Abstract
The first paper of Hubel and Wiesel in The Journal of Physiology in 1959 marked the beginning of an exciting chapter in the history of visual neuroscience. Through a collaboration that lasted 25 years, Hubel and Wiesel described the main response properties of visual cortical neurons, the functional architecture of visual cortex and the role of visual experience in shaping cortical architecture. The work of Hubel and Wiesel transformed the field not only through scientific discovery but also by touching the life and scientific careers of many students. Here, I describe my personal experience as a postdoctoral student with Torsten Wiesel and how this experience influenced my own work.
I read the first papers of Hubel and Wiesel when I was an undergraduate student at the University of Santiago de Compostela, in Spain. At that time, the laboratory of my advisor, Carlos Acuña, was recording from single neurons in visual cortex and I was assigned to read a selection of the Hubel and Wiesel papers in The Journal of Physiology (Hubel & Wiesel, 1959, 1962, 1963, 1968). I loved reading these papers. I felt that Hubel and Wiesel had started a very exciting journey that I wanted to join. In my years as a graduate student, I found the experience of recording from visual neurons fascinating and I kept waiting for a moment when I would find an unusual stimulus that would reveal something truly amazing about the cortex. I once heard Jonathan Horton say that being naive is almost a requirement at the beginning of a scientific career. I definitely met this requirement.
Sometime in the middle of my graduate studies, I decided that I had to pursue my postdoctoral work with Hubel and Wiesel. David Hubel was the closest one to my home town. He was doing a sabbatical in Oxford and my advisor invited him to visit the laboratory. Unfortunately, he cancelled the visit at the last minute for health reasons. About a year later, Torsten Wiesel was invited to give a plenary lecture at the Spanish Society for Neuroscience and I sought this opportunity to talk to him. Finding some time to talk with Torsten turned out to be quite difficult. The meeting was small but Torsten was always very busy and continuously surrounded by senior scientists. I was assigned to take him from the room where he gave his lecture to another room and I tried to impress him with my questions as much as I could but I was not very successful. Fortunately, soon after finishing my oral presentation at the meeting, I learned from my friend Javier Cudeiro (I will always be grateful for this) that Torsten was now taking time to meet students. I waited for my turn in line and was rewarded with a full 10 minutes of his time.
Torsten Wiesel and Rockefeller University
After our brief meeting, I continued to communicate with Torsten through ‘old-fashioned’ letters that I mailed to Rockefeller University and his secretary mailed to my address in Spain. The most important letter, where he told me that I had a position in his laboratory, travelled to Santiago de Chile, went back to New York, and then travelled to Santiago de Compostela in Spain. I still keep these letters, which have become a small treasure for me. They are about one to two pages long, which is at least an order of magnitude longer than Torsten's emails nowadays! Moreover, these letters prepared me very well for what I was going to find in Rockefeller University. In one letter, Torsten told me about his new work in cortical plasticity with Charles Gilbert and, in the next one, he told me that he was becoming the University's President. In a subsequent letter, he mentioned a young fellow in his laboratory called Clay Reid. Clay wrote the next and last old-fashioned letter. The rest were phone calls and faxes. Email would come later.
I arrived in New York City with a Fulbright fellowship that paid for my salary and a great variety of social events that made my postdoctoral years really fun. Fortunately, Torsten and Clay also helped me make the postdoctoral years very productive. When I arrived, the laboratory was not ready, which made me somewhat nervous. However, we worked really hard and we started doing experiments a few weeks later. Working with Clay was a terrific experience. He was clearly very smart, always supportive and explained the most difficult concepts with amazing clarity. Torsten was now the President of Rockefeller University and did not work in the laboratory. However, we saw him frequently, particularly when we had to write an abstract or put together a talk for a scientific congress.
The meetings with Torsten are impossible to forget. Before the meeting, everybody seemed quite nervous and all the materials for display had to look perfect. Once the meeting started, Torsten basically tore down our presentations and, after he left, multiple graphs, which seemed extremely nice just a few hours before the meeting, went directly to the trash without much hesitation. The ones that survived were the very best and would become the essence of our story.
At the end of one of these meetings, Clay asked me whether I would be willing to do two overnight experiments each week instead of one. The first experiment of the week would be to continue our project and the other to start a new project with Judith Hirsch. This was the beginning of a very productive time that would generate data for many future papers.
