Abstract
The hippocampus is critically involved in storing explicit memory such as memory for space. A defining feature of explicit memory storage is that it requires attention both for encoding and retrieval. Whereas, a great deal is now known about the mechanisms of storage, the mechanisms whereby attention modulates the encoding and retrieval of space and other hippocampus-dependent memory representations are not known. In this review we discuss recent studies, including our own, which show on the cellular level that attention is critical for the stabilization of spatial and reward-associated odour representations. Our findings support the view that in the hippocampus attention selects the reference frame for task-relevant information. This mechanism is in part mediated by dopamine acting through D1/D5 receptors and involves an increase in neuronal synchronization in the gamma band frequency. We propose that synchronous activity leads to enhancements in synaptic strength that mediate the stabilization of hippocampal representations.
One of Hubel and Wiesel's seminal contributions to our understanding of the cerebral cortex was the discovery fifty years ago, which we celebrate in this volume, that the striate cortex functions to transform the input it receives from the lateral geniculate nucleus. The neurons of the lateral geniculate nucleus have concentric receptive fields, and these are transformed to linear receptive fields with specific axes of orientation characteristic of cells in the superficial layers of the striate cortex (for review see Hubel & Wiesel, 1979, 1998). These remarkable findings focused the attention of the neuroscience community on how the neocortex acts to transform sensory information (see for example Corbetta et al. 1991; Colby & Goldberg, 1999; Bichot & Desimone, 2006; Goldberg et al. 2006).
In an extension of the Hubel–Wiesel thinking, the problem of the cortical transformation of sensory information is now also being addressed in the study of the archicortical representation of space and its role in explicit memory. Here we review the cellular studies of hippocampal function and illustrate how complex processes, such as attention and explicit memory, are reflected on the cellular level.
A defining feature of explicit memory, such as the hippocampal-dependent memory for place, is that it requires attention. The recruitment of attention is important not only for optimal encoding but also for subsequent retrieval (Schacter, 1996; Fernandes et al. 2005). Since the hippocampus receives multi-sensory information, the encoding of this information probably engages several brain structures, each of which might be the target of independent attentional modulation. This multimodal information is then integrated at the level of the hippocampus, where the contributing sensory modalities are brought together into a unified representation.
We propose that attention acts on these unified percepts in the temporal lobe to modulate the long-term stability of explicit memory. In support of this idea, we first describe data showing that attention serves to switch between different reference frames used to discriminate task-relevant information. Second, we discuss evidence showing that attention is necessary for the consolidation of long-term memory. Third, we suggest the physiological mechanism that could mediate these attentional effects at the level of hippocampal networks. Finally, we review neuroanatomical data indicating that attentional processing can occur at the level of the hippocampus to modulate memory encoding and retrieval.
A hippocampal representation of space
A new era of research in the hippocampus was opened up in 1971, when John O’Keefe at University College London made an amazing discovery about how the hippocampus processes sensory information. He found that neurons in the hippocampus of the rat register information not about a single sensory modality – sight, sound, touch, or pain – but about the space surrounding the animal, a modality that depends on information from several senses (O’Keefe & Dostrovsky, 1971). The pattern of action potentials in the hippocampal pyramidal neurons is so distinctively associated with a particular area of the spatial environment that O’Keefe referred to them as ‘place cells’. Based on these findings, O’Keefe & Nadel (1978) suggested that the hippocampus contains an allocentric representation of space, a cognitive map of the external environment that combines inputs from several sensory modalities which the animal uses to navigate (O’Keefe & Conway, 1978). These ideas were supported by experiments with rodents and people showing that damage to the hippocampus severely compromises the animal's ability to learn a task that relies on spatial information (Schenk & Morris, 1985). Together, these findings indicated that the spatial map played a central role in spatial navigation, our awareness of the environment around us.
Work in the hippocampal formation following O’Keefe's initial groundbreaking discovery has led to the delineation of four basic cell types that together provide all the requisite information for accurate spatial navigation: (1) place cells in the hippocampus, which fire when the animal is in a particular position in the environment (O’Keefe & Dostrovsky, 1971); (2) head direction cells in the pre-subiculum, which fire when the animal's head points in a particular direction (Taube, 1998); (3) grid cells in the entorhinal cortex, which fire in a grid-like hexagonal pattern that conveys information about position, direction, and distance (Hafting et al. 2005); and most recently, (4) border cells (also in the entorhinal cortex), which fire when the animal is close to the borders of the environment (Solstad et al. 2008).
As the study of sensory processing in the visual system was later used to understand visual perception (Engel et al. 1991, 2001), the analysis of the different hippocampal cell types has been used to understand two cognitive functions: spatial navigation and memory encoding. While other researchers pursued spatial navigation by examining the computations involved in transforming sensory-motor cues into a spatial map (for review see (O’Keefe, 1979; Leutgeb et al. 2005; McNaughton et al. 2006), our laboratory focused on bridging the gap between a spatial map and the well-described mnemonic functions of the hippocampal formation in long-term explicit memory.
We began to think about the spatial map in 1992, wondering how it is formed and maintained, and how attention directs these processes. We were struck by the fact that the spatial map of even a simple locale does not form instantaneously but requires several minutes to develop (Bostock et al. 1991; Wilson & McNaughton, 1993). This suggested to us, as well as others (Touretzky & Redish, 1996; Redish & Touretzky, 1997; Shapiro, 2001), but see (Samsonovich & McNaughton, 1997), that the formation of the map is a learning process: practice makes perfect also for space. Furthermore, much like a memory process, the spatial map, once fully formed, under optimal circumstances remains stable for weeks or even months (Thompson & Best, 1989).
Unlike vision, studied by Hubel and Wiesel, or somatosensory maps, studied by Mountcastle, which are based on Kantian a priori knowledge largely set by a predetermined pattern of cortical organization, the spatial map presents us with a new type of representation, one based on a combination of predetermined characteristics and learning. The general capability for forming spatial maps is built into the brain, but the particular map is not. Unlike neurons in a sensory system, place cells of the hippocampus are not purely driven by sensory information but by a combination of external and internal cues that represent the characteristics of the external world (for review see McNaughton et al. 2006). This information is not encoded in the hippocampus as a topographic map; rather, it is represented by the activity of different ensembles of active cells elicited by distinct animals’ experiences. The collective activity of these neuronal ensembles more closely represents the location where the animal perceives it is, even when it does not agree with some of the sensory input (Rotenberg & Muller, 1997). Since the perception of space involves information acquired through several sensory modalities, the process by which these modalities are brought together raises several questions: How are the relevant aspects that comprise space selected and unified in a single construct? What aspects of the animal's perception are represented in the hippocampus? How is the map formed and maintained?
We started investigating these questions by focusing on the molecular and physiological mechanisms necessary for the formation and maintenance of place cells. Although the discovery of place cells by O'Keefe & Dostrovsky (1971) and the discovery of long-term potentiation (LTP) in the hippocampus by Bliss & Lomo (1973)– a cellular model of memory formation and consolidation – there was no published experimental work connecting the two findings when we began studying place cell maps in 1992. At that point, even though almost nothing was known about the molecular steps underlying the formation of place fields, many important molecular steps involved in LTP had been worked out and their role in spatial memory had been validated by behavioural studies (Morris et al. 1990; Grant et al. 1992; Silva et al. 1992a,b; Barnes et al. 1994; Collingridge & Bliss, 1995; Huang et al. 1996). We thought that if learning affected the formation of a place cell map, then the physiological mechanisms involved in the formation and consolidation of hippocampal place fields might require the same molecular cascades necessary for the consolidation of memory and LTP (Fig. 1A). To explore this possibility, we initiated a collaboration with the laboratory of Robert Muller, whose laboratory had recently shown the effects of repeated experience on the re-mapping of place fields (Bostock et al. 1991).
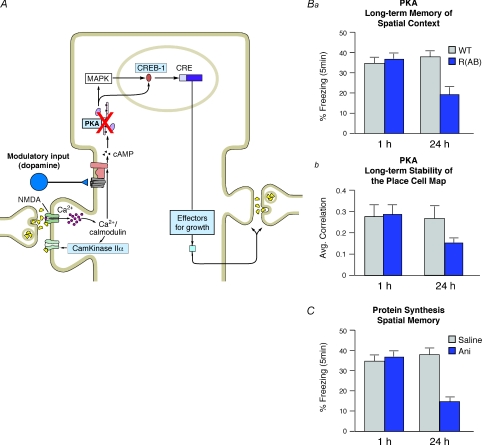
Figure 1. Signal transduction pathways involved in place field stabilization and memory consolidation.
A, schematic representation showing signalling cascades leading to activation of protein synthesis necessary for place field stability and memory consolidation. Protein kinase A (PKA) activity is necessary for the activation of protein synthesis-dependent processes. B, long-term memory of spatial context (Ba) and long-term stability of place fields (Bb) are compromised in R(AB) mice, where PKA activation is disrupted. C, long-term memory is disrupted in the presence of anisomycin, a protein synthesis inhibitor. WT, wild-type; R(AB) mice, mice expressing an inhibitory form of the RIα subunit of PKA; ANI, anisomycin.
