Abstract
Carotid body (CB) glomus cells from rat express a TASK-like background K+ channel that is believed to play a critical role in the regulation of excitability and hypoxia-induced increase in respiration. Here we studied the kinetic behaviour of single channel openings from rat CB cells to determine the molecular identity of the ‘TASK-like’ K+ channels. In outside-out patches, the TASK-like background K+ channel in CB cells was inhibited >90% by a reduction of pHo from 7.3 to 5.8. In cell-attached patches with 140 mm KCl and 1 mm Mg2+ in the bath and pipette solutions, two main open levels with conductance levels of ∼14 pS and ∼32 pS were recorded at a membrane potential of −60 mV. The K+ channels showed kinetic properties similar to TASK-1 (∼14 pS), TASK-3 (∼32 pS) and TASK-1/3 heteromer (∼32 pS). The presence of three TASK isoforms was tested by reducing [Mg2+]o to ∼0 mm, which had no effect on the conductance of TASK-1, but increased those of TASK-1/3 and TASK-3 to 42 pS and 74 pS, respectively. In CB cells, the reduction of [Mg2+]o to ∼0 mm also caused the appearance of ∼42 pS (TASK-1/3-like) and ∼74 pS (TASK-3-like) channels, in addition to the ∼14 pS (TASK-1-like) channel. The 42 pS channel was the most abundant, contributing ∼75% of the current produced by TASK-like channels. Ruthenium red (5 μm) had no effect on TASK-1 and TASK-1/3, but inhibited TASK-3 by 87%. In CB cells, ruthenium red caused ∼12% inhibition of TASK-like activity. Methanandamide reduced the activity of all three TASKs by 80–90%, and that of TASK-like channels in CB cell also by ∼80%. In CB cells, hypoxia caused inhibition of TASK-like channels, including TASK-1/3-like channels. These results show that TASK-1, TASK-1/3 and TASK-3 are all functionally expressed in isolated CB cells, and that the TASK-1/3 heteromer provides the major part of the oxygen-sensitive TASK-like background K+ conductance.
Carotid body (CB) glomus cells detect hypoxia and transmit the signal to brainstem respiratory centres to adjust ventilation. The generally accepted ‘membrane’ model of oxygen sensing states that hypoxia causes inhibition of K+ channels that leads to depolarization, increased Ca2+ influx via Ca2+ channels, release of neurotransmitters from CB glomus cells, and activation of afferent nerves to increase ventilation (Lopez-Barneo et al. 2008). The mechanism by which hypoxia causes inhibition of K+ current is yet to be clearly identified, but recent studies suggest that multiple pathways may converge on the K+ channels (Prabhakar, 2006). K+ channels such as large-conductance Ca2+-activated (BK), voltage-gated K+ (Kv) and background (leak) K+ channels are expressed in CB cells and inhibited by hypoxia, and therefore may serve as oxygen sensors (Ganfornina & Lopez-Barneo, 1992; Donnelly, 1999; Patel & Honore, 2001; Conforti et al. 2003; Buckler, 2007; Peers & Wyatt, 2007). The specific types of K+ channels regulated by hypoxia may be species dependent. Earlier studies in the rat showed that hypoxia inhibits BK channels to cause depolarization of glomus cells (Wyatt & Peers, 1995). However, studies by other investigators showed that inhibition of a background K+ conductance that has the characteristics of TASKs is important for hypoxia-induced depolarization (Buckler, 1999; Buckler et al. 2000). These findings suggest that both BK and background K+ channels may be targets of hypoxia in the rat.
In rat CB glomus cells, whole-cell current recordings first showed the presence of O2-sensitive K+ current that was not inhibited by tetraethylammonium, 4-aminopyridine and charybdotoxin (Buckler, 1997). This current was found to be contributed by a ∼14 pS K+ channel whose kinetic properties were similar to those of TASK-1. Like TASK-1 whose unitary conductance is ∼14 pS, the 14 pS channel in CB cells showed flickery openings of short duration, mild inward rectification, and inhibition by acid, zinc, quinidine and bipuvacaine (Buckler et al. 2000). In a subsequent study, further characterization of the TASK-like K+ channel in rat CB cells showed that a ∼14–16 pS channel was sensitive to changes in extracellular [Mg2+] (Williams & Buckler, 2004). Because TASK-1 is insensitive to [Mg2+], this suggested that the properties of the 14 pS channel in CB glomus cells were not consistent with TASK-1. Therefore, although the CB K+ channels appear to be TASK-like, their molecular identities are still uncertain. It is possible that a K+ channel with conductance and pH sensitivity similar to those of TASKs is expressed in CB glomus cells, as reported in other neurons (Han et al. 2002, 2003).
The goal of this study was to re-examine and further characterize the TASK-like K+ channels in rat CB glomus cells to help identify their molecular correlates. Therefore, single channel kinetics of TASK-like K+ channels in CB cells were compared with those of TASK-1, TASK-3 and TASK-1/3 heteromer under conditions that would distinguish among the three isoforms. Inhibitors such as ruthenium red and methanandamide have been reported to be selective for TASK-3 and TASK-1, respectively (Maingret et al. 2001; Czirjak & Enyedi, 2003). Therefore, these inhibitors were employed further to help separate TASK-like channels in CB cells. In addition to TASK-like channels, the presence of non-TASK background K+ channels that open near the resting membrane potential was examined, as mRNA transcripts of other two-pore domain K+ channels (such as TREK/TRAAK and TASK-2) are also expressed in CB cells (Yamamoto et al. 2002).
Methods
Carotid body cell isolation
The use of animals in this study was approved by the Animal Care and Use Committee of Rosalind Franklin University. Rats (postnatal 7–14; 352 rats) were anaesthetized with isoflurane and decapitated, and the head placed in ice-cold buffered saline solution (118 mm NaCl, 23 mm NaHCO3, 3 mm KCl, 2 mm KH2PO4, 1.2 mm CaCl2, 1 mm MgCl2, 10 mm glucose, pH 7.2). CBs from both sides were dissected and placed in ice-cold low-Ca2+, low-Mg2+ phosphate buffered saline solution (PBS: 137 mm NaCl, 2.8 mm KCl, 2 mm KH2PO4, 0.07 mm CaCl2, 0.05 mm MgCl2, pH 7.4). Each CB was cut into three to four pieces and placed in a solution containing trypsin (400 μg ml−1) and collagenase (400 μg ml−1) in low Ca2+/Mg2+ PBS and incubated at 37°C for 20–25 min. CBs were gently triturated using a fire polished Pasteur pipette to mechanically dissociate the cells. Enzymatic digestion was continued for an additional few minutes when necessary. CB growth medium (Ham's F-12, 10% fetal bovine serum, 23 mm glucose, 200 mm l-glutamine, 10 kunits penicillin–streptomycin, and 300 μg ml−1 insulin) was added to stop enzyme activity. After brief trituration, the solution containing the digested CBs was centrifuged for 4 min at ∼6000 rpm (∼2000 g) using a microcentrifuge. Supernatant was removed and warm CB growth medium added to gently resuspend the pellet. This step was repeated to remove traces of enzymes. Suspended CB cells were placed on glass coverslips coated with poly-l-lysine, and incubated at 37°C for 50 min to allow settling and attachment of the cells. CB growth medium was further added to the plate containing the coverslips and then incubated at 37°C for an additional 2 h. Coverslips were then transferred to the recording chamber for electrophysiological experiments.
