Abstract
Two cellular proteins, stromal interaction molecule 1 (STIM1) and Orai1, are recently discovered essential components of the Ca2+ release activated Ca2+ (CRAC) channel. Orai1 polypeptides form the pore of the CRAC channel, while STIM1 plays the role of the endoplasmic reticulum Ca2+ sensor required for activation of CRAC current (ICRAC) by store depletion. It is not known, however, if the role of STIM1 is limited exclusively to Ca2+ sensing, or whether interaction between Orai1 and STIM1, either direct or indirect, also defines the properties of ICRAC. In this study we investigated how the relative expression levels of ectopic Orai1 and STIM1 affect the properties of ICRAC. The results show that cells expressing low Orai1 : STIM1 ratios produce ICRAC with strong fast Ca2+-dependent inactivation, while cells expressing high Orai1 : STIM1 ratios produce ICRAC with strong activation at negative potentials. Moreover, the expression ratio of Orai1 and STIM1 affects Ca2+, Ba2+ and Sr2+ conductance, but has no effect on the current in the absence of divalent cations. The results suggest that several key properties of Ca2+ channels formed by Orai1 depend on its interaction with STIM1, and that the stoichiometry of this interaction may vary depending on the relative expression levels of these proteins.
A two decade long search for the molecular components of store-operated Ca2+ entry (SOCE) and Ca2+ release activated Ca2+ (CRAC) channel in particular has been rewarded by the discovery of two cellular proteins, stromal interaction molecule 1 (STIM1) and Orai1 (Liou et al. 2005; Roos et al. 2005; Zhang et al. 2005; Feske et al. 2006; Vig et al. 2006b). STIM1 is localised both on the plasma membrane and the endoplasmic reticulum (ER) (Manji et al. 2000; Liou et al. 2005; Spassova et al. 2006). STIM1 that is expressed in the ER plays the role of the Ca2+ sensor in the ER lumen, while Orai1 is expressed on the plasma membrane and contributes to the Ca2+ selective pore of the channel (Prakriya et al. 2006; Taylor, 2006; Yeromin et al. 2006; Putney, 2007). Knockdown of either STIM1 or Orai1 by siRNA leads to a suppression of the native ICRAC in hematopoietic and other cell types, and a missense mutation in the gene encoding Orai1 has been linked to one form of hereditary severe combined immune deficiency (SCID) syndrome, associated with a reduced SOCE in lymphocytes (Roos et al. 2005; Feske et al. 2006; Litjens et al. 2007; Luik & Lewis, 2007). Ectopic expression of either STIM1 or Orai1 alone has little effect on the amplitude of the native ICRAC, but ectopic expression of both proteins together produces a large store-operated Ca2+ current with properties very similar to those of ICRAC (Roos et al. 2005; Peinelt et al. 2006; Soboloff et al. 2006; Vig et al. 2006b).
Present understanding of the SOCE mechanism suggests that depletion of intracellular Ca2+ stores causes STIM1 to accumulate in junctional ER, which is located in close proximity to the plasma membrane, while Orai1 accumulates in the areas of plasma membrane apposed STIM1 puncta (Luik et al. 2006; Varnai et al. 2007). Interaction between STIM1 and Orai1, either direct through cytoplasmic coiled-coil regions, or indirect through other binding partners results in activation of Ca2+ entry (Huang et al. 2006; Li et al. 2007; Varnai et al. 2007; Muik et al. 2008). Accumulation of STIM1 into puncta near the plasma membrane is essential for activation of the current through the channels formed by the Orai1 peptides, and reversal of STIM1 localisation by either replenishment of Ca2+ stores, or pharmacologically, terminates Ca2+ entry (Smyth et al. 2008).
Site-directed mutagenesis has been used to confirm basic functions and to localise some functionally important regions of STIM1 and Orai1. Several mutations in the putative EF-hand Ca2+-sensing domain of STIM1 have been shown to produce constitutively active Ca2+ currents, confirming that STIM1 is the Ca2+ sensor that communicates the filling state of Ca2+ stores to the plasma membrane (Zhang et al. 2005; Spassova et al. 2006). The observation that mutations of several acidic residues in Orai1 result in significant changes in the selectivity of Orai1/STIM1 mediated current supported the notion that Orai1 forms the pore of the CRAC channel (Prakriya et al. 2006; Vig et al. 2006a; Yeromin et al. 2006). Recent investigations of the stoichiometry of the Orai1 pore and stoichiometry of interaction between Orai1 and STIM1 suggested that the pore of the channel is formed by two Orai1 dimers, while two STIM1 peptides are required for activation of one channel (Mignen et al. 2008; Ji et al. 2008; Penna et al. 2008). It is not yet clear, however, whether 4 Orai1 : 2 STIM1 is the only stoichiometry that produces a functional channel, and whether STIM1, apart from being Ca2+ sensor in the ER, plays any role in defining the properties of the Orai1 mediated current.
In the present study we expressed Orai1 and STIM1 at various ratios in a heterologous expression system and investigated the properties of the resulting store-operated currents. Transfection of H4IIE liver cells with STIM1 and Orai1 plasmids mixed in different proportions revealed that cells transfected with an excess of STIM1 were more likely to express currents showing fast Ca2+-dependent inactivation, while cells transfected with an excess of Orai1 produced currents that showed little inactivation, or even activation at negative potentials. Furthermore, expression ratios of Orai1 and STIM1 affected Ca2+, Ba2+ and Sr2+ conductance and potentiation of the currents by 2-APB. This suggests that some key properties of Orai1/STIM1 mediated currents that have been used to identify ICRAC in the past, including the kinetics of fast Ca2+ dependent inactivation, arise from interaction between STIM1 and Orai1 and can not be attributed to Orai1 alone.
Methods
Cell culture and transfections
H4IIE (ATCC CRL 1548) cells and HEK293 cells were cultured at 37°C in 5% (v/v) CO2 in air in Dulbecco's modified Eagle's medium (DMEM) supplemented with 100 μm minimal essential medium (MEM) non-essential amino acids, 2 mm l-glutamine and 10% (v/v) fetal bovine serum (FBS) (complete DMEM). To express Orai1 and STIM1 proteins, H4IIE cells growing on glass coverslips at a 10–20% density were transfected using PolyFect transfection reagent (Qiagen, Germany) according to the manufacturer specifications.
Subcloning of STIM1 and Orai1
Full-length cDNAs for hOrai1 (NM_032790) and hSTIM1 (NM_003156) were purchased from OpenBioSystems (Huntsville, AL, USA). The Orai1 and STIM1 open reading frames were then PCR amplified and subcloned into the expression vector pCMV-Sport6 (OpenBioSystems) and the green fluorescent protein (GFP)-coexpresssing vector pAdTrack-CMV (a gift from Drs Lei Zhang and S. Brookes, Flinders University, Adelaide). The GFP fused Orai1 and STIM1 were expressed from constructs pEX-GFP-Myc-Orai1 (a gift from Prof. R. Lewis and Dr. R. Dolmetsch, Stanford University, USA) and pApuro-GFP-STIM1 (a gift from Dr. T. Kurosaki, Research Centre for Allergy and Immunology, Kanagawa, Japan), respectively. Integrity of the constructs was verified by DNA sequencing.