An unusual plan and my first grant
A few years later, when Clay moved to Harvard Medical School, Torsten had to renew his grant and he revealed an unusual plan. I would become the Principal Investigator and he would become the Co-Principal Investigator in the grant. This was a terrific arrangement for me. Writing the renewal of Torsten's grant resulted in more frequent meetings that helped me in many different areas of my scientific training, and made me work harder on my poor writing skills. The grant did not do very well and had to be resubmitted, which extended my learning experience (although at that time it felt like a curse!). Currently, my main RO1 grant is still the continuation of Torsten's grant, which is now in its 22nd year.
Since now you know the unusual story of my grant, I thought I would provide you with a brief sample of what I did with it over the past years. A main theme of my laboratory has revolved around the work that I started with Torsten and Clay at Rockefeller University. I was always fascinated by the intricacies of the neuronal circuits and I thought that there was a need for a detailed study of specific connections across the visual pathway and their role in generating neuronal response properties. Clay Reid had taught me the main tools that I needed and he was always very generous at answering questions. Basically, I had to record from neurons that were monosynaptically connected or shared a monosynaptic connection and compare their response properties with techniques of automatic receptive field mapping (Jones & Palmer, 1987; Reid et al. 1997). The monosynaptic connections had to be identified extracellularly with techniques of cross-correlation analysis, an approach that only works with fairly strong connections. Therefore, I used this method to study receptive field transformations in retinogeniculate connections, geniculocortical connections and the strong intracortical connections that link neurons from the middle layers of the cortex with neurons in the superficial layers.
Retinogeniculate connections
While working with Clay Reid at Rockefeller University, I noticed that some neighbouring geniculate cells had very similar receptive fields and often generated spikes within 1 ms of each other. This precise synchrony could be seen as a narrow peak centred at zero in the correlogram obtained after cross-correlating the firing patterns of the two geniculate cells. In some experiments, we were able to record simultaneously from a pair of synchronous geniculate cells together with the excitatory postsynaptic potential generated by one of the retinogeniculate connections, commonly know as the s-potential. These triplet recordings demonstrated that the precise 1 ms synchrony was generated by strong retinal inputs shared by the two geniculate cells. Clay Reid, Martin Usrey and I put these results together in a paper that first described this precise geniculate synchrony, showed that synchronous geniculate cells converged at the same cortical target and demonstrated that the synchronous spikes were especially effective at driving the cortical target to threshold (Alonso et al. 1996).
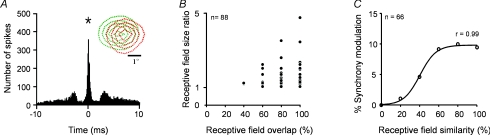
A few years later, in my own laboratory at the University of Connecticut, I decided to initiate a large-scale comparison of the receptive field properties from synchronous geniculate cells. Two graduate students, Chun-I Yeh and Carl Stoelzel, recorded from 372 pairs of geniculate cells with overlapping receptive fields and found precise 1 ms synchrony in 88 of them. Figure 1A illustrates one of the cell pairs that showed the strongest synchrony in our sample. The correlogram has a narrow 1 ms peak characteristic of geniculate cells sharing a retinal afferent. The receptive fields are both off-centre and have very similar position, size and response latency, although they are not identical.
Figure 1. Geniculate synchrony generated by inputs from shared retinal afferents.
A, correlogram showing a narrow 1 ms peak centred at zero (asterisk) and the receptive field centres of two geniculate cells (shown in different colours for each cell; dotted lines indicate off-responses). B, cell pairs with total receptive field overlap (100%) showed a wider range of mismatches in receptive field size than those with partial overlap. C, synchrony was more strongly modulated by stimuli in cell pairs with the most similar receptive fields. Reprinted with permission from Alonso et al. (2008).
Subtle receptive field mismatches were commonly found in synchronous geniculate cells and were not random. When we plotted receptive field overlap against the ratio of receptive field size, all synchronous geniculate cells fell into a triangular region of the plot. Cells with complete receptive field overlap, which were more numerous, showed a wider range of receptive field mismatches in size (Fig. 1B) and response latency (not shown) than those with partial receptive field overlap.