Since NMDA receptors are critically involved in LTP and memory, one of our first experiments used a pharmacological approach to determine the effects of NMDA receptor blockade on the formation of the spatial map. We found that blocking NMDA receptors while placing a rat into a novel environment specifically ablated the long-term stability of the newly formed map without affecting a previously formed map (Kentros et al. 1998). Thus, in the absence of NMDA receptor activity, newly formed maps remapped each time the animal was placed in the same environment. These findings closely paralleled results showing that NMDA receptor blockade had no effect on previously learned locations or previously induced LTP (for review see Morris et al. 1990).
We also knew that protein kinase A (PKA) activates the transcription factor CREB and the genes coding proteins necessary for the late phase of LTP (Abel et al. 1997). We therefore investigated whether PKA and protein synthesis were necessary to consolidate the spatial map. Similar to our results with the NMDA receptor blockade, we found that although neither PKA nor protein synthesis is needed for the initial formation of a map, they are both essential for the map to become stable in the long term, so that the mouse can recall the same map every time it enters the same space (Rotenberg et al. 2000; Agnihotri et al. 2004; Fig. 1Bb). Furthermore, our findings showed that blockade of PKA activity or inhibition of protein synthesis affected not only the long-term stability of the spatial map but also the ability to retain long-term spatial memories (Fig. 1Ba and C). This provided evidence that the spatial map is an actual mnemonic process.
In the three decades since the description of the cognitive map theory, four sets of findings have further supported the idea that place cells are indeed correlates of explicit memory. First, as we previously mentioned, the unique construct that represents a contextual experience is acquired over a period of time through learning (Bostock et al. 1991; Mehta et al. 1997; Kentros et al. 2004; but see Samsonovich & McNaughton, 1997). Moreover, similar to the experience-dependent plasticity observed during memory retrieval (McGaugh, 1966), spatial representations undergo experience-dependent modifications as the animal repeatedly processes information within the same context (Mehta et al. 1997), and this plasticity is sensitive to the same molecular cascades necessary for LTP (Ekstrom et al. 2001). Second, as with memory formation, task-contingencies modulate what is encoded in the hippocampus by increasing neuronal responses to task-relevant information (Markus et al. 1995; Wiener et al. 1995; Wood et al. 1999). Third, pyramidal neurons that encode space are not only driven by spatial cues but also by auditory and olfactory cues (Kuperstein & Eichenbaum, 1985; Eichenbaum et al. 1987; Sakurai, 1996; Wood et al. 1999). These distinct representations appear to be constituents of different ‘maps’ that animals use in specific situations to manage task-relevant information critical for explicit memory storage (Knierim et al. 1995; Markus et al. 1995; Gothard et al. 1996; Barnes et al. 1997; Gothard et al. 2001; Olypher et al. 2002). Interestingly, some of these ‘maps’ appear to be modulated by time as it has been demonstrated in the firing of hippocampal cells in response to past (retrospective) or future (prospective) events (Frank et al. 2000; Wood et al. 2000; Ferbinteanu & Shapiro, 2003), indicating that these cells process episodic memory traces. Fourth, hippocampal cells fire in structured patterns that indicate ‘memory replay’ of previous experiences when the animal is stationary. For example, place cells appear to sweep forward as the animal pauses at a choice point in a maze (Johnson & Redish, 2007) and both forward and reverse ‘replay’ have been observed in the ripple events as the animal feeds at the ends of a linear track (Foster & Wilson, 2006; Diba & Buzsaki, 2007). In a striking recent example, Pastolokova et al. (2008) found that hippocampal neurons fired in a stereotyped sequence as the animal ran in a fixed wheel during the delay period of a memory task. These sequences predicted the future choice as well as the behavioural errors the animals made in the task indicating that hippocampal neurons can support episodic recall that participates in the planning of future behavioural events (Pastalkova et al. 2008). Together, these findings demonstrate that hippocampal pyramidal cells can code a variety of complex mnemonic representations, some of which are not restricted to space or a particular time frame.
The reference frames that organize the complex information that reaches the hippocampus are thought to dynamically control neuronal activity by modulating the importance of reliable signals that are available to the animal at any given time (Gothard et al. 2001). For example, animals shift reference frames in conditions where there are changes in task contingencies (Ferbinteanu & Shapiro, 2003), the reliability of the external or internal cues is compromised (Knierim et al. 1995; Zinyuk et al. 2000), or there are changes in the environment (Gothard et al. 2001) or reward locations (Breese et al. 1989). These findings raise two further questions: (1) What determines which particular reference frame the animal uses to process and store information at any given time? (2) What are the physiological consequences of focusing on a particular reference frame?
Insights into the first question were first provided by the landmark study conducted by Markus et al. (1995), who found that when rats learned several tasks in either the open field or the radial arm maze, the spatial representations were much more directional when animals searched for food in a fixed goal location than when the food was scattered throughout the environment. The authors suggested that using planned routes to retrieve a reward might engage attentional mechanism that were probably not required during random forageing in the environment. Furthermore, this attentional mechanisms could serve to switch between different reference frames determined by distinct task-contingencies (Markus et al. 1995).
Olypher and collaborators (2002) later expanded this idea and provided an answer for the second question: What are the physiological consequences of focusing on a particular reference frame? The authors approached this question by studying the ‘overdispersion’ of place fields – the variability in place cell firing produced as animals walk through the same spatial locations – while animals performed an active avoidance task that required the use of an arena and room-based reference frames. To explain the differences in overdispersion observed when animals switched between these reference frames, the authors developed a model which suggested that changes in the variability of place cell firing reflected switches in attentional focus (Olypher et al. 2002). Jackson & Redish (2007) later provided further insight into overdispersion by showing that the changes in place field variability were produced by a global network mechanism that switched reference frames according to the animal's goals during reward-directed behaviours. These data suggest that attention modulates the variability of spatial representations through a global network mechanism that switches reference frames in response to task-relevant information (recently reviewed by (Johnson et al. 2009).
Attention modulates the long-term stability of hippocampal representations
These several experiments and models strongly implicate attention as a modulator of place cell activity. However, these studies did not assess whether attention directly influences long-term memory encoding. This question was recently tested in our laboratory by a series of experiments, conducted by Kentros and collaborators (2004), evaluating how differential degrees of attention to environmental cues affect the stability of place fields in mice.
Kentros and collaborators found that when mice freely explore an environment under no task contingencies, place fields are not stable. This finding, at first glance, appears to contradict the established idea from the rat literature that, once formed, place fields are always fixed to a particular spatial location (Thompson & Best, 1989). We realized, however, that this difference in stability might simply reflect the fact that these measures have been taken differently in rats and in mice, although this does not rule out neuroethological differences between the species. Rats do not freely explore an open field in the absence of food reinforcement and, therefore, cannot be used to study spatial representations without additional motivation. As a result, rats are normally tested during forageing activities to encourage complete sampling of the arena (Thompson & Best, 1989). Mice, by contrast, exhibit high levels of spontaneous activity, and can be tested during free exploration without the presence of motivational rewards (Kentros et al. 2004; Muzzio et al. 2009). This characteristic led us to think that the mouse might be a highly tractable model system for studying the interplay between attention and memory at both the behavioural and physiological levels.
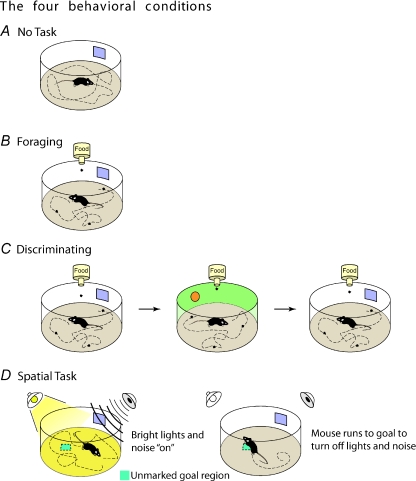
To study the effects of differential attentional engagement on long-term spatial memory retrieval, we exposed mice to four different behavioural conditions that varied in terms of the degrees of attentional demands that were placed on the animals and measured the stability of spatial representations over time (see Fig. 2). In the no-task condition, animals freely navigated in the environment under no task contingencies. In the forageing condition, animals searched for food pellets randomly scattered in the environment. In the discriminating condition, animals were exposed to a novel environment in between forageing sessions. Finally, in the spatial task condition, animals performed a spatial task that required finding an unmarked goal region in the environment in order to turn off aversive lights and sounds. The spatial task was similar to the Morris water maze, where animals have to use environmental cues to find a goal location to escape from an aversive stimulus (e.g. cold water).
Figure 2. Four tasks requiring different degrees of attention to the visuospatial environment.
A, No Task animals were repeatedly placed into the recording environment under no-task contingencies. B, Forageing animals were food-deprived and trained to search for randomly dropped food pellets. C, Discriminating animals were treated exactly as the Forageing animals, except that these animals were placed into a novel environment between familiar forageing sessions. D, Spatial Task animals performed an operant place preference task that required to find an unmarked goal region in order to turn off aversive stimuli (bright lights and loud noise). Published with permission of Neuron.
