Abstract
In maize (Zea mays), abscisic acid (ABA)-induced H2O2 production activates a 46 kDa mitogen-activated protein kinase (p46MAPK), and the activation of p46MAPK also regulates the production of H2O2. However, the mechanism for the regulation of H2O2 production by MAPK in ABA signalling remains to be elucidated. In this study, four reactive oxygen species (ROS)-producing NADPH oxidase (rboh) genes (ZmrbohA–D) were isolated and characterized in maize leaves. ABA treatment induced a biphasic response (phase I and phase II) in the expression of ZmrbohA–D and the activity of NADPH oxidase. Phase II induced by ABA was blocked by pretreatments with two MAPK kinase (MPKKK) inhibitors and two H2O2 scavengers, but phase I was not affected by these inhibitors or scavengers. Treatment with H2O2 alone also only induced phase II, and the induction was arrested by the MAPKK inhibitors. Furthermore, the ABA-activated p46MAPK was partially purified. Using primers corresponding to the sequences of internal tryptic peptides, the p46MAPK gene was cloned. Analysis of the tryptic peptides and the p46MAPK sequence indicate it is the known ZmMPK5. Treatments with ABA and H2O2 led to a significant increase in the activity of ZmMPK5, although ABA treatment only induced a slight increase in the expression of ZmMPK5. The data indicate that H2O2-activated ZmMPK5 is involved in the activation of phase II in ABA signalling, but not in phase I. The results suggest that there is a positive feedback loop involving NADPH oxidase, H2O2, and ZmMPK5 in ABA signalling.
Keywords: Abscisic acid, feedback regulation, hydrogen peroxide, mitogen-activated protein kinase, NADPH oxidase, signal transduction, Zea mays
Introduction
NADPH oxidase catalyses the production of superoxide (O2−) by transferring electrons from NADPH to molecular oxygen, followed by dismutation of O2− to H2O2. The mammalian NADPH oxidase consists of two plasma membrane proteins, gp91phox and p22phox (phox, phagocyte oxidase). The cytosolic regulatory proteins, p47phox, p67phox, and p40phox, and the small G protein Rac2 translocate to the plasma membrane after stimulation to form the active complex (Babior, 2004). Plant NADPH oxidases, termed rboh (respiratory burst oxidase homologue, a homologue of gp91phox, which is the catalytic subunit of phagocyte NADPH oxidase), have been isolated from many plant species, including rice (Oryza sativa; Groom et al., 1996), Arabidopsis thaliana (Keller et al., 1998; Torres et al., 1998), tobacco (Nicotiana benthamiana; Yoshioka et al., 2003), and potato (Solanum tuberosum; Yoshioka et al., 2001; Yamamizo et al., 2007). The Arabidopsis genome contains 10 rboh genes (Torres and Dangl, 2005). These plant rboh proteins are predicted to contain cytosolic FAD- and NADPH-binding domains and six conserved transmembrane helices that correspond to those identified in gp91phox, and carry an N-terminal extension comprising two Ca2+-binding EF-hand motifs (Torres and Dangl, 2005; Sagi and Fluhr, 2006). The activity of NADPH oxidase can be regulated by Ca2+, calcium-dependent protein kinase (CDPK), and Rac GTPase (Sagi and Fluhr, 2001; Kobayashi et al., 2007; Wong et al., 2007; Ogasawara et al., 2008; Takeda et al., 2008). Genetic evidence shows that reactive oxygen species (ROS) generated by NADPH oxidase play important roles in plant defence response, cell death, abiotic stress, hormonal response, stomatal closure, and root hair development (Torres et al., 2002; Foreman et al., 2003; Kwak et al., 2003, 2006; Yoshioka et al., 2003; Sagi et al., 2004; Torres and Dangl, 2005; Gapper and Dolan, 2006; Sagi and Fluhr, 2006; Takeda et al., 2008).
The mitogen-activated protein kinase (MAPK) cascade is one of the major pathways by which extracellular stimuli are transduced into intracellular responses in all eukaryotic cells (Tena et al., 2001; Zhang and Klessig, 2001; Jonak et al., 2002; Nakagami et al., 2005; Pitzschke and Hirt, 2006). MAPK and immediate upstream activators, MAPK kinase (MAPKK) and MAPKK kinase (MAPKKK), constitute a functionally interlinked MAPK cascade. Activated MAPK can phosphorylate a variety of substrates including transcription factors, other protein kinases, and cytoskeleton-associated proteins (Nakagami et al., 2005; Pitzschke and Hirt, 2006). It has been shown that MAPKs are involved in plant signal transduction in response to pathogens, drought, salinity, cold, wounding, heavy metals, O3, ROS, UV-B, and hormone stimuli (Tena et al., 2001; Zhang and Klessig, 2001; Jonak et al., 2002; Mittler, 2002; Mittler et al., 2004; Nakagami et al., 2005, 2006; Pitzschke and Hirt, 2006).
Both NADPH oxidase and MAPK have been shown to be involved in the plant hormone abscisic acid (ABA) signal transduction. ABA treatment induces the expression of AtrbohD and AtrbohF genes in Arabidopsis guard cells (Kwak et al., 2003), and enhances the activity of NADPH oxidase in maize leaves (Jiang and Zhang, 2002a, 2003). AtrbohD and AtrbohF mediate ABA-induced ROS production, ABA activation of Ca2+-permeable channels, and ABA-induced stomatal closure (Kwak et al., 2003). Meanwhile, ABA treatment also activates AtMPK3 (Lu et al., 2002), AtMPK6, and AtMPK4 (Xing et al., 2008) in Arabidopsis, OsMAPK5 in rice (Xiong and Yang, 2003), p45MAPK in pea (Pisum sativum; Burnett et al., 2000), and p46MAPK in maize (Zea mays; Zhang et al., 2006). The activation of MAPKs can regulate plant development and plant responses to multiple stresses (Lu et al., 2002; Xiong and Yang, 2003; Nakagami et al., 2005, 2006; Pitzschke and Hirt, 2006; Xing et al., 2008). However, the relationship between NADPH oxidase and MAPK in ABA signalling is not yet clear.
In a previous study, results showed that ABA activates a 46 kDa MAPK (p46MAPK), which is involved in ABA-induced antioxidant defence and acts downstream of H2O2 production in maize leaves (Zhang et al., 2006). The activation of MAPK can also enhance H2O2 production. These findings allow the causal relationship between NADPH oxidase and MAPK in ABA signalling to be investigated. First, four maize rboh genes, called ZmrbohA, ZmrbohB, ZmrbohC, and ZmrbohD, were isolated and then their expression was studied in maize leaves treated with ABA. The effects of MAPKK inhibitors and H2O2 scavengers on the expression of these rboh genes and the activity of NADPH oxidase induced by ABA were also investigated. Furthermore, ABA-activated p46MAPK, designated ZmMPK5, was partially purified and identified, and the p46MAPK gene cloned. The data showed that ABA induces a biphasic response in the expression of rboh genes and the activity of NADPH oxidase and phase II is regulated by H2O2 and ZmMPK5. The results suggest that there is a positive feedback regulation involving NADPH oxidase, H2O2, and MAPK in ABA signalling.
Materials and methods
Plant material and treatments
Seeds of maize (Zea mays L. cv. Nongda 108; from Nanjing Agricultural University, China) were sown in trays of sand in a light chamber at a temperature of 22–28 °C, photosynthetic active radiation (PAR) of 200 μmol m−2 s−1, and a photoperiod of 14/10 h (day/night), and watered daily. When the second leaf was fully expanded, they were collected and used for all investigations.
The plants were treated as described previously (Zhang et al., 2006). In order to study the effects of various inhibitors or scavengers, the detached plants were pretreated with 100 μM 2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one (PD98059), 10 μM 1,4-diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto) butadiene (U0126), 400 U catalase (CAT), 5 mM dimethylthiourea (DMTU), and 100 μM diphenylene iodonium (DPI) for 6 h, and then exposed to 100 μM ABA or 10 mM H2O2 treatment. Previous studies showed that the concentrations and the time of pretreatments with these inhibitors or scavengers are suitable for investigating the role of MAPK or H2O2 in ABA signalling in the leaves of maize plants (Jiang and Zhang, 2002b; Zhang et al., 2006, 2007). After treating the detached maize plants, the second leaves were sampled and immediately frozen under liquid N2 for further analysis.
RNA preparation and cDNA synthesis
Total RNA was isolated from leaves using Plant RNA Purification Reagent (Tiangen Biotech Co., Ltd, Beijing, China) according to the manufacturer's instructions. DNase treatment was included in the isolation step using the RNase-free DNase (TaKaRa Bio Inc., China). Approximately 2 μg of total RNA were reverse transcribed using oligo d(T)16 primer and M-MLV reverse transcriptase (Promega, Madison, WI, USA) at 42 °C for 60 min.
Isolation of gp91phox homologues from maize
To amplify partial sequences of putative gp91phox homologues (Zmrbohs) from maize, degenerate oligonucleotides Pzrb5 and Pzrb6 (Pzrb5 MRTTYGARTGGCATCC; Pzrb6 CATIAYDYCYTTRAACCA) were designed and synthesized, based on the conserved amino acid and nucleotide sequences of plant rboh genes. The resulting major fragment was cloned into pMD18-T vector (TaKaRa Bio Inc., China) and three clones were sequenced in both directions. A 5′ rapid amplification of cDNA ends approach (Invitrogen 5′ RACE kit) was used to isolate the unknown 5′-region of the Zmrbohs according to the manufacturer's recommendations. The 3′-full RACE Core Set Ver. 2.0 Kit (TaKaRa Bio Inc., China) was used to isolate the unknown 3′-region of the Zmrbohs according to the manufacturer's recommendations. Following amplification, RACE products were ligated into pMD18-T vector, cloned, and sequenced.
