate immune response and stomatal development is disrupted. Inactivation of certain MAPKs is achieved by a key component of MAPK signalling – AP2C1 which results in the inactivation of MPK4 and MPK6 (Schweighofer et al., 2007). This may result in the decoupling of the activated MPK3/6 pairing. As MPKs are specifically activated by MKKs (Asai et al., 2002), inactivation of the paired MPK3/6 may disrupt the regulation of the upstream paired MKK4/5 function, potentially allowing for the initiation of stomatal development. Furthermore, stomatal numbers responding to changes in CO2 concentration and relative humidity, show a positive correlation with ABA (Lake and Woodward, 2008); ABA also activates MAPK3 with the negative regulator of ABA, ABI1, antagonizing MAPK6 (Leung et al., 2006).
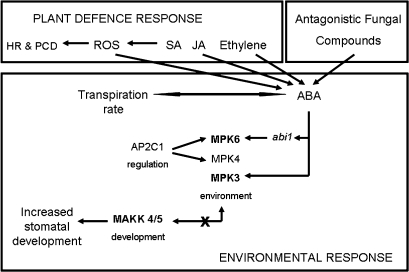
A putative mechanism for a systemic pathogen signalling system to increase stomatal numbers is proposed in Fig. 5. JA, SA, and ethylene constitute part of the defence response, resulting in localized hypersensitive responses (HR) and programmed cell death (PCD) (Reyna and Yang, 2006). ABA interacts with both JA and ethylene via pathway cross-talk (Leung et al., 2006) and is induced in Arabidopsis when challenged by the pathogens Xanthamonas campestris and Psuedomonas syringae (Robert-Seilaniantz et al., 2007). A mechanism involving the interaction of ABA, ABI1, and AP2C1 may be employed to inactivate the pairing of environmentally sensitive MAPKs 3/6, which in concert with an upstream loss of function of the pairing of MKKs 5/4 (Wang et al., 2007; Menges et al., 2008) results in increased stomatal initiation, development, and density. MAPK4 also functions as a regulator of pathogen defence responses, repressing SA and initiating JA-dependent gene expression (Andreasson et al., 2005)—cross-talk between hormones (SA, JA, ethylene, and ABA) and MPK cascades may allow for differential responses to specific biotic interactions.
Fig. 5.
Stomatal density response to pathogen challenge. Putative mechanism of long-distance signalling in response to pathogen infection to effect stomatal density increases. Induction of defence responses (hypersensitive responses, HR, reactive oxygen species, ROS; programmed cell death, PCD) by salicylic acid (SA), jasmonic acid (JA), and ethylene in mature leaves simultaneously triggers signal cross-talk with ABA (Schmelz et al., 2003). Environmentally sensitive Mitogen Activated Protein Kinases (MPK3/MPK6) are de-coupled through interaction with ABA, its negative regulator ABI1, and AP2C1. Decoupling disrupts upstream the specific kinase cascade MKK4/5 resulting in a loss of function and therefore increased stomatal initiation. The role of ABA antagonistic compounds [fusicocin, oligogalacturonic acid (OGA) and chitosan] in stomatal developmental signalling mechanisms are unknown.
It is demonstrated here, for the first time, that susceptibility of A. thaliana to a powdery mildew is enhanced under ECO2 concentrations and shows how, with a reversal of the plants normal physiological (epidermal) responses, may contribute, together with changes in source/sink relations, gene expression and phytohormone levels, to make the plant a better host. Whilst this study has a focus on morphological responses which will impinge on fundamental physiological processes (stomatal conductance, water loss, and carbon gain), it identifies the pressing need for experimental research which couples molecular techniques to whole plant physiology. The use of Arabidopsis for the elucidation of plant–pathogen interactions utilizing biochemical mutants together with the application and measured effects of fungal compounds will provide further insights into signalling systems involved. Such research will allow targeted analyses of crop–pathogen interactions and, ultimately, assist prediction of future outcomes of such interactions under regional and global climate change scenarios.
Acknowledgments
We thank the Royal Society, UK for funding JAL, P Swarbrick for training in pathogen techniques, and FI Woodward, D Cameron, B Lomax, and DJ Read for comments on this and earlier manuscripts.