Transfection in HeLa cells
Full length rat TASK-1, TASK-3 and TASK-2 DNA coding sequences were cloned previously in this laboratory and inserted into the pcDNA3.1 vector. TASK-1/3 heteromer (rat) was a gift from Dr D. A Bayliss (Univ. of Virginia), and contains a linker (Gly-Ser-Ala) between the end of the C-terminus of TASK-1 and the beginning of TASK-3. HeLa cells were seeded at a density of 2 × 105 cells per 35 mm dish 24 h prior to transfection in 10% bovine serum in Dulbecco's modified Eagle's medium (DMEM). HeLa cells were co-transfected with DNA fragments encoding a K2P channel and green fluorescent protein (GFP) in pcDNA3.1 using LipofectAMINE2000 and OPTI-MEM I Reduced Serum Medium (Invitrogen). Green fluorescence from cells expressing GFP was detected with the aid of a Nikon microscope equipped with a mercury lamp light source. Cells were used 2–3 days after transfection.
Electrophysiological studies
Electrophysiological recording was performed using a patch clamp amplifier (Axopatch 200, Axon Instruments, Union City, CA, USA). Channel current was filtered at 3 kHz using an 8-pole Bessel filter (−3 dB; Frequency Devices, Haverhill, MA, USA) and transferred to a computer using the Digidata 1320 interface (Axon Instruments) at a sampling rate of 20 kHz. Single-channel currents were analysed with the pCLAMP program (Version 10). For single channel analysis, the minimum duration was set at 0.05 ms. Channel openings were analysed to obtain channel activity (NPo, where N is the number of channels in the patch, and Po is the probability of a channel being open). NPo was determined from ∼30 s to 1 min of current recording. The single channel current tracings shown in the figures were filtered at 1 kHz. In experiments using cell-attached and inside-out patches, pipette and bath solutions contained (mm): 140 KCl, 1 MgCl2, 5 EGTA, 10 glucose and 10 Hepes (pH 7.3). [Mg2+] was altered to desired levels when needed. In other cell-attached recordings the bath solution contained (mm): 135 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 glucose and 10 Hepes (pH 7.3). An outside-out patch was formed from the whole-cell configuration by gently lifting up the pipette. Na2ATP (1 mm) was added to the pipette solution when using outside-out patches. For statistics, Student's t-test (for comparison of two sets of data) and one-way analysis of variance with Bonferroni's correction (for comparison of three data sets) were used with P < 0.05 as the criterion for significance. Data were analysed using the Origin program (OriginLab Corp., Northampton, MA, USA) and represented as means ±s.d. Initial experiments showed that TASK-like channel activity in CB cells was not sensitive to temperature (24°C vs. 36°C) in cell-attached patches, as reported previously for cloned TASKs (Kang et al. 2005).Therefore, all subsequent experiments were done at room temperature of ∼24°C.
Hypoxia studies
Cell-attached patches were formed on CB cells and perfused with a bicarbonate-buffered solution containing 117 mm KCl, 23 mm KHCO3, 1 mm MgCl2, 11 mm glucose and 10 mm Hepes and gassed with 5% CO2–95% air mixture (normoxia) for at least 60 min. After steady state channel activity was obtained, the perfusion solution was switched to solution gassed (for at least 60 min) with 5% CO2–95% N2 mixture (hypoxia) for ∼3–5 min and then back to normoxic solution. The pipette solution contained (mm): 140 KCl, 0 or 1 MgCl2, 5 EGTA, 10 glucose and 10 Hepes (pH 7.3). The temperature of the perfusion solutions was kept at 32 ± 1°C, and the rate of perfusion was ∼2.2 ml min−1. Oxygen content of the solutions was checked using an oxygen meter (ISO2, WPI, Sarasota, FL, USA) that was calibrated to 0% with solution gassed with pure nitrogen for 60 min and to 21% with solution gassed with air for 60 min at 32°C. The reading on the meter for the hypoxic solution inside the recording chamber used in this study ranged from 2 to 3%.
Results
Single K+ channels in CB cells
Enzymatic dissociation of rat CB yielded isolated single round cells as well as clusters of 3–10 cells consisting of cells of slightly different diameters. Relatively large round cells within each cluster were chosen, and cell-attached patches were formed with pipette solution containing 140 mm KCl and bath solution containing 5 mm KCl. At the pipette potential of 0 mV, single channel openings in the inward current direction were present in nearly all patches (Fig. 1A). At the pipette potential of −60 mV, no measurable channel current was present, indicating that the resting membrane potentials of the cells were close to −60 mV (range was −55 to −60 mV). At the pipette potential of −120 mV (membrane potential would be ∼+60 mV), outward currents of smaller unitary conductance were recorded. Expanded current tracings at three pipette potentials are shown in Fig. 1A. In these initial experiments, many of the patches exhibited single channel openings with multiple conductance levels, as illustrated by the expanded current tracings. For the sake of clarity, we will hereafter use the estimated membrane potential values, rather than the pipette potential values, assuming that the resting Em is −60 mV and 0 mV in 5 mm and 140 mm external KCl, respectively.
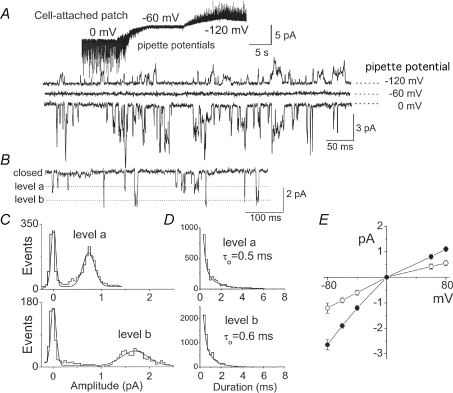
Figure 1. Properties of single channel openings in cell-attached patches.
A, current recording from a cell-attached patch formed in bath solution containing 5 mm KCl and pipette solution containing 140 mm K+. The pipette potential was initially set at 0 mV and changed to −60 mV and −120 mV to record inward and outward currents. Expanded current tracings recorded at the three pipette potentials are shown. B, current recording from a cell-attached patch with lower channel activity showing two open levels designated ‘level a’ and ‘level b’. Bath and pipette solutions contained 140 mm KCl, and the membrane potential was set at −60 mV to record inward current. C, amplitude histograms of channel openings of the two open levels were analysed separately. D, open time duration histograms of channel openings of the two open levels. E, current–voltage relationships of the two open levels. Each point is the mean ±s.d. of 4 determinations.
