Abstract
N-Methyl-d-aspartate (NMDA) receptors are widely studied because of their importance in synaptic plasticity and excitotoxic cell death. Here we report a novel method of potentiating NMDA receptors with fluorescence excited by blue (480 nm) light. In the presence of 300 nm of a (7-nitro-2,1,3-benzoxadiazol-4-yl) amino (NBD)-tagged neuroactive steroid carrier C2-NBD-(3α,5α)-3-hydroxypregnan-20-one (C2-NBD 3α5αP), responses of cultured hippocampal neurons to 10 μm NMDA were potentiated to 219.2 ± 9.2% of the baseline response (100%) by a 30 s exposure to 480 nm light. The potentiation decayed back to baseline with a time constant of 80.6 s. Responses to 1 μm and 100 μm NMDA were potentiated to 147.9 ± 9.6% and 174.1 ± 15.6% of baseline, respectively, suggesting that visible-light potentiation is relatively insensitive to NMDA concentration. Peak autaptic NMDA responses were potentiated to 178.9 ± 22.4% of baseline. Similar potentiation was seen with 10 μm NBD-lysine, suggesting that visible-light potentiation is not a steroid effect. Potentiation was also seen with a steroid analogue in which the NBD was replaced with fluorescein, suggesting that NBD is not the only fluorophore capable of supporting visible-light potentiation. UV light and redox potentiation of NMDA receptors largely occluded subsequent blue light potentiation (127.7 ± 7.4% and 120.2 ± 6.2% of baseline, respectively). The NR1a(C744A,C798A) mutant that is insensitive to redox and UV potentiation was also largely unaffected by visible-light potentiation (135.0 ± 10.0% of baseline). Finally, we found that the singlet oxygen scavenger furfuryl alcohol decreased visible-light potentiation. Collectively, these data suggest that visible-light potentiation of NMDA receptors by fluorescence excitation shares mechanisms with UV and redox potentiation and may involve singlet oxygen production.
N-Methyl-d-aspartate (NMDA) receptors are a subtype of glutamate-gated ion channels widely expressed in the central nervous system and vitally important to both synaptic plasticity and the excitotoxic cell death associated with multiple neurological disorders (Dingledine et al. 1999; Paoletti & Neyton, 2007). As a result, a great deal of effort has been devoted to better understanding the role and function of NMDA receptors. This focus has resulted in the identification of numerous NMDA antagonists, some of which have been developed as clinical agents. Despite this intense scrutiny, relatively few positive modulators have been identified, although NMDA potentiators have been shown to enhance long term potentiation (Sliwinski et al. 2004) and have drawn interest as cognitive enhancers (Flood et al. 1992; Akwa et al. 2001).
We previously reported that blue (480 nm) light potentiates GABAA receptors in the presence of C2-NBD 3α5αP, a (7-nitro-2,1,3-benzoxadiazol-4-yl) amino (NBD)-tagged neuroactive steroid based on the structure of the neurosteroid (3α,5α)-3-hydroxypregnan-20-one (3α5αP, allopregnanolone) (Eisenman et al. 2007). We demonstrated spatially selective receptor potentiation with these minimally disruptive light wavelengths that elicit fluorescence, suggesting that this compound is a useful experimental tool with possible clinical utility. Although C2-NBD 3α5αP was designed based on GABA-active neurosteroid structure, the photoactivation is apparently not steroid dependent, since other NBD-tagged compounds also elicit weak photoactivation. Visible-light potentiation in the presence of NBD-tagged compounds may therefore be relevant to other receptors besides GABAA receptors.
Here we report that 480 nm light in the presence of C2-NBD 3α5αP potentiates NMDA receptors. As in the case of GABAA receptors, visible-light potentiation does not appear to be steroid dependent, but steroids appear to serve as efficient carriers, presumably because of their hydrophobicity. At high concentrations other NBD-tagged compounds with unrelated structures also support visible-light potentiation. In addition, a steroid analogue in which the NBD group was replaced with fluorescein also supported visible-light potentiation. Visible-light potentiation is occluded by ultraviolet (UV) light potentiation and by redox potentiation and does not occur in recombinant NR1a/NR2B NMDA receptors containing a NR1a(C744A,C798A) mutant that is resistant to UV and redox modulation. Finally, visible-light potentiation is attenuated by the singlet oxygen scavenger furfuryl alcohol. We conclude that the mechanism of action of visible-light potentiation in the presence of C2-NBD 3α5αP is common to UV light and redox potentiation of NMDA receptors and may be mediated by generation of singlet oxygen.
Methods
Ethical approval
This work was performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and with the approval of the Washington University Animal Studies Committee.
Hippocampal microcultures
Primary microcultures of hippocampal cells were prepared from 1- to 3-day postnatal Sprague–Dawley rats (Mennerick et al. 1995). Briefly, isoflurane-anaesthetized rats were decapitated and the hippocampi removed. Hippocampi were cut into 500 μm-thick transverse slices. Single-cell suspensions were prepared with 1 mg ml−1 papain digestion in oxygenated Leibovitz L-15 medium, followed by mechanical trituration in modified Eagle's medium containing 5% horse serum, 5% fetal calf serum, 17 mm d-glucose, 400 μm glutamine, 50 U ml−1 penicillin and 50 μg ml−1 streptomycin. Cells were plated in the modified Eagle's medium at a density of 75 cells mm−2 onto 35 mm plastic culture dishes pre-coated with collagen microdroplets sprayed on a layer of 0.15% agarose. Cytosine arabinoside (5–10 μm) was added on the third day after plating to halt glial proliferation. Electrophysiology was performed 4–8 days following plating.
Cell culture and transfection
HEK 293 cells were grown in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum and 1 mm glutamine in 35 mm culture dishes. Cells were transfected with either wild-type or dithiothreitol (DTT)-insensitive mutant NR1a(C744A,C798A) subunits along with wild-type NR2B subunits using Lipofectamine2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. NR1a subunits were packaged in pRc/CMV vectors. NR2B subunits were packaged in a pcDNA1 vector. DsRed (Clontech Laboratories, Mountain View, CA, USA) was used as a positive transfection marker. Each dish was transfected with 0.34 μg NR1a, 1.01 μg NR2B and 0.05 μg DsRed cDNA. Following transfection, 300 μm ketamine was added to the medium to prevent excitotoxic cell death (Boeckman & Aizenman, 1996). Electrophysiology was performed 36–72 h following transfection.