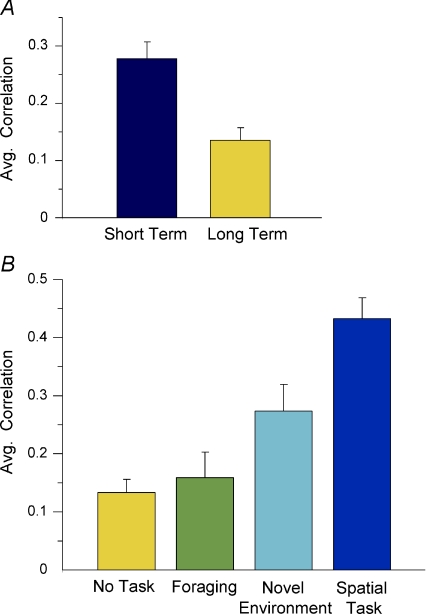
We found that the degree of place cell stability in area CA1 of the hippocampus correlated with the degree to which the animal assigned behavioural significance to the visuospatial landmarks in the training environments. For example, the animals assigned to the no-task condition displayed poor place field stability, while the animals accurately performing a spatial task displayed stable place fields. Thus, the hippocampal representation of the environment was not automatically stable but instead depended upon the animal's behavioural context: place fields were stable only when the task required mice to attend to the spatial layout of the environment (Fig. 3).
Figure 3. Behavioural relevance modulates hippocampal place field stability.
A, in the No Task group the short-term stability of place fields calculated between sessions recorded every 30 min was high, indicating that in this group the place cells fired in the same circumscribed locations after a short-term delay. However, the long-term stability recorded every 6 h was low, indicating that the place cells fired in different regions of the environment after a long-term delay. B, average similarity scores for place field recorded in the same familiar environment in the 4 behavioural tasks (Fig. 2A–D). There was a gradual increase in place field stability that was maximal in cells recorded from animals performing the spatial task. Published with permission of Neuron.
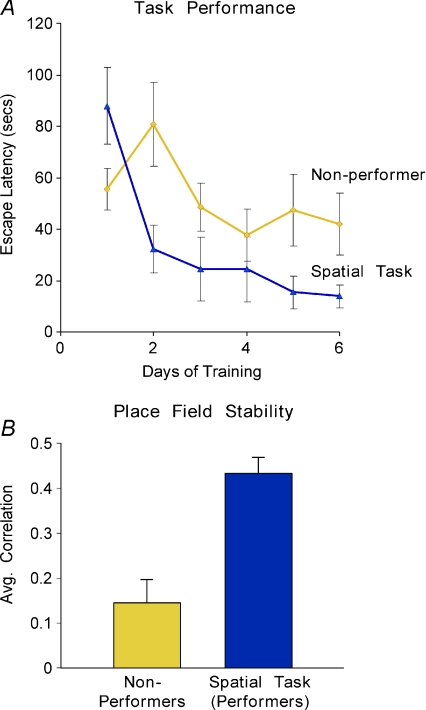
Interestingly, some animals trained in the spatial task failed to learn it. In these non-performing animals the place fields were far less stable than those of their littermates who performed the task well, showing that the accuracy of spatial memory retrieval directly correlated with task performance: only those animals that assigned significance and attended to the visuospatial environment displayed stable retrieval of the spatial map (Fig. 4).
Figure 4. Spatial task performance correlates with the degree of place field stability.
A, average latency to turn off aversive stimuli in the spatial task. Animals were divided into two groups, those that learned the spatial task (performers) and those that did not (non-performers). B, only animals that learn the task (performers) displayed high place field stability. Published with permission of Neuron.
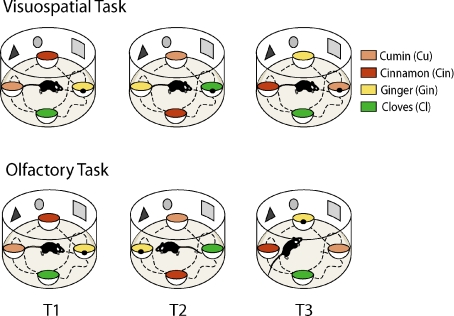
The findings by Kentros et al. (2004) showing that stable retrieval of the place cell maps requires attention raised the following questions: Is the non-selective attentional state generated by the arousal resulting from performing a goal-oriented task sufficient to stabilize the place field map? Or does this process require selective attention to space? To address these questions, Muzzio and collaborators (2009) developed two versions of a goal-oriented task that differ with respect to whether the animal must pay attention to fixed visuospatial cues or a spatially shifting olfactory cue. In the visuospatial version, mice had to associate a specific spatial location with the reward, independent of the odour covering the reward. Thus, in each trial the same location was rewarded but the odours covering the reward changed. In the non-spatial olfactory version, mice had to associate a specific odour with the food reward, independent of spatial location. In each trial the location of the cup containing the rewarded odour shifted semi-randomly among the four-cup locations (Fig. 5). We reasoned that if a general state of arousal was sufficient to produce place field stability, then both the visuospatial and olfactory groups should display stable place fields. By contrast, if selective attention to a particular spatial reference frame was important, then only the visuospatial animals should display place field stability. In this context, selective attention was defined as the process by which animals ‘selected’ stimulus features that were task-relevant from a rich environmental background that contained relevant and irrelevant information (Driver, 2001). Our measure of whether animals attended to this information was the accuracy and stability of the retrieved memories.
Figure 5. Visuospatial and olfactory goal-oriented tasks.
Animals were trained to retrieve a food reward hidden inside a cup filled with scented bedding. In the visuospatial group, animals had to attend to the visuospatial cues placed on the wall of the cylinder and ignore the odours placed on each cup to find the food reward. In the olfactory group, animals had to attend to an odour that shifted locations on every trial and ignore the visuospatial cues on the wall of the cylinder to retrieve the food reward. Animals were trained for 3 days receiving two 3-trial sessions per day. Adapted from Muzzio et al. 2009.
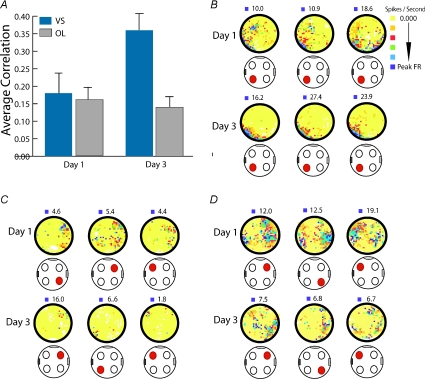
Using this approach, we found that only animals that selectively attended to the visuospatial environment displayed both short- and long-term stability of place fields in the hippocampal area CA1. The place fields recorded from these animals were not only stable but also organized and coherent, which reflected a smooth distribution of firing activity within the fields. Conversely, the place field stability of cells from animals that attended to a spatially shifting olfactory cue was dramatically reduced. In this group, the place fields were disorganized and dispersed in the environment. Interestingly, the lack of stability and coherence of place fields in the olfactory group was paralleled by the emergence of representations that re-mapped according to the position of the reward-associated odour in every trial. These olfactory representations were more consistently retrieved during periods of sniffing and digging when animals were restricted to the cup locations in close proximity to the odours and reward, and were not processing space (Muzzio et al. 2009). These data suggest that attention acts as a switch that can shift the processing of visuospatial representations in favour of reward-associated odour representations by modulating the encoding of task-relevant non-spatial aspects of the environment (Fig. 6).
Figure 6. Only attention to the fixed visuospatial environment leads to stable spatial representations.
A, long-term stability on days 1 and 3 during training in the visuospatial and olfactory tasks. Only animals that learned to attend to space (visuospatial group) displayed place field stability on Day 3. B–D, examples of rate maps from cells recorded in the visuospatial (B) and the olfactory groups (C and D) during training. B, on day 1 the depicted cell displays a place field with low coherence and stability. However, the same cell displays a highly stable place field on day 3 after the animal has learned to attend to space. C and D, examples of cells in the olfactory group where the fields become locked to the reward-associated odour (C) and the fields become highly unstable and unorganized (D). Red circle in the cartoon below each map indicates position of the reward. Purple square on top of each map indicates peak firing rate. Colour map next to panel B indicates how firing activity was represented in the maps. Adapted from Muzzio et al. 2009.
These findings also illustrate two important points. First, during navigation, the primary firing mode of hippocampal cells is spatial (O’Keefe & Nadel, 1978). Under these conditions, animals will display spatial representations during both attentive and inattentive states. However, the stability and coherence of the spatial map requires selective attention to space. Second, when animals are not navigating, the retrieval of non-spatial cues is more prominent. These results are in agreement with findings showing that hippocampal cells can code odours during brief periods of sniffing (Wiener et al. 1989; Wood et al. 1999), temporal relationships during classical conditioning protocols (Weiss et al. 1996; McEchron & Disterhoft, 1999; McEchron et al. 2003), and the negative valence of a learned fear stimulus (Moita et al. 2003). In all these cases, hippocampal cells code the task-relevant information that is critical for the animal by changing firing rate responses. Thus, it has been suggested that the hippocampus codes some salient aspects of the environment through changes in firing rate that later are bound together in a spatial context to form an explicit memory (Huxter et al. 2003). In this context, hippocampal rate coding resembles the process of ‘bias competition’ described in the visual system (Desimone & Duncan, 1995), where changes in firing rate serve to bias information processing by favouring critical over irrelevant information – a mechanism of visual perception that also has been associated with attention (Desimone, 1996; Chelazzi et al. 1998; Duncan, 1998).
