Abstract
Developmental refinement of synaptic transmission can occur via changes in several pre- and postsynaptic factors, but it has been unknown whether the intrinsic Ca2+ sensitivity of vesicle fusion in the nerve terminal can be regulated during development. Using the calyx of Held, a giant synapse in the auditory pathway, we studied the presynaptic mechanisms underlying the developmental regulation of Ca2+–secretion coupling, comparing a time period before, and shortly after the onset of hearing in rats. We found an ∼2-fold leftward shift in the relationship between EPSC amplitude and presynaptic Ca2+ current charge (QCa), indicating that brief presynaptic Ca2+ currents become significantly more efficient in driving release. Using a Ca2+ tail current protocol, we also found that the high cooperativity between EPSC amplitude and QCa was slightly reduced with development. In contrast, in presynaptic Ca2+ uncaging experiments, the intrinsic Ca2+ cooperativity of vesicle fusion was identical, and the intrinsic Ca2+ sensitivity was slightly reduced with development. This indicates that the significantly enhanced release efficiency of brief Ca2+ currents must be caused by a tighter co-localization of Ca2+ channels and readily releasable vesicles, but not by changes in the intrinsic properties of Ca2+-dependent release. Using the parameters of the intrinsic Ca2+ sensitivity measured at each developmental stage, we estimate that during a presynaptic action potential (AP), a given readily releasable vesicle experiences an about 1.3-fold higher ‘local’ intracellular Ca2+ concentration ([Ca2+]i) signal with development. Thus, the data indicate a tightening in the Ca2+ channel–vesicle co-localization during development, without a major change in the intrinsic Ca2+ sensitivity of vesicle fusion.
For the correct functioning of neuronal networks, it is crucial that synaptic connections are formed with the appropriate strength and speed, properties which are set up during brain development. At the calyx of Held, a large glutamatergic relay synapse in the auditory brainstem, high speed of synaptic signalling is crucial for auditory information processing (Trussell, 1999), and high speed is enabled via the near-synchronous release of hundreds of transmitter quanta from the large nerve terminal. Calyces of Held are formed at postnatal days (P) ∼2–3 (Kandler & Friauf, 1993; Hoffpauir et al. 2006; Rodriguez-Contreras et al. 2008), but airborne hearing starts in rodents only at ∼P12 (Jewett & Romano, 1972; Geal-Dor et al. 1993). Interestingly, when comparing synaptic function before hearing onset (at ∼P5–P9) and shortly after hearing onset (at P12–P15 or older), it has been found that several pre- and postsynaptic developmental refinements occur which contribute to enhance fast signalling at the calyx of Held synapse. Thus, the presynaptic AP in the nerve terminal becomes shorter (Taschenberger & von Gersdorff, 2000; Yang & Wang, 2006), the kinetics of AMPA receptor-mediated EPSCs are speeded up (Taschenberger & von Gersdorff, 2000; Iwasaki & Takahashi, 2001) and the NMDA receptor-mediated component of the EPSC is strongly reduced with development (Futai et al. 2001; Joshi & Wang, 2002), although signalling via NMDA receptors remains functional (Steinert et al. 2008).
There is also evidence for a tightening in presynaptic Ca2+ channel–vesicle coupling at around the onset of hearing in mice (Fedchyshyn & Wang, 2005), based on the finding that synaptic transmission becomes less sensitive to exogenously added EGTA, a Ca2+ buffer with slow Ca2+ binding kinetics (Neher, 1998). In addition, the steepness in log–log plots of the EPSC amplitude versus presynaptic Ca2+ current charge (called the ‘Ca2+ current–release cooperativity’ in what follows) can be used as an indicator of how many local Ca2+ channels contribute to the release control of a given vesicle. Varying the number of Ca2+ channels when many Ca2+ channels control the release of a given vesicle will create a graded variation of the ‘local’[Ca2+]i signal. In this case, the Ca2+ current–release cooperativity will be equally high as the intrinsic (‘biochemical’) cooperativity with which Ca2+ induces release (‘domain overlap’; Borst & Sakmann, 1999). With fewer Ca2+ channels contributing to release control, however, the Ca2+ current–release cooperativity drops, until a value of 1 is reached in the limit of a ‘single-channel’ control mechanism (Yoshikami et al. 1989; Augustine et al. 1991; Meinrenken et al. 2002; Brandt et al. 2005; Gentile & Stanley, 2005). Indeed, it was found that the Ca2+ current–release cooperativity was reduced with development at the mouse calyx of Held, which probably indicates that fewer Ca2+ channels contribute to the release control of a given readily releasable vesicle (Fedchyshyn & Wang, 2005). However, it has been unknown whether the intrinsic Ca2+ cooperativity and sensitivity of vesicle fusion might change during development.