The in silico extension was also done to speed up the cloning process. All sequences of PCR products were used for BLAST searches in the NCBI database (www.ncbi.nlm.nih.gov) and the TIGR database (www.tigr.org) for more information. Some overlapping sequences were retrieved and experimentally verified by amplifying them with gene-specific primers by RT-PCR.
Real-time quantitative RT-PCR expression analysis
Real-time quantification RT-PCR reactions were performed in a DNA Engine Opticon 2 real-time PCR detection system (Bio-Rad Laboratories Inc., USA) using the SYBR® Premix Ex Taq™ (TaKaRa Bio Inc., China) according to the manufacturer's instructions. cDNA was amplified by PCR using the following primers: ZmrbohA, forward ATGACATTCTTCTGCTTATTGGC and reverse ATGCTTCCCACCTCTTCGTT; ZmrbohB, forward ACCCTTTGAATGGCATCCG and reverse AAGGAGTTGCACCAATCCCTAAT; ZmrbohC, forward GAATACGAAAGCTGCACGGGCATT and reverse CCAAAGTATTTGCGCAGTGGAGCA; ZmrbohD, forward GCGGGCAGTACATCTTCGT and reverse GCGGGCAGTACATCTTCGT; actin, forward GATTCCTGGGATTGCCGAT and reverse TCTGCTGCTGAAAAGTGCTGAG.
Each PCR reaction (20 μl) contained 10 μl 2× real-time PCR Mix (containing SYBR Green I), 0.2 μM of each primer, and appropriately diluted cDNA. The thermal cycling conditions were 95 °C for 10 s followed by 40 cycles of 94 °C for 5 s, 62 °C for 10 s, and 72 °C for 15 s. To standardize the data, the ratio of the absolute transcript level of the Zmrboh genes to the absolute transcript level of Zmactin was calculated for each sample. The relative expression levels of Zmrboh genes were calculated as γ-fold changes relative to the appropriate control experiment for the different chemical treatments.
Plasma membrane isolation
Plasma membrane of leaves was isolated according to Yan et al. (1998) with some modifications. Leaves were cut and ground with a mortar and pestle in ice-cold homogenization buffer, made up of 250 mM sucrose, 250 mM KI, 2 mM ethylene glycol-bis(beta-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 10% (v/v) glycerol, 0.5% (w/v) bovine serum albumin (BSA), 2 mM dithiothreitol (DTT), 1 mM phenylmethylsulphonyl fluoride (PMSF), 5 mM 2-mercaptoethanol, and 50 mM 1,3-bis[tris(hydroxymethyl)methylamino] propane, adjusted to pH 7.8 with MES. The homogenate was filtered through two layers of Miracloth and centrifuged in a swinging bucket rotor at 11 500 g for 10 min at 0 °C. The supernatants were centrifuged at 87 000 g for 35 min. The microsomal pellets were resuspended in phase buffer (250 mM sucrose, 3 mM KCl, and 5 mM KH2PO4, pH 7.8).
The microsomal membrane preparation was fractionated by two-phase partitioning in aqueous Dextran T-500 and polyethylene glycol (PEG 3350) according to the method of Larsson (1987). Protein was quantified according to the method of Bradford (1976) using BSA as a standard.
Determination of NADPH oxidase activity of plasma membranes
The NADPH-dependent O2–-generating activity in isolated plasma membrane vesicles was determined by following the reduction of sodium, 3′-[1-(phenylamino-carbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzenesulfonic acid hydrate (XTT) by O2– (Sagi and Fluhr, 2001).
Partial purification of p46MAPK
Purification of p46MAPK was performed by monitoring its activity with an in-gel kinase assay and in-solution kinase assay using myelin basic protein (MBP) as substrate. Protein extracts were prepared from maize leaves (1000 g) as described in Zhang and Klessig (1997). The protein extracts were loaded onto a 40 ml Q-Sepharose fast flow column equilibrated with buffer A as described by Zhang and Klessig (1997) plus 50 mM NaCl. The kinase activity eluted at 310 mM NaCI in buffer A. The highest kinase activities were adjusted to a final concentration of 300 mM NaCI, and loaded onto a 20 ml phenyl-Sepharose fast flow column equilibrated with buffer A plus 300 mM NaCl. The active fractions (eluting at 48% ethylene glycol) were pooled and exchanged with buffer A plus 50 mM NaCl, and then loaded onto a 6 ml Resource Q column equilibrated with buffer A plus 50 mM NaCl. The kinase activity eluted at 288 mM in buffer A. The active fractions were exchanged with buffer A plus 145 mM NaCl, and then loaded onto a 1 ml Mono Q™ 5/50 GL column equilibrated with buffer A plus 145 mM NaCl. The kinase activity eluted at 263 mM in buffer A. The highest kinase peak fractions were adjusted to a final concentration of 10 mM MgCl2, and exchanged with buffer B described by Zhang and Klessig (1997) plus 145 mM NaCI, and then loaded onto a 3.5 ml poly-L-lysine–agarose column (Sigma). The kinase activity eluted at 760 mM in buffer B. The active fractions were pooled and concentrated. To purify the p46MAPK further, the above-mentioned concentrated sample was loaded onto a Superdex 75 prep grade column equilibrated with buffer B plus 250 mM NaCI, and the column was eluted with the same buffer. The partially purified kinase from the Superdex 75 prep grade step was stored at –80 °C in buffer B plus 50% glycerol.
In-gel kinase activity assay
In-gel kinase activity assays were performed using the method described by Zhang and Klessig (1997).
In-solution kinase activity assay
Assays were performed at room temperature for 30 min in a final volume of 40 μl containing 0.5 mg ml−1 MBP, 50 μM [γ-32P]ATP, 25 mM TRIS, pH 7.5, 5 mM MgCl2, 1 mM EGTA, 1 mM DTT, and enzyme. At the end of the incubation period, 20 μl reaction mixtures were spotted on 1 cm×1 cm Whatman P81 phosphocellulose paper pieces. These were then washed three times with 150 mM H3PO4 (for 10 min), rinsed for 5 min in ethanol, air dried, placed in vials with scintillation liquid, and the levels of radioactivity were determined.
Analysis of proteins by mass spectrometry
Proteins to be analysed by mass spectrometry (MS) were separated by 12% PAGE. Gels were stained with Coomassie Brilliant Blue R250, and the 46 kDa band was excised and sent to the National Center of Biomedical Analysis, Academy of Military Medical Sciences (Beijing, China) for mass spectrometry analyses. Briefly, protein in the gel fragment was digested with trypsin and the tryptic digest was analysed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Ultraflex, Brucker Daltonics, Bremen, Germany) using BioTools software, version 3.0 to search the NCBI database using the online program Mascot (from Matrix Science at http://www.matrixscience.com).
Cloning of the p46MAPK gene
Two primers, 5′-AARATHGCNAANGCNTTYGA-3′ and 5′-GTDACVACRTAYTCMGTCAT-3′ (where Y is T and C; R is A and G; V is A, C, and G; N is A, T, C, and G; M is A and C; and D is A, T, and G), which correspond to the purified p46MAPK peptide; KIANAFD- and -MTEYVVTRW-, respectively, were used to PCR amplify the cDNA, encoding p46MAPK from maize treated with ABA. The first-strand cDNA was obtained by RT using SUPERSCRIPT II (Invitrogen) from total RNA of maize seedlings treated for 2 h with 100 μM ABA. Using the cDNA as the template, the main part of cDNA was amplified in a PCR reaction. PCR products were cloned into pMD-19T plasmid (TAKARA) and were confirmed by sequence analysis. 5′-RACE PCR was performed by using a nested PCR with gene-specific primers derived from the cloned p46MAPK 5′ end (reverse primer 1, 5′-GGTGACGAGGAAGTTGG-3′; reverse primer 2, 5′-TGCGAGCAAGCCCAAA-3′; reverse primer 3, 5′-GCGTTGGCGACCTTCT-3′), and a UAP and AUAP provided within the 5′-full RACE kit (Invitrogen) as forward primer. 3′-RACE PCR was done with gene-specific primers derived from the cloned p46MAPK 3′ end (forward primer 1, 5′-CGCCACATGGACCACGAGA-3′; forward primer 2, 5′-CTCCTGCGCAGAGGGCT-3′) and 3′-RACE outer primer, 3′-RACE inner primer provided within 3′-full RACE core set kit (Takara) as reverse primers. 5′-RACE and 3′-RACE products were cloned into pMD-19T plasmid (TAKARA) and were sequenced. Splicing three fragments, and thus designing a pair of specific primers (forward primer, 5′-ATGGACGGCGGGGGGCAGCC-3′; reverse primer, 5′-TCTGATAATCAGGGTTGAATG-3′), an open reading frame (ORF) of cDNA encoding p46MAPK was obtained by PCR amplification.
Semiquantitative RT-PCR expression analysis
Total RNA was isolated from root, stem, and leaf, respectively, and subjected to RT-PCR amplification with 25 cycles; actin gene was used as the control to show the normalization of the amount of templates in PCR reactions. The two primers for P46MAPK were: forward GCCGCAGCAGCCACTGCC; reverse TGAATGCAGCCCTCTGCGC.
Antibody production and immunoprecipitation kinase activity assay
The peptides for ZmMPK5-C (EEQMKDLIYQEALAFNPDYQ) corresponding to the carboxy terminus of ZmMPK5 were synthesized as described in Berberich et al. (1999) and conjugated to the keyhole limpet haemacyanin carrier. The ZmMPK5 polyclonal antibody was raised in rabbits and purified by affinity chromatography. The specificity of the antibody for ZmMAPK5 was proven earlier by Berberich et al. (1999).
Protein extract (100 μg) was incubated with anti-ZmMPK5 antibody (diluted 1:10000, v/v) in an immunoprecipitation buffer as described previously (Zhang et al., 2006). Kinase activity in the immuno-complex was determined by an in-gel kinase assay as described above.