References
- Adam L, Ellwood S, Wilson I, Saenz G, Xioa S, Oliver RP, Turner JG, Somerville S. Comparison of Erysiphe cichoracearum and E. cruciferarum and a survey of 360 Arabidopsis thaliana accessions for resistance to these two powdery mildew pathogens. Molecular Plant–Microbe Interactions. 1999;12:1031–1043. doi: 10.1094/MPMI.1999.12.12.1031. [DOI] [PubMed] [Google Scholar]
- Agrios GN. Plant pathology. 4th edn. San Diego, USA: Academic Press; 1997. [Google Scholar]
- Ainsworth EA, Rogers A. The response of photosynthesis and stomatal conductance to rising CO2: mechanisms and environmental interactions. Plant, Cell and Environment. 2007;30:258–270. doi: 10.1111/j.1365-3040.2007.01641.x. [DOI] [PubMed] [Google Scholar]
- Andreasson E, Jenkins T, Brodersen P, et al. The MAP kinase MKS1 is a regulator of plant defence responses. The EMBO Journal. 2005;24:2579–2589. doi: 10.1038/sj.emboj.7600737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chui WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. MAP kinase signaling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- Berger S, Sinha AK, Roitsch T. Plant physiology meets phytopathology: plant primary metabolism and plant–pathogen interactions. Journal of Experimental Botany. 2007;58:4019–4026. doi: 10.1093/jxb/erm298. [DOI] [PubMed] [Google Scholar]
- Bruce TJA, Pickett JA. Plant defence signaling induced by biotic attacks. Current Opinion in Plant Biology. 2007;10:387–392. doi: 10.1016/j.pbi.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Burdon JJ, Trall PH, Ericson L. The current and future dynamics of disease in plant communities. Annual Review of Phytopathology. 2006;44:19–39. doi: 10.1146/annurev.phyto.43.040204.140238. [DOI] [PubMed] [Google Scholar]
- Calo L, Garcia I, Gotor C, Romero LC. Leaf hairs influence phytopathogenic fungus infection and confer an increased resistance when expressing a Trichoderma α 1,3-glucanase. Journal of Experimental Botany. 2006;57:3911–3920. doi: 10.1093/jxb/erl155. [DOI] [PubMed] [Google Scholar]
- Casson S, Gray JE. Influence of environmental factors on stomatal development. New Phytologist. 2008;178:9–23. doi: 10.1111/j.1469-8137.2007.02351.x. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Datta S. How will plant pathogens adapt to host plant resistance at elevated CO2 under a changing climate? New Phytologist. 2003;159:733–742. doi: 10.1046/j.1469-8137.2003.00842.x. [DOI] [PubMed] [Google Scholar]
- Chassot C, Buchala A, Schoonbeek H, Metraux JP, Lamotte O. Wounding of Arabidopsis leaves causes a powerful but transient protection against Botrytis infection. The Plant Journal. 2008;55:555–567. doi: 10.1111/j.1365-313X.2008.03540.x. [DOI] [PubMed] [Google Scholar]
- De Costa DM, Rathnayake RMPS, De Costa WAJM, Kumari WMD, Dissanayake DMN. Variation of phyllosphere microflora of different rice varieties in Sri Lanka and its relationship to leaf anatomical and physiological characters. Journal of Agronomy and Crop Science. 2006;192:209–220. [Google Scholar]
- Evans N, Baierl A, Semenov MA, Gladders P, Fitt Bruce DL. Range and severity of a plant disease increased by global warming. Journal of the Royal Society Interface. 2008;5:525–531. doi: 10.1098/rsif.2007.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann F, Koehl J, Portz R, Beltrame AB, Osswald W. Physiological changes of Fagus sylvatica seedlings infected with Phytophthora citricola and the contribution of its elicitin ‘Citricolin’ to pathogenesis. Plant Biology. 2005;7:650–658. doi: 10.1055/s-2005-872891. [DOI] [PubMed] [Google Scholar]
- Garrett KA, Dendy SP, Frank EE, Rouse MN, Travers SE. Climate change effects on plant disease: genomes to ecosystems. Annual Review of Phytopathology. 2006;44:489–509. doi: 10.1146/annurev.phyto.44.070505.143420. [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Ton J. Biotic interactions: recurring themes and expanding scales. Current Opinion in Plant Biology. 2007;10:331–334. [Google Scholar]