At the calyx of Held, presynaptic Ca2+ uncaging has shown a relatively high intracellular Ca2+ sensitivity of vesicle fusion, compatible with amplitudes of ‘local’ intracellular Ca2+ signals at the sites of vesicle fusion of only ∼10 μm[Ca2+]i (Bollmann et al. 2000), or ∼25 μm[Ca2+]i (Schneggenburger & Neher, 2000; Lou et al. 2005). However, Ca2+ uncaging has been limited to young rats (see references above) or mice (Sun et al. 2007) of about 7–10 days postnatally (P7–P10). To investigate a possible change in the intracellular Ca2+ sensitivity of vesicle fusion during development, we have established presynaptic Ca2+ uncaging experiments at a more mature stage of development in rats (P12–P15); a developmental stage that immediately follows the onset of hearing at ∼P12 in rats (Jewett & Romano, 1972). When comparing the presynaptic Ca2+–secretion coupling between P8–P9 and P12–P15, we found a pronounced increase in the efficiency of brief Ca2+ currents in inducing release. On the other hand, the intracellular Ca2+ sensitivity of vesicle fusion as measured by Ca2+ uncaging was unchanged, or even slightly reduced, in agreement with a recent report in mice (Wang et al. 2008). Thus, the developmental enhancement of Ca2+–secretion coupling must be caused by a tighter co-localization of readily releasable vesicles with voltage-gated Ca2+ channels.
Methods
Slice electrophysiology
Transverse brainstem slices of 180–200 μm thickness containing the medial nucleus of the trapezoid body (MNTB) were prepared as described previously (von Gersdorff et al. 1997), using a Leica VT 1000S slicer (Leica Microsystems, Wetzlar, Germany). For the dissection of the brainstem, a rat was killed quickly by decapitation without prior anaesthesia, in a procedure approved by the Veterinary office of the Canton of Vaud, Switzerland (authorization 1864). We studied two age groups of Wistar rats: postnatal days 8–9 (P8–P9, ‘young’ age group), and P12–P15 (‘more mature’ age group), with the day of birth referred to as P0. For the experiments shown in Fig. 2, transverse slices with a tilted angle of about 30 deg were made, in an attempt to cut the axons close to the calyces of Held thereby improving presynaptic voltage-clamp conditions (Borst & Sakmann, 1998). Slices were kept in an incubation chamber with a bicarbonate-buffered solution containing (in mm): 125 NaCl, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 25 glucose, 0.4 ascorbic acid, 3 myo-inositol, 2 sodium pyruvate, 2 CaCl2 and 1 MgCl2 (pH 7.4, when bubbled with 95% O2–5% CO2). The extracellular solution during the experiments had the same composition; tetraethylammonium chloride (TEA, 10 mm), tetrodotoxin (TTX, 1 μm), d-2-amino-5-phosphonopentanoic acid (d-AP5, 50 μm), γ-d-glutamylglycine (γ-DGG, 2 mm) and cyclothiazide (CTZ, 100 μm) were added for paired recordings (Figs 1 and 3). For the experiments in Fig. 2, γ-DGG was omitted. For postsynaptic recordings after afferent fibre stimulation (Fig. 6), d-AP5 (50 μm), CTZ (100 μm), bicuculine (10 μm) and strychnine (2 μm) were added to the extracellular solution. Afferent stimulation of presynaptic axons was performed with a concentric bipolar stimulation electrode (MCE-100; Rhodes Medical Instruments, Woodland Hills, CA, USA) placed medially to the MNTB. Experiments were done at room temperature (21–24°C).