Electrophysiology
Whole-cell recordings were performed using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA) interfaced to a Pentium-IV-based computer via a Digidata acquisition board (Axon Instruments). At the time of the experiments, the culture medium was replaced with an extracellular recording solution consisting of (in mm): 138 NaCl, 4 KCl, 0.5 CaCl2, 10 glucose, 10 Hepes (pH 7.25). In order to prevent excitotoxic damage to neurons between recordings, 10 μm d-2-amino-5-phosphonovalerate (d-APV) was also included in the recording solution. Recordings were at room temperature. The standard pipette solution contained (in mm): 130 caesium methanesulfonate, 4 NaCl, 0.5 CaCl2, 5 EGTA, 10 Hepes (pH 7.25). Exogenous drugs were applied with a multi-barrel pipette coupled to miniature solenoid valves that allowed rapid switching (∼100 ms on whole cells). d-APV was excluded from all solutions applied via the multi-barrel pipette. As illustrated in Fig. 1B, the experimental protocol included 5 s of baseline perfusion to remove APV locally prior to any drug application. For experiments on hippocampal neurons, 1 μm 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) and 10 μm bicuculline were included in all solutions to block AMPA and GABAA receptor-mediated synaptic currents. For the synaptic experiments in Fig. 2D, the calcium in the extracellular solution was increased to 2 mm and the caesium methanesulfonate in the internal solution was replaced with 140 mm potassium gluconate.
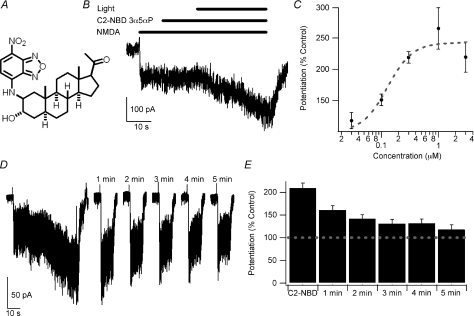
Figure 1. Blue-light potentiation of NMDA responses.
A, structure of C2-NBD 3α5αP. B, example of blue-light potentiation of the response to 10 μm NMDA with 300 nm C2-NBD 3α5αP. C, concentration–response curve of 0.03 μm (n= 5), 0.1 μm (n= 5), 0.3 μm (n= 58), 1 μm (n= 5) and 3 μm (n= 5) C2-NBD 3α5αP potentiation of 10 μm NMDA-induced currents. The dashed line represents a fit of the Hill equation with EC50= 0.13 μm and n= 2.0. Note that the fit was performed with the baseline fixed at 100% and that the fit is empirical only and does not necessarily imply that a typical drug–receptor interaction mediates the light-induced potentiation. D, the left portion of the panel illustrates blue-light potentiation using the same protocol depicted in panel B. The cell was then re-challenged with a 10 s application of 10 μm NMDA once every minute for 5 min. E, summary bar graph of potentiation immediately after light exposure and for every re-challenge (n= 8). Error bars represent s.e.m. Baseline response is 100% (dashed line). Note the slow decay of the potentiation. Responses following light exposure and at 1 and 2 min were significantly different from current immediately prior to activation of the light (repeated-measures ANOVA followed by Dunnett's test, P < 0.05). Recordings were performed with CsMeSO4 internal solution at −60 mV in cultured hippocampal neurons.
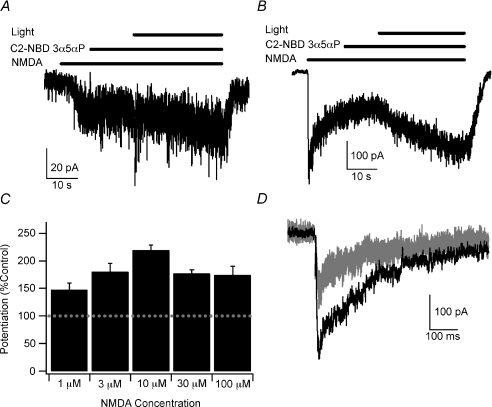
Figure 2. Blue-light potentiation of responses to 1–100 μm NMDA and synaptic NMDA responses.
A, example of blue-light potentiation of the response to 1 μm NMDA with 300 nm C2-NBD 3α5αP. B, example of blue-light potentiation of the response to 100 μm NMDA with 300 nm C2-NBD 3α5αP. C, summary bar graph of average potentiation with 1 μm, 3 μm, 10 μm, 30 μm and 100 μm NMDA in the presence of 300 nm C2-NBD 3α5αP. Error bars represent s.e.m. Baseline response is 100% (dashed line). Potentiated currents at each NMDA concentration were significantly different from the current prior to activation of the light (paired t test, P < 0.05; n= 5, 5, 58, 4 and 5, respectively). There was no statistically significant difference between NMDA concentrations (one-way ANOVA, P > 0.05). Recordings were performed with CsMeSO4 internal solution at −60 mV in cultured hippocampal neurons. D, example of blue-light potentiation of autaptic NMDA responses. Three baseline responses (superimposed grey traces) were recorded prior to a 30 s exposure to C2-NBD 3α5αP and blue light. Another autaptic response (black trace) was recorded immediately after washing out C2-NBD 3α5αP. Peak responses were potentiated to 178.9 ± 22.4% of baseline (P < 0.05, paired t test, n= 9). Stimulus artifact was blanked for clarity. Recordings were made in cultured hippocampal neurons at a holding potential of −60 mV with 2 mm CaCl2 in the external solution and with potassium gluconate replacing CsMeSO4 in the internal solution.
Photoactivation
For photoactivation, cells were epifluorescently illuminated using a metal halide lamp. Light was routed through Chroma filter set 41001 (Chroma Technology Corp., Rockingham, VT, USA), employing an HQ480 nm/40 nm excitation filter and an HQ535 nm/50 nm emission filter. Light then passed through a ×40, 0.6 NA objective mounted on a Nikon TE2000 inverted microscope to cells that were plated on a polystyrene culture dish (Falcon 353001). Using the ×40 objective and optics described above, light intensity was approximately 156 mW mm−2 (Eisenman et al. 2007). For UV potentiation light was applied as above, but was routed through a Semrock DAPI 5060A filter set (Semrock, Rochester, NY, USA) employing a 377 nm/50 nm excitation filter and a 447 nm/60 nm emission filter.