- Guo YP, Guo DP, Peng Y, Chen JS. Photosynthetic responses of radish (Raphanus sativus var. longipinnatus) plants to infection by turnip mosaic virus. Photosynthetica. 2005;43:457–462. [Google Scholar]
- Holley SR, Roopa D, Yalamanchili RD, Moura DS, Ryan CA, Stratmann JW. Convergence of signalling pathways induced by systemin, oligosaccharide elicitors and ultraviolet-B radiation at the level of mitogen-activated protein kinases in Lycopersicon peruvianum suspension-cultured cells. Plant Physiology. 2003;132:1728–1738. doi: 10.1104/pp.103.024414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC. Climate change 2007: the physical science basis. Contribution of Working Group I to the 4th Assessment Report of the IPCC. UK: Cambridge University Press; 2007. [Google Scholar]
- Kobayashi T, Ishiguro K, Nakajima T, Kim HY, Okada M, Kobayashi K. Effects of elevated atmospheric CO2 concentration on the infection of rice-blast and sheath blight. Phytopathology. 2006;96:425–431. doi: 10.1094/PHYTO-96-0425. [DOI] [PubMed] [Google Scholar]
- Koh S, Andre A, Edwards H, Ehrhardt D, Somerville S. Arabidopsis thaliana subcellular responses to compatible Erysiphe cicohoracearum infections. The Plant Journal. 2005;44:516–529. doi: 10.1111/j.1365-313X.2005.02545.x. [DOI] [PubMed] [Google Scholar]
- Lake JA, Quick WP, Beerling DJ, Woodward FI. Plant development: signals from mature to new leaves. Nature. 2001;411:154. doi: 10.1038/35075660. [DOI] [PubMed] [Google Scholar]
- Lake JA, Woodward FI, Quick WP. Long-distance CO2 signalling in plants. Journal of Experimental Botany. 2002;53:183–193. doi: 10.1093/jexbot/53.367.183. [DOI] [PubMed] [Google Scholar]
- Lake JA, Woodward FI. Response of stomatal numbers to CO2 and humidity: control by transpiration rate and abscisic acid. New Phytologist. 2008;179:397–404. doi: 10.1111/j.1469-8137.2008.02485.x. [DOI] [PubMed] [Google Scholar]
- Leung J, Orfanidi S, Chefdor F, Meszaros T, Bolte S, Mizoguchi T, Shinozaki K, Giraudat J, Bogre L. Anatgonistic interaction between MAP kinase and protein phosporylase 2C in stress recovery. Plant Science. 2006;171:596–606. [Google Scholar]
- Martin C, Glover BJ. Functional aspects of cell patterning in aerial epidermis. Current Opinion in Plant Biology. 2007;10:70–82. doi: 10.1016/j.pbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Matros A, Amme S, Kettig B, Buck-Sorlin GH, Sonnewald U, Mock HP. Growth at elevated CO2 concentrations leads to modified profiles of secondary metabolites in tobacco cv. SamsunNN and to increased resistance against infection with potato virus Y. Plant, Cell and Environment. 2006;29:126–137. doi: 10.1111/j.1365-3040.2005.01406.x. [DOI] [PubMed] [Google Scholar]
- Menges M, Doczi R, Okresz L, Morandini P, Mizzi L, Soloviev M, Murray JAH, Bogre L. Comprehensive gene expression atlas for the Arabidopsis MAP kinase signaling pathways. New Phytologist. 2008;179:643–662. doi: 10.1111/j.1469-8137.2008.02552.x. [DOI] [PubMed] [Google Scholar]
- Mmbaga MT, Steadman JR, Roberts JJ. Interaction of bean leaf pubescence with rust Urediniospore deposition and subsequent infection density. Annals of Applied Biology. 1994;125:243–254. [Google Scholar]
- Monier JM, Lindow SE. Frequency, size and localization of bacterial aggregates on bean leaf surfaces. Applied Environmental Microbiology. 2004;70:346–355. doi: 10.1128/AEM.70.1.346-355.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oerke EC, Dehne HW, Schonbeck F, Weber A. Crop production and crop protection: estimated losses in major food and cash crops. Amsterdam: Elsevier; 1994. [Google Scholar]
- Oerke EC, Steiner U, Dehne HW, Lindenthal M. Thermal imaging of cucumber leaves affected by downy mildew and environmental conditions. Journal of Experimental Botany. 2006;57:2121–2132. doi: 10.1093/jxb/erj170. [DOI] [PubMed] [Google Scholar]