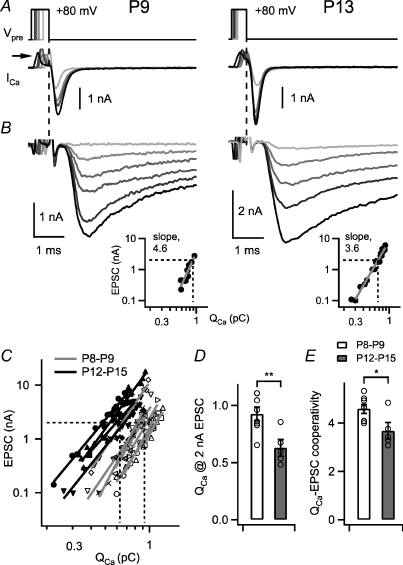
Figure 2. A slight decrease in the Ca2+ current–release cooperativity during developmental maturation.
A and B, presynaptic Ca2+ tail currents (A) and the corresponding postsynaptic EPSCs (B) in a P9 (left) and a P13 calyx of Held synapse (right), with presynaptic depolarizations to +80 mV of varying durations. The insets in B show plots of the EPSC amplitude versus QCa for the corresponding cells on the same scales, with slope values as indicated. C, plot of the EPSC amplitudes as a function of the presynaptic Ca2+ charge for n= 8 cells at P8–P9 (open symbols) and for n= 5 cells at P12–P15 (filled symbols). Each logarithmized data set was fitted with a line to yield the individual slope values. Note the leftward shift, and the trend towards shallower slopes in the more mature age group (P12–P15). D, average and individual values of the presynaptic Ca2+ charge needed to evoke an EPSC of 2 nA. Note the significant difference between age groups (P= 0.007). E, the slope values obtained from line fits to log–log plots of EPSC amplitude versus QCa. Note the slight, but statistically significant (P= 0.04) reduction of the Ca2+ current–release cooperativity with development.
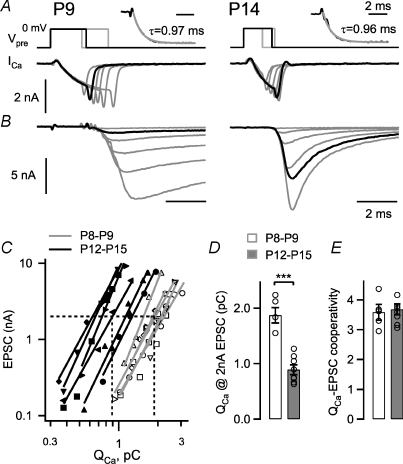
Figure 1. A pronounced leftward shift in the EPSC–presynaptic Ca2+ charge relation during developmental maturation.
A and B, presynaptic Ca2+ currents (A) and the corresponding postsynaptic EPSCs (B) in a P9 (left) and a P14 calyx of Held synapse, with presynaptic depolarizations to 0 mV of increasing lengths. The insets in A show an exponential fit (grey line) to the rising phase of the Ca2+ currents, with time constants as indicated. The traces highlighted by black lines show presynaptic Ca2+ currents with similar charge transfer, which evoke an ∼9-fold larger EPSC in the P14 synapse. C, plot of the EPSC amplitudes as a function of the presynaptic Ca2+ charge, for n= 7 cells at P8–P9 (open symbols) and for n= 6 cells at P12–P15 (filled symbols). The logarithmized data set of each cell was fitted with a line, which is superimposed. Note that the data from the older age group is clearly leftward shifted. D, average and individual values of the presynaptic Ca2+ charge needed to evoke an EPSC of 2 nA. E, the slope values of the line fits to the EPSC amplitude versus QCa data in double-logarithmic coordinates (‘Ca2+ current–release cooperativity’). Note the unchanged value between the two age groups (P= 0.7).
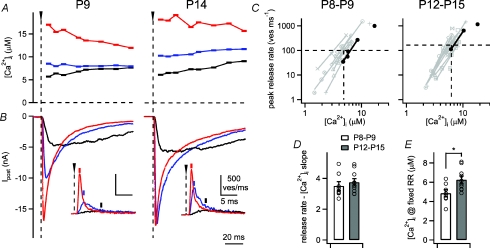
Figure 3. Ca2+ uncaging experiments measure the intracellular Ca2+ sensitivity and Ca2+ cooperativity of vesicle fusion at two developmental stages.