Western blotting
HEK293 cells transfected with pEX-GFP-Myc-Orai1 and pApuro-GFP-STIM1 were harvested and incubated on ice for 30 min in the lysis buffer containing 150 mm NaCl, 50 mm Tris-HCl (pH 7.4), 1 mm EDTA (pH 8.2), 0.25% sodium deoxycholate, 1% NonIdet P-40, 0.1% SDS and 0.1% protease inhibitor cocktail (Sigma). The concentration of crude proteins was measured using the BCA Protein Determination Kit (Sigma). Equal amounts of protein were separated on 10% SDS-PAGE and transferred to nitrocellulose membranes. The blots were then blocked for 1 h in Tris-buffered saline with Tween-20 (TBST) buffer (150 mm NaCl, 25 mm Tris, pH 7.4, 0.1% Tween-20) containing 5% non-fat milk powder and incubated in the blocking solution for 1 h with the appropriate primary antibody, including anti-STIM1 monoclonal antibody (1 : 1000 diluted, BD Biosciences), anti-GFP polyclonal antibody Ab290 (1 : 10 000 diluted, Abcam) and anti-GAPDH (1 : 500 diluted, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). After four washes in TBST buffer and 1 h incubation with the secondary antibodies (horseradish peroxidase-conjugated anti-mouse IgG (1 : 5000 diluted, Sigma) or anti-Rabbit IgG (1 : 1000 diluted, Rockland Immunochemicals Inc., Gilbertsville, PA, USA)) the protein bands were visualised with an enhanced chemiluminescence detection system (Amersham Biosciences/GE Healthcare). The autoradiographs were scanned using a GS-800 densitometer (Bio-Rad Laboratories, Hercules, CA, USA) and the band intensities quantified using Quantity One software version 4.3.1 (Bio-Rad Laboratories).
Patch clamping
Whole-cell patch clamping was performed at room temperature using a computer-based patch-clamp amplifier (EPC-9, HEKA Electronics, Germany) and PULSE software (HEKA Elektronik, Lambrecht/Pfalz, Germany). The bath solution contained (mm): NaCl, 140; CsCl, 4; CaCl2, 10; MgCl2, 2; glucose 10; and Hepes, 10; adjusted to pH 7.4 with NaOH. The internal solution contained (mm): caesium glutamate, 130; CaCl2 5; MgCl2 5; MgATP 1; EGTA, 10; and Hepes, 10; adjusted to pH 7.2 with NaOH. The calculated internal free Ca2+ concentration was about 100 nm (EQCAL, Biosoft, Cambridge, UK). Depletion of the intracellular Ca2+ stores was achieved by addition of 20 μm InsP3 (Amersham) to the internal solution. Patch pipettes were pulled from borosilicate glass and fire-polished; pipette resistance ranged between 3 and 5 MΩ. Series resistance did not exceed 10 MΩ and was compensated by 50–70%. In order to monitor the development of ISOC, 100 ms voltage ramps between −138 and +102 mV were applied every 2 s, starting immediately after achieving the whole-cell configuration. Acquired currents were filtered at 2.7 kHz and sampled at 10 kHz. All voltages shown have been corrected for the liquid junction potential of −18 mV between the bath and electrode solutions (estimated by JPCalc; Barry, 1994). The holding potential was −18 mV throughout. Cell capacitance was compensated automatically by the EPC9 amplifier.
Data analysis
Normalised instantaneous tail currents for voltage steps to −118 mV after test pulses in the range of −158 to 102 mV were used to produce the apparent open probability (Po) curves by fitting with the Boltzmann distribution with an offset of the form:
| (1) |
where Pmin is an offset, V is the membrane potential, V1/2 is the half-maximal activation potential, and k is the slope factor.
To determine the amplitude of the instantaneous tail currents and to minimise the error due to the cell capacitance, the tail currents were fitted with a single exponential function to the beginning of the −118 mV pulse.
To obtain bell-shaped apparent Po curves to fit some of the experimental data, we used a multiple of two Boltzmann distributions with independent parameters:
| (2) |
Where P1o (V) and P2o (V) are independent Boltzmann distributions of the form shown by eqn (1). Equation (1) assumes that there is a voltage-dependent gate present in the channel, while eqn (2) assumes the presence of two such voltage-dependent gates independent of each other. Nether of these statements is necessarily correct for the channel under study, as voltage dependence of the open probability does not necessarily imply presence of a voltage sensor in the channel. Nevertheless, these equations provide the means for quantitative description of the data.
Results
Ectopic expression of Orai1 and STIM1 in H4IIE liver cells
In order to investigate the properties of the heterologously expressed Orai1/STIM1 mediated current, we co-transfected plasmids containing STIM1 and Orai1 cDNA at a 1 : 1 ratio into H4IIE liver cells. To facilitate identification of the transfected cells, either the STIM1 or Orai1 plasmid contained cDNA encoding enhanced green fluorescent protein (eGFP). The time course of activation of Orai1/STIM1 mediated currents varied between transfected cells, with some cells showing no constitutively active currents and relatively slow activation by intracellular InsP3 (Fig. 1A, cell 1), while others showed very large initial currents that inactivated to a steady state (Fig. 1A, cell 4). The majority of transfected cells showed intermediate patterns, with some exhibiting a constitutively active current (Fig. 1A, cells 2 and 3). The presence of an apparently constitutively active current at the start of the recording could be a result of very fast activation, within 2–3 s of achieving whole cell configuration, due to InsP3 diffusing from the pipette. However, when transfected cells were patched with a pipette containing no InsP3 and Ca2+ buffered to 120 nm, a constitutively active current was still present in a high proportion of cells (∼30%, not shown), suggesting that fast diffusion of InsP3 is an unlikely explanation. Similar constitutively active Orai1/STIM1 currents were reported in a recent study (Zhang et al. 2008).
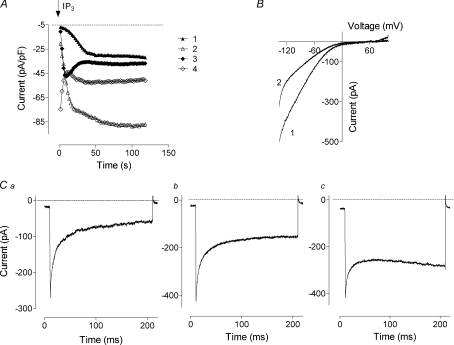
Figure 1. Overexpression of STIM1 and Orai1 in H4IIE cells.
A, examples of development of the Orai1/STIM1 mediated current. Each point represents the amplitude of the current at −118 mV measured from the responses to voltage ramps ranging from −138 mV to 102 mV and applied every 2 s. B, examples of the I–V plots obtained from cells expressing both STIM1 and Orai1. C, examples of the currents obtained in response to 200 ms steps to −138 mV. Traces were taken 40–60 s after obtaining whole-cell configuration when the amplitude of the current reached steady-state. Note significant variations in current kinetics. Cells were transfected with Orai1/STIM1 in a molar ratio of 1 : 1. Leakage was determined by applying 50 μm 2-APB at the end of the experiment and was corrected for in panels B and C, but not A.
The I–V plots in all transfected cells showed strong inward rectification and very positive reversal potential, similar to that reported in other studies with ectopic expression of Orai1 and STIM1 (Peinelt et al. 2006; Soboloff et al. 2006). The shape of I–V plots varied between cells, with some showing stronger inward rectification at potentials below −100 mV than others (Fig. 1B).