The conductance levels of single channel openings could be more clearly determined by forming cell-attached patches using pipettes with smaller tip diameter and thus with lower channel activity (Fig. 1B). In these experiments, both the bath and pipette solutions contained 140 mm KCl, and therefore the reversal potential recorded was always ∼0 mV. The presence of two conductance levels was obvious from such tracings recorded at the membrane potential of −60 mV, and are indicated by ‘level a’ and ‘level b’. Amplitude histograms of the two levels analysed separately yielded unitary conductance levels of ∼14 ± 1 pS and ∼32 ± 2 pS (Fig. 1C). Mean open times obtained from duration histograms were 0.5 ± 0.1 ms and 0.6 ± 0.1 ms for the 14 pS and 32 pS channels, respectively (Fig. 1D). The current–voltage relationships obtained at 140 mm KCl were mildly inward rectifying for both conductance levels (Fig. 1E). Both channels were K+ selective, as judged by the 16 ± 2 mV and 34 ± 3 mV shifts when the [K+] in the pipette solution was reduced from 140 mm to 70 mm and 35 mm, respectively. These kinetic properties are similar to those of cloned TASK-1 and TASK-3 characterized previously (Duprat et al. 1997; Kim et al. 1999, 2000). These K+ channels in CB cells are hereafter referred to as TASK-like K+ channels.
pH sensitivity of TASK-like K+ channels
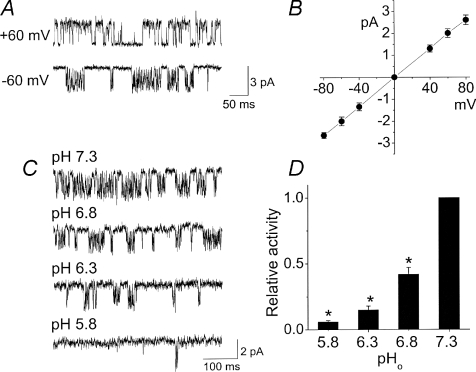
A well-defined property of TASKs is their high sensitivity to changes in extracellular pH (pHo) especially in the acidic range (Duprat et al. 1997; Kang et al. 2004). As this property has not been tested previously at the single channel level, the pHo sensitivity of TASK-like K+ channels in CB cells was studied using outside-out patches held at −60 mV, and switching the pH of the bath solution from 7.3 to 5.8, 6.3 and then back to 7.3 (Fig. 2A). In other patches, pH of the bath solution was switched from 7.3 to 6.8, and then to pH 7.3. Channel openings at expanded scale are shown in Fig. 2B. The relative activity of the channels present in a patch was determined by setting the threshold for channel analysis to catch ∼14 pS or ∼32 pS channel, normalizing the values obtained by multiplying by respective amplitudes (0.9 pA and 1.9 pA), and then adding the final two results. The plot of channel activity as a function of pHo showed that the TASK-like K+ channels were strongly inhibited by acid (Fig. 2C). These results show that TASK-like K+ channels in CB cells possess the pH sensitivity of the TASKs, and support the earlier identification of the pH-sensitive whole-cell current in these cells (Buckler et al. 2000).
Figure 2. Extracellular pH dependence of TASK-like channels.
A, current recording from an outside-out patch showing TASK-like channels. pH of the bath solution was switched as shown. Membrane potential was set at −60 mV to record inward current. B, expanded current tracings at different pH values. Dotted lines indicate two conductance levels. C, channel activity plotted as a function of extracellular pH. Asterisk indicates significance difference from the control value obtained at pH 7.0 (P < 0.05).
Modulation of single channel conductance levels by extracellular Mg2+ concentration
The single channel conductance of TASK-1 is relatively insensitive to changes in extracellular [Mg2+], whereas that of TASK-3 is strongly affected (Musset et al. 2006). These properties of TASKs were used to further determine the molecular identity of TASK-like K+ channels in CB cells. First, single channel conductance levels of cloned rat TASK-1, TASK-3 and TASK-1/3 heteromer were determined at three different extracellular [Mg2+] in the pipette of cell-attached patches in bath solution containing 140 mm KCl (Fig. 3). The membrane potential was held at −60 mV. As reported previously, the single channel amplitude of TASK-1 was insensitive to changes in [Mg2+], staying at ∼0.9 pA (∼15 pS; Fig. 1A). In contrast, the mean amplitudes of TASK-1/3 heteromer were 2.5 pA, 1.9 pA and 1.6 pA when [Mg2+] in the pipette solution was 0 mm, 1 mm and 2 mm, respectively (Fig. 3B). For TASK-3, the amplitudes were 4.2 pA, 1.9 pA and 1.4 pA when [Mg2+] in the pipette solution was 0 mm, 1 mm and 2 mm, respectively (Fig. 3C). Thus, the conductance levels of both TASK-1/3 and TASK-3 were significantly altered by changes in [Mg2+], with greater changes observed with TASK-3.
Figure 3. Mg2+ dependence of unitary conductance of TASKs.
A, channel openings recorded from cell-attached patches with pipette solution containing 0, 1 or 2 mm Mg2+ for TASK-1 expressed in HeLa cells. Bath and pipette solutions contained 140 mm KCl, and the membrane potential was held at −60 mV. Dotted lines show open levels. Amplitude histograms were obtained for each recording and the mean amplitude plotted as a function of [Mg2+]. Each bar is the mean ±s.d. of 5 determinations. Asterisks indicate significant difference from the value observed at 1 mm Mg2+ at the level of 0.05. B, same for TASK-1/3 heteromer. C, same for TASK-3.
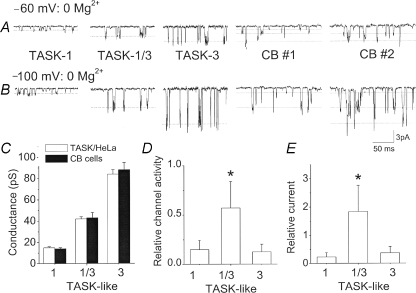
The largest differences in conductance levels among the three TASK isoforms are observed at 0 mm Mg2+. To determine which TASK isoform represents the TASK-like K+ channel in CB cells, single channels from cell-attached patches of CB cells were recorded with pipette solution containing 0 mm Mg2+ at membrane potentials of −60 and −100 mV, and compared to those of three TASK isoforms (Fig. 4). Each TASK isoform exhibited one main amplitude level at each membrane potential, and the amplitude at −100 mV was significantly greater than at −60 mV as expected. Single channel recordings from cell-attached patches formed on CB cells showed three different levels, corresponding to the amplitude levels of the three TASK isoforms. For example, in CB cell no. 1 at −60 mV, three different levels are observed as indicated by dotted lines. At −100 mV, CB cell no. 1 clearly shows channel openings whose amplitude is similar to those of TASK-1/3 heteromer and TASK-1. In CB cell no. 2, amplitudes similar to those of TASK-1/3 and TASK-3 are present. The three conductance levels of the TASK-like channels in CB cells were similar to those of cloned TASKs expressed in HeLa cells (Fig. 4C). The three different conductance levels in CB cells were analysed separately to obtain channel activities for each level by setting the amplitude range to be within ±25% of each mean level. Results of recordings from 14 CB cells showed that the amplitude corresponding to TASK-1/3 heteromer was most frequently observed, as shown in Fig. 4D, which plots the channel activity of the three conductance levels. Multiplying channel activity (NPo) by the current amplitude (i) provides an estimate of the channel current (I) contributed by each level. Determination of the channel current contributed by the three conductance levels showed that the TASK-1-like, TASK-1/3-like and TASK-3-like channels contribute 10%, 75% and 15% of the total TASK-like current measured at hyperpolarized membrane potentials (Fig. 4D).The contributions of TASK-1/3-like channels were similar in CB cells prepared from 7- to 8-day-old (76 ± 5%) and from 12- to 14-day-old rats (74 ± 4%). Although outward currents at depolarized potentials were not analysed due to difficulty in separating the three conductance levels, the relative contributions are likely to be similar, as the ratio of outward current at +60 mV to inward current at −60 mV is similar for the three TASK channels (Kang et al. 2004). These results provide evidence that the TASK-1/3 heteromer is the major TASK isoform present in the enzymatically isolated CB cells used in this study.