Physiological mechanisms of attentional modulation of spatial memories
Even though firing rate changes may serve to increase the saliency of particular stimuli or some task-relevant aspects of the environment, firing rate mechanisms alone are probably not sufficient to modulate the saliency of a complex representation such as space. Since encoding of a spatial construct involves the integration of many inputs representing several multimodal sensory contributions, e.g. proprioception, visual perception of colour, shapes and distance, it is likely that an attentional mechanism that modulates this type of representation would produce network changes that simultaneously affect all the inputs giving rise to this representation. Such a mechanism could ensure that all aspects of the spatial representation are modulated simultaneously and that all the salient features are integrated. This view is supported by the findings, discussed previously, that the attentional switches in reference frames appear to require global network modulation (Jackson & Redish, 2007).
A possible network mechanism for physiological integration of sensory stimuli in cortical sensory areas is neuronal synchronization in the gamma band (Singer & Gray, 1995; Womelsdorf et al. 2006; Womelsdorf & Fries, 2007). In sensory areas, gamma synchronization participates in the encoding of complex stimuli such as composite odours (Laurent, 1996; Stopfer et al. 1997) or different features of complex visual cues (e.g. distance, continuity, colinearity, etc. (Gray et al. 1989; Engel et al. 1991; Kreiter & Singer, 1996). In all of these cases, synchronization simultaneously raises the saliency of the relevant stimulus features, thereby defining which responses should be integrated for further processing (‘feature binding’; for review see Singer & Gray, 1995). As a result, the gamma synchronization involved in ‘feature selection’ has also been strongly associated with attention in both animals and humans (Gruber et al. 1999; Fries et al. 2001, 2002; Niebur et al. 2002). Indeed, gamma synchronization increases in the presence of attended stimuli, decreases in the presence of unattended ones and is diminished by distracters (Fries et al. 2001; Taylor et al. 2005) – a common test of attentional disengagement (Reynolds & Chelazzi, 2004).
Neuronal synchronization can be manifested in one of two ways: by an increase in the power of a specific frequency, which reflects that more neurons are synchronized, or by an increase in spike phase locking at a particular frequency. In both cases the result reveals that the population oscillatory response is more synchronized at a specific frequency band, which increases the chances that the population of active neurons could have an effect on the postsynaptic cells (Axmacher et al. 2006). Such a mechanism has strong implications for neuronal processing and memory consolidation because it facilitates amplification of critical information on downstream targets (Salinas & Sejnowski, 2001). Specifically, gamma oscillations could enhance synaptic potentiation. For example, the time windows between connected neurons that lead to optimal plastic changes range from 10 to 30 ms. These are the same time intervals that characterize the cycling patterns of low gamma oscillations (30–60 Hz). Furthermore, within these time windows, discharges that coincide with the peak depolarizing phase of the gamma cycle lead to synaptic potentiation and those coinciding with the trough to synaptic depression (Wespatat et al. 2004). By increasing or decreasing the strength of information on postsynaptic targets, this mechanism gives gamma oscillation control over the selection of task relevant information. As a corollary, gamma oscillation could provide a physiological mechanism through which attention selects information for long-term memory storage.
At the level of the hippocampus, the possible link between an attentional process mediated by gamma synchronization and memory formation is supported by several studies using intracranial electrophysiological recordings in epileptic patients (Fell et al. 2003; Sederberg et al. 2003, 2007a,b). These studies showed that increases in gamma activity in the hippocampus not only correlate with encoding but also with the retrieval of correct memories (Sederberg et al. 2007a). Furthermore, gamma synchronization between the hippocampus and the entorhinal cortex also predicted successful memory encoding (Fell et al. 2003), indicating that synchronous coupling between structures involved in memory encoding is critical for successful memory formation.
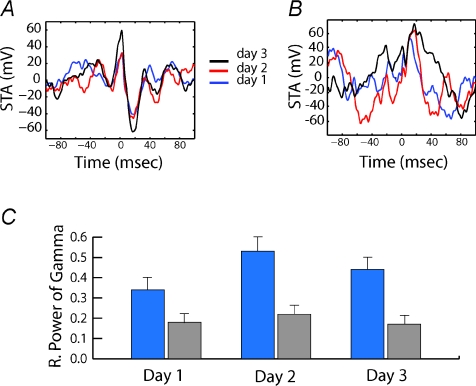
The earlier studies evaluated gamma synchronization in the context of semantic memory. To determine if gamma synchronization also influenced the encoding and retrieval of long-term multimodal spatial memories, our laboratory examined neuronal synchronization during acquisition of the visuospatial and olfactory tasks (Fig. 5). We found that at the beginning of each trial when animals were actively searching for the reward, there was an increase in neuronal synchronization only in animals that attended to the visuospatial environment. This increase in neuronal synchronization was produced by a gradual enhancement in phase locking in the low gamma range (20–60 Hz) (Fig. 7). No changes in synchronization, in either the visuospatial or the olfactory group, were observed in the theta range. Importantly, the enhanced synchronization in the gamma range was restricted to the periods when animals in the visuospatial group were searching for the reward and disappeared at the end of the trials when animals were not attending to the environmental task-contingencies. Furthermore, the synchronization was diminished by a distracter, suggesting that hippocampal gamma synchronization shares features with the attentional physiological processes described in cortical areas (Fries et al. 2001). Similar to the human studies described above, the enhancement in neuronal synchronization reported in this study correlated with the increase in place field stability, suggesting that a physiological attentional mechanism of signal amplification is necessary for stable spatial memory retrieval (Muzzio et al. 2009).
Figure 7. Gamma synchronization is enhanced in animals that attend to space during periods of navigation.
A and B, spike-triggered average (STA) across three training days in an animal trained in the visuospatial group (A) and one trained in the olfactory group (B). High synchronicity is only observed in the visuospatial animal. C, relative power of Gamma shows that gamma synchronization is only enhanced in the visuospatial group on days 2 and 3. Adapted from Muzzio et al. 2009.
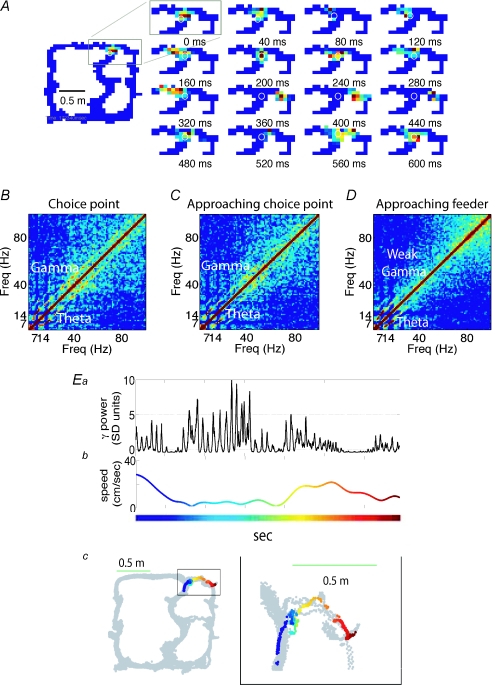
Recent studies in the rat have also reported increases in gamma oscillations in the context of spatial navigation. Montgomery et al. (2008) trained rats in a delayed alternation T-maze and recorded dendritic and somatic local field potentials from several areas of the hippocampus. The authors found increases in gamma power when animals approached the T-junction – a point where animals have to evaluate choices. Johnson & Redish (2007) recently reported similar results in a multiple T-maze task. At the last junction where animals had to decide whether to turn right or left, reconstruction of the firing patterns at fast time scales showed projections of locations ahead of the animal (‘sweep forward’; Fig. 8A). These sweeps went first down one path and then the other. During these periods when animals deliberated about turning right or left, the authors suggested that animals were evaluating possible outcomes by vicarious trial and error. Interestingly, during these periods of cognitive processing there was an increase in gamma and theta power (Johnson & Redish, 2007, Fig. 8B and Ea). We suggest that the increase in gamma activity at the juncture points described in these studies results from increasing attentional resources to task-relevant information.
Figure 8. Gamma power is increased during vicarious trial and error.
A, sweeps recorded at the final T choice point showing spatial representations ahead of the animal's position. These representations sampled each arm and were obtained through reconstruction from the firing patterns of the neuronal ensemble at fast time scales. B–D, average cross frequency correlations at choice point (B), approaching the choice point (C) and approaching the feeders (D). Note the enhanced average gamma at the choice point in comparison to the other locations. Ea, gamma power recorded from an animal as it made a turn to the right. Note the enhanced gamma power at the choice point. Eb, speed of movement. Ec, schematic representation of the maze showing all positions sampled during the session (grey) and the path of the animal during the time Gamma was measured (coloured points). Adapted from Johnson & Redish (2007); published with permission of the authors and the JNS. Published with permission of J Neurosci and David Reddish.