Results
Cloning of the cDNA encoding Zmrbohs
In a previous study, one rboh gene (ZmrbohB) in maize was reported (Lin et al., 2009). Here, an attempt was made to clone other Zmrboh genes from maize.
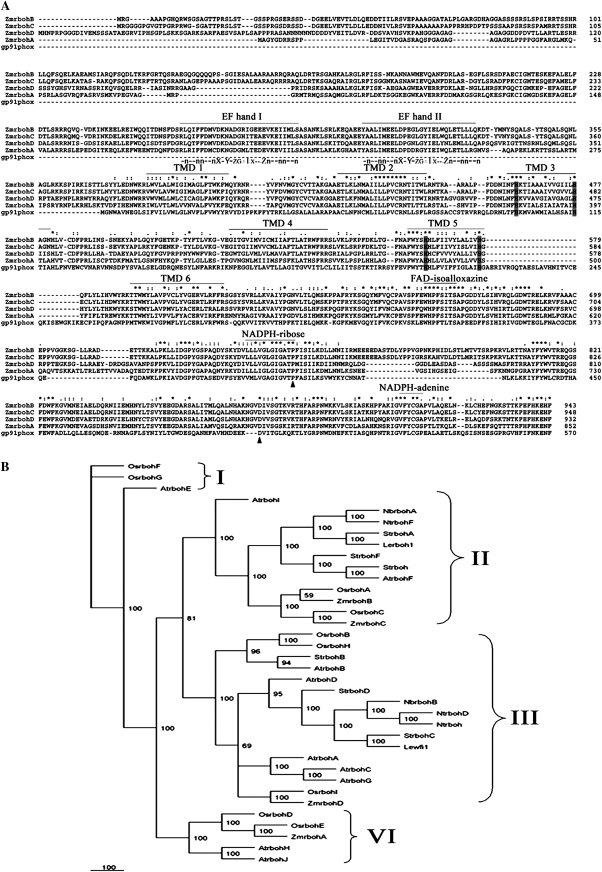
A combination of an in silico comparative cloning with a PCR-based strategy was developed to obtain new Zmrboh genes. Three novel maize rbohs were identified, designated ZmrbohA, ZmrbohC, and ZmrbohD (Fig. 1A). The ZmrbohA cDNA sequence was 3604 bp in length and had an ORF of 2559 nucleotides, encoding a protein of 852 amino acids with a predicted molecular mass (MW) of ∼95.9 kDa and a pI at 9.51. The ZmrbohC cDNA sequence (3317 bp) had an ORF of 2847 nucleotides, encoding a protein of 948 amino acids. The calculated MW and pI of the protein were 106.9 kDa and 9.42, respectively. A 2972 bp ZmrbohD transcript was also cloned, which had an ORF of 2799 nucleotides, encoding a protein of 932 amino acids with a predicted MW of 104.5 kDa and a pI at 9.36. However, it is still possible that more Zmrboh genes exist.
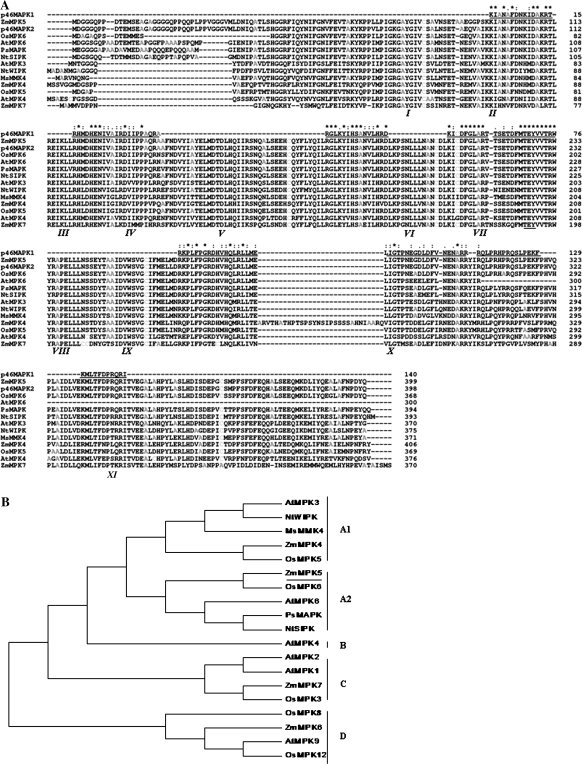
Fig. 1.
Alignment and phylogenetic relationship of ZmrbohA–D and its related members of the rboh family. (A) Alignment of the predicted amino acid sequences of ZmrbohA–D and human gp91phox. Multiple alignment of the predicted ZmrbohA–D and gp91phox protein was made with the CLUSTAL-W program. Asterisks (*) indicate the strictly conserved amino acid residues. Amino acid similarity (: and .) is based on the CLUSTAL-X convention and dashes indicate gaps in the sequence to allow for maximal alignment. Six potential transmembrane-spanning domains (TMD 1 to TMD 6) are indicated with overlines. Histidine residues involved in haem binding are boxed in grey scale. Solid triangles under the sequences indicate amino acid residues that are required for the human NADPH oxidase function and conserved between gp91phox and the rboh proteins. EF hand motifs in the N-terminal domain of rboh are overlined. Beneath these motifs is the sequence of a canonical EF hand. n is usually a hydrophobic residue. Dashes indicate variable amino acid residues. X, Y, Z, and -X, contain oxygen within their side chains. Carbonyl oxygen of # serves as a ligand. -Z is usually glutamic acid. (B) Unrooted phylogenetic tree of various plant rbohs. Bootstrap values of 50% or higher are shown on significant nodes. Species names: At, Arabidopsis thaliana; Le, Lycopersicon esculentum; Nb, Nicotiana benthamiana; Nt, Nicotiana tabacum; Os, Oryza sativa; St, Solanum tuberosum; Zm, Zea mays.
To investigate further the sequence features of ZmrbohA, C, and D, their deduced amino acid sequences were compared and aligned with human gp91phox and ZmrbohB. The results revealed that they were highly similar to each other and many structural features were characteristics of plant rboh. The C-terminal region of each Zmrboh contained conserved FAD and NADPH binding sites, which were likely to be located in the cytoplasm (Sagi and Fluhr, 2006). Moreover, the N-terminal regions of these proteins were hydrophilic and contained two Ca2+-binding EF-hand motifs, which play a key role in the regulation of rboh (Sagi and Fluhr, 2001; Wong et al., 2007; Ogasawara et al., 2008; Takeda et al., 2008). The topology analysis showed that six transmembrane-spanning domains (TMD1–6), usually identified in human gp91phox and plant rbohs, were also conserved in the Zmrboh sequence (Fig. 1A). Likewise, TMD3 and TMD5 contained pairs of histidine residues that are important for haem binding in human gp91phox (Finegold et al., 1996; Fig. 1A). Moreover, human gp91phox amino acid residues Pro-415 and Asp-500, which are indispensable for the catalytic activity (Segal et al., 1992), were also conserved in these Zmrbohs (Fig. 1A).
Figure 1B showed a phylogenetic tree for polypeptides of ZmrbohA–D and the 32 reported plant rboh proteins. The deduced amino acid sequence of ZmrbohA was most similar to rice OsrbohE (88.9% identity), followed by rice OsrbohD (74.1%). ZmrbohB was most similar to OsrbohA from rice (88.7% identity). It also had high sequence identity to ZmrbohC (84.9% identity). ZmrbohC and ZmrbohD had maximal sequence identity to OsrbohC (89.6% identity) and OsrbohI (85.2% identity), respectively. From this tree, four main clusters could be distinguished. The first contains AtrbohE, OsrbohF, and OsrbohG. OsrbohF is a defence-related gene (Yoshie et al., 2005), which was deposited in the database by Wong et al. (2007) and is identical to the clone OsrbohE obtained by Yoshie et al. (2005). The second major cluster includes NbrbohA and Lerboh1, which are constitutively expressed in tobacco and tomato (Lycopersicon esculentum=Solanum lycopersicum), respectively (Amicucci et al., 1999; Yoshioka et al., 2003). But this cluster also contains rice OsrbohA (Yoshie et al., 2005), Arabidopsis AtrbohF (Kwak et al., 2003), and potato StrbohA (Kumar et al., 2007), which are inducible genes. Both ZmrbohB and ZmrbohC were classified in this cluster. The third major cluster contains StrbohB–D (Yoshioka et al., 2001; Yamamizo et al., 2007), NbrbohB (Yoshioka et al., 2003), NtrbohD (Simon-Plas et al., 2002), and AtrbohD (Deskian et al., 1998; Kwak et al., 2003) proteins whose transcription has been shown to be induced by biotic and abiotic stresses. The ZmrbohD sequence was closely related to these sequences. The fourth major cluster consists of the newly identified ZmrbohA and the four proteins of unknown function AtrbohH, AtrbohJ, OsrbohD, and OsrbohE (Sagi and Fluhr, 2006).