Data analysis and statistics
Data acquisition was performed with pCLAMP (Axon Instruments, CA, USA). Data analysis was performed using pCLAMP, Igor (Wavemetrics, Lake Oswego, OR, USA) and Excel (Microsoft, Redmond, WA, USA). Potentiation was calculated as the ratio of the current measured at the end of the light exposure compared to the current measured immediately before light exposure. For the synaptic experiments in Fig. 2D, three baseline responses were averaged and the average compared to the response immediately following light potentiation. Charge transfer was calculated by measuring the area under the autaptic response after subtracting the holding current. A double exponential (A0+A1e−t/τ1+A2e−t/τ2) was fitted to the decay phase of the autaptic response starting at the point where the response had decayed to 90% of the peak. The weighted time constant was calculated as a weighted sum of the two time constants from the double exponential fit (τw= (A1τ1+A2τ2)/(A1+A2). Data plotting was done with Igor. Data are presented in the text and figures as mean ± standard error of the mean (s.e.m.). Statistical differences were determined using Student's two-tailed t test or one-way analysis of variance followed by post hoc Student–Newman–Keuls test for comparisons between more than two groups. For the experiment in Fig. 1E, data were analysed with a repeated-measures analysis of variance followed by Dunnett's test. For all statistics, a P value less than 0.05 was considered significant.
Compounds
All commercially available drugs were from Sigma (St Louis, MO, USA) except NBD-lysine (Anaspec, San Jose, CA, USA). C2-NBD 3α5αP was synthesized by reacting 4-chloro-7-nitro-2,1,3-benzoxadiazole with (2β,3α,5α)-2-amino-3-hydroxypregnan-20-one. C2-fluorescein 3α5αP was synthesized by reacting fluoroscein-5-isothiocyanate (FITC isomer I) with (2β, 3α,5α)-2-amino-3-hydroxypregnan-20-one. Synthetic and spectroscopic details for C2-NBD 3α5αP and C2-fluorescein 3α5αP will be published elsewhere. Steroid stock solutions were prepared at 1–10 mm in DMSO, and diluted in the bath solution on the day of the experiment with final DMSO concentrations ≤ 0.1%.
Results
NMDA receptors are potentiated by blue light in the presence of fluorescent analogues of neuroactive steroids
In the process of developing NBD-tagged fluorescent analogues of the neuroactive steroid 3α5αP, we serendipitously discovered that the analogue C2-NBD 3α5αP (Fig. 1A) potentiates GABAA receptors when illuminated with 480 nm light (Eisenman et al. 2007). Control experiments demonstrated that C2-NBD 3α5αP had no effect on AMPA-mediated synaptic currents. Although this result excludes strong presynaptic effects and effects on some classes of excitatory receptor channels, the result does not exclude the possibility that NMDA receptors might be modulated by C2-NBD 3α5αP. Given the sensitivity of both GABAA and NMDA receptors to ultraviolet wavelengths in the absence of any fluorophore (Leszkiewicz et al. 2000; Chang et al. 2001; Leszkiewicz & Aizenman, 2003), we anticipated that NMDA receptors may also be sensitive to blue-light potentiation in the presence of C2-NBD 3α5αP. We used the same protocol developed for blue-light potentiation of GABAA receptors to test the response of NMDA receptors in cultured hippocampal neurons. C2-NBD 3α5αP, 300 nm, had no effect on NMDA currents in the dark, but potentiated responses to 10 μm NMDA to 219.2 ± 9.2% (Fig. 1B, n= 58) of the baseline response to NMDA (baseline = 100%). In control experiments, 480 nm light without C2-NBD 3α5αP (110.7 ± 3.3%, n= 6), 300 nm 3α5αP (101.6 ± 1.0%, n= 5) and 480 nm light in the presence of 300 nm 3α5αP (114.9 ± 13.7%, n= 5) had no significant effect on NMDA currents. The current resulting from blue-light potentiation was blocked by 50 μm d-2-amino-5-phosphonovalerate, a competitive NMDA receptor antagonist. Following application of d-APV, only 3.3 ± 0.9% of the potentiated current remained (n= 5), consistent with the potentiated current being mediated by NMDA receptors rather than activation of other receptors or channels.
We next characterized the concentration–response relationship for C2-NBD 3α5αP in the presence of 10 μm NMDA. The dashed line shown in Fig. 1C represents a fit of the Hill equation with EC50= 0.13 μm and n= 2.0. Note that the fit is empirical only and does not necessarily imply that a typical drug–receptor interaction mediates the light-induced potentiation. Since 300 nm C2-NBD 3α5αP appears to be a near-maximal concentration, 300 nm was used for subsequent experiments. We also sought to explore the longevity of the potentiation using the protocol illustrated in Fig. 1D. Following blue-light potentiation using the protocol illustrated in Fig. 1B, 10 μm NMDA was reapplied for 10 s every minute for five trials and the responses measured. These data are summarized in Fig. 1E. Similar to blue-light potentiation of GABA receptors (Eisenman et al. 2007), blue-light potentiation of NMDA receptors decays slowly (time constant = 80.6 s based on a fit to a single exponential with the response immediately following light exposure considered time zero). Control experiments with light but no C2-NBD 3α5αP showed no potentiation and no evidence of rundown (data not shown).