A and B, presynaptic [Ca2+]i steps produced by Ca2+ uncaging with different UV light intensities (A) and the resulting EPSCs in the postsynaptic cell (B), for recordings in a P9 rat (left) and a P14 rat (right). The insets in B show transmitter release rates as obtained by EPSC deconvolution. C, plot of the peak release rates as a function of the corresponding [Ca2+]i steps on double-logarithmic coordinates. The values for [Ca2+]i steps below 10 μm amplitude were fitted by lines. The data obtained from the example cells (A and B) are shown by black symbols and lines; data points and fits for all other cells obtained with 2 mm DM-nitrophen are shown by grey symbols and lines. D, average slope values derived from fitting the peak release rate–[Ca2+]i relation (see C). There was no significant change (P= 0.49), indicating that the intrinsic Ca2+ cooperativity of release was unchanged during development. E, plot of the average interpolated [Ca2+]i value needed to evoke a peak transmitter release rate of 100 ves ms−1 (at P8–P9), or a pool-corrected value of 160 ves ms−1 at P12–P15 group (dashed lines in C). There was a 1.29-fold increase of this [Ca2+]i value, indicating a slightly lower Ca2+ sensitivity with development (P= 0.012).
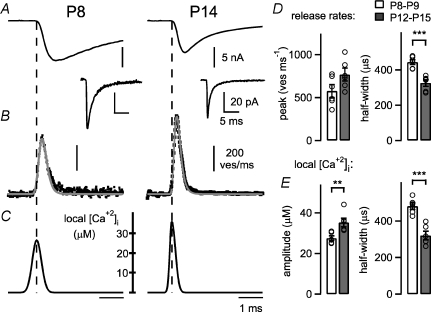
Figure 6. The ‘local’[Ca2+]i signal experienced by readily releasable vesicles during AP-evoked release increases with development.
A, EPSCs (average of n= 5 traces each) evoked by afferent fibre stimulation in a P8 and a P14 synapse, in the presence of cyclothiazide (CTZ). The average mEPSC waveform recorded in each cell is also shown (lower trace; note different scales). B, transmitter release rates (average of n= 5 single sweeps; black data points) for the EPSCs shown in A, as obtained by deconvolution of the evoked EPSC with the mEPSC waveform (see Methods). The transmitter release rates predicted by the ‘local’[Ca2+]i waveforms shown in C are superimposed (grey traces). C, the iteratively refined local [Ca2+]i waveforms which best predicted the observed transmitter release rates shown in B. D, average peak transmitter release rates (left) and their half-widths (right) for each developmental stage. Note the highly significant (P < 0.001) reduction in the half-width of AP-evoked transmitter release with development (right panel). E, average amplitude (left) and half-widths (right) of the back-calculated local [Ca2+]i transients for both developmental stages. Note that the local [Ca2+]i signal is significantly higher (P= 0.009, left), and at the same time briefer in more mature animals (P < 0.001, right).
Whole-cell recordings of visually identified presynaptic calyces and/or postsynaptic principal neurons of the MNTB were made with EPC-9/2 or EPC-10/2 double patch-clamp amplifiers (HEKA Elektronik, Lambrecht/Pfalz, Germany) in set-ups with upright microscopes under infrared gradient contrast illumination (Luigs and Neumann, Ratingen, Germany). The intracellular (pipette) solutions contained (in mm): 135 caesium gluconate, 20 TEA, 10 Hepes, 5 Na2-phosphocreatine, 4 MgATP, 0.3 Na2GTP, pH 7.2, to which either 100 μm or 5 mm EGTA was added for pre- or postsynaptic recordings, respectively. Pre- and postsynaptic series resistances were 15–25 MΩ (using up to 60% compensation) and 3–8 MΩ (compensation up to 85%), respectively. EPSC traces were off-line corrected for the remaining Rs error, and presynaptic Ca2+ currents were corrected for leak and capacitative currents with a P/5 protocol.