Induction of the Zmrbohs by ABA
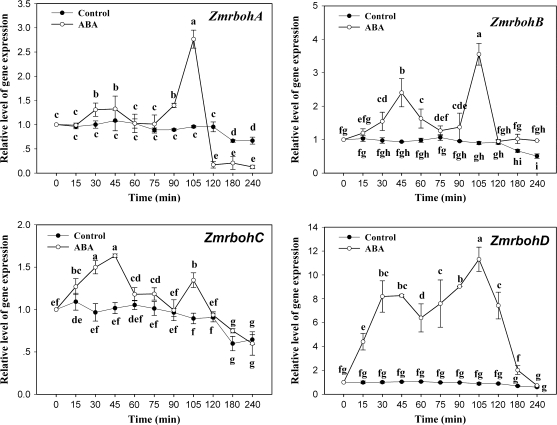
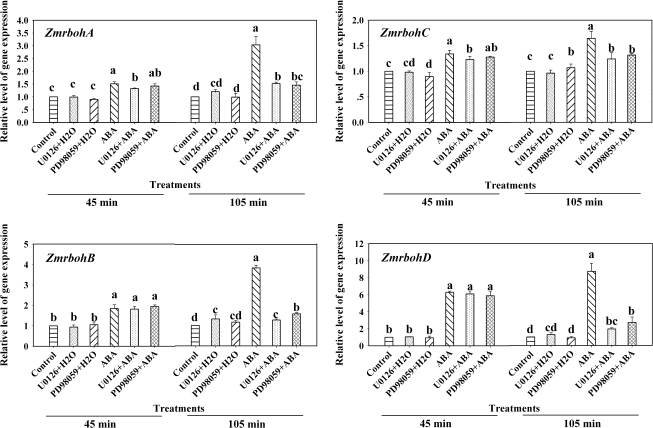
In the Arabidopsis genome, there are 10 rboh genes (AtrbohA–J). Genechip microarray expression analyses showed that ABA treatment only induced the expression of AtrbohD and AtrbohF in guard cells and AtrbohD in leaves of Arabidopsis (Kwak et al., 2003). To investigate the effects of ABA on the induction of ZmrbohA–D genes in leaves of maize seedlings, relative quantitative real-time PCR analysis was performed on total RNA isolated from maize plants treated with ABA. The results showed that these genes had similar expression profiles after ABA treatment (Fig. 2). In detail, ABA treatment induced a biphasic response, in which the first peak (phase I) occurred after 30–45 min of ABA treatment, and the second peak (phase II) appeared within 105 min of ABA treatment, in the expression of ZmrbohA–D. However, there were some slight differences in the expression of these Zmrbohs induced by ABA. The expression of ZmrbohA in phase I and ZmrbohC in phase II induced by ABA was slight, but the expression of ZmrbohD in both phase I and phase II induced by ABA was the highest among these Zmrbohs genes.
Fig. 2.
Expression analysis of ZmrbohA–D in leaves of maize plants exposed to ABA treatment. The detached plants were treated with 100 μM ABA for various times as indicated. The plants treated with distilled water under the same conditions during the whole period served as controls. Relative expression levels of Zmrboh genes, analysed by real-time quantitative PCR, are presented as values relative to those of the controls at 0 min, defined as 1, after normalization to Zmactin transcript levels. Values are means ±standard error (n=3). Means denoted by the same letter did not significantly differ at P <0.05 according to Duncan's multiple range test.
Involvement of H2O2 in the induction of Zmrbohs by ABA
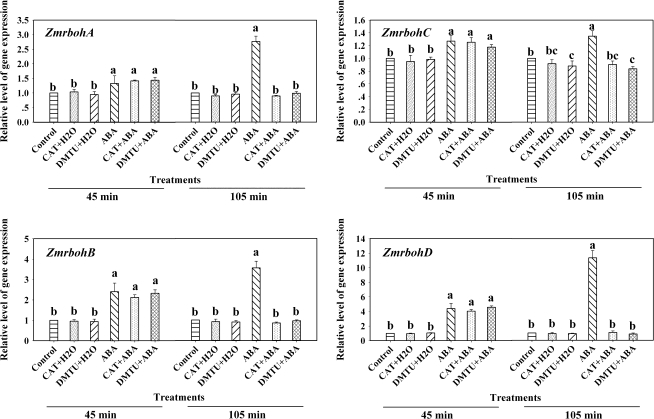
Previous studies have shown that H2O2 can regulate the expression of AtrbohD (Deskian et al., 1998), and ABA can induce H2O2 production (Jiang and Zhang, 2002b; Zhang et al., 2006; Hu et al., 2007). To establish a link between H2O2 and the expression of Zmrbohs in ABA signalling, the detached plants were pretreated with the two H2O2 scavengers, catalase (CAT) and dimethylthiourea (DMTU), which were shown to block almost fully the production of H2O2 induced by ABA in maize leaves (Jiang and Zhang, 2002b; Hu et al., 2006), and then exposed to ABA treatment. Experimental results showed that pretreatments with the two H2O2 scavengers dramatically abolished phase II induced by ABA, but did not affect phase I (Fig. 3), indicating that H2O2 is required for the ABA-induced up-regulation in the expression of ZmrbohA–D in phase II.
Fig. 3.
Effects of pretreatments with the H2O2 scavengers CAT and DMTU on the expression of ZmrbohA–D in leaves of maize plants exposed to ABA treatment. The detached plants were pretreated with distilled water, CAT (400 U) and DMTU (5 mM) for 6 h, respectively, and then exposed to 100 μM ABA treatment for 45 min and 105 min, respectively. The plants treated with distilled water under the same conditions during the whole period served as controls. Relative expression levels of Zmrboh genes, analysed by real-time quantitative PCR, are presented as values relative to those of the controls, defined as 1, after normalization to Zmactin transcript levels. Values are means ±standard error (n=3). Means were compared by Duncan's multiple range test at 45 min and 105 min, respectively. Means denoted by the same letter do not differ significantly at P <0.05.
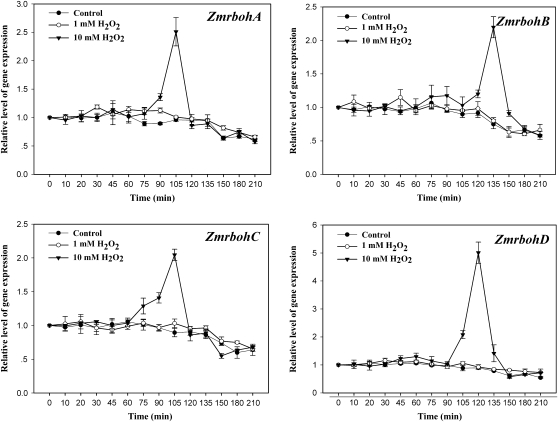
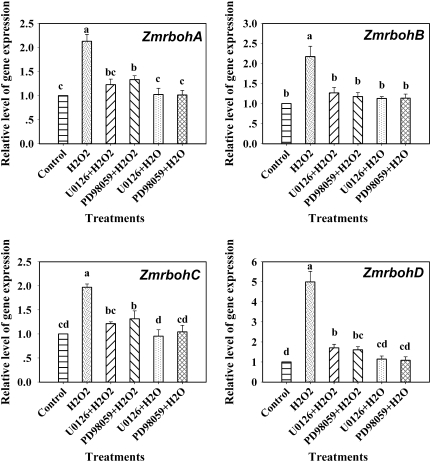
To determine further whether it is H2O2 that is involved in ABA-induced ZmrbohA–D expression, the effect of exogenously applied H2O2 on the activation of the transcript levels of ZmrbohA–D was examined. Treatment with 10 mM H2O2 resulted in a transient increase in phase II in the expression of ZmrbohA–D in leaves of maize plants, peaking after 105–135 min of H2O2 treatment, but treatment with 1 mM H2O2 had very little effect on the expression of these Zmrbohs (Fig. 4).
Fig. 4.
Expression analysis of ZmrbohA–D in leaves of maize plants exposed to H2O2 treatment. The detached plants were treated with H2O2 (1 mM and 10 mM) for various times as indicated. The plants treated with distilled water under the same conditions during the whole period served as controls. Relative expression levels of Zmrboh genes, analysed by real-time quantitative PCR, are presented as values relative to those of the controls at 0 min, defined as 1, after normalization to Zmactin transcript levels. Values are means ±standard error (n=3).
MAPK is required for the expression of Zmrbohs induced by ABA and H2O2
In maize, it has been reported that the MAPK cascade-dependent increase in ABA-induced H2O2 production could be an amplification loop in ABA signalling (Zhang et al., 2006). In order to investigate whether ABA-induced expression of Zmrboh genes is related to the activation of MAPK, the detached maize plants were pretreated with the widely used MAPKK inhibitors PD98059 and U0126, which have been shown to reduce substantially the ABA-induced activation of MAPK in maize leaves (Zhang et al., 2006), and then exposed to ABA (100 μM) or H2O2 (10 mM) treatment. Experimental results showed that pretreatments with PD98059 (100 μM) and U0126 (10 μM) almost completely blocked the phase II increase in the expression of ZmrbohA–D induced by ABA, but had very little effect on the phase I increase induced by ABA (Fig. 5). These results suggest that the phase II increase in the expression of ZmrbohA–D induced by ABA requires MAPK activation. Moreover, pretreatments with MAPKK inhibitors also almost completely arrested the H2O2-induced increases in the expression of these Zmrbohs (Fig. 6), suggesting that MAPK activation is also required for the H2O2-induced increase in the expression of ZmrbohA–D in maize leaves.
Fig. 5.
Effects of pretreatment with the MAPKK inhibitors PD98059 and U0126 on the expression of ZmrbohA–D in leaves of maize plants exposed to ABA treatment. The detached plants were pretreated with distilled water, U0126 (10 μM) and PD98059 (100 μM) for 6 h, respectively, and then exposed to ABA (100 μM) for 45 min and 105 min, respectively. The plants treated with distilled water under the same conditions during the whole period served as controls for the above. Relative expression levels of Zmrboh genes, analysed by real-time quantitative PCR, are presented as values relative to those of the controls, defined as 1, after normalization to Zmactin transcript levels. Values are means ±standard error (n=3). Means were compared by Duncan's multiple range test at 45 min and 105 min, respectively. Means denoted by the same letter did not significantly differ at P <0.05.
Fig. 6.