These subtle receptive field mismatches were large enough to cause stimulus-dependent changes in synchrony. Paradoxically, the most pronounced synchrony changes were found in geniculate cells with the most similar receptive fields. Geniculate cells with large receptive field mismatches showed weak 1 ms synchrony, which could not be made stronger with visual stimulation. In contrast, geniculate cells with similar receptive fields showed strong synchrony, which could be considerably reduced with appropriate stimuli. In the cells from Fig. 1A, a bar sweeping from left to right at high speed generated two transient responses that did not overlap in time, due to the small horizontal displacement of the receptive fields. The relation between the average receptive field similarity and average synchrony modulation by stimuli could be described with a sigmoidal function, as illustrated in Fig. 1C.
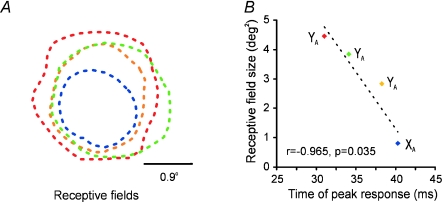
The consequences of retinogeniculate divergence can be appreciated more directly in recordings from three or four synchronous geniculate cells. Figure 2 illustrates an example from a quadruplet recording in which almost all cell combinations showed precise 1 ms synchrony. Notice that all receptive fields are of the same sign (off-centre) and are completely overlapping; however, they differ in size and temporal latency. Another graduate student in my lab, Chong Weng, noticed that receptive field size and response latency were strongly correlated in these multi-cell recordings: the larger the receptive field was, the faster the response latency (Fig. 2B). This correlation between size and timing suggests that the visual information feeding the cortex improves its spatial resolution as time progresses, within a narrow window of about 10 ms (Weng et al. 2005).
Figure 2. Quadruplet recording from synchronous geniculate cells.
A, the receptive field centres are completely overlapping and are all of the same sign (off, illustrated as dotted lines). Each cell is illustrated in a different colour. B, there is a strong correlation between receptive field size and response latency. YA: Y cell in layer A of the geniculate nucleus. XA: X cell in layer A. Reprinted with permission from Weng et al. (2005).
The results on synchronous geniculate cells demonstrate that the retinal receptive field array is diversified at the level of the thalamus across multiple parameter dimensions including position, size and timing. This increase in receptive field diversity could be used to build cortical receptive fields more efficiently and could signal small stimulus variations to the cortex through changes in the geniculate synchrony (Alonso et al. 2006). The work of Barlow, Kuffler, Hubel and Wiesel (Barlow et al. 1957; Hubel, 1960; Wiesel, 1960) described the basic receptive field structure of retinal ganglion cells and geniculate cells. Now, with the aid of multielectrode arrays, we have shown how the receptive field structure of a retinal ganglion cell is diversified in space and timing through neuronal divergence at the level of the geniculate.
Geniculocortical connections
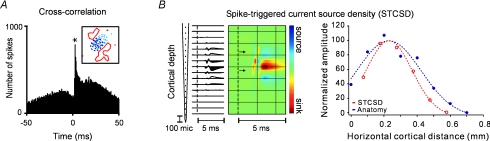
Geniculocortical connections are weaker and less temporally precise than retinogeniculate connections. And yet, they are remarkably specific. Figure 3A shows one of the strongest geniculocortical connections that we measured at Rockefeller University (Reid & Alonso, 1995; Alonso et al. 2001). The correlogram has a peak displaced from zero, which is smaller and wider than the peak illustrated in Fig. 1, as would be expected from weaker and less temporally precise connections (notice the difference in time scale between Figs 1A and 3A). The receptive fields are illustrated in the inset: the on-centre receptive field from the geniculate cell is superimposed on the on-subregion from the cortical simple cell.
Figure 3. Two methods to investigate geniculocortical connections.
A, cross-correlation analysis. Correlogram and receptive fields illustrating a strong connection between a geniculate cell and a cortical simple cell. Data from Reid & Alonso (1995) and Alonso et al. (2001). B, STCSD. This method measures the current sinks generated by single geniculate afferents in the cortex. These current sinks are strong and spatially restricted. Left, sink of a geniculate afferent restricted to cortical layer 4 (arrows mark layer limits). The sink has three components: axonal response, synaptic delay and postsynaptic response. Right, horizontal cortical distribution of the current sink (red) and the synapses from a single X geniculate axon terminal (Humphrey et al. 1985). Reprinted with permission from Alonso & Swadlow (2005) and Jin et al. (2008b).