Further experiments showed that the variation in the shape of the I–V plots recorded from different cells was caused by the variations in the fast Ca2+-dependent inactivation of the currents at negative potentials. Thus we observed cells with very strong fast Ca2+ dependent inactivation (Fig. 1Ca), cells with very little or no inactivation (Fig. 1Cc), and all stages in between (Fig. 1Cb). Such variations in the kinetics of Orai1/STIM1 mediated currents suggest that fast Ca2+ dependent inactivation is not an intrinsic property that can be ascribed to Orai1 alone, otherwise it would be very similar in all transfected cells. Rather, the results suggest that inactivation arises from interaction of Orai1 with some other protein in the cell, the amount of which varies from cell to cell. The obvious candidate for such a protein that interacts with Orai1 and changes the kinetics of the current is STIM1.
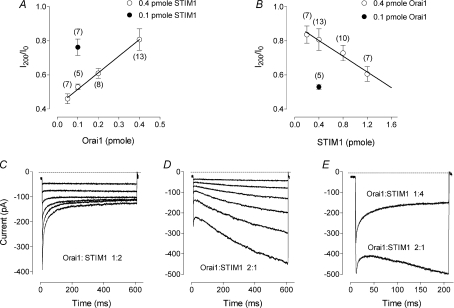
To investigate this possibility we varied the ratio between the plasmids in the transfection mixture in order to change the relative amounts of expressed Orai1 and STIM1 proteins in the cell. First, the amount of STIM1 plasmid in the transfection mixture was kept constant at 0.4 pmol and the amount of the plasmid containing Orai1 varied between 0.05 and 0.8 pmol. Subsequently, the amount of Orai1 plasmid in the transfection mixture was kept constant at 0.4 pmol and the amount of the plasmid containing STIM1 varied between 0.2 and 1.2 pmol. The volume of the transfection mixture was kept constant in all transfections. To compare inactivation of Orai1/STIM1 currents recorded from different cells, similar to those shown in Fig. 1C, we normalised amplitude of the current at the end of a 200 ms, −100 mV pulse (I200) to the amplitude of the current at the beginning of the pulse (I0). In cells transfected with 0.4 pmol STIM1 and 0.4 pmol Orai1 plasmids (1 : 1 ratio) the average extent of inactivation (I200/I0) was 0.80 ± 0.06 (n= 13) (Fig. 2A), although inactivation varied significantly between individual cells. With decreasing concentration of Orai1 plasmid in the transfection mixture, the inactivation became more pronounced and the variability between cells reduced. At the ratio of 1 : 8 (0.05 pmole Orai1/0.4 pmol STIM1) all cells produced inactivating currents with an average I200/I0 of 0.46 ± 0.03 (n= 7) (Fig. 2A). Similarly, the inactivation became more pronounced when the concentration of Orai1 plasmid was kept constant and the concentration of STIM1 plasmid increased (Fig. 2B). Comparison of the results obtained with different amounts of each plasmid revealed that the extent of inactivation depended on the ratio between the amounts of the plasmids, but not on their absolute amounts (Fig. 2A, B).
Figure 2. The kinetics of the Orai1/STIM1 mediated current under different transfection conditions.
A, dependence of the inactivation at negative potentials on the amount of the Orai1 plasmid. Each point represents the relative amplitude of the Orai1/STIM1 mediated current at the end of the 200 ms step to −118 mV (I200) normalised to the peak current at the beginning of the step (I0). Open circles represent averaged data for the 0.4 pmol of STIM1. For comparison, the averaged data for 0.1 pmol STIM1 and 0.1 pmol Orai1 is presented (filled circle). Number of cells is indicated in brackets. Note that the extent of the current inactivation in the cells transfected with 0.1 pmol STIM1 and 0.1 pmol Orai1 is not different from that in cells transfected with 0.4 pmol STIM1 and 0.4 pmol Orai1 (P= 0.6). Continuous line represents linear regression for the data obtained with the range of Orai1 concentrations and 0.4 pmol STIM1 (r2= 0.48; P < 0.0001). B, dependence of the inactivation at negative potentials on the amount of the STIM1 plasmid. Continuous line represents linear regression for the data obtained with the range of STIM1 concentrations and 0.4 pmol Orai1 (r2= 0.98; P < 0.01). C, example of the currents recorded from a cell transfected with 1 Orai1/2 (STIM1+GFP) mixture. D, example of the currents recorded from a cell transfected with 2 (Orai1+GFP)/1 STIM1 mixture. Current traces were obtained in response to 600 ms steps ranging from −138 mV to −38 mV in 20 mV increments. E, example of the currents obtained in HEK293 cells using similar transfection mixtures.
In these experiments, we have found that more consistent results were obtained if the plasmid containing the GFP sequence was in excess. Therefore, in all subsequent experiments we transfected cells with mixtures of 1 : 2 or 1 : 4 Orai1/(STIM1 + GFP) and 2 : 1 or 4 : 1 (Orai1 + GFP)/STIM1 to investigate the properties of inactivating and non-inactivating currents, respectively. Examples of such currents are shown in Fig. 2C and D. With longer hyperpolarising pulses, most cells transfected with an excess of Orai1 showed strongly activating currents at negative potentials (Fig. 2D).
We have shown previously that H4IIE cells express ISOC with properties virtually identical to those of ICRAC in hematopoietic cells (Litjens et al. 2004; Aromataris et al. 2008). The amplitude of the native ISOC in H4IIE cells (∼2.5 pA pF−1 at −118 mV) is at least severalfold smaller than the amplitude of the currents mediated by ectopically expressed Orai1/STIM1 (15–60 pA pF−1). Therefore, the presence of native STIM1 and Orai in H4IIE cells was unlikely to have a significant impact on the results. Nevertheless, to investigate the possibility that the kinetics of the currents mediated by ectopically expressed Orai1/STIM1 was influenced by the presence of the endogenous ISOC or some other proteins in H4IIE cells we used HEK293 cells, which have very small native ISOC (Mercer et al. 2006; Soboloff et al. 2006). Similarly to H4IIE cells, HEK293 cells produced inactivating currents when transfected with an excess of STIM1 and activating currents when transfected with an excess of Orai1 (Fig. 2E). Furthermore, results similar to those shown in Fig. 2B, C and D were obtained using plasmids containing GFP fused Orai1 and STIM1 (GFP-Orai1 and GFP-STIM1, not shown).
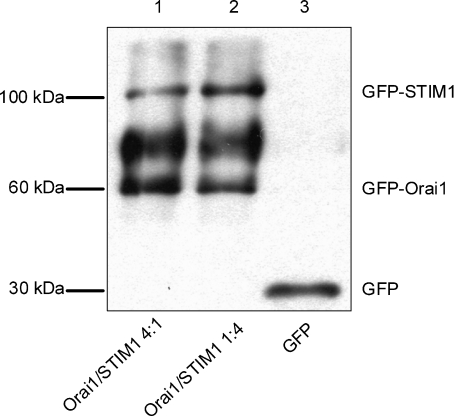
In the next set of experiments we transfected HEK293 cells with GFP-STIM1 and GFP-Orai1, and used Western blots probed with anti-GFP to estimate the relative amounts of the STIM1 and Orai1 proteins expressed under the transfection conditions used in patch clamping. GFP-STIM1 appeared on a Western blot as a clear single band above 100 kDa (Fig. 3). The same single band was obtained if we used anti-STIM1 (not shown). GFP-Orai1, however, appeared as a double band above 60 kDa (Fig. 3). The wide band of higher molecular weight for GFP-Orai1 was likely to be the result of post-translational modification of Orai1 (glycosylation) (Gwack et al. 2007). On average, the intensities of the bands corresponded well with the amounts of the transfected plasmids. For the 4 Orai1 : 1 STIM1 transfection (Fig. 3, first lane) the intensity ratio between the GFP-Orai1 single band of lower molecular weight and the GFP-STIM1 band was 5.75 ± 0.47 (n= 3), while for the 1 Orai1 : 4 STIM1 transfection (Fig. 3, second line) this ratio was 0.48 ± 0.01 (n= 3).