Neuroanatomical substrates of hippocampal attentional modulation
To understand how attention might modulate complex multimodal representations during encoding and retrieval, it will be important to determine whether the neuronatomical interconnectivity of this area provides a basis for an attentional process to occur (Rowland & Kentros, 2008). In this section we will first review the connectivity within the hippocampal formation comprising three regions: the entorhinal cortex (EC), the input region to the hippocampus proper; the hippocampus proper; and the subicular complex, the output region. The pathways connecting these areas display a unique arrangement that supports the role of attention in the selection of novel and/or critical information. Then, we will review the connectivity between the hippocampus and cortical and subcortical areas involved in attention, which further suggests that the hippocampus is in a central position to modulate the interplay between attention and memory.
Entorhinal-hippocampal circuitry supports selection of critical information
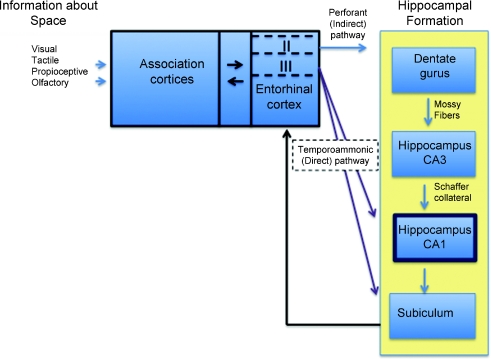
The flow of information into the hippocampal formation starts in the entorhinal cortex, which is the major input to the hippocampus. The entorhinal cortex is divided into medial and lateral regions, each of which receives polymodal information from associational areas including the parahippocampal (postrhinal in rodents) and perirhinal cortices (Witter, 1986; Amaral & Witter, 1989). Within the medial entorhinal area, the dorsolateral region contains grid cells – multipeaked grid cells (see page 2 and Fyhn et al. 2004; Hafting et al. 2005). The lateral subdivision primarily conveys non-spatial information including olfactory information from the perirhinal cortex (Burwell, 2000). The entorhinal cortex displays a laminar organization consisting of six layers that are interconnected by axon collaterals. The superficial layers II and III, with minor contributions from the deep layers, send information to the hippocampus proper, and completing the loop, the deep layers V and VI receive processed information from the subiculum – the major output structure of the hippocampus – back into the entorhinal cortex (Witter et al. 1989).
The hippocampus proper consists of the dentate gyrus and areas CA3 and CA1. These areas receive inputs from different layers of entorhinal cortex. Layer II projects to the dentate gyrus and region CA3 via the perforant pathway, whereas layer III projects directly to CA1 and the subiculum via the temporoammonic pathway (Witter & Amaral, 2004). Information that reaches the dentate gyrus from layer II reaches area CA1 indirectly by means of a series of interconnected axonal paths known as the trisynaptic loop including the perforant path, the mossy fibres, and the Schaffer collaterals. Thus, CA1 receives information via two routes, a direct one arising from layer III of the entorhinal cortex and an indirect one arising from CA3 via the trisynaptic loop (Fig. 9). The direct route terminates on the distal portions of the apical dendrites, whereas the indirect route terminates on the proximal dendrites.
Figure 9. Neural circuitry important for processing and storing polymodal representations.
Information converges on the entorhinal cortex. This information reaches area CA1 via two routes, an indirect pathway that reaches CA1 via a series of synaptic connections (blue arrows, trisynaptic loop) and a direct route through the temporoammonic pathway (purple arrows). Some processed information is redirected to the entorhinal cortex via the subicular output (black arrow).
This neuroanatomical arrangement places the CA1 region in a unique position to compare the information that has been processed through the trisynaptic loop with the new information arriving directly from cortex. This arrangement has inspired models predicting that the area CA1 serves as a novelty detector (Lisman & Grace, 2005) or an attentional gate (Vinogradova, 2001). Both of these ideas would require that information be differentially integrated along the apical dendrites. This is supported by the differential ion channel distribution in these dendrites (Hoffman et al. 1997; Lorincz et al. 2002), which has been shown to produce differential regional excitability (Nolan et al. 2004). This characteristic might be essential for an attentional gating system that relies on integrative properties.
Cortical and subcortical connections with the attentional networks
Neurotransmitter systems
More than 35 years ago, Michael Posner proposed that attention is not a single entity but consists of several processes that are functionally and neuroanatomically distinct (Posner & Petersen, 1990). For example, different attentional mechanisms are likely to be required for maintaining an alert state, for orienting to targets, and for exerting executive control of thoughts and behaviour (Fan & Posner, 2004). Neuroimageing and pharmacological studies later confirmed that each of these attentional networks requires a distinct neuroanatomical substrate that is modulated by a different neurotransmitter system (Marrocco et al. 1994; Fan & Posner, 2004; Raz, 2004). Orienting requires the posterior parietal cortex (PPC) and is modulated by the cholinergic system. Alerting relies heavily on the thalamus and some cortical areas including superior parietal lobe, superior temporal lobe, and frontal eye fields, and is mediated by the noradrenaline system in the locus coeruleous. The executive network requires the anterior cingulate and lateral parts of the prefrontal cortex and mainly relies on the dopaminergic system (Raz, 2004; Posner & Rothbart, 2007).
Each of the main neuromodulatory systems involved in attention sends inputs to the hippocampus. The cholinergic input reaches the temporal lobe via the medial septum and the diagonal band of Broca (Witter et al. 1989), the noradrenaline input originates in the locus coeruleous and projects heavily to the dentate gyrus (Haring & Davis, 1985), and the dopaminergic input originates in the ventral tegmental area and projects heavily to the ventral CA1 area and the entorhinal cortex (Gasbarri et al. 1994, 1996, 1997; Lisman & Grace, 2005). Disturbance in the activity of several of these neurotransmitter systems has been implicated in a variety of clinical disorders characterized by an inability to focus attention, such as attention deficit disorder, schizophrenia, senile dementia, and Parkinson's disease (Clark et al. 1987; Politis et al. 2004; Oades et al. 2005; Martin & Freedman, 2007; Kim et al. 2008; Scarr & Dean, 2008).
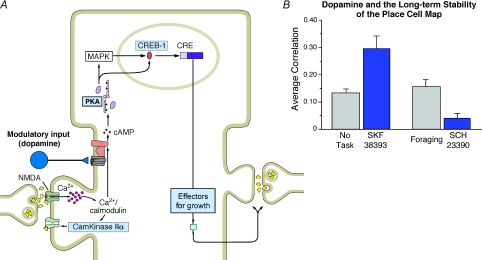
Even though the role of these neurotransmitter systems has been extensively studied in relation to neuropsychiatric disorders (Sarter et al. 2007), their effect on place cell firing has been explored in very few studies. As a result, we investigated the effects of D1/D5 agonists and antagonists, two major dopamine receptors expressed in the hippocampal formation, on place field stability (Kentros et al. 2004). Consistent with a role for dopamine in attentional processes within the hippocampus, we found that place field stability was increased by systemic applications of a D1/D5 receptor agonist and compromised by a D1/D5 antagonist (Fig. 10B). These results closely parallel findings showing that lesions of the mesohippocampal dopaminergic pathway affect the ability to acquire and store information in the Morris water maze (Gasbarri et al. 1996) and the effects of dopamine agonists and antagonists on LTP (Huang & Kandel, 1995) and long-term memory in ageing mice (Bach et al. 1999).
Figure 10. Dopamine modulation of place field stability.
A, schematic representation showing signalling cascades leading protein synthesis. Dopamine and Ca2+ increase levels of cAMP, which activates PKA and MAPK. These kinases translocate to the nucleus to activate CREB and initiate transcription processes that lead to synthesis of new proteins. B, animals in the No Task condition (Fig. 2A) were injected with the D1/D5 receptor agonist SKF 38393. Animals in the Forageing condition (Fig. 2B) were injected with the D1/D5 receptor antagonist SCH 2390. Place field correlations between sessions were calculated prior to drug injection and 6 h post injection. The agonist-injected animals displayed higher stability than the no task animals that did not receive the drug, whereas the antagonist-injected animals displayed lower place field stability than the forageing animals that did not receive the drug. Tasks are described in the section ‘Attention modulates the long-term stability of hippocampal representations’ and Fig. 2.
Our studies could not distinguish between the D1 and D5 receptor-mediated modulation. However, a recent study using a selective blockade of the D1 receptor in rats found that when animals were tested in an unchanged environment, place field stability was not affected. However, the re-mapping observed after a contextual change involving recordings in complete darkness was exacerbated (Gill & Mizumori, 2006). Since the contextual change from a lit to a dark environment requires a switch in reference frames from an allocentric (driven by external cues) to an egocentric (driven by internal cues) standpoint, the D1 receptor might be involved in the attentional process that is necessary to successfully switch reference frames. Interestingly, the D1 receptor is more abundant in the entorhinal cortex, whereas the D5 receptor predominates in the hippocampus (Montague et al. 2001). This suggests that the attention involved in reference frame switching might require the entorhinal cortex, whereas the D5 receptor may be selectively involved in place cell stability.