A question that haunted us for many years was: how does a cortical simple cell become connected to the ‘right’ geniculate inputs? Is there a selection based on Hebbian mechanisms or is it a consequence of random wiring? Cross-correlation analysis could not be used to address this question because it was technically very difficult to measure more than a handful of geniculate inputs per cortical cell. We needed a new technique that would allow us to measure multiple, neighbouring, geniculocortical connections.
In my years at the University of Connecticut I made a terrific friend and collaborator who came up with the right tool to approach this question. Harvey Swadlow developed a method to identify multiple thalamocortical connections in a 300 μm cortical cylinder. By combining methods of spike-trigger averaging and current source density analysis (CSD), he measured for the first time the current sinks generated by a single thalamic afferent through the depths of the somatosensory cortex (Swadlow et al. 2002). These current sinks turned out to be unusually strong and had a characteristic triphasic temporal profile that was a reliable marker of a single thalamocortical connection, just as a refractory period is a reliable marker of a single unit in extracellular recordings. The triphasic profile corresponded to the axon terminal response, a synaptic delay of 0.5 ms and the postsynaptic sink caused by the thalamocortical connection in the somatosensory cortex. Following Harvey's lead, we later used this method to measure the current sinks generated by single geniculocortical connections in the cat visual cortex (Fig. 3B).
The current sinks measured with this method of spike-triggered CSD (or STCSD) were remarkably restricted in cortical space. They were restricted to specific layers or sublayers of the visual cortex and, horizontally, they were restricted to a region that was equivalent in size or smaller than the region covered by a geniculate axon arbor (Fig. 3B).
We have recently used this technique to demonstrate that on and off geniculate afferents are segregated in cat visual cortex and that off geniculate afferents dominate the cortical representation of the area centralis. This study was led by a postdoctoral student in my lab, Jianzhong Jin, and published together with Harvey Swadlow, who developed the STCSD method, and Michael Stryker, Josh Gordon and Edward Ruthazer, who used recordings from muscimol-silenced cortex to also demonstrate the on/off segregation (Jin et al. 2008b). These results provide strong support to computational models that predict a role for on/off segregation in building orientation maps in visual cortex (Miller, 1994; Nakagama et al. 2000; Ringach, 2004). Moreover, the finding that off-centre geniculate afferents dominate the cortical representation of the area centralis suggest an important difference in how dark and light features are processed in visual scenes. We wonder whether our predilection to read black letters in light backgrounds (in books and visual acuity charts) has something to do with our finding. We are also working on a new paper that will provide evidence for a relation between on/off segregation and orientation preference in visual cortex and will address the question of connection specificity raised above (Jin et al. 2008a).
An important prediction from Hubel and Wiesel's work is that geniculate afferents play a major role in building orientation columns in visual cortex (Hubel & Wiesel, 1962, 1968). The work that I did with Clay Reid (Reid & Alonso, 1995) and, more recently with Harvey Swadlow (Jin et al. 2008a,b) is heavily inspired by this prediction.
Corticocortical connections
Another discussion that I remember from my days at Rockefeller University relates to the connections between simple cells and complex cells. We were wondering why these connections had not been demonstrated with techniques of cross-correlation analysis. A common answer was that the connections were too weak to be measured. This answer was consistent with a careful study from Joseph Malpeli showing that complex cells in layers 2 + 3 of the cortex remained active when the main geniculocortical inputs to cortical area 17 were blocked and simple cells in layer 4 were silenced (Malpeli et al. 1986).
I thought that Malpeli's result could be explained by the weakness of intracortical connections and the recent demonstration of rapid plasticity in the superficial layers of the cortex (Gilbert & Wiesel, 1992). However, while the correlated firing generated by horizontal connections had been measured by Dan Ts’o, Charles Gilbert and Torsten Wiesel (Ts’o et al. 1986), I could not find any systematic measurement of correlated firing between vertically aligned layer 4 simple cells and layer 2 + 3 complex cells. I thought that it was important to address this knowledge gap.