Ca2+ uncaging and Ca2+ imaging
Ca2+ uncaging experiments were similar to those described previously (Wölfel et al. 2007). In brief, the presynaptic intracellular solutions contained (in mm): 130 caesium gluconate, 20 TEA-Cl, 20 Hepes, 5 Na2-phosphocreatine, 5 Na2ATP, 0.3 Na2GTP, 0.5 MgCl2, 0.1 fura-2FF (TefLabs, Austin, TX, USA), DM-nitrophen (Calbiochem; 2 mm, with 1.75 mm CaCl2 added); pH 7.2, 310 mosmol l−1. In some experiments, a higher DM-nitrophen concentration (5 mm, with 4.52 mm CaCl2 added) was used to produce post-flash [Ca2+]i values up to 90 μm. An SP-20 flash lamp (Rapp OptoElectronic, Hamburg, Germany) produced a brief single flash of light (∼450 μs half-width). Simulations showed that this light pulse should produce a step-like cellular [Ca2+]i elevation with a 20–80% rise time of 240 μs. A dual-port epifluorescence condenser (TILL Photonics, Gräfelfing, Germany) in the microscope selected 60% of the flash lamp light and 40% of the monochromator light used to excite fura-2FF (at 350 and 380 nm) for the ratiometric measurement of post-flash [Ca2+]i. The relatively high intensity of the monochromator (Polychrome V, TILL Photonics) caused a slow [Ca2+]i rise following weak flashes (see Fig. 3A and B; black [Ca2+]i traces), and delayed the decay of [Ca2+]i following flashes to higher [Ca2+]i.
Data analysis and model calculations
The presynaptic Ca2+ influx charge (QCa) during Ca2+ currents (Fig. 1) was obtained by integrating the Ca2+ current traces, starting from the zero intercept point during the pulse to 0 mV, until 3 ×τfast of a fit of the Ca2+ tail current decay with a double-exponential function. For the protocol using steps to +80 mV (Fig. 2), only the charge of the Ca2+ tail current was analysed up to a time value of 3 ×τfast.
To obtain transmitter release rates from paired recordings (Figs 3 and 4, obtained in the presence of 100 μm CTZ and 2 mmγ-DGG), the Rs-corrected EPSC traces were deconvolved, assuming a bi-exponential decay of the mEPSC (Neher & Sakaba, 2001). We used a standard mEPSC amplitude (q) of 15 pA for all cells in each age group. This reflects our finding that the mEPSC amplitudes were not significantly changed in this age range (see below; but see Taschenberger et al. 2005), and that the blocking effect of 2 mmγ-DGG in the presence of 100 μm CTZ is ∼50% (see Wölfel et al. 2007; their Fig. 2). The deconvolution analysis assumes that the EPSC is composed of the sum of release-evoked mEPSCs, and a late current mediated by glutamate spill-over. During deconvolution, the late current is calculated based on previous release events and a model of glutamate diffusion (Neher & Sakaba, 2001). The parameters for the glutamate diffusion model were found by deconvolving the EPSC obtained in response to ‘fitting protocol’ stimuli (see e.g. Figs 6 and 12 of Neher & Sakaba, 2001), which were given at the start of each paired recording.
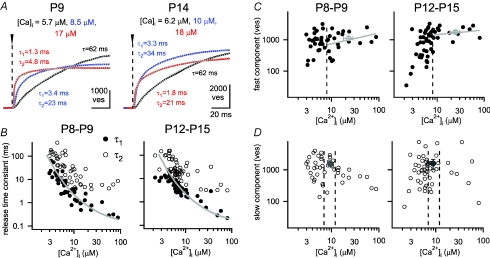
Figure 4. Ca2+ uncaging evokes a fast and a slow component of transmitter release at both developmental stages.
A, integrated release rate traces for a P9 (left) and a P14 (right) synapse evoked by step-like elevations of [Ca2+]i (traces are from the same cells and in colours corresponding to the examples shown in Fig. 3A and B). The post-flash [Ca2+]i value for each response is indicated. The cumulative release rate traces were fitted with various exponential fit functions (see Methods), and the best-fit traces are shown superimposed (grey dotted lines): single exponentials for the lowest [Ca2+]i steps (black traces, time constant τ indicated), or double exponentials, with the indicated values for τ1 and τ2. B, the two time constants (τ1 and τ2) of the fast and the slow release component were plotted against the corresponding post-flash [Ca2+]i values at P8–P9 (left panel) and at P12–P15 (right panel). The prediction of the five-site model of Ca2+ binding and vesicle fusion, which effectively models the fast release component, is superimposed (grey line; parameters as given for the P8–P9 age group in the legend to Fig. 5). Note that this data set, and the data shown in C and D also include experiments done with 5 mm DM-nitrophen, which allowed us to obtain higher post-flash [Ca2+]i values of up to 90 μm. C and D, the number of vesicles released in each kinetic component of release as a function of post-flash [Ca2+]i, plotted separately for P8–P9 (left panels) and for P12–P15 rats (right panels). The numbers were estimated by the amplitude values of the fast and the slow time constants in fits of the cumulative release rate traces (A and B), when the best-fit functions were either double-exponentials, double-exponentials plus line, or triple exponential functions. The average number of vesicles released in the fast component by [Ca2+]i steps above 8 μm is indicated by the grey average symbol. For [Ca2+]i > 8 μm, the data for the fast release component were fitted by lines (grey lines in C). The average number of slowly released vesicles was estimated in the [Ca2+]i range of 7–12 μm (see dotted lines and grey average symbols in D), because the number of slowly released vesicles showed a tendency to decrease with [Ca2+]i steps > 15 μm.