The potentiation of NMDA receptors is less efficacious than the potentiation of GABAA receptors at low agonist concentrations. One possible explanation is that 10 μm NMDA elicits a larger fraction of the maximal NMDA current than the 0.5 μm GABA used to characterize potentiation of GABAA receptors (Eisenman et al. 2007). We explored this hypothesis using 1 μm NMDA and found that responses were potentiated to 147.9 ± 9.6% (n= 5) of the baseline response (Fig. 2A and C). The similarity of blue-light potentiation of responses to both 1 and 10 μm NMDA supports the conclusion that blue-light potentiation of NMDA receptors is less effective than blue-light potentiation of GABAA receptors. This also suggests that blue-light potentiation of NMDA receptors is not strongly dependent on NMDA concentration. We performed further tests of the relative insensitivity of blue-light potentiation to NMDA concentration by testing 3 μm, 30 μm and 100 μm NMDA. We found that responses were potentiated to 180.3 ± 14.9%, 176.7 ± 7.1% and 174.1 ± 15.6%, respectively, of the current immediately prior to activating the light (n= 5, 4 and 5 respectively; Fig. 2B and C). The increasing potentiation with increasing NMDA concentration up to 10 μm with slightly lower potentiation of responses to 30 μm and 100 μm NMDA may reflect some non-linear sensitivity of potentiation to NMDA concentration. Alternatively, the lower potentiation at higher NMDA concentrations may be the result of continuing desensitization of the receptors upon which the potentiated response was superimposed. In either case, the measured potentiation was relatively insensitive to NMDA concentration over the range tested and statistically showed no dependence of potentiation on NMDA concentration (Fig. 2).
The relative insensitivity of blue-light potentiation to NMDA concentration suggests that peak synaptic NMDA responses, which are responses to locally high agonist concentrations largely uninfluenced by receptor desensitization, may also be potentiated. To test this hypothesis, we selected single neuron islands in our cultures and measured evoked autaptic responses. After establishing a stable baseline EPSC, we applied C2-NBD 3α5αP (without NMDA) and blue light for 30 s. After washing away C2-NBD 3α5αP, we again evoked a synaptic response. We found that the peak of the autaptic response was potentiated to 178.9 ± 22.4% (n= 9) of baseline. A sample result is illustrated in Fig. 2D. Charge transfer was 226.0 ± 35.6% of baseline, a trend level increase compared to the increase in the peak current (P= 0.09, paired t test). Similarly, the weighted time constant calculated from a double exponential fit of the decay phase of the autaptic responses showed a non-significant increase from 243 ± 20.4 ms to 574.3 ± 195.2 ms (P= 0.12, paired t test). Of note, the bi-exponential time constants of the control responses (73 ± 4 ms and 453 ± 32 ms) were consistent with prior observations (Lester & Jahr, 1992; Mennerick & Zorumski, 1994). Thus, blue-light potentiation of synaptic NMDA receptors results in an increase in the peak synaptic response with weaker effects on the time course of the response. In contrast, blue-light potentiation of synaptic GABAA receptors results in a prolongation of the decay of the synaptic response with minimal effect on the peak amplitude of the response (Eisenman et al. 2007). The observation that the potentiation of peak amplitude of the autaptic NMDA response is comparable to the potentiation of responses to exogenously applied 100 μm NMDA provides additional support to the conclusion that blue-light potentiation is relatively insensitive to agonist concentration over a wide range of agonist concentrations.
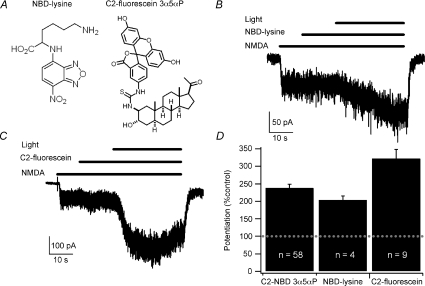
We next examined whether the presence of the steroid is required or, as with GABAA receptors, the presence of the NBD moiety is critical. We tested the ability of 480 nm light in the presence of 10 μm NBD-lysine (Fig. 3A) to potentiate the response to 10 μm NMDA and found that responses were enhanced to 203.2 ± 12.4% of the baseline NMDA response (n= 4; Fig. 3B and D). In contrast, 1 μm NBD-lysine did not support robust blue-light potentiation of the responses to 10 μm NMDA (115.3 ± 5.1%, n= 4). The blue-light potentiation in the presence of NBD-lysine and the observation that 3α5αP does not potentiate NMDA receptors strongly suggest that the potentiation is not mediated by binding to a steroid site in the NMDA receptor, although the greater potency of C2-NBD 3α5αP compared to NBD-lysine suggests that 3α5αP is a more efficient carrier of NBD. Thus blue-light potentiation of NMDA receptors is qualitatively similar to blue-light potentiation of GABAA receptors.
Figure 3. Blue-light potentiation of NMDA responses does not require the steroid backbone or the NBD moiety.
A, structures of NBD-lysine and C2-fluorescein 3α5αP. B, example of blue-light potentiation of the response to 10 μm NMDA in the presence of 10 μm NBD-lysine. C, example of blue-light potentiation of the response to 10 μm NMDA in the presence of 300 nm C2-fluorescein 3α5αP. D, summary bar graph of average potentiation. Error bars represent s.e.m. Baseline response is 100% (dashed line). Potentiated currents for each fluorophore were significantly different from current prior to activation of the light (paired t test, P < 0.05). Potentiation with C2-fluorescein 3α5αP was significantly different from potentiation with either NBD-lysine or C2-NBD 3α5αP while NBD-lysine and C2-NBD 3α5αP were not significantly different (one-way ANOVA followed by Student–Newman–Keuls test, P < 0.05). Recordings were performed with CsMeSO4 internal solution at −60 mV in cultured hippocampal neurons.
We also tested the necessity of using NBD as the fluorophore by replacing the NBD group in C2-NBD 3α5αP with a fluorescein group (Fig. 3A). As shown in Fig. 3C, 300 nm C2-fluorescein 3α5αP also supports robust blue-light potentiation (322.7 ± 26.0%, n= 9). In several cases, potentiation with C2-fluorescein 3α5αP resulted in apparent desensitization during the light exposure, potentially complicating the quantification of effects. For this reason, subsequent experiments were performed with C2-NBD 3α5αP.