Effects of pretreatment with the MAPKK inhibitors PD98059 and U0126 on the expression of ZmrbohA–D in leaves of maize plants exposed to H2O2 treatment. The detached plants were pretreated with distilled water, U0126 (10 μM) and PD98059 (100 μM) for 6 h, respectively, and then exposed to H2O2 (10 mM) treatment for 105 min (ZmrbohA), 135 min (ZmrbohB), 105 min (ZmrbohC), and 120 min (ZmrbohD), respectively. The plants treated with distilled water under the same conditions during the whole period served as controls for the above. Relative expression levels of Zmrboh genes, analysed by real-time quantitative PCR, are presented as values relative to those of the controls, defined as 1, after normalization to Zmactin transcript levels. Values are means ±standard error (n=3). Means denoted by the same letter did not significantly differ at P <0.05 according to Duncan's multiple range test.
Regulation of NADPH oxidase activity by ABA, H2O2, and MAPK
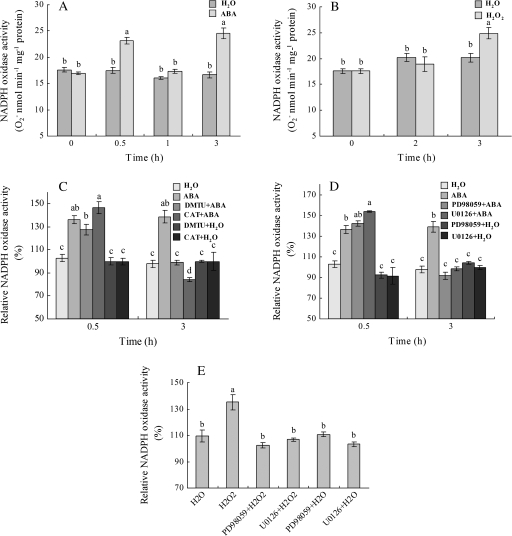
To investigate the effects of ABA, H2O2, and MAPK on the activity of NADPH oxidase in leaves of maize plants, the leaf plasma membranes were isolated and the activity of the plasma-membrane NADPH oxidase was determined. A biphasic response in the activity of NADPH oxidase was observed in the ABA-treated leaves, with the first increase (phase I) occurring within 30 min of ABA treatment, followed by a decrease (Fig. 7A). The second increase (phase II) in the activity of NADPH oxidase appeared after 3 h of ABA treatment. Pretreatments with the MAPKK inhibitors PD98059 and U0126 and the H2O2 scavengers CAT and DMTU fully blocked the ABA-induced phase II increase in the activity of NADPH oxidase, but had little effect on the ABA-induced phase I increase (Fig. 7C, D), suggesting that MAPK and H2O2 are required for the phase II increase in the activity of NADPH oxidase induced by ABA. Treatment with H2O2 alone only induced the phase II increase in the activity of NADPH oxidase after 3 h of treatment (Fig. 7B), and the increase induced by H2O2 was substantially reduced by the pretreatments with the MAPKK inhibitors PD98059 and U0126 (Fig. 7E), suggesting that MAPK is also required for the H2O2-induced increase in the activity of NADPH oxidase in maize leaves.
Fig. 7.
Regulation of plasma-membrane NADPH oxidase activity by ABA, H2O2, and MAPK in maize leaves. (A) ABA-induced activation of NADPH oxidase. The detached plants were treated with 100 μM ABA for various times as indicated. (B) H2O2-induced activation of NADPH oxidase. The detached plants were treated with 10 mM H2O2 for various times as indicated. (C) Effects of pretreatments with the H2O2 scavengers CAT and DMTU on the activity of NADPH oxidase in the leaves of maize plants exposed to ABA treatment. The detached plants were pretreated with CAT (400 U) and DMTU (5 mM) for 6 h, respectively, and then exposed to ABA (100 μM) treatment for 0.5 h and 3 h, respectively. (D) Effects of pretreatments with the MAPKK inhibitors PD98059 and U0126 on the activity of NADPH oxidase in the leaves of maize plants exposed to ABA treatment. The detached plants were pretreated with PD98059 (100 μM) and U0126 (10 μM) for 6 h, respectively, and then exposed to ABA (100 μM) treatment for 0.5 h and 3 h, respectively. (E) Effects of pretreatments with the MAPKK inhibitors PD98059 and U0126 on the activity of NADPH oxidase in the leaves of maize plants exposed to H2O2 treatment. The detached plants were pretreated with PD98059 (100 μM) and U0126 (10 μM) for 6 h, respectively, and then exposed to H2O2 (10 mM) treatment for 3 h. The plants treated with distilled water under the same conditions during the whole period served as controls for the above. Values are means ±standard error (n=6). Means denoted by the same letter do not differ significantly at P <0.05 according to Duncan's multiple range test.
Partial purification and identification of p46MAPK
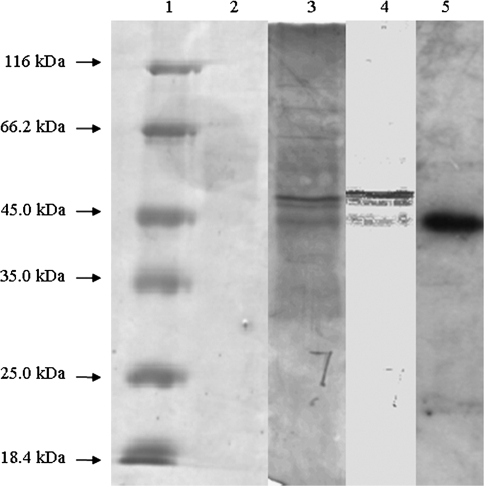
In a previous study, it was reported that ABA activates a 46 kDa MAPK (p46MAPK), which is involved in ABA-induced antioxidant defence in maize leaves (Zhang et al., 2006). Here the p46MAPK was partially purified from leaves of maize plants. At each step, p46MAPK activity was assayed with an in-gel kinase assay and an in-solution kinase assay using MBP as a substrate. Maize p46MAPK was purified to near-homogeneity by (NH4)2SO4 fractionation, ultracentrifugation, and six column chromatography steps (see Materials and methods for details). The purification procedure is summarized in Table 1. The 0–30% (NH4)2SO4 fraction contained almost all of the p46MAPK activity (data not shown). In all of the subsequent chromatographic steps, only a single major kinase activity peak was detected by the in-solution assay, using MBP as the substrate (data not shown). The enzyme from Superdex 75 prep grade column was purified about 10 210-fold, showing three bands in the 45–66.2 kDa range after SDS–PAGE and silver staining (Fig. 8). Only a 45.4 kDa protein band is near to molecular weight of p46MAPK. To ensure that the 45.4 kDa protein band is the kinase required, in-gel kinase assays were performed with MBP as a substrate. Only one band of kinase activity was detected, and its MW was 45.4 kDa, which correlated with the 45.4 kDa protein detected by silver staining (Fig. 8). These results indicate that this polypeptide is p46MAPK.
Table 1.
Partial purification of p46MAPK from maize leaves
| Step of purification | Protein (mg) | Total activity (pmol min−1) | Specific activity (pmol min−1 mg−1) | Fold of purification | Recovery (%) |
| Homogenatea | 3740 | 7106 | 2 | 1 | 100.0 |
| 30% (NH4)2SO4 | 258 | 4489 | 17 | 9 | 63.2 |
| 100 000 g | 171 | 3454 | 20 | 10 | 48.6 |
| Q-Sepharose FF | 11.56 | 983 | 85 | 43 | 13.8 |
| Phenyl-Sepharose FF (HS) | 1.73 | 415 | 240 | 120 | 5.8 |
| Resource Q | 0.368 | 307 | 834 | 417 | 4.3 |
| Mono Q™ 5/50 GLb | 0.138 | 249 | 1 804 | 902 | 3.5 |
| Poly-L-lysine-agaroseb | 0.021 | 189 | 9 005 | 4 502 | 2.7 |
| Hiload 16/60Superdex 75pgb | 0.004 | 82 | 20 420 | 10 210 | 1.1 |
The starting homogenate was prepared from 1000 g of maize leaves treated with 100 μM ABA for 2 h. MBP was used as substrate in the kinase assays. Total protein amount was measured according to Bradford (1976).
The amount of protein was estimated by comparison with known bovine serum albumin on a Coomassie-stained SDS–polyacrylamide gel.
Fig. 8.
SDS–PAGE analysis and in-gel kinase assay of p46MAPK from the Hiload 16/60 Superdex 75pg column. Protein from the Hiload 16/60 Superdex 75pg column was separated on a 12% SDS–polyacrylamide gel. Lane 1, molecular mass markers in kilodaltons; lane 2, SDS–PAGE was stained with Coomassie blue; lane 3, SDS–PAGE was stained with silver; lane 4, lane 3 analysed by Quantity One software; lane 5, in-gel kinases assay of purified protein kinase. Protein from Hiload 16/60 Superdex 75pg column was loaded onto a 12% SDS–polyacrylamide gel embedded with MBP.
In order to identify p46MAPK, the purified 45.4 kDa protein from SDS/PAGE was digested by trypsin, followed by MALDI-TOF-TOF-MS/MS analyses. The resulting spectrum was used to search for matching proteins in the NCBI database, using the Mascot search program (MS/MS ion search). The search yielded a top score of 207 for gi|4239889, MAP kinase 5 from Zea mays (data not shown). The amino acid residues identified by MALDI are shown in Fig. 9A. Furthermore, the selected tryptic peptide (m/z 1779.841) sequenced by MS/MS revealed an amino acid sequence of TTSETDFMTEYVVTR, corresponding to residues 218–232 of ZmMAPK5 (data not show). These results suggest that the ABA-activated p46MAPK is ZmMAPK5, first isolated by Berberich et al. (1999).
Fig. 9.