Figure 4. Mg2+ dependence of unitary conductance of TASK-like channels in carotid body cells.
A, channel openings recorded from cell-attached patches with pipette solution containing no Mg2+ for TASK-1, TASK-1/3 heteromer, TASK-3 and CB cells at membrane potential of −60 mV as shown. Single channel openings from two CB cells are shown. Bath and pipette solutions contained 140 mm KCl. B, same as in A except that the membrane potential was set at −100 mV. C, three conductance levels of TASK isoforms expressed in HeLa cells, and those determined from CB cells. Each bar is the mean ±s.d. of 6 determinations. No significant difference was present within each group (P > 0.05). D, channel amplitudes in CB cells determined and binned according to their amplitude levels. Channels showing amplitude levels similar to TASK-1 (0.6–1.0 pA at −60 mV; 1.0–1.7 pA at −100 mV), TASK-1/3 heteromer (2.0–3.0 pA at −60 mV; 3.5–5.5 pA at −100 mV) and TASK-3 (3.5–4.8 pA at −60 mV; 7.0–10 pA at −100 mV) were analysed for channel activity (NPo). Each bar is the mean ±s.d. of 14 determinations. Significance is denoted by an asterisk (P < 0.05). E, channel activity determined from D multiplied by the single channel amplitude (i) at −60 mV to estimate the relative current (iNPo) contributed by each type of TASK-like channels in CB cells at −60 mV. Each bar is the mean ±s.d. of 14 determinations. Significance is denoted by an asterisk (P < 0.05).
Inhibition of TASK-like K+ channels by ruthenium red and methanandamide
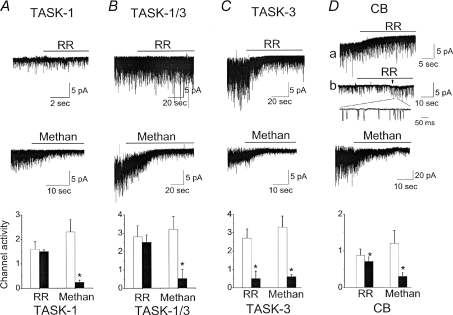
Earlier studies showed that TASK-1 and TASK-1/3 are insensitive to ruthenium red, whereas TASK-3 is strongly inhibited due to the presence of negatively charged residues at the extracellular region between the first transmembrane segment and the first pore region of TASK-3 (Czirjak & Enyedi, 2003; Kang et al. 2004). These effects of ruthenium red (5 μm) on TASK isoforms were confirmed in outside-out patches from HeLa cells expressing each TASK isoform (Fig. 5A–C). In CB cells, ruthenium red produced a small (18%), but significant, inhibition of the TASK-like channel activity, in keeping with our finding that TASK-3-like channels provide only a small fraction of the total TASK-like current. Figure 5D shows two outside-out patch recordings with high and low channel activities. In both patches (a and b), ruthenium red produced only a small inhibition, and the 32 pS channels were still active after ruthenium red application (shown in expanded tracing of patch b), indicating that the major isoform present in CB cells is the TASK-1/3 heteromer.
Figure 5. Effect of ruthenium red and methanandamide on TASKs and TASK-like channels in CB cells.
A, channels recorded from outside-out patches from HeLa cells expressing TASK-1. Membrane potential was held at −60 mV. Bath and pipette solution contained 140 mm KCl and 1 mm Mg2+. Ruthenium red (RR; 5 μm) or methanandamide (Methan; 5 μm) was applied to the bath perfusion solution as shown. Bar graph shows channel activity before (open bars) and after drug application (filled bars). Each bar is the mean ±s.d. of 5 determinations, and significance (P < 0.05) is denoted with an asterisk. Paired t tests were used. B–C, same as in A except that the cells express TASK-1/3 heteromer (B) or TASK-3 (C). D, same as in A except that CB cells are used. Two tracings for RR are shown (a and b). In the upper tracing, some patches have baseline currents that are not close to zero, probably due to some membrane leak causing a DC shift. RR reduced these baseline currents. In the bottom tracing (b), the membrane potential was changed from −60 mV to −100 mV during application with RR at arrow. Expanded current tracing is shown in the inset.
Anandamide and its non-metabolizable derivative methanandamide were initially reported to be potent inhibitors of TASK-1 but not TASK-3 (Maingret et al. 2001). A later study reported, however, that methanandamide was a potent blocker of TASK-3 as well (Veale et al. 2007). Our own test of the effect of methanandamide of the three TASK isoforms showed that it is a potent inhibitor of all three isoforms (Fig. 5). Therefore, methanandamide was not a selective inhibitor of TASK-1 and could not be used to test for the presence of TASK-1. In agreement with these findings, methanandamide produced a strong inhibition of TASK-like channels in CB cells when applied to the bath solution in outside-out patches (Fig. 5D). As expected of a lipid soluble molecule, methanadamide produced a similar degree of inhibition of TASKs when applied to the bath solution in inside-out patches or cell-attached patches (not shown). The inhibition of all TASK-like channels by methanandamide is consistent with the earlier observation that methanandamide abolished the hypoxia-sensitive leak current in CB type I cells (Wyatt & Buckler, 2003).
Inhibition of TASK-like channels in CB cells by hypoxia
The hypoxic sensitivity of TASK-like channels expressed in CB cells was first studied by perfusion of cell-attached patches with pipette solution containing 1 mm Mg2+. After a steady state channel activity was reached in normoxic medium (∼3 min of perfusion), the perfusion was switched to the hypoxic medium (Fig. 6A). This resulted in a decrease in channel activity that occurred over several seconds and remained decreased as long as hypoxia was present. Channel activity recovered after switching back to the normoxic medium. Expanded single channel openings ∼30 s before and ∼30 min after application of the hypoxic medium are shown in Fig. 6B. The hypoxic medium produced a strong inhibition of all TASK-like channels, showing that hypoxia inhibited all isoforms of TASKs including heteromeric TASK-1/3. Hypoxia reduced the ∼14 pS channel (TASK-1) and ∼32 pS channel (TASK-1/3 and TASK-3) to 23 ± 5% and 18 ± 4% (n= 4) of control levels observed in normoxic solution, respectively. As the majority (75%) of the ∼30 pS channel openings represents the TASK-1/3 heteromer in the CB cells used in this study, this showed that hypoxia produced a strong inhibition of the heteromer. When the pipette solution contained no Mg2+, TASK-1/3-like channels represented by ∼70 pS channels (dotted line) were also strongly inhibited by the hypoxic solution (Fig. 6C).