Blue-light potentiation shares a common mechanism with UV light and redox potentiation
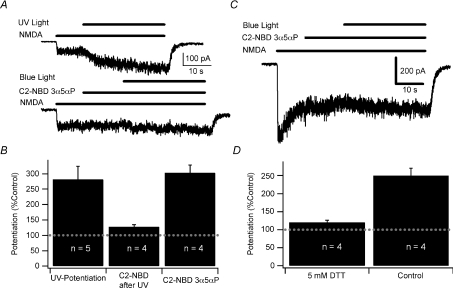
While there are numerous classes of potentiators of GABAA receptors, there are relatively few NMDA receptor potentiators. As noted above, both GABAA and NMDA receptors are potentiated by 350 nm ultraviolet (UV) light (Leszkiewicz et al. 2000; Chang et al. 2001; Leszkiewicz & Aizenman, 2003). Although the mechanism of UV potentiation is uncertain, we hypothesized that there might be a common mechanism for both UV and blue-light potentiation of NMDA receptors. We tested this hypothesis by potentiating receptors with a 30 s exposure to UV light in the presence of 10 μm NMDA. We then tested the ability of the same receptors to undergo 480 nm light potentiation in the presence of fluorophore. Controls consisting of blue-light potentiation without prior UV potentiation were interleaved in the same culture dishes using the same solutions. UV light potentiated NMDA currents to 281.7 ± 42.8% (n= 5) of the baseline. Subsequent blue-light potentiation of the same neurons resulted in an additional 27.7 ± 7.4% potentiation (n= 4, Fig. 4A) compared to 303.3 ± 25.5% overall enhancement by C2-NBD 3α5αP in control neurons in the same cultures (n= 4; P= 0.004, Student's t test; Fig. 4B). These results indicate that UV potentiation significantly occludes subsequent blue-light potentiation, consistent with the hypothesis that UV and fluorophore-assisted blue-light potentiation share a common mechanism. However, it is also difficult to exclude the possibility that occlusion represents a ceiling effect in which potentiation by UV light maximized the response, preventing further potentiation.
Figure 4. UV and DTT potentiation occlude blue-light potentiation of NMDA receptors.
A, sample traces showing UV potentiation of the response to 10 μm NMDA (upper trace) followed by blue-light potentiation using 300 nm C2-NBD 3α5αP in the same cell. Traces were shifted for clarity. B, summary bar graph of UV potentiation of responses to 10 μm NMDA, blue-light potentiation following UV potentiation, and the blue-light potentiation of NMDA responses in control cells from the same cultures. Note that the fluorophore-assisted blue-light potentiation following UV potentiation was calculated from the NMDA current measured immediately before activating the blue light. Baseline response is 100%. Potentiated currents were significantly different from current prior to activation of the light (paired t test, P < 0.05). Potentiation with UV and with C2-NBD 3α5αP were both significantly different from potentiation with C2-NBD 3α5αP following UV potentiation but were not significantly different from each other (one-way ANOVA followed by Student–Newman–Keuls test, P < 0.05). C, sample trace showing blue-light potentiation of the response to 10 μm NMDA from a culture treated with 5 mm DTT for 10 min. D, summary bar graph of blue-light potentiation of DTT-treated cells compared to controls from an untreated sister culture. Error bars represent s.e.m. Baseline response is 100% (dashed line). Potentiated currents were significantly different from current prior to activation of the light (paired t test, P < 0.05). Potentiation in the presence of DTT was significantly less than control potentiation (Student's t test, P < 0.05). Recordings were performed with CsMeSO4 internal solution at −60 mV in cultured hippocampal neurons.
Prior studies indicate that UV potentiation and dithiothreitol (DTT) potentiation of NMDA receptors share a common mechanism (Leszkiewicz & Aizenman, 2002) based in part on occlusion experiments similar in concept to those of Fig. 4A and B. We therefore examined the ability of DTT to occlude blue-light potentiation of NMDA receptors. Pretreating neurons with 5 mm DTT for 10 min prior to testing significantly occluded blue-light potentiation of responses to 10 μm NMDA (120.2 ± 6.2%, n= 4; Fig. 4C). An untreated sister culture, tested with the same solutions immediately after the DTT-treated culture was tested, exhibited 249.5 ± 20.9% potentiation (n= 4; P= 0.006; Student's t test; Fig. 4D). As noted above, the possibility of saturation of potentiation cannot be completely excluded. However, because photopotentiation is still robust at nearly saturating NMDA concentrations and because DTT-potentiated NMDA responses are further potentiated by sulfated steroids (Park-Chung et al. 1997), we favour an explanation of a common mechanism.
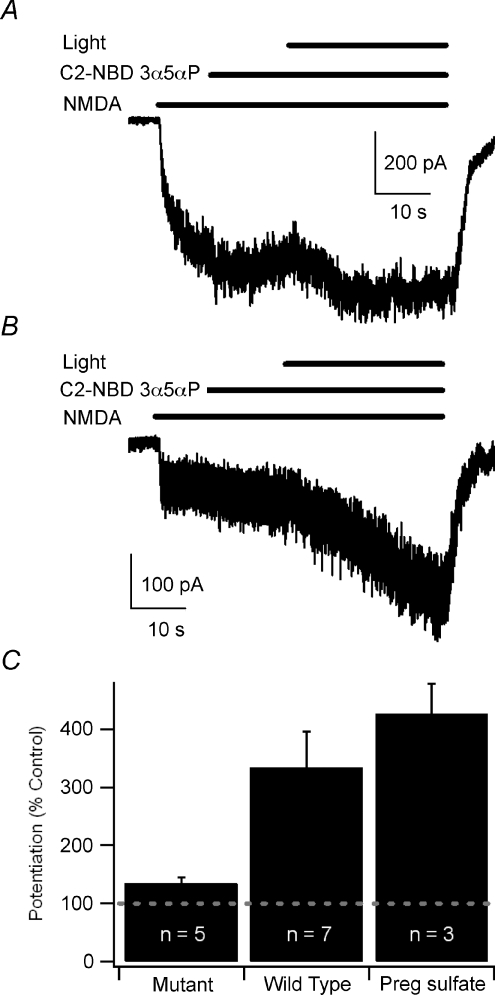
Although these data support the hypothesis that blue-light potentiation of NMDA receptors shares a common mechanism with UV potentiation and redox potentiation of NMDA receptors, saturation of potentiation rather than occlusion due to a common mechanism cannot be completely excluded. We, therefore, sought another method to test the hypothesis of a common mechanism of potentiation. In earlier studies, additional evidence suggesting that UV and redox potentiation of NMDA receptors share a common mechanism came from the demonstration that the DTT-resistant NR1a(C744A,C798A) mutant (Sullivan et al. 1994) is also resistant to UV potentiation when co-expressed with wild-type NR2B subunits (Leszkiewicz & Aizenman, 2002). We sought to test further the possibility of a common mechanism of blue-light potentiation by testing blue-light potentiation of the NR1a(C744A,C798A) mutant. Mutant NR1a(C744A,C798A) receptors co-expressed with NR2B subunits exhibited only a small degree of blue-light potentiation (135.0 ± 10.0%; n= 5; Fig. 5A and C) while wild-type receptors were robustly potentiated by 480 nm light (333.8 ± 62.2%; n= 7; P= 0.02, Student's t test; Fig. 5B and C). As a positive control to ensure that mutant NR1a(C744A,C798A) receptors can be positively modulated, we tested the ability of 50 μm pregnenolone sulfate (PS) to potentiate the mutant receptors. PS was chosen because it is thought to potentiate NMDA receptors through a mechanism different to redox modulation (Park-Chung et al. 1997) and is an inhibitor of GABA currents (Majewska et al. 1988; Eisenman et al. 2003). PS elicited robust potentiation of NMDA currents mediated by the NR1a(C744A,C798A) mutant (426.1 ± 52.4%; n= 3; P= 0.00046; Student's t test). Collectively, these data suggest that blue-light potentiation of NMDA receptors could share a common mechanism with UV light potentiation and DTT potentiation.