The effects of the cholinergic system on place cells were investigated in one study in rats using scopolamine, a specific antagonist of the muscarinic receptor. Blocking muscarinic transmission reduced place cell firing rate, decreased the spatial coherence, and reduced the stability of place fields (Brazhnik et al. 2003). Similarly, Tanila (2001) found that modulating noradrenaline release by using agonists and antagonists of the α2-autoreceptors destabilized place fields as well. These results suggested that place cell stability might be vulnerable to more than one neuromodulatory system. It remains to be determined whether these neurotransmitter systems affect place cell stability through their contributions to a hippocampus-mediated attentional process.
Hippocampal connections with attentional networks
In addition to receiving subcortical inputs from all the neurotransmitter systems involved in attention, the hippocampus has direct and indirect connections with several brain areas involved in attentional processing. For example, the posterior parietal cortex, an area that participates in attention and planning (Coulthard et al. 2008), has recently been proposed to be the key structure that translates spatial information arising from the temporal lobe into actual plans for locomotion (Whitlock et al. 2008). Therefore, the interaction between information that undergoes attentional selection in both structures might be of fundamental importance for proper navigation. The hippocampus does not have direct connections with the posterior parietal cortex; however, interaction between these areas may occur via the postrhinal and retrosplenial cortices, which are heavily connected to the posterior parietal cortex (Burwell & Amaral, 1998; Kerr et al. 2007). By contrast, the hippocampus sends strong projections to the medial prefrontal cortex, but this area does not project back to the hippocampus and only has few and weak projections to the entorhinal cortex (Swanson, 1981; Jay et al. 1989; Sesack et al. 1989). The prefrontal feedback to the temporal lobe is thought to occur via the nucleus reuniens of the thalamus, which is the major thalamic projection to the hippocampus (Vertes et al. 2007). Since the thalamus participates in orienting attention as well as arousal (Schiff, 2008), the interconnectivity with the medial prefrontal cortex places it in a unique position to convey different sources of attentional information to the temporal lobe. Finally, the anterior cingulate – a critical area for executive attention – is connected to the entorhinal cortex and subiculum, providing an additional indirect pathway through which the hippocampus could receive attentional information (Jones & Witter, 2007). In summary, the neuroanatomical connections between the hippocampus and cortical and subcortical areas involved in attention place the entorhinal cortex and hippocampus in a central position to further modulate attentional processes.
An overall view
The pioneering work of Hubel and Wiesel in understanding vision has encouraged the application of their single cell approach to higher-order association cortical areas. Using this approach, there have been important advances over the past 50 years in defining how sensory information is represented and modulated by attentional mechanisms. Here we present evidence that attentional processes contribute to the stabilization of spatial representations in the dorsal hippocampus by enhancing processing of task-relevant information. We show that attention in the dorsal hippocampus can act on the retrieval of spatial information in the same manner as selective attention in cortical areas modulates sensory perception. In each case, attention increases the strength and reduces the variability of the encoded signal. This is particularly evident in the increased coherence and reduced variability observed in tasks that require directionality or goal-directed behaviour. We also provide evidence that the attentional process necessary for the long-term stability of hippocampal representations is mediated by increased synchronicity in the low gamma frequency. Such a global network mechanism could ensure that the critical aspects of the multimodal representation of space are simultaneously selected and encoded in long-term memory. Finally, we suggest that neuroanatomical connections support the idea that an attentional process could occur at the level of the hippocampus and that a component contributing to hippocampal attentional modulation is mediated by dopamine and other neuromodulatory systems.
These several findings are consistent with the idea that attention is not a unitary process since it is neither a property of a single structure nor a function of the brain as a whole (Posner & Petersen, 1990). Rather, attention represents a family of processes that functions in different behavioural contexts, with different time frames and at different hierarchal levels of the central nervous system.
References
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Agnihotri NT, Hawkins RD, Kandel ER, Kentros C. The long-term stability of new hippocampal place fields requires new protein synthesis. Proc Natl Acad Sci U S A. 2004;101:3656–3661. doi: 10.1073/pnas.0400385101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Mormann F, Fernandez G, Elger CE, Fell J. Memory formation by neuronal synchronization. Brain Res Rev. 2006;52:170–182. doi: 10.1016/j.brainresrev.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci U S A. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, Jung MW, McNaughton BL, Korol DL, Andreasson K, Worley PF. LTP saturation and spatial learning disruption: effects of task variables and saturation levels. J Neurosci. 1994;14:5793–5806. doi: 10.1523/JNEUROSCI.14-10-05793.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, Suster MS, Shen J, McNaughton BL. Multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388:272–275. doi: 10.1038/40859. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Desimone R. Finding a face in the crowd: parallel and serial neural mechanisms of visual selection. Prog Brain Res. 2006;155:147–156. doi: 10.1016/S0079-6123(06)55009-5. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock E, Muller RU, Kubie JL. Experience- dependent modifications of hippocampal place cell firing. Hippocampus. 1991;1:193–205. doi: 10.1002/hipo.450010207. [DOI] [PubMed] [Google Scholar]
- Brazhnik ES, Muller RU, Fox SE. Muscarinic blockade slows and degrades the location-specific firing of hippocampal pyramidal cells. J Neurosci. 2003;23:611–621. doi: 10.1523/JNEUROSCI.23-02-00611.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese CR, Hampson RE, Deadwyler SA. Hippocampal place cells: stereotypy and plasticity. J Neurosci. 1989;9:1097–1111. doi: 10.1523/JNEUROSCI.09-04-01097.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD. The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Duncan J, Miller EK, Desimone R. Responses of neurons in inferior temporal cortex during memory-guided visual search. J Neurophysiol. 1998;80:2918–2940. doi: 10.1152/jn.1998.80.6.2918. [DOI] [PubMed] [Google Scholar]
- Clark CR, Geffen GM, Geffen LB. Catecholamines and attention. I: Animal and clinical studies. Neurosci Biobehav Rev. 1987;11:341–352. doi: 10.1016/s0149-7634(87)80006-4. [DOI] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Bliss TV. Memories of NMDA receptors and LTP. Trends Neurosci. 1995;18:54–56. [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Petersen SE. Selective attention modulates extrastriate visual regions in humans during visual feature discrimination and recognition. Ciba Found Symp. 1991;163:165–175. doi: 10.1002/9780470514184.ch10. discussion 175–180. [DOI] [PubMed] [Google Scholar]
- Coulthard EJ, Nachev P, Husain M. Control over conflict during movement preparation: role of posterior parietal cortex. Neuron. 2008;58:144–157. doi: 10.1016/j.neuron.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci U S A. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Diba K, Buzsaki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J. A selective review of selective attention research from the past century. Br J Psychol. 2001;92:53–78. [PubMed] [Google Scholar]
- Duncan J. Converging levels of analysis in the cognitive neuroscience of visual attention. Philos Trans R Soc Lond B Biol Sci. 1998;353:1307–1317. doi: 10.1098/rstb.1998.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Kuperstein M, Fagan A, Nagode J. Cue-sampling and goal-approach correlates of hippocampal unit activity in rats performing an odor-discrimination task. J Neurosci. 1987;7:716–732. doi: 10.1523/JNEUROSCI.07-03-00716.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Meltzer J, McNaughton BL, Barnes CA. NMDA receptor antagonism blocks experience-dependent expansion of hippocampal ‘place fields’. Neuron. 2001;31:631–638. doi: 10.1016/s0896-6273(01)00401-9. [DOI] [PubMed] [Google Scholar]
- Engel A, Konig P, Kreiter A, Singer W. Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science. 1991;252:1177–1179. doi: 10.1126/science.252.5009.1177. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Fan J, Posner M. Human attentional networks. Psychiatr Prax. 2004;31:S210–214. doi: 10.1055/s-2004-828484. [DOI] [PubMed] [Google Scholar]
- Fell J, Klaver P, Elfadil H, Schaller C, Elger CE, Fernandez G. Rhinal-hippocampal theta coherence during declarative memory formation: interaction with gamma synchronization? Eur J Neurosci. 2003;17:1082–1088. doi: 10.1046/j.1460-9568.2003.02522.x. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, Shapiro ML. Prospective and retrospective memory coding in the hippocampus. Neuron. 2003;40:1227–1239. doi: 10.1016/s0896-6273(03)00752-9. [DOI] [PubMed] [Google Scholar]