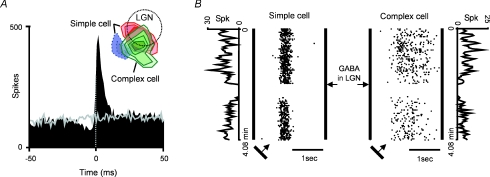
This reasoning led to the first experiments that I did when Clay Reid moved to Harvard Medical School and the experiments that I proposed for Torsten's grant renewal. The experiments turned out to be much more difficult than I thought and probably they would not have worked without the invaluable help of Luis Martinez, a postdoctoral student who arrived from Spain to join the lab. Figure 4 shows one of the strongest connections that we found in several years of recordings. In this example, the two cells had overlapping receptive fields, similar orientation preferences and the correlogram showed a peak displaced from zero, consistent with a monosynaptic connection (Fig. 4A). This peak is wider but similar in shape to the peak from geniculocortical connections (Fig. 3): it is asymmetric with respect to zero, has a fast rise time and a dip on the left side that matches the autocorrelogram of the presynaptic cell. Why do the peaks in the correlograms increase in width from retina to visual cortex (compare Figs 1, 3 and 4)? In a modelling study with Francisco Vico and Francisco Veredas, we showed that the differences in peak width could be explained by differences in the time course of the excitatory postsynaptic potentials from each connection (Veredas et al. 2005).
Figure 4. Intracortical connection between a layer 4 simple cell and a complex cell in layers 2 + 3 of primary visual cortex.
A, correlogram showing a peak consistent with a monosynaptic connection (grey line is the shuffle correlogram) and receptive fields from the complex cell (green) and simple cell (red: on-subregion; blue: off-subregion). The dotted circle is the receptive field from the multiunit activity recorded at the centre of the GABA injection in the lateral geniculate nucleus (LGN). B, a small injection of GABA in LGN blocked the activity of both the simple cell and complex cell. Reprinted with permission from Martinez & Alonso (2001).
The experiments that I did with Luis Martinez demonstrated for first time that the connections between simple cells and complex cells could be measured with cross-correlation analysis (Alonso & Martinez, 1998) and that, when a connection was demonstrated by this method, both the simple cell and the complex cell could be silenced by making a small injection of GABA in the geniculate nucleus (Martinez & Alonso, 2001) (Fig. 4B). These results provided strong support to a main prediction originating from the work of Hubel and Wiesel: that simple cells connect monosynaptically to complex cells and drive their visual responses (Hubel & Wiesel, 1962).
Other collaborations and future directions
Most of the connections that a neuron receives are weak and cannot be studied with the techniques described above. To learn more about the functional role of these weak but numerous connections, we started studying how the behavioural state modulates neuronal response properties in awake animals. In the laboratory of Harvey Swadlow at the University of Connecticut, two postdoctoral students, Tatyana Bezdudnaya and Monica Cano, studied how changes in arousal alter the response properties of geniculate and cortical neurons in the rabbit (Bezdudnaya et al. 2006; Cano et al. 2006). In my laboratory, currently at SUNY Optometry in New York, we are studying how changes in visual attention and task difficulty modulate neuronal responses in primary visual cortex of awake behaving primates.
Susana Martinez Conde and Steve Macknik generously provided their expertise to get the primate experiments going in my lab, by helping with the initial surgeries and with the installation of the equipment to control the behavioural task. Harvey Swadlow brought another powerful tool for these experiments. A few years ago, he developed an array of ultra-thin electrodes with independent microdrives to perform chronic recordings in awake rabbits. A main advantage of this array is that the electrodes are so thin that they can be moved through the same electrode track for months or years without causing visible tissue damage. A second advantage is that the electrodes and microdrives are very small and they are attached to the skull, providing excellent stability for single unit recording (Swadlow et al. 2005).
With the help of Harvey Swadlow and his postdoctoral student, Yulia Bereshpolova, we decided to make a bold move and adapt his ultra-thin arrays for recordings in awake primates. Our boldness is beginning to pay off. We are now able to obtain high-quality single unit recordings that are exceptionally stable in awake behaving primates. The high stability of the recordings allows us to study neurons for several hours and characterize in detail their response properties. In addition, we can measure how the neuronal responses change as the monkeys perform a simple detection task that can vary in the level of difficulty and the spatial location of attention.