Figure 5. The NMDA NR1a (C744A,C798A) mutant shows markedly diminished sensitivity to blue-light potentiation.
A, sample trace showing only a small degree of blue-light potentiation of the response to 10 μm NMDA in the presence of 300 nm C2-NBD 3α5αP in the NR1a (C744A,C798A)-NR2B receptors expressed in HEK 293 cells. B, sample trace showing blue-light potentiation of wild-type NR1a-NR2B NMDA receptors. C, summary bar graph of blue-light potentiation of mutant receptors, wild-type receptors and 50 μm pregnenolone sulfate (Preg sulfate) potentiation of mutant receptors. Error bars represent s.e.m. Baseline response is 100% (dashed line). Potentiated currents from both mutant and wild-type receptors were significantly different from current prior to activation of the light or application of pregnenolone sulfate (paired t test, P < 0.05). Potentiation of mutant receptors was significantly less than potentiation of wild-type receptors (Student's t test, P < 0.05). Recordings were performed with CsMeSO4 internal solution at −60 mV.
Reactive oxygen species may contribute to blue-light potentiation
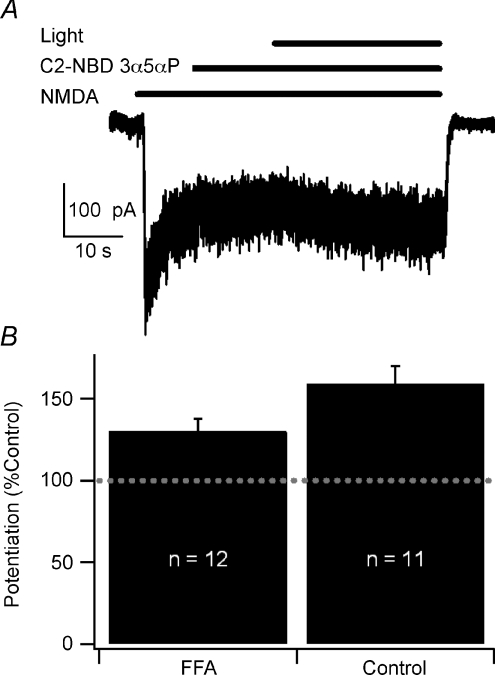
We next sought to identify mechanisms contributing to blue-light potentiation. It has been recently reported that exposing the fluorophore lucifer yellow to light results in the generation of superoxide which modulates both voltage- and ligand-gated channels (Takeuchi & Yoshii, 2008). Similarly, ‘chromophore-assisted laser inactivation’ uses light to inactivate fluorophore-tagged proteins via production of singlet oxygen (Horstkotte et al. 2005). Based on these observations, we hypothesized that excitation of the fluorophore with blue light results in the generation of reactive oxygen species (ROS) that in turn directly or indirectly alter the redox site on the NMDA receptor. We tested this hypothesis with the singlet oxygen scavenger furfuryl alcohol (FFA; Linetsky & Ortwerth, 1997) and found that 0.02% v/v FFA decreased blue-light potentiation compared to a sister culture control (130.2 ± 7.6%, n= 12 vs. 159.4 ± 10.7%, n= 11; P= 0.03; Student's t test; Fig. 6). Co-application of 0.02% v/v FFA with C2-NBD 3α5αP had no effect on NMDA receptor-mediated current in the absence of light (Fig. 6). However, higher concentrations of FFA did have direct effects on NMDA receptor-mediated current (data not shown), limiting the use of higher concentrations to more fully suppress blue-light potentiation. In contrast, the superoxide scavenger 1,2-dihydroxybenzene-3,5-disulfonate (Tiron; Miller & Macdowall, 1975) at 2 μm and 5 μm did not significantly inhibit blue-light potentiation (data not shown). These results suggest that blue-light potentiation may involve the generation of singlet oxygen.
Figure 6. Furfuryl alcohol attenuates blue-light potentiation.
A, sample trace showing blue-light potentiation in the presence of 0.02% v/v furfuryl alcohol co-applied with 300 nm C2-NBD 3α5αP. B, summary bar graph of blue-light potentiation in the presence and absence of furfuryl alcohol (FFA). Error bars represent s.e.m. Baseline response is 100% (dashed line). Potentiated currents from both control and FFA-treated neurons were significantly different from current prior to activation of the light (paired t test, P < 0.05). Potentiation of FFA-treated neurons was statistically significantly less than control potentiation (Student's t test, P < 0.05). Recordings were performed with CsMeSO4 internal solution at −60 mV in cultured hippocampal neurons.