Figure 3. Western blotting of the expressed GFP-labelled Orai1 and STIM1.
Each lane was loaded with lysates (2 μg of protein) of HEK293 cells transfected with GFP-Orai1 and GFP-STIM1 containing plasmids at a molar ratio of 4 : 1 (lane 1); GFP-Orai1 and GFP-STIM1 containing plasmids at a molar ratio of 1 : 4 (lane 2); or GFP alone containing plasmid (lane 3). The blot was probed with anti-GFP antibody. The relative amounts of the expressed Orai1 and STIM1 proteins in the same transfection and between transfections were estimated by measuring optical densities of the corresponding bands (n= 3).
A fourfold increase in the amount of a plasmid in the transfection mixture resulted in 3.65 ± 0.13 and 3.21 ± 0.27 (n= 3)-fold increase of GFP-STIM1 and GFP-Orai1 expression respectively (Fig. 3). Similar results for STIM1 were obtained when anti-STIM1 was used instead of anti-GFP (not shown). These results demonstrate that variations in the amounts of the STIM1 and Orai1 containing plasmids in the transfection mixtures were closely followed by the changes in the expression levels of the corresponding proteins.
Ca2+ dependence of the amplitude and kinetics of the Orai1/STIM1 mediated currents
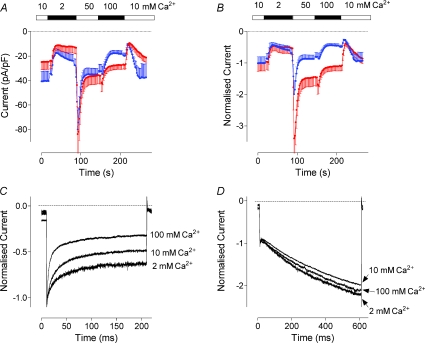
Reduction of the Ca2+ concentration in the bath solution from 10 to 2 mm resulted in the reduction of the amplitude by about 55% for both activating (4 : 1 Orai1/STIM1 ratio) and inactivating (1 : 4 Orai1/STIM1 ratio) Orai1/STIM1 mediated current (Fig. 4A and B). Increase of the Ca2+ concentration to 50 mm caused a sharp increase in the amplitude which was followed by an exponential decline. A further increase to 100 mm resulted in a small potentiation and further decline in the amplitude. Upon return to 10 mm Ca2+ the amplitude of the current was reduced and then slowly returned to the initial level. Qualitatively, dependence of the amplitude of inactivating and activating currents on Ca2+ concentration was similar and could be explained by slow feedback inhibition of the channel by Ca2+ entering the cell. Similar slow Ca2+-dependent inhibition has been previously described for ICRAC in lymphocytes (Zweifach & Lewis, 1995b). Quantitatively, however, the steady state amplitudes of the activating current in 50 mm and 100 mm Ca2+ were significantly larger than that in 10 mm Ca2+; while the steady state amplitudes of the inactivating current in 50 mm and 100 mm Ca2+ were significantly lower (Fig. 4A and B). The initial potentiation, both relative and absolute, of the current by higher Ca2+ concentrations was also significantly stronger for activating currents.
Figure 4. Dependence of the Orai1/STIM1 current on the external Ca2+ concentration.
A, amplitude of the Orai1/STIM1 mediated current at −118 mV at different Ca2+ concentrations. Data were obtained in cells transfected with Orai1 and STIM1 plasmids at a molar ratio of either 4 : 1 (blue trace) or 1 : 4 (red trace). Each point represents the amplitude of the current at −118 mV measured from the responses to voltage ramps ranging from −138 mV to 102 mV and applied every 2 s. Zero-time point on panels A and B corresponds to 60 s after obtaining whole cell configuration. B, the same data as in panel A normalised to the current amplitude in 10 mm Ca2+. C and D, dependence of current kinetics on the external Ca2+ concentration. All traces are recorded in response to voltage steps to −118 mV and normalised to the current amplitude at the beginning of the pulse. Traces were taken 30–40 s after changing Ca2+ concentration in the bath when the amplitude of the current reached steady-state (see panel A).
In the next experiment we compared Ca2+ dependence of the currents on a much shorter time scale. As expected, the kinetics and the extent of the fast Ca2+-dependent inactivation of the currents recorded from cells transfected with an excess of STIM1 were strongly dependent on the external Ca2+ concentration, similarly to that of native ISOC in H4IIE cells or ICRAC in hematopoietic cells (Fig. 4C) (Zweifach & Lewis, 1995a; Litjens et al. 2004). At higher Ca2+ concentrations the inactivation was faster and more complete. In contrast, the kinetics of activating currents recorded from cells transfected with an excess of Orai1 showed no dependence on the external Ca2+ concentration between 2 and 100 mm (Fig. 4D).
Selectivity for Ba2+ and Sr2+
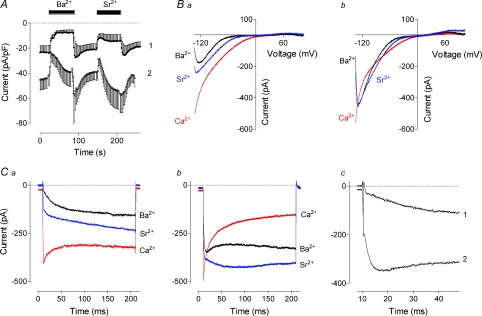
In cells transfected with an excess of Orai1 (4 : 1 Orai1/STIM1 ratio) that produced activating currents, replacement of Ca2+ with Ba2+ or Sr2+ in the bath solution led to a decrease of the current amplitude. On average, at −100 mV, Ba2+ current was ∼70% smaller than the Ca2+ current. Current carried by Sr2+ at −100 mV was ∼35% smaller than Ca2+ current (Fig. 5A). In the cells transfected with an excess of STIM1 (1 : 4 Orai1/STIM1 ratio) and producing inactivating currents, replacement of Ca2+ with Ba2+ or Sr2+ in the bath solution led to instantaneous reduction in the current amplitude followed by its gradual increase. On average, at −100 mV, Ba2+ current was just 10% smaller than the Ca2+ current, while current carried by Sr2+ at −100 mV was ∼40% larger than Ca2+ current (Fig. 5A). The shape of the I–V plots obtained in response to voltage ramps in the presence of Ba2+ and Sr2+ suggested a reduced open probability of the channel at holding potential and activation of the current by hyperpolarisation (Fig. 5B). This was confirmed by applying voltage steps to −100 mV (Fig. 5C). Replacement of Ca2+ with Ba2+ or Sr2+ in cells expressing either inactivating or activating Ca2+ currents produced currents activated by hyperpolarisation. The kinetics of the current activation at −100 mV in the presence of Ba2+ was significantly faster in cells with inactivating Ca2+ currents (Fig. 5C).
Figure 5. Effects of Ba2+ and Sr2+ on the Orai1/STIM1 currents obtained under different transfection conditions.