Figure 6. Sensitivity of TASK-like channels in CB cells to hypoxia.
A, cell-attached patch was perfused with normoxic, hypoxic and then back to normoxic solution. Pipette contained 140 mm K+ and 1 mm Mg2+, and bath solution contained 140 mm KHCO3. Cell membrane potential was held at −60 mV. B, expanded single channel openings in normoxic and hypoxic solutions. Pipette and bath solution contained 1 mm Mg2+. The bar graph shows normalized channel activity and standard deviation under two conditions at steady state. Significance indicated by an asterisk (P < 0.05). Dotted line indicates the ∼32 pS level. Cell membrane potential was held at −60 mV. C, expanded single channel openings in normoxic and hypoxic conditions. Pipette solution contained 0 mm Mg2+. Dotted line indicates the ∼42 pS level. Cell membrane potential was held at −60 mV.
Other background K+ channels in CB cells
In addition to TASK-like K+ channels, three other background K+ channels were recorded in cell-attached patches of CB cells in symmetrical 140 mm KCl: 34 pS,180 pS and 78 pS channels. The 34 pS channel was active in cell-attached and inside-out patches, and showed a conductance similar to that of TASK-3 when the current was inward. Therefore, it could easily be ignored if only the inward currents were recorded. Figure 7A shows the opening of the 34 pS channel in an inside-out patch after TASK activity had run down. This 34 pS K+ channel showed bursts of openings that were longer in duration than those of TASKs. The current–voltage relationship was linear (Fig. 7B), with the outward current openings showing longer open time durations and less open channel noise. These biophysical properties of the 34 pS K+ channel are similar to that of the previously reported cerebellar granule ‘Type 4 K+ channel’, which is highly sensitive to change in extracellular pH (Han et al. 2002). Test of the pH sensitivity of the 34 pS channel showed that indeed it was strongly inhibited by acid (Fig. 7C–D), similar to TASKs.
Figure 7. A non-TASK, pH-sensitive background K+ channel.
A, channels recorded from cell-attached patches with membrane potential of +60 mV and −60 mV in bath and pipette solutions containing 140 mm KCl. B, current–voltage relationship. C, channel current was recorded in outside-out patch at different extracellular pH. D, channel activity observed at pH 7.3 was taken as 1.0, and the relative activity recorded at different pH values is plotted. Each bar is the mean ±s.d. of 4 determinations. Significance from the control value obtained at 7.3 is denoted by an asterisk (P < 0.05).
The 34 pS channel was recorded in 7 of 122 patches (6%) and therefore is a minor background K+ channel in CB cells. Based on averaged channel activity of the 34 pS channel at −60 mV, its contribution was 2.2% of that of TASKs.
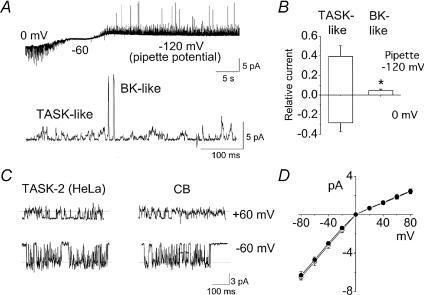
In cell-attached patches with pipette solution containing 140 mm KCl and bath solution containing 5 mm KCl, TASK-like K+ channels are observed in most patches as described above (see Fig. 1) and also shown in Fig. 8A. At the membrane potential of +60 mV (pipette potential of −120 mV), a 180 pS, large conductance channel was observed in 24% of patches (29/122 patches). No opening of such channel was ever observed at membrane potential negative to −60 mV (inward current). The frequency of opening of the 180 pS channel varied among patches, but was usually low compared with those of TASKs, as shown in the expanded tracing of Fig. 8A. This 180 pS channel was K+ selective, as judged by a −33 ± 2 mV shift in reversal potential by a reduction of [K+]o from 140 mm to 35 mm in outside-out patches. The 180 pS channel was inhibited by application of iberiotoxin (1 μm; n= 3), a specific blocker of the BK channel. Analysis of the channel currents (averaged NPoi) contributed by TASK-like and BK-like channels showed that the K+ current contributed by BK channel was ∼12% compared to that of TASKs at a membrane potential +60 mV positive to the reversal potential (Fig. 8B).
Figure 8. Large conductance Ca2+-activated K+(BK) channel and TASK-2-like channel in CB cells.
A, current recording from a cell-attached patch formed in bath solution containing 140 mm KCl and pipette solution containing 5 mm K+. The pipette potential was initially set at 0 mV (Em was −60 mV) and changed to −60 mV (Em was 0 mV) and −120 mV (Em was +60 mV). Large amplitude openings are BK channels and observed only when the current was outward, as shown in the expanded tracing below. B, channel activities of TASK-like channels and BK channels determined from currents recorded at +60 mV (outward current), and then multiplied by 0.8 pA (main amplitude of TASK) and 10 pA (amplitude of BK) to estimate the relative current (I) for the two channel types. Significance is denoted by an asterisk (P < 0.05). C, channels recorded from cell-attached patches of HeLa cells expressing cloned rat TASK-2 and in CB cells. D, current–voltage relationships of TASK-2 and TASK-2-like K+ channel in CB cells. Each point is the mean ±s.d. of 3 determinations.
The third K+ channel showed single channel properties similar to those of TASK-2, as judged by the comparison of the cloned TASK-2 expressed in HeLa cells and TASK-2-like channel in CB cells (Fig. 8C). In Fig. 8C, channel openings shown are from inside-out patches that illustrate more clearly the TASK-2-like channel after TASK channels have rundown. The single channel conductance levels of TASK-2 were 78 pS at +60 mV and 34 pS at −60 mV, producing a mildly inwardly rectifying K+ channel, as previously reported (Kang & Kim, 2004). TASK-2 also showed long opening bursts and fluctuating open level amplitude within each burst. These properties of TASK-2 closely resemble the 78 pS channels recorded in CB cells. TASK-2-like channels were recorded in only 5 out of 122 patches (4%), indicating that the TASK-2-like channel makes a negligible contribution to the net background K+ conductance. These findings show that TASK-like K+ channels are the major background K+ conductance in isolated rat CB cells used in this study. In CB cells, another ion channel with a unitary conductance of ∼8 pS at the membrane potential of −60 mV (inward current) and negligible outward current at +60 mV in cell-attached patches was also observed in 13% of patches (15 of 122 patches; Fig. 8E). Due to the low conductance and low expression level of this channel (usually one channel in the patch that expresses it), its contribution to the background K+ conductance would be negligible even if it were a K+ channel, and therefore the biophysical properties of this channel were not studied further.