For deconvolving the EPSCs evoked by afferent fibre stimulation (Fig. 6), we determined the mEPSC parameters (amplitude, and τfast and τslow of the mEPSC decay) from measurements of spontaneous mEPSCs in each cell, and no correction for glutamate spillover was made, as in Schneggenburger & Neher (2000). Detection of mEPSCs was done with a routine written in IgorPro. The mean (±s.e.m.) mEPSC amplitudes for the data obtained in Fig. 6 (in the presence of 100 μm CTZ) were 42.0 ± 3.8 pA at P8–P9 (n= 6), and 40.3 ± 3.4 pA at P12–P15 (n= 6), and therefore, not significantly different between the two age groups (P= 0.72).
The cumulative release rate traces after Ca2+ uncaging (Fig. 4A) were derived by integration of the release rate traces obtained by deconvolution, without correction for readily releasable pool recovery. The integrated release rate traces were fitted with five functions: single-exponential, exponential plus line, double-exponential, double-exponential plus line, and triple-exponential (Wölfel et al. 2007). The best-fitting model was selected based on the Bayesian information criterion (BIC; Schwarz, 1978) by the lowest BIC value. The BIC value was calculated as:  , where N is the number of fitted data points (yi),
, where N is the number of fitted data points (yi),  is the summed square of fit residuals, and M is the number of fit parameters used in the model. In most cases, the best-fit functions were double-exponential, double-exponential plus line, or triple-exponential functions. In these cases, we used the fast and slow time constants (τ1, τ2) as release time constants (Fig. 4B), and the corresponding amplitude values (A1, A2) as an estimate of the number of released vesicles in each kinetic component (Fig. 4C and D).
is the summed square of fit residuals, and M is the number of fit parameters used in the model. In most cases, the best-fit functions were double-exponential, double-exponential plus line, or triple-exponential functions. In these cases, we used the fast and slow time constants (τ1, τ2) as release time constants (Fig. 4B), and the corresponding amplitude values (A1, A2) as an estimate of the number of released vesicles in each kinetic component (Fig. 4C and D).
The five-site model of Ca2+ binding and vesicle fusion (Schneggenburger & Neher, 2000) was used to simultaneously fit the [Ca2+]i dependence of peak transmitter release rates, release delays and times to peak release rate (Fig. 5) by minimizing the sum of logarithmized fit residuals for each fitted parameter. A 5th order adaptable step size Runge–Kutta method was used to solve the system of differential equations. The model was driven with a realistic [Ca2+]i waveform simulated for the measured flash-lamp discharge. The values of interest (peak release rate, release delay at 5 released vesicles, time to peak release) were extracted from the simulated release rate trace at each [Ca2+]i. The vesicle pool sizes used for the fits were fixed to the average values of the fast release component (1109 and 1767 vesicles for the young, and the more mature age group; Fig. 4C).
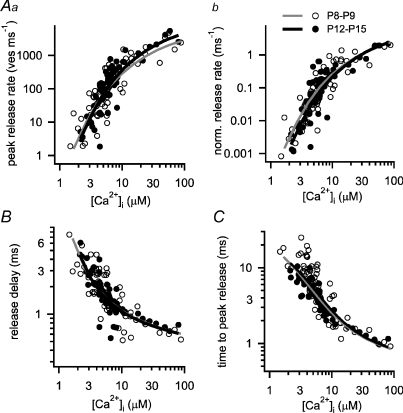
Figure 5. The intrinsic Ca2+ sensitivity of transmitter release is largely unchanged during developmental refinement of synaptic transmission.