A, dependence of the amplitude of the Orai1/STIM1-mediated current at −118 mV on Ba2+ and Sr2+. Data were obtained in cells transfected with Orai1 and STIM1 plasmids at a molar ratio of either 4 : 1 (trace 1) or 1 : 4 (trace 2). Each point represents the amplitude of the current at −118 mV measured from the responses to voltage ramps ranging from −138 mV to 102 mV and applied every 2 s. Zero-time point corresponds to 60 s after achieving whole cell. B, representative I–V plots of the data shown on panel A. a corresponds to trace 1, and b corresponds to trace 2 on panel A. C, representative current traces in response to steps to −118 mV of the data shown on panel A. a corresponds to trace 1, and b corresponds to trace 2 on panel A. c, the same Ba2+ traces as shown on panels a and b but on a shorter time scale. Traces shown on panels B and C were taken 40–60 s after applying Ba2+ or Sr2+ in the bath.
Voltage dependence of open probability
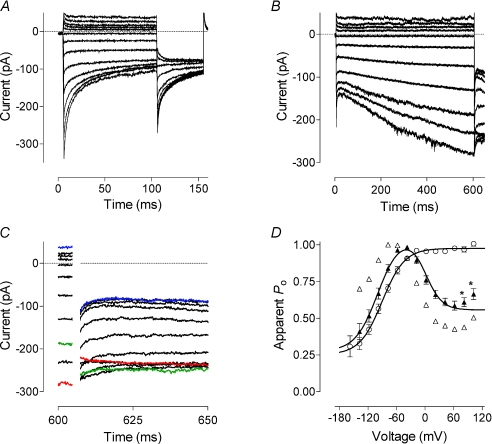
The change in the current kinetics from inactivating to activating suggests a change in the voltage dependence of the open probability of the channel. To quantify the relative open probability of the expressed channels under different conditions we used peak tail currents at −100 mV (see Methods, Fig. 6). In cells transfected with an excess of STIM1 and showing inactivating Ca2+ currents, the maximum open probability was at 0 mV and above, while the relative minimum open probability saturated at about 0.25 at potentials below −140 mV (Fig. 6A). The normalised amplitude of the peak tail currents plotted against voltage could be fitted with a single Boltzmann function (see Methods). Increasing the length of the voltage steps from 100 ms to 600 ms in these cells did not reveal any other type of voltage dependence of the current and did not shift the Boltzmann curve (not shown). The half-maximum activation voltage was −72 mV and the slope of the curve corresponded to a gating charge of approximately −1.
Figure 6. Voltage dependence of the Orai1/STIM1 mediated Ca2+ current.
A, current traces obtained in response to 100 ms voltage steps ranging from −158 to 102 mV in 20 mV increments followed by a step to −118 mV. H4IIE cells were transfected with Orai1 and STIM1 at a 1 : 4 molar ratio. B, current traces obtained in response to 600 ms voltage steps ranging from −138 to 102 mV in 20 mV increments followed by a step to −118 mV. H4IIE cells were transfected with Orai1 and STIM1 at a 4 : 1 molar ratio. C, tail currents shown on panel B on a shorter time scale. D, apparent open probability of the Orai1 channel obtained by normalising tail currents shown on panels A and B. Open circles represent averaged data (n= 6) obtained in cells expressing inactivating currents. Data from inactivating currents were fitted with a single Boltzmann function (eqn (1), Methods). Filled triangles represent averaged data (n= 9) obtained in cells expressing activating currents. Data from activating currents were fitted with a multiple of two Boltzmann functions (eqn (2), Methods). Data points marked with asterisks were excluded from fitting. Open triangles represent the data obtained from cell shown on panel B.
In cells expressing activating Ca2+ currents, the amplitude of the peak tail currents at −100 mV showed complex dependence on the voltage of the preceding steps. In general, relative open probability of activating currents showed bell-shaped voltage dependence between −120 and 80 mV with a maximum at about −30 mV (Fig. 6B–D). The voltage dependence of the open probability between −140 mV and −20 mV was very similar to that of the inactivating Ca2+ currents and presumably was due to the same gating process, while the open probability between −20 and 80 mV had the opposite voltage dependence (Fig. 6C). The data points could be well fitted with a multiple of two independent Boltzmann functions (eqn (2), Methods). Slight upward deviation of the points obtained after the steps to 100 and 120 mV could be due to a poor fit of very fast tail currents, or due to the activity of some native channels present in H4IIE cells at these potentials.
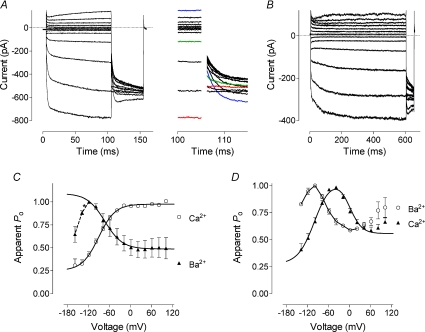
In the presence of Ba2+, voltage dependence of the inactivating currents was reversed (Fig. 7A and C). The data points between −100 and 120 mV could be fitted with a single Boltzmann function with the half-maximum activation voltage of −63 mV and the slope corresponding to a gating charge of 1. There was, however, evidence of an opposite voltage dependence at potentials below −100 mV as open probability started to decrease after that point (Fig. 7C). It was likely that in a wider range of voltages the open probability would be described by a bell shaped curve; however, it was impossible to obtain a reasonable number of points below −100 mV for proper fitting.
Figure 7. Voltage dependence of the Orai1/STIM1 mediated current in the presence of Ba2+.
A, current traces obtained in cells expressing inactivating Ca2+ currents (Orai1 and STIM1 at a 1 : 4 molar ratio) in response to 100 ms voltage steps ranging from −158 to 102 mV in 20 mV increments followed by a step to −118 mV. Calcium in the bath solution was replaced with 10 mm Ba2+. Inset shows tail currents shown on panel A at a shorter time scale. B, current traces obtained in cells expressing activating Ca2+ currents (Orai1 and STIM1 at a 4 : 1 molar ratio) in response to 600 ms voltage steps ranging from −138 to 102 mV in 20 mV increments followed by a step to −118 mV. Ca2+ in the bath solution was replaced with 10 mm Ba2+. C, apparent open probability of the Orai1 obtained by normalising tail currents shown on panel A. Filled triangles represent averaged data (n= 4) obtained in the presence of Ba2+ in cells expressing inactivating currents. Data were fitted with a single Boltzmann function (eqn (1), Methods). Data points marked with asterisks were excluded from fitting. For comparison, data obtained in the same cells in the presence of Ca2+ are shown (open circles). D, apparent open probability of the Orai1 obtained by normalising tail currents shown on panel B. Open circles represent averaged data (n= 3) obtained in the presence of Ba2+ in cells expressing activating currents. For comparison, data obtained in the same cells in the presence of Ca2+ are shown (filled triangles).
In cells expressing activating Ca2+ currents, replacement of Ca2+ with Ba2+ did not change the shape of the voltage dependence of open probability, but shifted it to more hyperpolarising potentials by approximately 60 mV (Fig. 7B and D).