- Fernandes MA, Moscovitch M, Ziegler M, Grady C. Brain regions associated with successful and unsuccessful retrieval of verbal episodic memory as revealed by divided attention. Neuropsychologia. 2005;43:1115–1127. doi: 10.1016/j.neuropsychologia.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- Frank LM, Brown EN, Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27:169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Fries P, Schroder JH, Roelfsema PR, Singer W, Engel AK. Oscillatory neuronal synchronization in primary visual cortex as a correlate of stimulus selection. J Neurosci. 2002;22:3739–3754. doi: 10.1523/JNEUROSCI.22-09-03739.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. Spatial representation in the entorhinal cortex. Science. 2004;305:1258–1264. doi: 10.1126/science.1099901. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Packard MG, Campana E, Pacitti C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res Bull. 1994;33:445–452. doi: 10.1016/0361-9230(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Innocenzi R, Pacitti C, Brioni JD. Spatial memory impairment induced by lesion of the mesohippocampal dopaminergic system in the rat. Neuroscience. 1996;74:1037–1044. doi: 10.1016/0306-4522(96)00202-3. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Packard MG. The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1–22. doi: 10.1016/s0278-5846(96)00157-1. [DOI] [PubMed] [Google Scholar]
- Gill KM, Mizumori SJ. Context-dependent modulation by D1 receptors: differential effects in hippocampus and striatum. Behav Neurosci. 2006;120:377–392. doi: 10.1037/0735-7044.120.2.377. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Bisley JW, Powell KD, Gottlieb J. Saccades, salience and attention: the role of the lateral intraparietal area in visual behavior. Prog Brain Res. 2006;155:157–175. doi: 10.1016/S0079-6123(06)55010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard KM, Hoffman KL, Battaglia FP, McNaughton BL. Dentate gyrus and CA1 ensemble activity during spatial reference frame shifts in the presence and absence of visual input. J Neurosci. 2001;21:7284–7292. doi: 10.1523/JNEUROSCI.21-18-07284.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard KM, Skaggs WE, Moore KM, McNaughton BL. Binding of hippocampal CA1 neural activity to multiple reference frames in a landmark-based navigation task. J Neurosci. 1996;16:823–835. doi: 10.1523/JNEUROSCI.16-02-00823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant SG, O’Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Gruber T, Muller MM, Keil A, Elbert T. Selective visual-spatial attention alters induced gamma band responses in the human EEG. Clin Neurophysiol. 1999;110:2074–2085. doi: 10.1016/s1388-2457(99)00176-5. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005 doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Haring JH, Davis JN. Differential distribution of locus coeruleus projections to the hippocampal formation: anatomical and biochemical evidence. Brain Res. 1985;325:366–369. doi: 10.1016/0006-8993(85)90342-7. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci U S A. 1995;92:2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Nguyen PV, Abel T, Kandel ER. Long- lasting forms of synaptic potentiation in the mammalian hippocampus. Learn Mem. 1996;3:74–85. doi: 10.1101/lm.3.2-3.74. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Brain mechanisms of vision. Sci Am. 1979;241:150–162. doi: 10.1038/scientificamerican0979-150. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Early exploration of the visual cortex. Neuron. 1998;20:401–412. doi: 10.1016/s0896-6273(00)80984-8. [DOI] [PubMed] [Google Scholar]
- Huxter J, Burgess N, O’Keefe J. Independent rate and temporal coding in hippocampal pyramidal cells. Nature. 2003;425:828–832. doi: 10.1038/nature02058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J, Redish AD. Network dynamics of hippocampal cell-assemblies resemble multiple spatial maps within single tasks. Hippocampus. 2007;17:1209–1229. doi: 10.1002/hipo.20359. [DOI] [PubMed] [Google Scholar]
- Jay TM, Glowinski J, Thierry AM. Selectivity of the hippocampal projection to the prelimbic area of the prefrontal cortex in the rat. Brain Res. 1989;505:337–340. doi: 10.1016/0006-8993(89)91464-9. [DOI] [PubMed] [Google Scholar]
- Johnson A, Fenton AA, Kentros C, Redish AD. Looking for cognition in the structure within the noise. Trends Cogn Sci. 2009;13:55–64. doi: 10.1016/j.tics.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci. 2007;27:12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BF, Witter MP. Cingulate cortex projections to the parahippocampal region and hippocampal formation in the rat. Hippocampus. 2007;17:957–976. doi: 10.1002/hipo.20330. [DOI] [PubMed] [Google Scholar]
- Kentros C, Hargreaves E, Hawkins RD, Kandel ER, Shapiro M, Muller RV. Abolition of long-term stability of new hippocampal place cell maps by NMDA receptor blockade. Science. 1998;280:2121–2126. doi: 10.1126/science.280.5372.2121. [DOI] [PubMed] [Google Scholar]
- Kentros CG, Agnihotri NT, Streater S, Hawkins RD, Kandel ER. Increased attention to spatial context increases both place field stability and spatial memory. Neuron. 2004;42:283–295. doi: 10.1016/s0896-6273(04)00192-8. [DOI] [PubMed] [Google Scholar]
- Kerr KM, Agster KL, Furtak SC, Burwell RD. Functional neuroanatomy of the parahippocampal region: the lateral and medial entorhinal areas. Hippocampus. 2007;17:697–708. doi: 10.1002/hipo.20315. [DOI] [PubMed] [Google Scholar]
- Kim CH, Waldman ID, Blakely RD, Kim KS. Functional gene variation in the human norepinephrine transporter: association with attention deficit hyperactivity disorder. Ann N Y Acad Sci. 2008;1129:256–260. doi: 10.1196/annals.1417.023. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Kudrimoti HS, McNaughton BL. Place cells, head direction cells, and the learning of landmark stability. J Neurosci. 1995;15:1648–1659. doi: 10.1523/JNEUROSCI.15-03-01648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiter AK, Singer W. Stimulus-dependent synchronization of neuronal responses in the visual cortex of the awake macaque monkey. J Neurosci. 1996;16:2381–2396. doi: 10.1523/JNEUROSCI.16-07-02381.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperstein M, Eichenbaum H. Unit activity, evoked potentials and slow waves in the rat hippocampus and olfactory bulb recorded with a 24-channel microelectrode. Neuroscience. 1985;15:703–712. doi: 10.1016/0306-4522(85)90072-7. [DOI] [PubMed] [Google Scholar]
- Laurent G. Dynamical representation of odors by oscillating and evolving neural assemblies. Trends Neurosci. 1996;19:489–496. doi: 10.1016/S0166-2236(96)10054-0. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Moser MB, Moser EI. Place cells, spatial maps and the population code for memory. Curr Opin Neurobiol. 2005;15:738–746. doi: 10.1016/j.conb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Lorincz A, Notomi T, Tamas G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci. 2002;5:1185–1193. doi: 10.1038/nn962. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Qin YL, Leonard B, Skaggs WE, McNaughton BL, Barnes CA. Interactions between location and task affect the spatial and directional firing of hippocampal neurons. J Neurosci. 1995;15:7079–7094. doi: 10.1523/JNEUROSCI.15-11-07079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco RT, Witte EA, Davidson MC. Arousal systems. Curr Opin Neurobiol. 1994;4:166–170. doi: 10.1016/0959-4388(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Martin LF, Freedman R. Schizophrenia and the α7 nicotinic acetylcholine receptor. Int Rev Neurobiol. 2007;78:225–246. doi: 10.1016/S0074-7742(06)78008-4. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Disterhoft JF. Hippocampal encoding of non-spatial trace conditioning. Hippocampus. 1999;9:385–396. doi: 10.1002/(SICI)1098-1063(1999)9:4<385::AID-HIPO5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Tseng W, Disterhoft JF. Single neurons in CA1 hippocampus encode trace interval duration during trace heart rate (fear) conditioning in rabbit. J Neurosci. 2003;23:1535–1547. doi: 10.1523/JNEUROSCI.23-04-01535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- Mehta MR, Barnes CA, McNaughton BL. Experience-dependent, asymmetric expansion of hippocampal place fields. Proc Natl Acad Sci U S A. 1997;94:8918–8921. doi: 10.1073/pnas.94.16.8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moita MA, Rosis S, Zhou Y, LeDoux JE, Blair HT. Hippocampal place cells acquire location-specific responses to the conditioned stimulus during auditory fear conditioning. Neuron. 2003;37:485–497. doi: 10.1016/s0896-6273(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Montague DM, Striplin CD, Overcash JS, Drago J, Lawler CP, Mailman RB. Quantification of D1B(D5) receptors in dopamine D1A receptor-deficient mice. Synapse. 2001;39:319–322. doi: 10.1002/1098-2396(20010315)39:4<319::AID-SYN1015>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Montgomery SM, Sirota A, Buzsaki G. Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep. J Neurosci. 2008;28:6731–6741. doi: 10.1523/JNEUROSCI.1227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Davis S, Butcher SP. Hippocampal synaptic plasticity and NMDA receptors: a role in information storage? Philos Trans R Soc Lond B Biol Sci. 1990;329:187–204. doi: 10.1098/rstb.1990.0164. [DOI] [PubMed] [Google Scholar]