Discussion
Similar to GABAA receptor currents (Eisenman et al. 2007), NMDA receptor function is potentiated by 480 nm light in the presence of C2-NBD 3α5αP. The potentiation is largely independent of NMDA concentration from 1 μm to 100 μm. Visible-light potentiation is unlikely to be a steroid effect because 3α5αP, the parent neurosteroid, does not potentiate NMDA receptors, and 3α5αP is not absolutely required as demonstrated by the ability of NBD-lysine to promote visible-light potentiation. Visible-light potentiation is also unlikely to be mediated through the sulfated steroid binding site of NMDA receptors because steroid moieties are not required for potentiation, and the DTT-, UV- and blue-light-insensitive mutant NMDA receptor is effectively potentiated by pregnenolone sulfate. Nevertheless, steroids appear to act as particularly effective carriers of the potentiating fluorophore, suggested by the low aqueous concentrations that support visible-light potentiation (almost two orders of magnitude lower than the required concentration of NBD-lysine).
Although visible-light potentiation of NMDA receptors is qualitatively similar to potentiation of GABAA receptors, potentiation of GABAA receptors is more robust. Maximum potentiation of GABAA receptors is nearly 20-fold at low GABA concentrations (Eisenman et al. 2007), while maximum potentiation of NMDA receptors (which appears to be largely independent of agonist concentration) is only about 2-fold. These considerations suggest that in the absence of antagonists, the GABAA receptor potentiation effect would overcome the NMDA receptor effect, resulting in a net depression of activity, consistent with our prior observation that C2-NBD 3α5αP has anticonvulsant and anaesthetic effects when activated by 480 nm light (Eisenman et al. 2007). However, visible-light potentiation of NMDA receptors could prove a useful research tool for providing spatially and temporally controlled potentiation of NMDA receptors.
Because of the small number of known common potentiators of NMDA and GABAA receptors, we have suggested that visible-light potentiation of NMDA receptors may share a common mechanism with UV-light potentiation, which in turn shares a common mechanism with redox modulation (Leszkiewicz et al. 2000; Leszkiewicz & Aizenman, 2002). We have demonstrated that both UV-light potentiation and redox potentiation with DTT significantly occlude subsequent blue-light potentiation, consistent with a common mechanism. We then tested blue-light potentiation on the UV- and DTT-resistant NR1a(C744A,C798A)/NR2B mutant and demonstrated that the mutant is also resistant to blue-light potentiation. Finally, we demonstrated that blue-light potentiation is partially blocked by the singlet oxygen scavenger, furfuryl alcohol. Collectively, these data support the hypothesis that visible-light potentiation shares a common mechanism with UV light and DTT potentiation of NMDA receptors and may be mediated by the generation of singlet oxygen.
While suggesting a common mechanism of visible-light potentiation, UV-light potentiation and DTT potentiation of NMDA receptors, these data leave a number of questions unanswered. In particular, what is the mechanism by which the potentiation occurs? Classically, modulation of receptors occurs when the modulator binds to a specific site on the receptor. However, visible-light potentiation seems more likely to result in a chemical change to the receptor. What is the nature of the chemical change? As has been pointed out for the case of UV potentiation of GABA receptors (Leszkiewicz & Aizenman, 2003), a mechanism involving the generation of high levels of reactive oxygen species (ROS) is problematic as ROS would be expected to oxidize the receptors resulting in depression rather than potentiation (Aizenman et al. 1990). However, the observation that the singlet oxygen scavenger furfuryl alcohol partially occludes blue-light potentiation suggests that generation of singlet oxygen contributes to the mechanism of potentiation. Currently, we cannot resolve this dilemma. However, it is possible that the difference in the level of singlet oxygen generated by the compounds used in this study and the photosensitizers used to generate singlet oxygen for photodynamic therapy may provide the key to solving this puzzle. The latter types of photosensitizers are designed to be highly efficient at generating singlet oxygen so that cells are killed by this oxidant. By contrast, the compounds used in this study would be expected to be only very weak photosensitizers for singlet oxygen formation because of the photochemical properties of the NBD and fluorescein groups. For example, fluorescein itself has an efficiency of only 0.03 for singlet oxygen formation because its singlet state fluorescence occurs at a much faster rate than the rate of intersystem crossing to the triplet state required for singlet oxygen production (Devanathan et al. 1990). Thus, determining whether low levels of singlet oxygen can initiate chemical pathways and/or cellular responses that lead to a transient increase in NMDA receptors with decreased disulfide bond content will require further study.
A related question is the nature of the change in receptor behaviour. Single channel analysis of the effect of UV light demonstrated an increased frequency of channel openings with no change in open duration or channel conductance (Leszkiewicz et al. 2000). However, this does not provide a complete explanation of the effect. Specifically, does the potentiation occur as a result of altered receptor desensitization, altered rates of opening and closing or some complicated combination of changes? A simple shift in the NMDA concentration–response curve seems unlikely as such a shift should exhibit sensitivity to NMDA concentration. In particular, responses to saturating concentrations of NMDA and peak synaptic responses would not be potentiated as a result of a simple leftward shift in the concentration–response curve. In addition, UV potentiation of NMDA receptors does not alter the NMDA EC50 (Leszkiewicz et al. 2000). Similarly, significant changes in receptor desensitization seem less likely given relatively small changes in the decay time constants of autaptic responses.
In summary, we demonstrate that NMDA receptors are potentiated by 480 nm light in the presence of fluorophores activated by blue light. The potentiation is dependent on the presence of the fluorescent moiety and not the specific carrier, although neurosteroid analogues serve as effective carriers. Visible-light potentiation of NMDA receptors is partially occluded by UV-light potentiation and by DTT potentiation. Potentiation is also eliminated by the DTT-resistant and UV-resistant NR1a(C744A,C798A)/NR2B mutant. Finally, the singlet oxygen scavenger furfuryl alcohol partially inhibits visible-light potentiation of NMDA receptors. Collectively these data suggest that visible-light potentiation shares a common mechanism with UV and DTT potentiation of NMDA receptors and may be mediated via the generation of singlet oxygen.
Acknowledgments
We thank A. Taylor and A. Benz for technical help and laboratory members for advice and discussion. This work was supported by a gift from the Bantly Foundation (C.F.Z.) and by US National Institutes of Health grants NS44041 (L.N.E), GM 47969 (D.F.C., C.F.Z), MH77791 (C.F.Z.), NS54174, AA12952 and MH78823 (S.M.), and an NIH Neuroscience Blueprint Core Grant P30NS057105 to Washington University.