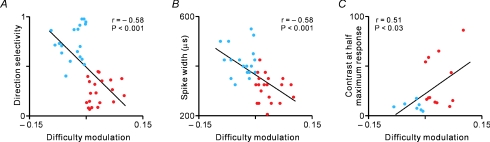
These experiments were led by Yao Chen, another postdoctoral student in my lab, and demonstrated the existence of two types of cells that we call difficulty-enhanced and difficulty-suppressed cells. The difficulty-enhanced cells enhance their responses at the focus of attention when the detection task becomes more difficult. In contrast, the difficulty-suppressed cells suppress their responses outside the focus of attention as difficulty increases. Interestingly, difficulty-suppressed neurons were more directionally selective (Fig. 5A), had wider spikes (Fig. 5B), higher contrast sensitivity (Fig. 5C) and generated more transient responses (not shown) than difficulty-enhanced neurons (Chen et al. 2008).
Figure 5. Difficulty-enhanced and difficulty-suppressed neurons have different response properties.
Difficulty-enhanced neurons (red circles) enhance their visual responses at the focus of attention when a detection task becomes increasingly difficult. Difficulty-suppressed neurons (blue circles) suppress their visual responses outside the focus of attention. Difficulty-suppressed neurons are more directionally selective (A), have wider spikes (B) and have higher contrast sensitivity (C) than difficulty-enhanced neurons. Difficulty modulation is measured by comparing the response during the hard task and the easy task. Positive and negative values indicate that the cell increased or decreased, respectively, the visual response as the task became more difficult. Reprinted with permission from Chen et al. (2008).
The response properties of difficulty-suppressed neurons are remarkably similar to those of V1 neurons projecting to area MT (Movshon & Newsome, 1996). We speculate that difficulty-suppressed neurons are part of a neuronal network that signals movement outside the focus of attention. Because peripheral movement is a powerful distracter (Yantis, 1996), the reduced activity of difficulty-suppressed neurons could help to prevent moving distracters from shifting the focus of attention and compromising the success of the difficult detection task.
More recently, I started another, very productive, collaboration with Garrett Stanley, in the Department of Biomedical Engineering at Georgia Tech and Emory University. Garrett and three members of his laboratory, Nicholas Lesica, Daniel Butts and Gaëlle Desbordes have injected new, fresh ideas into my laboratory and have made computational neuroscience very accessible to all of us. Their strong background in engineering has provided a new quantitative approach that was very much needed in my lab. With Garrett, we have begun a series of studies to investigate how natural scenes are represented by single neurons and neuronal populations in early visual processing (Lesica et al. 2007; Desbordes et al. 2008) and to study the role of temporal precision in these visual representations (Butts et al. 2007). Our collaborative team is working together to connect the original, classical ideas of early visual processing inspired by the work of Hubel and Wiesel to the natural visual world, within which elemental features such as contrast, temporal and spatial frequency, and orientation vary across the scene and change with time. More recently, Michael Black from Brown University has joined the team, bringing a formal connection between biological and computer vision.
Final thoughts
When I think back about my time at Rockefeller University, I feel extremely fortunate. Torsten was not only an inspiration as a scientist but also as a leader and as a person. He also seemed to enjoy every moment, sometimes by taking unusual approaches. In an inauguration of the child-care centre at Rockefeller University, he was photographed by the University magazine when he decided to try the new slide to verify that it truly worked! I also remember the day when he showed me the new space for his lab. As we were walking towards the lab entrance, we reached a platform about four feet high that seemed to require the use of stairs. I walked towards the stairs but Torsten did something different: he used his hands to pull himself up on to the platform. After seeing him, I turned around, replicated his move and felt quite accomplished; this feeling soon vanished when I remembered that Torsten was nearly 80 years old.
Just like the jump at the entrance of the lab, Torsten continuously challenged me to stretch myself, sometimes to the point where I almost break my bones. Fortunately, my bones are still intact! It has been a real honour to meet Torsten personally and enjoy his teachings and advice during my years at Rockefeller University. I wish him and David Hubel a joyful 50th anniversary!