Discussion
TASK-like background K+ channels are highly expressed in rat CB glomus cells, and their inhibition by hypoxia is believed to help elicit the cellular response necessary for excitation of afferent nerve fibres and regulation of respiration (Buckler, 2007). TASKs are also inhibited by acidosis and neurotransmitters via G proteins, and therefore may serve to regulate CB function under various physiological and pathophysiological conditions (Talley & Bayliss, 2002; Kim, 2005). Although the background K+ channel in isolated rat CB is TASK-like, it was reported to possess properties that are similar to neither TASK-1 nor TASK-3, suggesting that it could possibly be a different K+ channel. Therefore, the biophysical properties of the TASK-like K+ channels in rat CB neurons were re-examined using different concentrations of Mg2+, which alter the single channel conductance levels of TASKs, and using ruthenium red, which produces inhibition of TASK-1 but not TASK-3. From these experiments, evidence was obtained indicating that the TASK-like K+ channels in rat CB glomus cells consist of TASK-1, TASK-3 and TASK-1/3 heteromer. Our studies show that the channel with properties indistinguishable from those of TASK-1/3 heteromer is the major conductance present in these cells, contributing ∼75% of the total TASK-like K+ current measured in isolated CB cells. Furthermore, hypoxia strongly inhibited TASK-1/3-like channels in CB cells, as it did TASK-1-like channels.
Expression of TASK channels in CB cells
TASK-1 and TASK-3 are functional members of the two-pore domain, acid-sensitive K+ channel subfamily (Duprat et al. 1997; Kim et al. 1999). In situ hybridization and Northern blot analysis show that their mRNAs are expressed at low levels in many regions of the brain, but are highly expressed in such tissues as motor neurons, cerebellar granule neurons and the carotid body (Buckler et al. 2000; Brickley et al. 2001; Talley et al. 2001; Yamamoto et al. 2002). According to immunohistochemical analysis, TASK-1 immunoreactivity is expressed in nearly all cell types found in the CB, whereas that of TASK-3 is found only in Type I and vascular smooth muscle cells in CB (Yamamoto et al. 2002). The expression of TASK-1 and TASK-3 mRNA in rat CB was also shown by RT-PCR (Kim et al. 2006). Therefore, there is strong evidence showing that TASK-1 and TASK-3 are both expressed at the mRNA and protein levels in the rat CB.
Earlier studies reported the presence of an oxygen-sensitive, background K+ current in rat CB type I cells and the associated single channels that resembled TASK-1 (Buckler, 1997, 1999; Williams & Buckler, 2004). Like cloned TASK-1, the conductance of single channels recorded from CB glomus cells was ∼15 pS, and the channel openings were brief (Buckler et al. 2000; Williams & Buckler, 2004). Sensitivity of the TASK-like current in CB cells to acid, barium, zinc, bupivacaine, halothane and quinidine, or lack of sensitivity to tetraethylammonium, 4-aminopyridine and apamin was also similar to that reported for cloned TASK-1. Thus, it was justifiably concluded that the oxygen-sensitive K+ channel in rat CB glomus cells was likely to be TASK-1 expressed endogenously. Further characterization of the 15 pS channel in CB glomus cells, however, showed that the single channel conductance was increased to 28 pS by a reduction of external [Mg2+] to near 0 mm, indicating that the 15 pS channel was more like TASK-3 whose unitary conductance is dependent on divalent cation concentration (Musset et al. 2006). Thus, if the biophysical and pharmacological properties of the 15 pS channel are unlike those of TASK-1 and TASK-3, what is the true identity of the K+ channel in the rat CB cells?
In earlier studies (Buckler et al. 2000; Williams & Buckler, 2004), the single channel recordings were done in external solution containing 4 mm Mg2+. At this high [Mg2+], the single channel conductance of TASK-3 would be reduced to ∼15 pS, and resemble that of TASK-1. Therefore, the use of high [Mg2+] in earlier studies was probably the reason that different conductance levels of TASK isoforms were not identified. The results of our study performed at 0–2 mm Mg2+ provide evidence for the presence of both TASK-1- and TASK-3-like channels. The different sensitivity of the TASK isoforms to Mg2+ also allowed identification of TASK-1/3 heteromer-like channels in CB cells. Because TASK-1 and TASK-3 can heteromerize to form a functional isoform (Czirjak & Enyedi, 2002; Berg et al. 2004), it is not surprising that TASK-1/3-like channels are found in CB glomus cells. The heteromeric TASK-1/3 construct used here is not structurally identical to what is actually produced in the native CB cells. For example, the structure of the cytoplasmic regions would be constrained in the heteromeric constructs, because the end of the C-terminus was joined to the start of the N-terminus. Therefore, some caution is necessary in interpreting the data obtained using the TASK-1/3 heteromeric construct. Our results show that the channel with properties similar to the TASK-1/3 heteromer provides the major part of the TASK-like background conductance in CB glomus cells. This is further supported by the persistent opening of the 32 pS channel in the presence of ruthenium red that inhibits TASK-3 but not TASK-1/3 heteromer. These findings in CB cells are reminiscent of those observed in the cerebellar granule neurons that express both TASK-1 and TASK-3 mRNA transcripts and exhibit functional TASK-1/3 heteromeric channel activity (Kang et al. 2004).
In our study, the distinction between Type I (glomus) and Type II cells in the rat CB was based on earlier reports that relatively large diameter cells within a cluster of cells are mostly Type I cells, which are hypoxia sensitive (Urena et al. 1989). More than 90% of the cells chosen in such a way showed TASK-like activity in our studies. When membrane patches were depolarized to +60 mV, opening of BK channels was observed together with TASKs in some patches, also suggesting that TASKs are expressed in Type I cells, as BK is expressed in Type I cells (Wyatt & Peers, 1995). Immunohistochemical studies have shown that Type II cells do not express TASK-3 (Yamamoto et al. 2002), whereas most patches chosen in this study showed TASK-3-like channels. Therefore, although the intracellular Ca2+ response of each CB cell to hypoxia was not tested, the cells chosen are likely to be mainly Type I (glomus) cells. Although not tested extensively, some of the smaller diameter cells within the cell clusters also showed opening of TASK channels, perhaps suggesting that they are also of Type I origin. Further studies on the expression of TASKs and other ion channels in Type II cells would help us to better understand the differential expression of ion channels in the two types of cells.
Other K+ channels that contribute to the background K+ conductance in CB cells
CB expresses a number of other K+ channels including BK, TASK-2, TREK-1, HERG and Kir channels (Wyatt & Peers, 1994; Overholt et al. 2000; Yamamoto et al. 2002; Yamamoto & Taniguchi, 2006; Yamamoto et al. 2008). This would suggest that these K+ channels could be functionally expressed and provide part of the background K+ current. In CB cells used in this study, three additional K+ channels were found to be functionally active: a pH-sensitive channel (34 pS) whose molecular identity is not yet known, a BK-like channel (∼180 pS), and a TASK-2-like channel (∼78 pS), as shown in Figs 6 and 7. Among the three K+ channels, the 180 pS channel, identified as BK, was the most frequently observed, and showed significant activity at membrane potentials positive to resting Em. The activity of the BK-like K+ channel was voltage dependent, with increasing activation at more depolarized potentials. The BK channel has been reported to be sensitive to hypoxia and its inhibition to elicit excitation of the CB (Riesco-Fagundo et al. 2001; Lewis et al. 2002; Peers & Wyatt, 2007). However, the lack of effect of BK channel inhibitors on hypoxia-induced increase in [Ca2+] in CB glomus cells and in excitation of afferent nerve has also been reported (Buckler, 1997; Lahiri et al. 1998; Donnelly, 1999). Our finding that the BK activity is present, albeit smaller than those of TASKs, suggests that BK channels participate in hypoxic depolarization of CB cells, and their role becomes more important as the cell depolarizes. As TASKs and BK channels are the two most frequently recorded channels in our study, it seems quite likely that both channels are involved in hypoxia-induced depolarization, with the degree of contribution depending on the membrane potential and cytosolic [Ca2+]. The relative roles of TASK and BK channels in oxygen sensing will likely also depend on factors such as the age of the animal, species and degree of hypoxia. It is important to recognize that the results of our studies in isolated cells may not be the same as those in intact CB where the glomus cells fire spontaneously at some basal rate, and ion channels are under regulation by various neurotransmitters and second messengers present in the system (Peers & Wyatt, 2007).