- Muzzio I, Levita L, Kulkarni J, Monaco J, Kentros C, Stead M, Abbott L, Kandel E. Attention enhances the retrieval and stability of visuospatial and olfactory representations in the dorsal hippocampus. Plos Biology. 2009 doi: 10.1371/journal.pbio.1000140. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebur E, Hsiao SS, Johnson KO. Synchrony: a neuronal mechanism for attentional selection? Curr Opin Neurobiol. 2002;12:190–194. doi: 10.1016/s0959-4388(02)00310-0. [DOI] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsaki G, Siegelbaum SA, Kandel ER, Morozov A. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- O’Keefe J. A review of the hippocampal place cells. Prog Neurobiol. 1979;13:419–439. doi: 10.1016/0301-0082(79)90005-4. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The Hippocanpus as a Cognitive Map. Oxford, UK: Clarendon Press; 1978. [Google Scholar]
- O’Keefe J, Conway DH. Hippocampal place units in the freely moving rat: why they fire where they fire. Exp Brain Res. 1978;31:573–590. doi: 10.1007/BF00239813. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Oades RD, Sadile AG, Sagvolden T, Viggiano D, Zuddas A, Devoto P, Aase H, Johansen EB, Ruocco LA, Russell VA, Vallone D, Sadile A. The control of responsiveness in ADHD by catecholamines: evidence for dopaminergic, noradrenergic and interactive roles. Dysfunctions in dopamine systems and ADHD: evidence from animals and modeling. Dev Sci. 2005;8:122–131. doi: 10.1111/j.1467-7687.2005.00399.x. [DOI] [PubMed] [Google Scholar]
- Olypher AV, Lansky P, Fenton AA. Properties of the extra-positional signal in hippocampal place cell discharge derived from the overdispersion in location-specific firing. Neuroscience. 2002;111:553–566. doi: 10.1016/s0306-4522(01)00586-3. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politis A, Lykouras L, Mourtzouchou P, Christodoulou GN. Attentional disturbances in patients with unipolar psychotic depression: a selective and sustained attention study. Compr Psychiatry. 2004;45:452–459. doi: 10.1016/j.comppsych.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Raz A. Anatomy of attentional networks. Anat Rec B New Anat. 2004;281:21–36. doi: 10.1002/ar.b.20035. [DOI] [PubMed] [Google Scholar]
- Redish AD, Touretzky DS. Cognitive maps beyond the hippocampus. Hippocampus. 1997;7:15–35. doi: 10.1002/(SICI)1098-1063(1997)7:1<15::AID-HIPO3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- Rotenberg A, Abel T, Hawkins RD, Kandel ER, Muller RU. Parallel instabilities of long-term potentiation, place cells, and learning caused by decreased protein kinase A activity. J Neurosci. 2000;20:8096–8102. doi: 10.1523/JNEUROSCI.20-21-08096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg A, Muller RU. Variable place-cell coupling to a continuously viewed stimulus: evidence that the hippocampus acts as a perceptual system. Philos Trans R Soc Lond B Biol Sci. 1997;352:1505–1513. doi: 10.1098/rstb.1997.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland DC, Kentros CG. Potential anatomical basis for attentional modulation of hippocampal neurons. Ann N Y Acad Sci. 2008;1129:213–224. doi: 10.1196/annals.1417.014. [DOI] [PubMed] [Google Scholar]
- Sakurai Y. Hippocampal and neocortical cell assemblies encode memory processes for different types of stimuli in the rat. J Neurosci. 1996;16:2809–2819. doi: 10.1523/JNEUROSCI.16-08-02809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsonovich A, McNaughton BL. Path integration and cognitive mapping in a continuous attractor neural network model. J Neurosci. 1997;17:5900–5920. doi: 10.1523/JNEUROSCI.17-15-05900.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Parikh V. Abnormal neurotransmitter release underlying behavioral and cognitive disorders: toward concepts of dynamic and function-specific dysregulation. Neuropsychopharmacology. 2007;32:1452–1461. doi: 10.1038/sj.npp.1301285. [DOI] [PubMed] [Google Scholar]
- Scarr E, Dean B. Muscarinic receptors: do they have a role in the pathology and treatment of schizophrenia? J Neurochem. 2008;107:1188–1195. doi: 10.1111/j.1471-4159.2008.05711.x. [DOI] [PubMed] [Google Scholar]
- Schacter D. Search for Memory: The Brain, the Mind, and the Past. New York: Harper Collins/Basic Books; 1996. [Google Scholar]
- Schenk F, Morris RG. Dissociation between components of spatial memory in rats after recovery from the effects of retrohippocampal lesions. Exp Brain Res. 1985;58:11–28. doi: 10.1007/BF00238949. [DOI] [PubMed] [Google Scholar]
- Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci. 2008;1129:105–118. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci. 2003;23:10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, Litt B, Brandt A, Kahana MJ. Gamma oscillations distinguish true from false memories. Psychol Sci. 2007a;18:927–932. doi: 10.1111/j.1467-9280.2007.02003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, Tully MS, Kahana MJ. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb Cortex. 2007b;17:1190–1196. doi: 10.1093/cercor/bhl030. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shapiro M. Plasticity, hippocampal place cells, and cognitive maps. Arch Neurol. 2001;58:874–881. doi: 10.1001/archneur.58.6.874. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in α-calcium-calmodulin kinase II mutant mice. Science. 1992a;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in α-calcium-calmodulin kinase II mutant mice. Science. 1992b;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Solstad T, Boccara CN, Kropff E, Moser MB, Moser EI. Representation of geometric borders in the entorhinal cortex. Science. 2008;322:1865–1868. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- Swanson LW. A direct projection from Ammon's horn to prefrontal cortex in the rat. Brain Res. 1981;217:150–154. doi: 10.1016/0006-8993(81)90192-x. [DOI] [PubMed] [Google Scholar]
- Tanila H. Noradrenergic regulation of hippocampal place cells. Hippocampus. 2001;11:793–808. doi: 10.1002/hipo.1095. [DOI] [PubMed] [Google Scholar]
- Taube JS. Head direction cells and the neurophysiological basis for a sense of direction. Prog Neurobiol. 1998;55:225–256. doi: 10.1016/s0301-0082(98)00004-5. [DOI] [PubMed] [Google Scholar]
- Taylor K, Mandon S, Freiwald WA, Kreiter AK. Coherent oscillatory activity in monkey area v4 predicts successful allocation of attention. Cereb Cortex. 2005;15:1424–1437. doi: 10.1093/cercor/bhi023. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Best PJ. Place cells and silent cells in the hippocampus of freely-behaving rats. J Neurosci. 1989;9:2382–2390. doi: 10.1523/JNEUROSCI.09-07-02382.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touretzky DS, Redish AD. Theory of rodent navigation based on interacting representations of space. Hippocampus. 1996;6:247–270. doi: 10.1002/(SICI)1098-1063(1996)6:3<247::AID-HIPO4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C. Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res Bull. 2007;71:601–609. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova OS. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001;11:578–598. doi: 10.1002/hipo.1073. [DOI] [PubMed] [Google Scholar]
- Weiss C, Kronforst-Collins MA, Disterhoft JF. Activity of hippocampal pyramidal neurons during trace eyeblink conditioning. Hippocampus. 1996;6:192–209. doi: 10.1002/(SICI)1098-1063(1996)6:2<192::AID-HIPO9>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Wespatat V, Tennigkeit F, Singer W. Phase sensitivity of synaptic modifications in oscillating cells of rat visual cortex. J Neurosci. 2004;24:9067–9075. doi: 10.1523/JNEUROSCI.2221-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Sutherland RJ, Witter MP, Moser MB, Moser EI. Navigating from hippocampus to parietal cortex. Proc Natl Acad Sci U S A. 2008;105:14755–14762. doi: 10.1073/pnas.0804216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener SI, Korshunov VA, Garcia R, Berthoz A. Inertial, substratal and landmark cue control of hippocampal CA1 place cell activity. Eur J Neurosci. 1995;7:2206–2219. doi: 10.1111/j.1460-9568.1995.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Wiener SI, Paul CA, Eichenbaum H. Spatial and behavioral correlates of hippocampal neuronal activity. J Neurosci. 1989;9:2737–2763. doi: 10.1523/JNEUROSCI.09-08-02737.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Witter MP. A survey of the anatomy of the hippocampal formation, with emphasis on the septotemporal organization of its intrinsic and extrinsic connections. Adv Exp Med Biol. 1986;203:67–82. doi: 10.1007/978-1-4684-7971-3_5. [DOI] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Hippocampal formation. In: Paxinos G, editor. The Rat Nervous System. 3rd edn. San Diego, CA: Academinc Press; 2004. [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AH. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P. The role of neuronal synchronization in selective attention. Curr Opin Neurobiol. 2007;17:154–160. doi: 10.1016/j.conb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P, Mitra PP, Desimone R. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature. 2006;439:733–736. doi: 10.1038/nature04258. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Eichenbaum H. The global record of memory in hippocampal neuronal activity. Nature. 1999;397:613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Zinyuk L, Kubik S, Kaminsky Y, Fenton AA, Bures J. Understanding hippocampal activity by using purposeful behavior: place navigation induces place cell discharge in both task-relevant and task-irrelevant spatial reference frames. Proc Natl Acad Sci U S A. 2000;97:3771–3776. doi: 10.1073/pnas.050576397. [DOI] [PMC free article] [PubMed] [Google Scholar]