Acknowledgments
I would like to thank Harvey Swadlow, Garrett Stanley and Claudia Valencia for taking the time to read this manuscript and provide excellent comments. I would also like to thank NIH (NEI and NINDS) for funding the work that I presented here.
References
- Alonso JM, Martinez LM. Functional connectivity between simple cells and complex cells in cat striate cortex. Nat Neurosci. 1998;1:395–403. doi: 10.1038/1609. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Swadlow HA. Thalamocortical specificity and the synthesis of sensory cortical receptive fields. J Neurophysiol. 2005;94:26–32. doi: 10.1152/jn.01281.2004. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Usrey WM, Reid RC. Precisely correlated firing in cells of the lateral geniculate nucleus. Nature. 1996;383:815–819. doi: 10.1038/383815a0. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Usrey WM, Reid RC. Rules of connectivity between geniculate cells and simple cells in cat primary visual cortex. J Neurosci. 2001;21:4002–4015. doi: 10.1523/JNEUROSCI.21-11-04002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Yeh CI, Stoelzel CR. Visual stimuli modulate precise synchronous firing within the thalamus. (Special Issue in memory of Mircea Steriade) Thalamus Relat Syst. 2008;4:21–34. doi: 10.1017/S1472928807000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Yeh CI, Weng C, Stoelzel C. Retinogeniculate connections: a balancing act between connection specificity and receptive field diversity. Prog Brain Res. 2006;154:3–13. doi: 10.1016/S0079-6123(06)54001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Fitzhugh R, Kuffler SW. Dark adaptation, absolute threshold and Purkinje shift in single units of the cat's retina. J Physiol. 1957;137:327–337. doi: 10.1113/jphysiol.1957.sp005816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdudnaya T, Cano M, Bereshpolova Y, Stoelzel CR, Alonso JM, Swadlow HA. Thalamic burst mode and inattention in the awake LGNd. Neuron. 2006;49:421–432. doi: 10.1016/j.neuron.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Butts DA, Weng C, Jin J, Yeh CI, Lesica NA, Alonso JM, Stanley GB. Temporal precision in the neural code and the timescales of natural vision. Nature. 2007;449:92–95. doi: 10.1038/nature06105. [DOI] [PubMed] [Google Scholar]
- Cano M, Bezdudnaya T, Swadlow HA, Alonso JM. Brain state and contrast sensitivity in the awake visual thalamus. Nat Neurosci. 2006;9:1240–1242. doi: 10.1038/nn1760. [DOI] [PubMed] [Google Scholar]
- Chen Y, Martinez-Conde S, Macknik SL, Bereshpolova Y, Swadlow HA, Alonso JM. Task difficulty modulates the activity of specific neuronal populations in primary visual cortex. Nat Neurosci. 2008;11:974–982. doi: 10.1038/nn.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbordes G, Jin J, Weng C, Lesica NA, Stanley GB, Alonso JM. Timing precision in population coding of natural scenes in the early visual system. PLoS Biol. 2008;6:e324. doi: 10.1371/journal.pbio.0060324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Receptive field dynamics in adult primary visual cortex. Nature. 1992;356:150–152. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- Hubel DH. Single unit activity in lateral geniculate body and optic tract of unrestrained cats. J Physiol. 1960;150:91–104. doi: 10.1113/jphysiol.1960.sp006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat's striate cortex. J Physiol. 1959;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of cells in striate cortex of very young, visually inexperienced kittens. J Neurophysiol. 1963;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey AL, Sur M, Uhlrich DJ, Sherman SM. Projection patterns of individual X- and Y-cell axons from the lateral geniculate nucleus to cortical area 17 in the cat. J Comp Neurol. 1985;233:159–189. doi: 10.1002/cne.902330203. [DOI] [PubMed] [Google Scholar]
- Jin J, Wang Y, Chen Y, Swadlow HA, Alonso JM. Receptive field clustering of on and off geniculate afferents within a cortical orientation domain predicts the domain orientation preference. Abstract Viewer/Itinerary Planner, Society for Neuroscience, Washington, DC; Program No. 769.3.2008.