In addition to BK, a 34 pS K+ channel that has a linear current–voltage relationship was identified in CB cells, as shown in Fig. 6. Interestingly, the 34 pS K+ channel exhibited kinetic properties of a previously characterized K+ channel named ‘Type 4 K+ channel’ in cerebellar granule neurons (Han et al. 2002). Although the molecular identity of the Type 4 K+ channel is unknown, this K+ channel is highly sensitive to external pH, similar to that of TASKs. Unlike in CB cells, this 34 pS channel was a prominent background K+ channel in cerebellar granule neurons (Han et al. 2002). In CB cells from very young rats (P7–P14) used in this study, this 34 pS channel was a minor K+ channel, but whether its expression is increased in adults or in the newborn will need to be investigated.
Role of TASK-like K+ channels in the control of breathing
As TASKs are inhibited by hypoxia (Lewis et al. 2001; Buckler, 2007), it is reasonable to hypothesize that TASKs mediate hypoxia-induced depolarization and subsequent firing of afferent nerves to regulate ventilation. This hypothesis was recently tested using TASK-1−/− and TASK-3−/− mice in two studies. In the first study, control and double knockout mice were exposed to 10% oxygen to produce a hypoxic atmosphere; however, similar hyperventilation was observed in both groups, suggesting that TASKs are not required for the peripheral chemosensory response (Mulkey et al. 2007). In the second study, ventilatory response to 10% oxygen (hypoxia) was significantly reduced in TASK-1−/− mice but not in TASK-3−/− mice (Trapp et al. 2008). The peak firing rate of chemoafferent fibres from carotid body/sinus nerve preparation in response to hypoxia was also significantly reduced in TASK-1−/− mice compared with wild-type mice (Trapp et al. 2008). The discharge frequency of the chemoafferent fibres in response to hypoxia in double knockout mice was similar to that of TASK-1−/− mice. These results have led to the conclusion that TASK-1 and not TASK-3 is involved in peripheral chemoreception (Trapp et al. 2008). It is interesting that the CB response to hypoxia was blunted but not abolished even in double knockout mice, indicating that peripheral oxygen-sensing mechanisms other than inhibition of TASKs also contribute to the hypoxic response. In both studies, hypercapnic ventilatory response was found to be similar in control and double knockout mice, indicating that TASKs are not involved in central chemoreceptor function.
Because of different findings by the two studies regarding the role of TASKs in peripheral chemoreception, the role of TASKs is still uncertain. As suggested by Trapp et al. (2008), the ventilatory response measured at different baseline chemoafferent activities in the control and knockout mice could potentially account for the different results. A better animal model would be one in which TASKs are replaced with an oxygen-insensitive K+ channel so that the resting membrane potential and the baseline nerve activity are similar to those of control animals. As two studies used mice of different age groups (3–4 months vs. 4–8 months), it is possible that the expression of TASKs is age dependent, as in cerebellar granule neurons. Our studies in the rat show that all three TASK isoforms are functionally expressed in CB glomus cells, with a relatively high expression of the TASK-1/3 heteromer. Genetic deletion of TASK-1 would abolish the formation of the TASK-1/3 heteromer, and only TASK-3 would be functional. Similarly, genetic deletion of TASK-3 would result in functional expression of only TASK-1, as TASK-1/3 heteromer cannot form. Thus, one would predict that deletion of either TASK-1 or TASK-3 would reduce the sensitivity of CB glomus cells to hypoxia, if no other compensations were to occur. Perhaps the levels of expression of TASKs and other background K+ channels in the rat and mice are not the same, or other compensatory changes occur that have not been identified. Additional studies are needed to clearly identify background K+ channels in different species to better understand the role of TASKs in chemoreception.
Acknowledgments
This work was funded by NIH grant HL054621.
Author contributions
D.K. and E.J.C. designed and performed the experiments, analysed the data and wrote the manuscript (Rosalind Franklin University, North Chicago, IL, USA). J.L.C. and I.K. initiated the study of TASK channels in CB neurons, helped with the isolation procedure for CB cells, discussed and reviewed experiments and the manuscript throughout the study (University of Arkansas, Little Rock, AR, USA).