- Pangga IB, Chakraborty S, Yates D. Canopy size and induced resistance in Stylosanthes scabra determine Anthracnose severity at high CO2. Phytopathology. 2004;94:221–227. doi: 10.1094/PHYTO.2004.94.3.221. [DOI] [PubMed] [Google Scholar]
- Reyna NS, Yang Y. Molecular analysis of the rice MAP kinase gene family in relation to Magnaporthe grisea infection. Molecular Plant–Microbe Interactions. 2006;19:530–540. doi: 10.1094/MPMI-19-0530. [DOI] [PubMed] [Google Scholar]
- Robert-Seilaniantz A, Navarro L, Bari R, Jones JDG. Pathological hormone imbalances. Current Opinion in Plant Biology. 2007;10:372–379. doi: 10.1016/j.pbi.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Salinari F, Giosue S, Tubiello FN, Rettori A, Rossi V, Spanna F, Rosensweig C, Gullino ML. Downy mildew (Plasmopara viticola) epidemics on grapevine under climate change. Global Change Biology. 2006;12:1299–1307. [Google Scholar]
- Schultz-Lefert P, Robatzek S. Plant pathogens trick guard cells into opening the gates. Cell. 2006;126(5):831–834. doi: 10.1016/j.cell.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Engelberth J, Alborn HT, O'Donnell P, Sammons M, Toshima H, Tumlinson JH., III Simultaneous analysis of phytohormones, phytotoxins and volatile organic compounds in plants. Proceedings of the National Academy of Sciences, USA. 2003;100:10552–10557. doi: 10.1073/pnas.1633615100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer A, Kazanavicuite V, Scheikl E, et al. The PCC2C-type phosphatase APC21, which negatively regulates MPK4 and MPK6 modulates innate immunity, jasmonic acid, and ethylene levels in Arabidopsis. The Plant Cell. 2007;19:2213–2224. doi: 10.1105/tpc.106.049585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaik M. Race-non-specific resistance in bean cultivars to races of Uromyces appendiculatus var. appendiculatus and its correlation with leaf epidermal characteristics. Phytopathology. 1985;75:478–481. [Google Scholar]
- Stenglein SA, Arambarri AM, MdCM Sevillano, Balatti PA. Leaf epidermal characters related with plant's passive resistance to pathogens varies among accessions of wild beans Phaseolus vulgaris var. aborigineus (Leguminosae, Phaseoleae) Flora–Morphology, Distribution. Functional Ecology of Plants. 2005;200:285–295. [Google Scholar]
- Strengbom J, Reich PB. Elevated [CO2] and increased N supply reduce leaf disease and related photosynthetic impacts on Solidago rigida. Oecologia. 2006;149:519–525. doi: 10.1007/s00442-006-0458-4. [DOI] [PubMed] [Google Scholar]
- Swarbrick PJ, Schultz-Lefert P, Scholes JD. Metabolic consequences of susceptibility and resistance (rave-specific and broad-spectrum) in barley leaves challenged with powdery mildew. Plant, Cell and Environment. 2006;29:1061–1076. doi: 10.1111/j.1365-3040.2005.01472.x. [DOI] [PubMed] [Google Scholar]
- Thomas PW, Woodward FI, Quick WP. Systemic irradiance signalling in tobacco. New Phytologist. 2004;161:193–198. [Google Scholar]
- Thompson GB, Brown JKM, Woodward FI. The effects of host carbon dioxide, nitrogen and water supply on the infection of wheat by powdery mildew and aphids. Plant, Cell and Environment. 1993;16:687–694. [Google Scholar]
- Underwood W, Melotto M, He SY. Role of stomata in bacterial invasion. Cellular Microbiology. 2007;9:1621–1629. doi: 10.1111/j.1462-5822.2007.00938.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. The Plant Cell. 2007;19:63–73. doi: 10.1105/tpc.106.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward FI. Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature. 1987;327:617–618. [Google Scholar]
- Woodward FI, Lomas MR. Vegetation dynamics: simulating responses to climatic change. Biological Reviews. 2004;79:643–670. doi: 10.1017/s1464793103006419. [DOI] [PubMed] [Google Scholar]