Comparison of the maize p46MAPK with other MAPKs from higher plants. (A) Alignment of deduced amino acid sequence of the maize p46MAPK (p46MAPK2) with those of other members of the MAPK family. Asterisks (*) shows the strictly conserved amino acid residues. Amino acid similarity (·) is based on the CLUSTAL-X convention and dashes indicate gaps introduced to maximum alignment. The conserved TEY phosphorylation motif for MAPK is marked with the dotted line under the sequences. Roman numerals indicate the 11 major conserved subdomains of serine/threonine protein kinases. The peptide sequences obtained by microsequencing of the purified protein (called p46MAPK1) are shown above the ZmMPK5 sequence. (B) Phylogenetic relationship of ZmMPK5 with other plant MAPKs. Phylogenetic tree based on the genetic distance of the protein sequences was constructed by the neighbor-joining method using MEGA 3.1 software. Species names: Zm, Zea mays; At, Arabidopsis thaliana; Nt, Nicotiana tabacum; Os, Oryza sativa; Ps, Pisum sativum; Ms, Medicago sativa.
cDNA cloning of the p46MAPK gene
To confirm further that maize p46MAPK is ZmMPK5, the sequences from two of the peptides were used to design primers for reverse transcription (RT)-PCR. A full-length cDNA clone, which contains all of the peptide sequences obtained by microsequencing the purified protein (Fig. 9A), was obtained by screening a maize cDNA library. The cloned full-length cDNA of maize p46MAPK contained a 1197 bp ORF encoding a protein of 398 amino acids with a predicted MW of 44.9 kDa, and with a pI of 5.38. The p46MAPK shares 99.3% nucleotide sequence identity with ZmMPK5 isolated first by Berberich et al. (1999), and the amino acid sequence identity of these two genes is 97.7%. These results indicate that maize p46MAPK is ZmMPK5. Based on the sequence alignment a phylogenetic tree was constructed, which indicates that ZmMPK5 belongs to the A2 subgroup of the plant MAPK family and is most closely related to rice OsMPK6, Arabidopsis AtMPK6, pea PsMAPK, and tobacco NtSIPK (Fig. 9B).
Induction in the expression and the activity of ZmMPK5 by ABA and H2O2
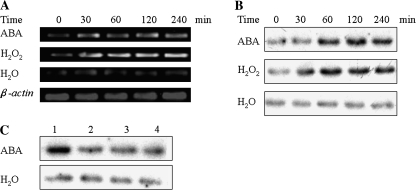
In a previous study, it was reported that ABA did not induce the expression of ZmMPK5 and the activity of ZmMPK5 in maize leaves (Berberich et al., 1999). In order to confirm whether p46MAPK (ZmMPK5) is regulated by ABA and H2O2, the expression and the activity of ZmMPK5 were analysed after ABA or H2O2 treatment using semiquantitative RT-PCR and immunocomplex kinase assay, respectively. Treatment with 100 μM ABA only slightly induced the expression of ZmMPK5 (Fig. 10A), but led to a significant increase in the activity of ZmMPK5 within 60 min, and maximized at 120 min (Fig. 10B). Treatment with 10 mM H2O2 induced both the expression (Fig. 10A) and the activity (Fig. 10B) of ZmMPK5 within 30 min, and remained high for 240 min. Pretreatments with the H2O2 scavengers DMTU and CAT and the NADPH oxidase inhibitor diphenylene iodonium (DPI) almost completely blocked the ABA-induced increase in the activity of ZmMPK5 in maize leaves, but these pretreatments alone did not affect the activity of ZmMPK5 in the control leaves (Fig. 10C). These results clearly indicate that ABA and H2O2 are involved in the activation of ZmMPK5 in maize leaves, and H2O2 is required for the ABA-induced activation of ZmMPK5.
Fig. 10.
Induction in the expression and the activity of ZmMPK5 by ABA and H2O2. (A) Effects of ABA and H2O2 on the expression of ZmMPK5 analysed by semi-quantitative RT-PCR. (B) Effects of ABA and H2O2 on the activity of ZmMPK5. The detached plants were treated with 100 μM ABA and 10 mM H2O2 for various times up to 4 h. The plants treated with distilled water under the same conditions during the whole period served as controls. (C) Effects of pretreatment with ROS scavengers or inhibitors on ABA-induced activation of ZmMPK5. The detached plants were pretreated with ROS scavengers or inhibitors as follows: 1, distilled water (control); 2, 5 mM DMTU; 3, 400 U CAT; 4, 100 μM DPI. The detached plants were pretreated for 6 h, and then exposed to distilled water or 100 μM ABA treatment for 2 h. (B, C) ZmMPK5 was immunoprecipitated from leaves after treatments. The ZmMPK5 activity was measured by immunoprecipitation kinase assay using MBP as a substrate. Experiments were repeated at least five times (A) or three times (B, C) with similar results.
Discussion
It has been well documented that ROS produced by NADPH oxidase play many important roles in plant development and plant responses to biotic and abiotic stresses (Torres and Dangl, 2005; Gapper and Dolan, 2006; Kwak et al., 2006; Sagi and Fluhr, 2006; Takeda et al., 2008). In the Arabidopsis genome, there are at least 10 rboh genes (AtrbohA–J). AtrbohC appears to be mainly involved in root-hair development, whereas AtrbohD and AtrbohF display pleiotropic functions following pathogen recognition and during plant hormone signalling. In Arabidopsis, the expression of AtrbohD and AtrbohF is up-regulated by ABA treatment in guard cells, but only AtrbohF is up-regulated by ABA in mesophyll cells and AtrbohD in leaves (Kwak et al., 2003). However, it is not clear whether such an expression model induced by ABA is also applicable to other plants. In this and another recent study (Lin et al., 2009), four rboh genes were isolated and characterized from maize leaves (ZmrbohA–D; Fig. 1A). The results of the present study showed that ABA not only induced the expression of ZmrbohB, C, and D (Fig. 2), which are similar to Arabidopsis AtrbohF and AtrbohD (Fig. 1B), respectively, but also induced the expression of ZmrbohA, which has a high homology to Arabidopsis AtrbohH and AtrbohJ. These data suggest that there are some differences between maize and Arabidopsis in the expression of rboh genes induced by ABA. More interestingly, the data showed that ABA treatment induced a biphasic response, in which the first peak (phase I) occurred after 30–45 min of ABA treatment, and the second peak (phase II) appeared within 105 min of ABA treatment, in the expression of ZmrbohA–D (Fig. 2). In Arabidopsis leaves, however, AtrbohD expression reached the highest induction by ABA at 30 min and then decreased back to basal levels after 120 min (Kwak et al., 2003). Meanwhile, the results showed that ABA also induced a biphasic response in the activity of NADPH oxidase in maize leaves (Fig. 7A). These results suggest that ABA might induce a biphasic ROS accumulation in maize leaves. A biphasic ROS accumulation in plant response to pathogens (Lamb and Dixon, 1997; Torres et al., 2006) and hyphal wall components from P. infestans (Yoshioka et al., 2001; Yamamizo et al., 2007) has been reported. It has been suggested that StrbohA and StrbohB–D contribute to phase I and phase II bursts, respectively (Yoshioka et al., 2001; Yamamizo et al., 2006). In barley aleurones, the two-phase kinetics for accumulation of H2O2 induced by ABA, with the first burst at 3 h after treatment of ABA and the second burst at 9 h following ABA treatment, has also been observed (Razem and Hill, 2007). However, the biphasic response in ABA-induced accumulation of H2O2 in maize leaves, detected by biochemical, histochemical, or cytochemical methods, had not been found in previous studies (Jiang and Zhang, 2001; Hu et al., 2005, 2007). One possible explanation for this disparity is that, in addition to NADPH oxidases, other sources of apoplastic H2O2 production, such as cell wall peroxidase and polyamine oxidase, are also involved in ABA-induced H2O2 production in maize leaves, and the ABA-induced H2O2 production from these sources is not synchronous with that from NADPH oxidase, as shown by a recent study by Xue et al. (2009).
The MAPK cascade is one of the major pathways by which extracellular stimuli are transduced into intracellular responses in plant cells (Tena et al., 2001; Zhang and Klessig, 2001; Jonak et al., 2002; Nakagami et al., 2005). MAPKs have been shown to be involved in ABA or ROS signalling (Kovtun et al., 2000; Lu et al., 2002; Mittler, 2002; Moon et al., 2003; Xiong and Yang, 2003; Desikan et al., 2004; Mittler et al., 2004; Rentel et al., 2004; Nakagami et al., 2005; Pitzschke and Hirt, 2006; Xing et al., 2008). In a previous study, it was found that ABA and H2O2 activates a 46 kDa MAPK (p46MAPK), which is involved in ABA-induced antioxidant defence in maize leaves (Zhang et al., 2006). In the present study, the p46MAPK was partially purified (Fig. 8) and partial amino acid sequences obtained by microsequencing some internal tryptic peptides (Fig. 9A), and the p46MAPK gene cloned using the sequences from two of the peptides to design primers (Fig. 9A). The results indicated that p46MAPK is ZmMPK5, first isolated by Berberich et al. (1999). Phylogenetic analysis suggests that ZmMPK5 is most closely related to rice OsMPK6, Arabidopsis AtMPK6, pea PsMAPK, and tobacco NtSIPK (Fig. 9B). In the study by Berberich et al. (1999), however, it was reported that ABA did not induce the expression of ZmMPK5 and the activity of ZmMPK5 in maize leaves. But in the present study, the data showed that ABA and H2O2 rapidly activated ZmMPK5 analysed by immunocomplex kinase activity assay (Fig. 10B), although ABA only slightly induced the expression of ZmMPK5 (Fig. 10A). It is not possible to give an explanation for this disparity because they did not give the detailed data in their paper. However, the data from the present study and a previous one by Zhang et al. (2006) provide unequivocal evidence for the involvement of ZmMPK5 in ABA and ROS signalling in maize leaves. In Arabidopsis, a recent study indicated that AtMPK6 is required for ABA-induced up-regulation of CAT1, encoding catalase 1 (Xing et al., 2008). In tobacco, SIPK has been shown to be involved in ROS signalling (Yoshioka et al., 2003; Asai et al., 2008). These results clearly suggest that ZmMPK5 or its orthologues in other plant species is an important component in ABA and ROS signalling in plants.