Selectivity in the absence of divalent cations
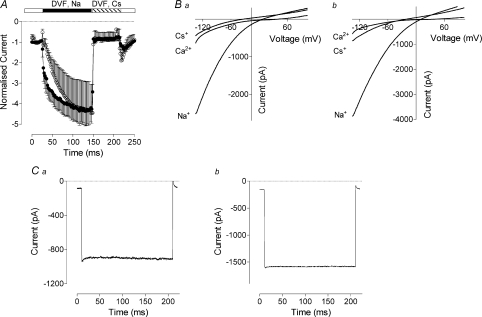
In the absence of divalent cations in the bath solution the pore of the channel formed by Orai1 peptides becomes permeable to monovalent cations (Mercer et al. 2006). In H4IIE cells expressing either activating or inactivating Orai1/STIM1 mediated Ca2+ currents, replacement of divalent cations in the bath solution with Na+ caused an approximate fourfold increase of the inward current amplitude (Fig. 8A, B). In the absence of divalent cations channels formed by Orai1 were more selective for Na+ than Cs+. The relative conductance for Na+ over Cs+ (GNa/GCs) was 4.9 ± 0.73 (n= 4) and 4.6 ± 0.57 (n= 6) for activating and inactivating currents, respectively (Fig. 8B). Application of the voltage protocol also used in Fig. 6A revealed that both activating and inactivating currents lost their voltage dependence in the absence of divalent cations (not shown), and that Na+ currents showed neither activation nor inactivation during steps to negative potentials (Fig. 8C).
Figure 8. Orai1/STIM1 mediated cation current in the absence of divalent cations.
A, normalised amplitude of the Orai1/STIM1 mediated currents in divalent cation free (DVF) solutions in the presence of Na+ or Cs+ in cells expressing inactivating (filled circles) or activating (open circles) Ca2+ currents. Zero-time point corresponds to 60 s after obtaining whole cell configuration. B, I–V plots of Ca2+, Na+ and Cs+ currents recorded in cells expressing inactivating (a) and activating (b) Orai1/STIM1 mediated Ca2+ currents. C, current traces recorded in response to 200 ms step to −118 mV in cells expressing inactivating (a) and activating (b) Orai1/STIM1 mediated Ca2+ currents in DVF solution containing Na+. Traces shown on panels B and C were taken 100–120 s after applying DVF solution in the bath.
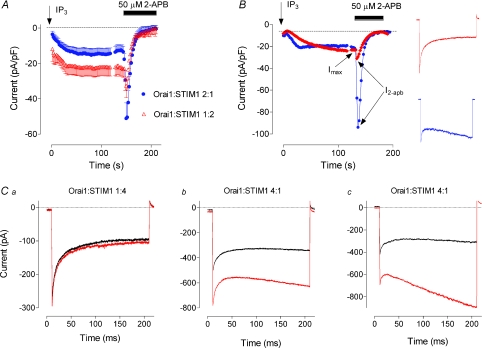
Differential effects of 2-APB
2-APB is a known inhibitor of endogenous ISOC and ectopically expressed Orai1/STIM1 mediated current (Gregory et al. 2001; Lis et al. 2007). It has complex effects on Orai2 and Orai3, but the mechanisms of these effects are not yet understood (Lis et al. 2007). In this section we investigated the effects of 2-APB on Orai1/STIM1 mediated currents of different kinetics. While 50 μm 2-APB blocked 100% of the Orai1/STIM1 mediated current irrespective of the current kinetics, the non-inactivating current showed a marked transient potentiation by 2-APB, while fast inactivating current showed much smaller or no potentiation by 2-APB (Fig. 9A, B). On average, potentiation of the amplitude of non-inactivating current by 50 μm 2-APB (Imax/I2-APB= 3.6 ± 0.65, n= 14) was 6 times stronger than for the inactivating current (Imax/I2-APB= 0.55 ± 0.13, n= 14, P < 0.0001). At the peak of potentiation by 2-APB, the kinetics of the strongly inactivating current was virtually unaffected (Fig. 9C). However, if the current showed little inactivation during 200 ms steps to −118 mV in the control solution, it showed strong activation at the peak of potentiation by 2-APB (Fig. 9C).
Figure 9. Effect of 2-APB on Orai1/STIM1 mediated current.
A, potentiation and block by 2-APB of Orai1/STIM1 mediated currents recorded under different transfection conditions. Each point represents the amplitude of the current at −118 mV measured from the responses to voltage ramps ranging from −138 mV to 102 mV and applied every 2 s. B, examples of 2-APB potentiation of size matched inactivating and non-inactivating Orai1/STIM1 currents. Inset shows current traces from the corresponding cells obtained before application of 2-APB. C, dependence of Orai1/STIM1 mediated current kinetics on 2-APB. Examples of current traces obtained in cells expressing different ratios of Orai1 and STIM1 in response to −118 mV steps before application of 2-APB (black) and at the peak of potentiation by 2-APB (red).
To exclude the possibility that 2-APB activates Orai1 directly in the absence of STIM1, we expressed these proteins separately and investigated the effect of 2-APB on the ISOC. Expression of Orai1 alone in H4IIE cells did not produce any store-operated current. Moreover, it resulted in a complete suppression of the native ISOC (not shown), as it does in HEK293 cells and rat basophilic leukaemia (RBL) cells (Soboloff et al. 2006). Application of 2-APB in H4IIE cells expressing Orai1 alone failed to enhance any current (not shown), suggesting that in the absence of sufficient amounts of STIM1 2-APB does not activate Orai1 even if the stores have been depleted.
Discussion
The main result of this study is that several key properties of Orai1/STIM1 mediated currents depend on the relative expression levels of STIM1 and Orai1 proteins. Increasing the Orai1 : STIM1 ratio in the transfection mixture from 1 : 4 to 4 : 1 drastically reduces the extent of fast Ca2+-dependent inactivation of the current, changes dependence of the whole cell Ca2+ conductance on external Ca2+, reduces relative Ba2+ and Sr2+ conductance, changes voltage dependence of the open probability, and increases potentiation by 2-APB.
There are several possible mechanisms which might explain the results of ectopic expression of Orai1 and STIM1. (i) The interaction between Orai1 and STIM1, either direct or through other binding partners, occurs at variable stoichiometry depending on their relative levels of expression. (ii) The stoichiometry of interaction between Orai1 and STIM1 is constant, but there are other binding partners that interact with STIM1 and Orai1. The amount of these other binding partners is limited. High concentrations of either STIM1 or Orai1 not partnered with each other reduce the availability of other binding partners for the functional Orai1/STIM1 complex. (iii) Overexpression of STIM1 and Orai1 modifies the junctional ER and therefore the structure of the microdomains where the functional interaction between them occurs.
Of these mechanisms, the second and the third seem less likely for the following reasons. If the properties of the Orai1/STIM1 currents were dependent on a binding partner present in the cell in limited amounts, which could bind to either STIM1 or Orai1, or both of them, the properties of the current would depend on the absolute amounts, rather then relative amounts, of the expressed proteins. We, however, observed clear dependence of the properties of the Orai1/STIM1 currents on the relative but not absolute expression levels of Orai1 and STIM1. It was possible to record currents with identical kinetics, either inactivating or activating, which differed by up to 10-fold in size, by maintaining the ratio of the plasmids in the transfection mixture but varying their absolute amounts. Furthermore, currents identical in size exhibited opposite voltage dependence if the ratios between Orai1 and STIM1 in the transfection mixture were reversed.
If the levels of expression of either STIM1 or Orai1 were affecting the properties of Orai1/STIM1 mediated current by affecting the properties of junctional ER (Luik et al. 2006), this effect would also depend on the absolute but not relative expression levels of these proteins. The only possibility for this mechanism to work would be if there was a particular component in junctional ER that regulated Orai1/STIM1 properties, which was affected by the expression of STIM1 and Orai1 in opposite ways. At present, there are no experimental data to support this idea.