References
- Berg AP, Talley EM, Manger JP, Bayliss DA. Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J Neurosci. 2004;24:6693–6702. doi: 10.1523/JNEUROSCI.1408-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Buckler KJ. A novel oxygen-sensitive potassium current in rat carotid body type I cells. J Physiol. 1997;498:649–662. doi: 10.1113/jphysiol.1997.sp021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ. Background leak K+-currents and oxygen sensing in carotid body type 1 cells. Respir Physiol. 1999;115:179–187. doi: 10.1016/s0034-5687(99)00015-8. [DOI] [PubMed] [Google Scholar]
- Buckler KJ. TASK-like potassium channels and oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2007;157:55–64. doi: 10.1016/j.resp.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Buckler KJ, Williams BA, Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525:135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L, Takimoto K, Petrovic M, Pongs O, Millhorn D. The pore region of the Kv1.2α subunit is an important component of recombinant Kv1.2 channel oxygen sensitivity. Biochem Biophys Res Commun. 2003;306:450–456. doi: 10.1016/s0006-291x(03)00989-6. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Enyedi P. Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore domain potassium channel subunits. J Biol Chem. 2002;277:5426–5432. doi: 10.1074/jbc.M107138200. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Enyedi P. Ruthenium red inhibits TASK-3 potassium channel by interconnecting glutamate 70 of the two subunits. Mol Pharmacol. 2003;63:646–652. doi: 10.1124/mol.63.3.646. [DOI] [PubMed] [Google Scholar]
- Donnelly DF. K+ currents of glomus cells and chemosensory functions of carotid body. Respir Physiol. 1999;115:151–160. doi: 10.1016/s0034-5687(99)00021-3. [DOI] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfornina MD, Lopez-Barneo J. Potassium channel types in arterial chemoreceptor cells and their selective modulation by oxygen. J Gen Physiol. 1992;100:401–426. doi: 10.1085/jgp.100.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Gnatenco C, Sladek CD, Kim D. Background and tandem-pore potassium channels in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 2003;546:625–639. doi: 10.1113/jphysiol.2002.032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Truell J, Gnatenco C, Kim D. Characterization of four types of background potassium channels in rat cerebellar granule neurons. J Physiol. 2002;542:431–444. doi: 10.1113/jphysiol.2002.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Choe C, Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J Physiol. 2005;564:103–116. doi: 10.1113/jphysiol.2004.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Han J, Talley EM, Bayliss DA, Kim D. Functional expression of TASK-1/TASK-3 heteromers in cerebellar granule cells. J Physiol. 2004;554:64–77. doi: 10.1113/jphysiol.2003.054387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DW, Kim D. Single-channel properties and pH sensitivity of two-pore domain K+ channels of the TALK family. Biochem Biophys Res Commun. 2004;315:836–844. doi: 10.1016/j.bbrc.2004.01.137. [DOI] [PubMed] [Google Scholar]
- Kim D. Physiology and pharmacology of two-pore domain potassium channels. Curr Pharm Des. 2005;11:2717–2736. doi: 10.2174/1381612054546824. [DOI] [PubMed] [Google Scholar]
- Kim I, Kim JH, Carroll JL. Postnatal changes in gene expression of subfamilies of TASK K+ channels in rat carotid body. Adv Exp Med Biol. 2006;580:43–47. doi: 10.1007/0-387-31311-7_7. discussion 351–359. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang H, Kim D. TBAK-1 and TASK-1, two-pore K+ channel subunits: kinetic properties and expression in rat heart. Am J Physiol Heart Circ Physiol. 1999;277:H1669–1678. doi: 10.1152/ajpheart.1999.277.5.H1669. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang H, Kim D. TASK-3, a new member of the tandem pore K+ channel family. J Biol Chem. 2000;275:9340–9347. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Roy A, Rozanov C, Mokashi A. K+-current modulated by PO2 in type I cells in rat carotid body is not a chemosensor. Brain Res. 1998;794:162–165. doi: 10.1016/s0006-8993(98)00276-5. [DOI] [PubMed] [Google Scholar]
- Lewis A, Hartness ME, Chapman CG, Fearon IM, Meadows HJ, Peers C, Kemp PJ. Recombinant hTASK1 is an O2-sensitive K+ channel. Biochem Biophys Res Commun. 2001;285:1290–1294. doi: 10.1006/bbrc.2001.5310. [DOI] [PubMed] [Google Scholar]
- Lewis A, Peers C, Ashford ML, Kemp PJ. Hypoxia inhibits human recombinant large conductance, Ca2+-activated K+ (maxi-K) channels by a mechanism which is membrane delimited and Ca2+ sensitive. J Physiol. 2002;540:771–780. doi: 10.1113/jphysiol.2001.013888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barneo J, Ortega-Saenz P, Pardal R, Pascual A, Piruat JI. Carotid body oxygen sensing. Eur Respir J. 2008;32:1386–1398. doi: 10.1183/09031936.00056408. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lazdunski M, Honore E. The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK-1. EMBO J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musset B, Meuth SG, Liu GX, Derst C, Wegner S, Pape HC, Budde T, Preisig-Muller R, Daut J. Effects of divalent cations and spermine on the K+ channel TASK-3 and on the outward current in thalamic neurons. J Physiol. 2006;572:639–657. doi: 10.1113/jphysiol.2006.106898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholt JL, Ficker E, Yang T, Shams H, Bright GR, Prabhakar NR. HERG-like potassium current regulates the resting membrane potential in glomus cells of the rabbit carotid body. J Neurophysiol. 2000;83:1150–1157. doi: 10.1152/jn.2000.83.3.1150. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E. Molecular physiology of oxygen-sensitive potassium channels. Eur Respir J. 2001;18:221–227. doi: 10.1183/09031936.01.00204001. [DOI] [PubMed] [Google Scholar]
- Peers C, Wyatt CN. The role of maxiK channels in carotid body chemotransduction. Respir Physiol Neurobiol. 2007;157:75–82. doi: 10.1016/j.resp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. O2 sensing at the mammalian carotid body: why multiple O2 sensors and multiple transmitters? Exp Physiol. 2006;91:17–23. doi: 10.1113/expphysiol.2005.031922. [DOI] [PubMed] [Google Scholar]
- Riesco-Fagundo AM, Perez-Garcia MT, Gonzalez C, Lopez-Lopez JR. O2 modulates large-conductance Ca2+-dependent K+ channels of rat chemoreceptor cells by a membrane-restricted and CO-sensitive mechanism. Circ Res. 2001;89:430–436. doi: 10.1161/hh1701.095632. [DOI] [PubMed] [Google Scholar]
- Talley EM, Bayliss DA. Modulation of TASK-1 (Kcnk3) and TASK-3 (Kcnk9) potassium channels: volatile anesthetics and neurotransmitters share a molecular site of action. J Biol Chem. 2002;277:17733–17742. doi: 10.1074/jbc.M200502200. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Aller MI, Wisden W, Gourine AV. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J Neurosci. 2008;28:8844–8850. doi: 10.1523/JNEUROSCI.1810-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urena J, Lopez-Lopez J, Gonzalez C, Lopez-Barneo J. Ionic currents in dispersed chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989;93:979–999. doi: 10.1085/jgp.93.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veale EL, Buswell R, Clarke CE, Mathie A. Identification of a region in the TASK3 two pore domain potassium channel that is critical for its blockade by methanandamide. Br J Pharmacol. 2007;152:778–786. doi: 10.1038/sj.bjp.0707436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BA, Buckler KJ. Biophysical properties and metabolic regulation of a TASK-like potassium channel in rat carotid body type 1 cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L221–230. doi: 10.1152/ajplung.00010.2003. [DOI] [PubMed] [Google Scholar]
- Wyatt CN, Buckler KJ. The effect of methanandamide on isolated type I cells. Adv Exp Med Biol. 2003;536:123–127. doi: 10.1007/978-1-4419-9280-2_15. [DOI] [PubMed] [Google Scholar]
- Wyatt CN, Peers C. Ca2+-activated K(+)-channels from isolated type I carotid body cells of the neonatal rat. Adv Exp Med Biol. 1994;360:159–161. doi: 10.1007/978-1-4615-2572-1_18. [DOI] [PubMed] [Google Scholar]
- Wyatt CN, Peers C. Ca2-activated K+ channels in isolated type I cells of the neonatal rat carotid body. J Physiol. 1995;483:559–565. doi: 10.1113/jphysiol.1995.sp020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Ishikawa R, Omoe K, Taniguchi K. Expression of inwardly rectifying K+ channels in the carotid body of rat. Histol Histopathol. 2008;23:799–806. doi: 10.14670/HH-23.799. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Kummer W, Atoji Y, Suzuki Y. TASK-1, TASK-2, TASK-3 and TRAAK immunoreactivities in the rat carotid body. Brain Res. 2002;950:304–307. doi: 10.1016/s0006-8993(02)03181-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Taniguchi K. Expression of tandem P domain K+ channel, TREK-1, in the rat carotid body. J Histochem Cytochem. 2006;54:467–472. doi: 10.1369/jhc.5A6755.2005. [DOI] [PubMed] [Google Scholar]