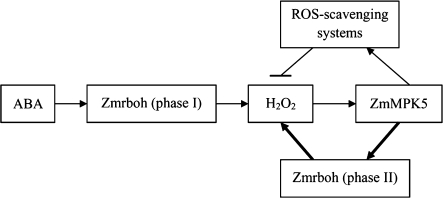
The question of the relationship between MAPK activation and ROS production in plants exposed to various stresses or stimuli appears to be particularly interesting. In a recent review, the relationship between MAPKs and ROS under stress has been considered as the relationship between chicken and egg (Pitzschke and Hirt, 2006). The stress-induced activation of MAPKs could be explained in most studies by the notion that ROS act upstream of MAPK pathways (Nakagami et al., 2005; Pitzschke and Hirt, 2006). However, some studies also showed that MAPKs could function in the upstream of ROS production (Yoshioka et al., 2003; Asai et al., 2008; Xing et al., 2008). In a previous study, using pharmacological and biochemical approaches, the data showed that ABA-induced H2O2 production activates p46MAPK, which in turn up-regulates the antioxidant defence systems in maize leaves (Zhang et al., 2006). The present results suggest that H2O2 acts upstream of the MAPK pathway in ABA signalling. In this study, the relationship was investigated further between NADPH oxidase, which has been shown to be the major source of H2O2 production induced by ABA in Arabidopsis guard cells (Kwak et al., 2003) and in maize leaves (Jiang and Zhang, 2002a; Hu et al., 2005, 2006), and ZmMPK5 in ABA signalling in maize leaves. Using the two MAPKK inhibitors PD98059 and U0126, which were shown to substantially reduce ABA-induced activation of MAPK in maize leaves (Zhang et al., 2006), and the two H2O2 scavengers, catalase (CAT) and dimethylthiourea (DMTU), which were shown to almost completely block production of H2O2 induced by ABA in maize leaves (Jiang and Zhang, 2002b; Hu et al., 2006), the present results showed that pretreatments with these inhibitors or scavengers nearly fully blocked phase II in the expression (Figs 3 and 5) and the activity of NADPH oxidase (Fig. 7C, D) induced by ABA, but did not affect phase I induced by ABA. These results suggest that MAPK and H2O2 are required for phase II in the expression and the activity of NADPH oxidase induced by ABA in maize leaves. Furthermore, H2O2 itself also only induced phase II in the expression (Fig. 4) and the activity (Fig. 7B) of NADPH oxidase in maize leaves, and the up-regulation in the expression and the activity of NADPH oxidase was arrested by the MAPKK inhibitors PD98059 and U0126 (Figs 6, 7E), suggesting that H2O2 induces phase II by activating the MAPK cascade. These results further support the conclusion from the previous study by Zhang et al. (2006). More importantly, the results indicate that the ABA-induced phase I and phase II in the expression and the activity of NADPH oxidase are regulated by different signalling pathways, and phase II seems to be dependent on the MAPK cascade activated by H2O2 in ABA signalling. The results suggest that there is a positive feedback loop involving NADPH oxidase, H2O2, and MAPK in ABA signalling (Fig. 11).
Fig. 11.
Model summarizing the interaction of NADPH oxidase (Zmrboh), H2O2, ZmMPK5, and antioxidant defence systems in ABA signalling in maize plants. Bold arrows indicate the positive feedback regulation involving Zmrboh, H2O2, and ZmMPK5 in ABA signalling.
In a recent study, however, it was shown that, in Arabidopsis, the AtMPK6 mutant almost completely blocked ABA-dependent H2O2 production and CAT1 expression (Xing et al., 2008). Combined with other evidence, the authors concluded that AtMKK1–AtMPK6 acts upstream of H2O2 production in the ABA signal transduction leading to up-regulation in the expression of CAT1. The conclusion seems to be contrary to the conclusion to this study and the previous study by Zhang et al. (2006). In fact, considering the positive feedback loop involving NADPH oxidase, H2O2, and MAPK in ABA signalling (Fig. 11), the results from the paper by Xing et al. (2008) and this study should be consistent. For example, in their study, ABA treatment for 4 h caused an increase in the content of H2O2 in leaves of wild-type, but no significant increase in leaves of the AtMPK6 mutant. In the authors' studies, phase II in the expression and the activity of NADPH oxidase (this study) and the content of H2O2 (Zhang et al., 2006) were nearly fully inhibited by pretreatment with the MAPKK inhibitors PD98059 and U0126 after 2 h of ABA treatment in maize leaves. Due to not considering the positive feedback loop involving H2O2 and MAPK in ABA signalling, however, the working models described by Xing et al. (2008) should only be a one-way model in ABA signalling networks.
What are the regulators for phase I in the expression and the activity of NADPH oxidase in ABA signalling? Recent studies have suggested that cytosolic calcium, CDPK, small GTPase Rac, pH, and calmodulin may be the candidates (Hu et al., 2007; Kobayashi et al., 2007; Wong et al., 2007; Ogasawara et al., 2008; Takeda et al., 2008; Van Breusegem et al., 2008). It was shown that activation of NADPH oxidase involves phosphorylation of two N-terminal Ser by a CDPK as well as interaction with Rac GTPase (Kobayashi et al., 2007; Wong et al., 2007). Ca2+-binding and phosphorylation synergistically activate the activity of NADPH oxidase (Ogasawara et al., 2008; Takeda et al., 2008). Moreover, calmodulin has also been shown to be necessary for ABA-induced H2O2 production (Hu et al., 2007). However, the exact regulatory mechanisms for phase I in the expression and the activity of NADPH oxidase in ABA signalling remain to be determined.
In conclusion, the present data indicate that ABA induces a biphasic response in the expression and the activity of NADPH oxidase in maize leaves, and phase I and phase II are regulated by different signalling pathways. Phase II is regulated by ZmMPK5 activated by H2O2 in ABA signalling, but phase I is mediated by an unknown signalling pathway in which ZmMPK5 and H2O2 are not involved. The results suggest that there is a positive feedback loop involving NADPH oxidase, H2O2, and MAPK in ABA signalling in maize leaves.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant nos. 30571122 and 30671247 to MJ), the Universities Qing-Lan Project of Jiangsu Province (to MJ), the Science Foundation for New Teachers of Doctoral Subject Point of Chinese Ministry of Education (grant no. 20070307018 to AZ), the Graduate Research and Innovation Plan of Jiangsu Province (grant no. CX07B_052Z to HD), and the Open Project of the National Key Laboratory of Crop Genetics and Germplasm Enhancement of Nanjing Agricultural University (grant no. ZW2007002 to MJ).