Overall, of all possibilities, variable stoichiometry of binding between Orai1 and STIM1 seems most plausible. There is ample experimental support for the hypothesis that Orai1 and STIM1 interact to form functional complexes that mediate Ca2+ entry (Ji et al. 2008; Muik et al. 2008; Zhang et al. 2008). Whether there are any other binding partners in those complexes is still a mater of debate (Li et al. 2007; Varnai et al. 2007). Recent evidence suggests that ectopically expressed Orai1 form homotetramers and each tetramer interacts with two STIM1 peptides (Mignen et al. 2008; Penna et al. 2008; Zhang et al. 2008). Results presented by Zhang et al. (2008) are consistent with the notions that stoichiometry of Orai1/STIM1 interaction in the complex varies depending on the relative expression levels of these proteins, and that at least two different stoichiometries are possible.
Regardless of the mechanism, it is clear that relative expression levels of Orai1 and STIM1 affect regulation of the channel by Ca2+ coming through the pore. In the absence of divalent cations in the external medium there was no difference between Orai1/STIM1 mediated currents recorded in cells under different transfection conditions. Both inactivation and activation were absent, and the relative sizes of Na+ conductance were identical, as was the selectivity for Na+ over Cs+. This suggests that the relative expression levels of Orai1 and STIM1 do not affect the properties of the pore but rather modify the regulation of open probability of the channel by Ca2+.
The difference between inactivating and activating currents in regard to regulation by extracellular Ca2+ is evident at both fast and slow time scale. Qualitatively, dependence of the Ca2+ conductance on extracellular Ca2+ is very similar in cells expressing inactivating and activating Orai1/STIM1 currents. However, compared to cells expressing inactivating currents, cells expressing activating current show significantly larger Ca2+ currents at 50 and 100 mm external Ca2+, which is consistent with a reduction of feedback inhibition by Ca2+.
On a shorter time scale, as expected, kinetics of fast inactivation of Orai1/STIM1 mediated current in cells expressing an excess of STIM1 strongly depended on the external Ca2+ concentration between 2 mm and 100 mm. In contrast, in this range of concentrations, kinetics of activation of the current in cells expressing an excess of Orai1 was independent of extracellular Ca2+. However, the activation was removed in the absence of the divalent cations in the bath. This suggests that the site responsible for current activation in cells expressing an excess of Orai1 does depend on Ca2+, but at a much lower concentration range than the site responsible for fast Ca2+-dependent inactivation in cells expressing an excess of STIM1.
The fast Ca2+-dependent inactivation of the native ICRAC and the ectopically expressed Orai1/STIM1 current is thought to be due to Ca2+ binding to a regulatory site somewhere close to the internal mouth of the pore. Using general assumptions about Ca2+ diffusion in the cytoplasmic space it has been suggested that the Ca2+ binding site responsible for fast inactivation is likely to be 3–4 nm away from the intracellular mouth of the pore and therefore is likely to be a part of the channel itself (Zweifach & Lewis, 1995a). On the other hand, recent investigation of the properties of Orai1 pore mutants suggests that the role of the inactivation gate is played by residues forming the selectivity centre within the pore region of Orai1 (Yamashita et al. 2007; Spassova et al. 2008). At present it is not yet known if voltage dependence of the current is conferred by the voltage-dependent binding of Ca2+ to a regulatory site, or by voltage-dependent movement of a part of the Orai1/STIM1 complex with Ca2+ bound to it, or both. While this paper was under review, Park et al. (2009) produced convincing evidence that there is a direct binding between STIM1 and Orai1 (Park et al. 2009). These authors show that the so-called CRAC activation domain (CAD) of STIM1 binds to both C and N termini of Orai1 and activates ICRAC, and that tetramers of CAD bind to multiple sites on the CRAC channels and create large clusters of Orai1 (Park et al. 2009). Moreover, apparently, current activated by CAD lacks fast Ca2+-dependent inactivation, but inactivation is restored by increasing the length of C terminus of the peptide (Park et al. 2009). This supports the notion that the function of the Ca2+ binding site responsible for fast Ca2+-dependent inactivation depends on the interaction between STIM1 and Orai1. The results presented in this paper suggest that this interaction between STIM1 and Orai1, and therefore the properties of the regulatory Ca2+ binding site(s), or the conformational change within the Orai1/STIM1 complex following Ca2+ binding to this site, are affected by the relative expression levels of Orai1 and STIM1. With eight potential binding sites for STIM1 present in Orai1 tetramer, relative expression levels of Orai1 and STIM1 may affect the way the Orai1/STIM1 complex is formed, and therefore, the properties of the regulatory Ca2+ binding site. This can explain the observed complex dependence of the channel open probability on voltage and divalent cations.
Lack of inactivation in the presence of Ba2+ or Sr2+ reported here can be a result of a lower affinity of these cations to the Ca2+ binding site involved in inactivation, or their lower efficacy. Similarly, lack of inactivation in cells expressing an excess of Orai1 can be explained by lower affinity of this site for Ca2+, or its lower efficacy. Consistent with that, in the cells where Orai1/STIM1 mediated current showed little inactivation at negative potentials, Ba2+ had little effect on current kinetics and the apparent open probability curve, just shifting it to more negative potentials, while in cells expressing Orai1/STIM1 current showing strong inactivation, Ba2+ abolished most of the inactivation and revealed a different type of gating that opened the channel at negative potentials.
Interestingly, Ba2+ has been shown to have different effects on native ICRAC in different cell types. In RBL cells at potentials below −80 mV, Ba2+ current through CRAC channels was bigger than Ca2+ current, while in Jurkat cells Ba2+ current was significantly smaller at all potentials (Hoth, 1995). This difference in Ba2+ permeability led to a suggestion that there might be different subtypes of CRAC channels expressed in different cell types. Our data suggest that different cell types that express the same isoforms of Orai and STIM still can have ICRAC with different properties if the ratios of expression of Orai1 and STIM1 are different.
Another salient property of ICRAC is potentiation by lower and inhibition by higher concentrations of 2-APB (Prakriya & Lewis, 2001). It has been suggested that 2-APB potentiates ICRAC by increasing the number of active CRAC channels on the plasma membrane (Prakriya & Lewis, 2006). Potentiation by 2-APB of Orai1/STIM1 mediated currents has been reported in some over-expression studies but not in others (Mercer et al. 2006; Lis et al. 2007). Here we show that the extent of potentiation of the Orai1/STIM1 current by 2-APB depends on relative levels of expression of Orai1 and STIM1 and correlates with the kinetics of the current. As this potentiation is much greater when the size of the current is limited by the expression of STIM1, one can speculate that 2-APB increases the probability of STIM1 interacting with Orai1, thus increasing the number of active channels on the membrane. With Orai1 in excess this would favour formation of Orai1/STIM1 complexes with fewer STIM1 bound. Alternatively, 2-APB could facilitate opening of the pre-formed channels with fewer STIM1 in the complex. Both these notions are consistent with the observation that potentiation of the amplitude of the Orai1/STIM1 current by 2-APB in cells expressing an excess of Orai1 was accompanied by an increase of activating component of the current recorded at negative voltage steps. Such strong activation of the current at negative potentials could also be achieved in the absence of 2-APB in some cells where, apparently, expression of Orai1 relative to STIM1 was exceptionally high.
In conclusion, we have shown that relative expression levels of Orai1 and STIM1 affect the properties of the ectopically expressed CRAC channels – the fast Ca2+-dependent inactivation, relative Ba2+ conductance, and potentiation by 2-APB. These data provide an explanation for the previously observed variations in the properties of native ICRAC in different cell types, and for some apparent inconsistencies of over-expression studies where both inactivating and non-inactivating Orai1/STIM1 mediated currents were reported. We believe that relative expression levels of Orai1 and STIM1 affect the stoichiometry of their assembly, and therefore, the properties of the Ca2+ binding site responsible for inactivation.