References
- Aizenman E, Hartnett KA, Reynolds IJ. Oxygen free radicals regulate NMDA receptor function via a redox modulatory site. Neuron. 1990;5:841–846. doi: 10.1016/0896-6273(90)90343-e. [DOI] [PubMed] [Google Scholar]
- Akwa Y, Ladurelle N, Covey DF, Baulieu EE. The synthetic enantiomer of pregnenolone sulfate is very active on memory in rats and mice, even more so than its physiological neurosteroid counterpart: distinct mechanisms? Proc Natl Acad Sci U S A. 2001;98:14033–14037. doi: 10.1073/pnas.241503698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckman FA, Aizenman E. Pharmacological properties of acquired excitotoxicity in Chinese hamster ovary cells transfected with N-methyl-d-aspartate receptor subunits. J Pharmacol Exp Ther. 1996;279:515–523. [PubMed] [Google Scholar]
- Chang Y, Xie Y, Weiss DS. Positive allosteric modulation by ultraviolet irradiation on GABAA, but not GABAC, receptors expressed in Xenopus oocytes. J Physiol. 2001;536:471–478. doi: 10.1111/j.1469-7793.2001.0471c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanathan S, Dahl TA, Midden WR, Neckers DC. Readily available fluorescein isothiocyanate-conjugated antibodies can be easily converted into targeted phototoxic agents for antibacterial, antiviral, and anticancer therapy. Proc Natl Acad Sci U S A. 1990;87:2980–2984. doi: 10.1073/pnas.87.8.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Eisenman LN, He Y, Fields C, Zorumski CF, Mennerick S. Activation-dependent properties of pregnenolone sulfate inhibition of GABAA receptor-mediated current. J Physiol. 2003;550:679–691. doi: 10.1113/jphysiol.2003.043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman LN, Shu HJ, Akk G, Wang C, Manion BD, Kress GJ, Evers AS, Steinbach JH, Covey DF, Zorumski CF, Mennerick S. Anticonvulsant and anesthetic effects of a fluorescent neurosteroid analog activated by visible light. Nat Neurosci. 2007;10:523–530. doi: 10.1038/nn1862. [DOI] [PubMed] [Google Scholar]
- Flood JF, Morley JE, Roberts E. Memory-enhancing effects in male mice of pregnenolone and steroids metabolically derived from it. Proc Natl Acad Sci U S A. 1992;89:1567–1571. doi: 10.1073/pnas.89.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstkotte E, Schroder T, Niewohner J, Thiel E, Jay DG, Henning SW. Toward understanding the mechanism of chromophore-assisted laser inactivation – evidence for the primary photochemical steps. Photochem Photobiol. 2005;81:358–366. doi: 10.1562/2004-07-22-RA-240. [DOI] [PubMed] [Google Scholar]
- Lester RA, Jahr CE. NMDA channel behavior depends on agonist affinity. J Neurosci. 1992;12:635–643. doi: 10.1523/JNEUROSCI.12-02-00635.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszkiewicz D, Aizenman E. A role for the redox site in the modulation of the NMDA receptor by light. J Physiol. 2002;545:435–440. doi: 10.1113/jphysiol.2002.032755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszkiewicz DN, Aizenman E. Reversible modulation of GABAA receptor-mediated currents by light is dependent on the redox state of the receptor. Eur J Neurosci. 2003;17:2077–2083. doi: 10.1046/j.1460-9568.2003.02656.x. [DOI] [PubMed] [Google Scholar]
- Leszkiewicz DN, Kandler K, Aizenman E. Enhancement of NMDA receptor-mediated currents by light in rat neurones in vitro. J Physiol. 2000;524:365–374. doi: 10.1111/j.1469-7793.2000.t01-1-00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linetsky M, Ortwerth BJ. Quantitation of the singlet oxygen produced by UVA irradiation of human lens proteins. Photochem Photobiol. 1997;65:522–529. doi: 10.1111/j.1751-1097.1997.tb08598.x. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Mienville JM, Vicini S. Neurosteroid pregnenolone sulfate antagonizes electrophysiological responses to GABA in neurons. Neurosci Lett. 1988;90:279–284. doi: 10.1016/0304-3940(88)90202-9. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Que J, Benz A, Zorumski CF. Passive and synaptic properties of hippocampal neurons grown in microcultures and in mass cultures. J Neurophysiol. 1995;73:320–332. doi: 10.1152/jn.1995.73.1.320. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Zorumski CF. Glial contributions to excitatory neurotransmission in cultured hippocampal cells. Nature. 1994;368:59–62. doi: 10.1038/368059a0. [DOI] [PubMed] [Google Scholar]
- Miller RW, Macdowall FD. The Tiron free radical as a sensitive indicator of chloroplastic photoautoxidation. Biochim Biophys Acta. 1975;387:176–187. doi: 10.1016/0005-2728(75)90062-6. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Wu FS, Purdy RH, Malayev AA, Gibbs TT, Farb DH. Distinct sites for inverse modulation of N-methyl-d-aspartate receptors by sulfated steroids. Mol Pharmacol. 1997;52:1113–1123. doi: 10.1124/mol.52.6.1113. [DOI] [PubMed] [Google Scholar]
- Sliwinski A, Monnet FP, Schumacher M, Morin-Surun MP. Pregnenolone sulfate enhances long-term potentiation in CA1 in rat hippocampus slices through the modulation of N-methyl-d-aspartate receptors. J Neurosci Res. 2004;78:691–701. doi: 10.1002/jnr.20332. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Traynelis SF, Chen HS, Escobar W, Heinemann SF, Lipton SA. Identification of two cysteine residues that are required for redox modulation of the NMDA subtype of glutamate receptor. Neuron. 1994;13:929–936. doi: 10.1016/0896-6273(94)90258-5. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Yoshii K. Superoxide modifies AMPA receptors and voltage-gated K+ channels of mouse hippocampal neurons. Brain Res. 2008;1236:49–56. doi: 10.1016/j.brainres.2008.08.008. [DOI] [PubMed] [Google Scholar]