- Jin JZ, Weng C, Yeh CI, Gordon JA, Ruthazer ES, Stryker MP, Swadlow HA, Alonso JM. On and off domains of geniculate afferents in cat primary visual cortex. Nat Neurosci. 2008b;11:88–94. doi: 10.1038/nn2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JP, Palmer LA. The two-dimensional spatial structure of simple receptive fields in cat striate cortex. J Neurophysiol. 1987;58:1187–1211. doi: 10.1152/jn.1987.58.6.1187. [DOI] [PubMed] [Google Scholar]
- Lesica NA, Jin J, Weng C, Yeh CI, Butts DA, Stanley GB, Alonso JM. Adaptation to stimulus contrast and correlations during natural visual stimulation. Neuron. 2007;55:479–491. doi: 10.1016/j.neuron.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpeli JG, Lee C, Schwark HD, Weyand TG. Cat area 17. I. Pattern of thalamic control of cortical layers. J Neurophysiol. 1986;56:1062–1073. doi: 10.1152/jn.1986.56.4.1062. [DOI] [PubMed] [Google Scholar]
- Martinez LM, Alonso JM. Construction of complex receptive fields in cat primary visual cortex. Neuron. 2001;32:515–525. doi: 10.1016/s0896-6273(01)00489-5. [DOI] [PubMed] [Google Scholar]
- Miller KD. A model for the development of simple cell receptive fields and the ordered arrangement of orientation columns through activity-dependent competition between ON- and OFF-center inputs. J Neurosci. 1994;14:409–441. doi: 10.1523/JNEUROSCI.14-01-00409.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon JA, Newsome WT. Visual response properties of striate cortical neurons projecting to area MT in macaque monkeys. J Neurosci. 1996;16:7733–7741. doi: 10.1523/JNEUROSCI.16-23-07733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagama H, Saito T, Tanaka S. Effect of imbalance in activities between ON- and OFF-center LGN cells on orientation map formation. Biol Cybern. 2000;83:85–92. doi: 10.1007/s004220000148. [DOI] [PubMed] [Google Scholar]
- Reid RC, Alonso JM. Specificity of monosynaptic connections from thalamus to visual cortex. Nature. 1995;378:281–284. doi: 10.1038/378281a0. [DOI] [PubMed] [Google Scholar]
- Reid RC, Victor JD, Shapley RM. The use of m-sequences in the analysis of visual neurons: linear receptive field properties. Vis Neurosci. 1997;14:1015–1027. doi: 10.1017/s0952523800011743. [DOI] [PubMed] [Google Scholar]
- Ringach DL. Haphazard wiring of simple receptive fields and orientation columns in visual cortex. J Neurophysiol. 2004;92:468–476. doi: 10.1152/jn.01202.2003. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Bereshpolova Y, Bezdudnaya T, Cano M, Stoelzel CR. A multi-channel, implantable microdrive system for use with sharp, ultra-fine “Reitboeck” microelectrodes. J Neurophysiol. 2005;93:2959–2965. doi: 10.1152/jn.01141.2004. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Gusev AG, Bezdudnaya T. Activation of a cortical column by a thalamocortical impulse. J Neurosci. 2002;22:7766–7773. doi: 10.1523/JNEUROSCI.22-17-07766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ts’o DY, Gilbert CD, Wiesel TN. Relationships between horizontal interactions and functional architecture in cat striate cortex as revealed by cross-correlation analysis. J Neurosci. 1986;6:1160–1170. doi: 10.1523/JNEUROSCI.06-04-01160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veredas FJ, Vico FJ, Alonso JM. Factors determining the precision of the correlated firing generated by a monosynaptic connection in the cat visual pathway. J Physiol. 2005;567:1057–1078. doi: 10.1113/jphysiol.2005.092882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng C, Yeh CI, Stoelzel CR, Alonso JM. Receptive field size and response latency are correlated within the cat visual thalamus. J Neurophysiol. 2005;93:3537–3547. doi: 10.1152/jn.00847.2004. [DOI] [PubMed] [Google Scholar]
- Wiesel TN. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S. Attentional capture in vision. In: Kramer AF, Coles GH, editors. Converging Operations in the Study of Selective Attention. Washington, DC, USA: American Psychological Association; 1996. pp. 45–76. [Google Scholar]