Acknowledgments
This work has been supported by the Australian Research Council and NHMRC Australia.
Glossary
Abbreviations
- 2-APB
2-aminoethoxydiphenyl borate
- CAD
CRAC activation domain
- CRAC
Ca2+-release activated Ca2+
- DVF
divalent free
- eGFP
enhanced green fluorescent protein
- ER
endoplasmic reticulum
- ICRAC
Ca2+-release activated Ca2+ current
- Ins3P
inositiol 1,4,5-trisphosphate
- ISOC
store operated Ca2+ current
- I–V
current–voltage
- GFP
green fluorescent protein
- RBL
rat basophilic leukaemia
- SCID
severe combined immunodeficiency syndrome
- siRNA
short interfering RNA
- SOCE
store operated Ca2+ entry
- STIM1
stromal interaction molecule 1
Author contributions
N.S.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; T.L.: conception and design, collection and assembly of data; L.M.: collection and assembly of data, data analysis and interpretation; G.J.B.: conception and design, data interpretation and manuscript writing; G.Y.R.: conception and design, collection of data, data analysis and interpretation, manuscript writing. All authors contributed to final approval of the manuscript. The experiments were conducted in the Laboratory of Cellular Physiology, School of Molecular and Biomedical Science, University of Adelaide.
References
- Aromataris EC, Castro J, Rychkov GY, Barritt GJ. Store-operated Ca2+ channels and Stromal Interaction Molecule 1 (STIM1) are targets for the actions of bile acids on liver cells. Biochim Biophys Acta. 2008;1783:874–885. doi: 10.1016/j.bbamcr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Gregory RB, Rychkov G, Barritt GJ. Evidence that 2-aminoethyl diphenylborate is a novel inhibitor of store-operated Ca2+ channels in liver cells, and acts through a mechanism which does not involve inositol trisphosphate receptors. Biochem J. 2001;354:285–290. doi: 10.1042/0264-6021:3540285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-hora M, Neems DS, Hogan PG, Rao A. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007;282:16232–16243. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- Hoth M. Calcium and barium permeation through calcium release-activated calcium (CRAC) channels. Pflugers Arch. 1995;430:315–322. doi: 10.1007/BF00373905. [DOI] [PubMed] [Google Scholar]
- Huang GN, Zeng WZ, Kim JY, Yuan JP, Han LH, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, ICRAC and TRPC1 channels. Nat Cell Biol. 2006;8:1003–U1096. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- Ji W, Xu P, Li Z, Lu J, Liu L, Zhan Y, Chen Y, Hille B, Xu T, Chen L. Functional stoichiometry of the unitary calcium-release-activated calcium channel. Proc Nat Acad Sci U S A. 2008;105:13668–13673. doi: 10.1073/pnas.0806499105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZZ, Lu JZ, Xu PY, Xie XY, Chen LY, Xu T. Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J Biol Chem. 2007;282:29448–29456. doi: 10.1074/jbc.M703573200. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Do Heo W, Jones JT, Myers JW, Ferrell J, James E, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Zoller M, Fleig A, Penner R. CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr Biol. 2007;17:794–800. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litjens T, Harland ML, Roberts ML, Barritt GJ, Rychkov GY. Fast Ca2+-dependent inactivation of the store-operated Ca2+ current (ISOC) in liver cells: a role for calmodulin. J Physiol. 2004;558:85–97. doi: 10.1113/jphysiol.2004.065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litjens T, Nguyen T, Castro J, Aromataris EC, Jones L, Barritt GJ, Rychkov GY. Phospholipase C-gamma1 is required for the activation of store-operated Ca2+ channels in liver cells. Biochem J. 2007;405:269–276. doi: 10.1042/BJ20061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik RM, Lewis RS. New insights into the molecular mechanisms of store-operated Ca2+ signaling in T cells. Trends Mol Med. 2007;13:103–107. doi: 10.1016/j.molmed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji SS, Parker NJ, Williams RT, van Stekelenburg L, Pearson RB, Dziadek M, Smith PJ. STIM1: a novel phosphoprotein located at the cell surface. Biochim Biophys Acta. 2000;1481:147–155. doi: 10.1016/s0167-4838(00)00105-9. [DOI] [PubMed] [Google Scholar]
- Mercer JC, DeHaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, STIM1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignen O, Thompson JL, Shuttleworth TJ. Orai1 subunit stoichiometry of the mammalian CRAC channel pore. J Physiol. 2008;586:419–425. doi: 10.1113/jphysiol.2007.147249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, Kahr H, Madl J, Gruber H, Groschner K, Romanin C. Dynamic coupling of the putative coiled-coil domain of Orai1 with STIM1 mediates Orai1 channel activation. J Biol Chem. 2008;283:8014–8022. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJS, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna A, Demuro A, Yeromin AV, Zhang SL, Safrina O, Parker I, Cahalan MD. The CRAC channel consists of a tetramer formed by STIM-induced dimerization of Orai dimers. Nature. 2008;456:116–120. doi: 10.1038/nature07338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. Regulation of CRAC channel activity by recruitment of silent channels to a high open-probability gating mode. J Gen Physiol. 2006;128:373–386. doi: 10.1085/jgp.200609588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW. New molecular players in capacitative Ca2+ entry. J Cell Sci. 2007;120:1959–1965. doi: 10.1242/jcs.03462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JT, Dehaven WI, Bird GS, Putney JW., Jr Ca2+-store-dependent and -independent reversal of STIM1 localization and function. J Cell Sci. 2008;121:762–772. doi: 10.1242/jcs.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- Spassova MA, Hewavitharana T, Fandino RA, Kaya A, Tanaka J, Gill DL. Voltage gating at the selectivity filter of the Ca2+ release-activated Ca2+ channel induced by mutation of the Orai1 protein. J Biol Chem. 2008;283:14938–14945. doi: 10.1074/jbc.M702208200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassova MA, Soboloff J, He L-P, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natl Acad Sci U S A. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW. Store-operated Ca2+ entry: a STIMulating stOrai. Trends Biochem Sci. 2006;31:597–601. doi: 10.1016/j.tibs.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Varnai P, Toth B, Toth DJ, Hunyady L, Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 complex. J Biol Chem. 2007;282:29678–29690. doi: 10.1074/jbc.M704339200. [DOI] [PubMed] [Google Scholar]
- Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006a;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006b;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Navarro-Borelly L, McNally BA, Prakriya M. Orai1 mutations alter ion permeation and Ca2+-dependent fast inactivation of CRAC channels: evidence for coupling of permeation and gating. J Gen Physiol. 2007;130:525–540. doi: 10.1085/jgp.200709872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeromin AV, Zhang SYL, Jiang WH, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Kozak JA, Jiang W, Yeromin AV, Chen J, Yu Y, Penna A, Shen W, Chi V, Cahalan MD. Store-dependent and -independent modes regulating Ca2+ release-activated Ca2+ channel activity of human Orai1 and Orai3. J Biol Chem. 2008;283:17662–17671. doi: 10.1074/jbc.M801536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS. Rapid inactivation of depletion-activated calcium current (ICRAC) due to local calcium feedback. J Gen Physiol. 1995a;105:209–226. doi: 10.1085/jgp.105.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS. Slow calcium-dependent inactivation of depletion-activated calcium current. J Biol Chem. 1995b;270:14445–14451. doi: 10.1074/jbc.270.24.14445. [DOI] [PubMed] [Google Scholar]