References
- Amicucci E, Gaschler K, Ward JM. NADPH oxidase genes from tomato (Lycopersicon esculentum) and curly-leaf pondweed (Potamogeton crispus) Plant Biology. 1999;1:524–528. [Google Scholar]
- Asai S, Ohta K, Yoshioka H. MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. The Plant Cell. 2008;20:1390–1406. doi: 10.1105/tpc.107.055855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior BM. NADPH oxidase. Current Opinion in Immunology. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Berberich T, Sano H, Kusano T. Involvement of a MAP kinase, ZmMPK5, in senescence and recovery from low-temperature stress in maize. Molecular and General Genetics. 1999;262:534–542. doi: 10.1007/s004380051115. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnett EC, Desikan R, Moser RC, Neill SJ. ABA activation of an MBP kinase in Pisum sativum epidermal peels correlates with stomatal responses to ABA. Journal of Experimental Botany. 2000;51:197–205. doi: 10.1093/jexbot/51.343.197. [DOI] [PubMed] [Google Scholar]
- Deskian R, Burnett EC, Hancock JT, Neill SJ. Harpin and hydrogen peroxide induce the expression of a homologue of gp91-phox in Arabidopsis thaliana suspension cultures. Journal of Experimental Botany. 1998;49:1767–1771. [Google Scholar]
- Desikan R, Cheung M-K, Bright J, Henson D, Hancock JT, Neill SJ. ABA, hydrogen peroxide and nitric oxide signaling in stomatal guard cells. Journal of Experimental Botany. 2004;55:205–212. doi: 10.1093/jxb/erh033. [DOI] [PubMed] [Google Scholar]
- Finegold AA, Shatwell KP, Segal AW, Klausner RD, Dancis A. Intramembrane bis-heme motif for transmembrane electron transport conserved in a yeast iron reductase and the human NADPH oxidase. Journal of Biological Chemistry. 1996;271:31021–31024. doi: 10.1074/jbc.271.49.31021. [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JH, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- Gapper C, Dolan L. Control of plant development by reactive oxygen species. Plant Physiology. 2006;141:341–345. doi: 10.1104/pp.106.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom QJ, Torres MA, Fordham-Skelton AP, Hammond-Kosack KE, Robinson NJ, Jones JD. rbohA, a rice homologue of the mammalian gp91phox respiratory burst oxidase gene. The Plant Journal. 1996;10:515–522. doi: 10.1046/j.1365-313x.1996.10030515.x. [DOI] [PubMed] [Google Scholar]
- Hu X, Jiang M, Zhang A, Lu J. Abscisic acid-induced apoplastic H2O2 accumulation up-regulates the activities of chloroplastic and cytosolic antioxidant enzymes in maize leaves. Planta. 2005;223:57–68. doi: 10.1007/s00425-005-0068-0. [DOI] [PubMed] [Google Scholar]
- Hu X, Jiang M, Zhang J, Zhang A, Lin F, Tan M. Calcium-calmodulin is required for abscisic acid-induced antioxidant defense and functions both upstream and downstream of H2O2 production in leaves of maize (Zea mays) plants. New Phytologist. 2007;173:27–38. doi: 10.1111/j.1469-8137.2006.01888.x. [DOI] [PubMed] [Google Scholar]
- Hu X, Zhang A, Zhang J, Jiang M. Abscisic acid is a key inducer of hydrogen peroxide production in leaves of maize plants exposed to water stress. Plant and Cell Physiology. 2006;47:1484–1495. doi: 10.1093/pcp/pcl014. [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant and Cell Physiology. 2001;42:1265–1273. doi: 10.1093/pcp/pce162. [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J. Involvement of plasma membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defense in leaves of maize seedlings. Planta. 2002a;215:1022–1030. doi: 10.1007/s00425-002-0829-y. [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. Journal of Experimental Botany. 2002b;53:2401–2410. doi: 10.1093/jxb/erf090. [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J. Cross-talk between calcium and reactive oxygen species originated from NADPH oxidase in abscisic acid-induced antioxidant defense in leaves of maize seedlings. Plant, Cell & Environment. 2003;26:929–939. doi: 10.1046/j.1365-3040.2003.01025.x. [DOI] [PubMed] [Google Scholar]
- Jonak C, Ökrész L, Bögre L, Hirt H. Complexity, cross talk and integration of plant MAP kinase signaling. Current Opinion in Plant Biology. 2002;5:415–424. doi: 10.1016/s1369-5266(02)00285-6. [DOI] [PubMed] [Google Scholar]
- Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C. A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. The Plant Cell. 1998;10:255–266. doi: 10.1105/tpc.10.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, Doke N, Yoshioka H. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. The Plant Cell. 2007;19:1065–1080. doi: 10.1105/tpc.106.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun Y, Chiu W-L, Tena G, Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proceedings of the National Academy of Sciences, USA. 2000;97:2940–2945. doi: 10.1073/pnas.97.6.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar GN, Iyer S, Knowles NR. Strboh A homologue of NADPH oxidase regulates wound-induced oxidative burst and facilitates wound-healing in potato tubers. Planta. 2007;227:25–36. doi: 10.1007/s00425-007-0589-9. [DOI] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei Z-M, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO Journal. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Nguyen V, Schroeder JI. The role of reactive oxygen species in hormonal responses. Plant Physiology. 2006;141:323–329. doi: 10.1104/pp.106.079004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Larsson C, Widell S, Kjellbom P. Preparation of high-purity plasma membranes. Methods in Enzymology. 1987;148:558–568. [Google Scholar]
- Lin F, Zhang Y, Jiang M. Alternative splicing and differential expression of two transcripts of nicotine adenine dinucleotide phosphate oxidase B gene from Zea mays. Journal of Integrative Plant Biology. 2009;51:287–298. doi: 10.1111/j.1744-7909.2008.00808.x. [DOI] [PubMed] [Google Scholar]
- Lu C, Han MH, Guevara-Garcia A, Fedoroff NV. Mitogen-activated protein kinase signaling in postgermination arrest of development by abscisic acid. Proceedings of the National Academy of Sciences, USA. 2002;99:15812–15817. doi: 10.1073/pnas.242607499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends in Plant Science. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Moon H, Lee B, Choi G, et al. NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants. Proceedings of the National Academy of Sciences, USA. 2003;100:358–363. doi: 10.1073/pnas.252641899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami H, Pitzschke A, Hirt H. Emerging MAP kinase pathways in plant stress signaling. Trends in Plant Science. 2005;10:339–346. doi: 10.1016/j.tplants.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Nakagami H, Soukupova H, Schikora A, Zarsky V, Hirt H. A mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis. Journal of Biological Chemistry. 2006;281:38697–38704. doi: 10.1074/jbc.M605293200. [DOI] [PubMed] [Google Scholar]
- Ogasawara Y, Kaya H, Hiraoka G, et al. Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. Journal of Biological Chemistry. 2008;283:8885–8892. doi: 10.1074/jbc.M708106200. [DOI] [PubMed] [Google Scholar]
- Pitzschke A, Hirt H. Mitogen-activated protein kinase and reactive oxygen species signaling in plants. Plant Physiology. 2006;141:351–356. doi: 10.1104/pp.106.079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razem FA, Hill RD. Hydrogen peroxide affects abscisic acid binding to ABAP1 in barley aleurones. Biochemistry and Cell Biology. 2007;85:628–637. doi: 10.1139/o07-107. [DOI] [PubMed] [Google Scholar]
- Rentel MC, Lecourieux D, Ouaked F, et al. OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature. 2004;427:858–861. doi: 10.1038/nature02353. [DOI] [PubMed] [Google Scholar]
- Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, Fluhr R. Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. The Plant Cell. 2004;16:616–628. doi: 10.1105/tpc.019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Fluhr R. Superoxide production by plant homologues of the gp91(phox) NADPH oxidase: modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiology. 2001;126:1281–1290. doi: 10.1104/pp.126.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Fluhr R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiology. 2006;141:336–340. doi: 10.1104/pp.106.078089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal AW, West I, Wientjes F, Nugent JH, Chavan AJ, Haley B, Garcia RC, Rosen H, Scrace G. Cytochrome b-245 is a flavocytochrome containing FAD and the NADPH-binding site of the microbicidal oxidase of phagocytes. Biochemical Journal. 1992;284:781–788. doi: 10.1042/bj2840781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Plas F, Elmayan T, Blein JP. The plasma membrane oxidase NtrbohD is responsible for AOS production in elicited tobacco cells. The Plant Journal. 2002;31:137–147. doi: 10.1046/j.1365-313x.2002.01342.x. [DOI] [PubMed] [Google Scholar]
- Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L. Local positive feedback regulation determines cell shape in root hair cells. Science. 2008;319:1241–1244. doi: 10.1126/science.1152505. [DOI] [PubMed] [Google Scholar]
- Tena G, Asai T, Chiu WL, Sheen J. Plant mitogen-activated protein kinase signaling cascades. Current Opinion in Plant Biology. 2001;4:392–400. doi: 10.1016/s1369-5266(00)00191-6. [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Current Opinion in Plant Biology. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proceedings of the National Academy of Sciences, USA. 2002;99:517–522. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Jones JDG, Dangl JL. Reactive oxygen species signaling in response to pathogens. Plant Physiology. 2006;141:373–378. doi: 10.1104/pp.106.079467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Onouchi H, Hamada S, Machida C, Hammond-Kosack KE, Jones JDG. Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox) The Plant Journal. 1998;14:365–370. doi: 10.1046/j.1365-313x.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- Van Breusegem F, Bailey-Serres J, Mittler R. Unraveling the tapestry of networks involving reactive oxygen species in plants. Plant Physiology. 2008;147:978–984. doi: 10.1104/pp.108.122325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HL, Pinontoan R, Hayashi K, et al. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. The Plant Cell. 2007;19:4022–4034. doi: 10.1105/tpc.107.055624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Jia W, Zhang J. AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. The Plant Journal. 2008;54:440–451. doi: 10.1111/j.1365-313X.2008.03433.x. [DOI] [PubMed] [Google Scholar]
- Xiong L, Yang Y. Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid-inducible mitogen-activated protein kinase. The Plant Cell. 2003;15:745–759. doi: 10.1105/tpc.008714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Zhang A, Jiang M. Involvement of polyamine oxidase in abscisic acid-induced cytosolic antioxidant defense in leaves of maize. Journal of Integrative Plant Biology. 2009;51:225–234. doi: 10.1111/j.1744-7909.2008.00766.x. [DOI] [PubMed] [Google Scholar]
- Yamamizo C, Doke N, Yoshioka H, Kawakita K. Involvement of mitogen-activated protein kinase in the induction of StrbohC and StrbohD genes in response to pathogen signals in potato. Journal of General Plant Pathology. 2007;73:304–313. [Google Scholar]
- Yan F, Feuerle R, Schaffer S, Fortmeier H, Schubert S. Adaptation of active proton pumping and plasmalemma ATPase activity of corn roots to low root medium pH. Plant Physiology. 1998;117:311–319. doi: 10.1104/pp.117.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshie Y, Goto K, Takai R, Iwano M, Takayama S, Isogai A, Che FS. Function of the rice gp91phox homologs OsrbohA and OsrbohE genes in ROS-dependent plant immune responses. Plant Biotechnology. 2005;22:127–135. [Google Scholar]
- Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JDG, Doke N. Nicotiana benthamiana gp91ph°x homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. The Plant Cell. 2003;15:706–718. doi: 10.1105/tpc.008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H, Sugie K, Park HJ, Maeda H, Tsuda N, Kawakita K, Doke N. Induction of plant gp91 phox homolog by fungal cell wall, arachidonic acid, and salicylic acid in potato. Molecular Plant–Microbe Interactions. 2001;14:725–736. doi: 10.1094/MPMI.2001.14.6.725. [DOI] [PubMed] [Google Scholar]
- Zhang A, Jiang M, Zhang J, Ding H, Xu S, Hu X, Tan M. Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytologist. 2007;175:36–50. doi: 10.1111/j.1469-8137.2007.02071.x. [DOI] [PubMed] [Google Scholar]
- Zhang A, Jiang M, Zhang J, Tan M, Hu X. Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiology. 2006;141:475–487. doi: 10.1104/pp.105.075416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Klessig DF. Salicylic acid activates a 48-kD MAP kinase in tobacco. The Plant Cell. 1997;9:809–824. doi: 10.1105/tpc.9.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Klessig DF. MAPK cascades in plant defense signaling. Trends in Plant Science. 2001;6:520–527. doi: 10.1016/s1360-1385(01)02103-3. [DOI] [PubMed] [Google Scholar]