A–C, plots of the peak transmitter release rates (Aa), the pool-corrected peak release rate (Ab), the minimal release delay (B), and the time to the peak of the transmitter release rate (C) as a function of presynaptic [Ca2+]i reached after flashes for both developmental groups (P8–P9, open symbols; P12–P15, filled symbols). In Ab, the peak release rates were normalized by the pool size estimate (FRP) of each individual cell. The data points for the two developmental groups largely overlap for all analysed parameters. The data sets in Aa, B and C were simultaneously fitted with the five-site model of cooperative Ca2+ binding and vesicle fusion (Schneggenburger & Neher, 2000), yielding the following parameters for each age group: kon= 1.21 × 108m−1 s−1, koff= 6500 s−1, b= 0.26, γ= 6960 s−1 (P8–P9 age group; grey fit lines); and kon= 1.15 × 108m−1 s−1, koff= 7900 s−1, b= 0.26, γ= 6960 s−1 (P12–P15 age group; black fit lines). In Ab, the fit predictions were normalized by the average FRP pool size values obtained in young synapses (1109 vesicles; P8–P9) and in more mature synapses (1767 vesicles; see Results). A small, constant delay (0.4 ms) was added to the model predictions of release delay (B) and time to peak release (C), indicating a time delay not explained by the model (see also Bollmann et al. 2000; Felmy et al. 2003).
The back-calculation of the ‘local’[Ca2+]i signal (Fig. 6C) used a two-sided Gaussian waveform (Schneggenburger & Neher, 2000) with parameters A, peak local [Ca2+]i; t0, time of peak; and σrise, σdecay determining the rise and decay rates of the local [Ca2+]i. The parameters were varied until the predicted release rate matched the observed one, as determined by minimizing the weighted χ2 value. Weighting was done with the fitted release rate trace taken to the power 0.25, which was found to improve the convergence of the fit around the peak of the release rate. The parameter σrise was constrained not to be smaller than the 20–80% rise time of the AP-evoked Ca2+ current in each age group. For the young age group, this value was 154 μs as measured experimentally (Müller et al. 2008). For the more mature age group, a value of 69 μs was used, which was found by the Hodgkin–Huxley simulation of the Ca2+ current during an AP (see Supplemental Fig. 3, available online only).
Data analysis and numerical simulations were performed using routines written in IgorPro 5.05 (Wavemetrics, Lake Oswego, OR, USA). Average values are reported as mean ±s.e.m., and statistical significance was assessed with a two-sample unpaired two-tailed Student's t test unless otherwise indicated. In the figures, a statistically significant difference between two sample groups is indicated by asterisks (*P < 0.05; **P < 0.01; ***P < 0.001).
Results
A leftward shift in the EPSC–presynaptic Ca2+ charge relation during development
We started by investigating how brief presynaptic Ca2+ currents become more efficient in triggering transmitter release during development of the calyx of Held synapse. During a presynaptic AP, a brief (< 0.5 ms half-width) Ca2+ current with a total charge of about 1 pC flows into the rat calyx of Held (Borst & Sakmann, 1998). Here, we used rectangular voltage-clamp pulses of varying lengths in the presynaptic nerve terminal (to either 0 mV, or to +80 mV in a ‘tail’ current protocol; see Figs 1 and 2, respectively), to measure the relationship between EPSC amplitude, and the presynaptic Ca2+ current integral (EPSC–QCa relationship). We wanted to investigate whether the developmental increase in the release efficiency of brief Ca2+ currents (Taschenberger et al. 2002; Fedchyshyn & Wang, 2005) is also apparent as an absolute leftward-shift in the EPSC–QCa relationship. Throughout this study, we compared two developmental age groups: synapses in P8–P9 rats, and in P12–P15 rats, which correspond to time intervals shortly before, and shortly after hearing onset, respectively (Jewett & Romano, 1972; Geal-Dor et al. 1993).
Figure 1A and B shows experiments from a P9 rat (left panel) and from a P14 rat (right panel), representative for the two age groups studied here. Ca2+ currents evoked by voltage steps of increasing lengths evoked EPSCs of increasing amplitudes (Fig. 1B). In the cell pair from the older age group, EPSCs rose and decayed faster (Fig. 1B, right). As highlighted by the black traces in Fig. 1A and B, Ca2+ currents with similar Ca2+ charge transfer (∼1.7 pC in this example) evoked a much larger EPSC in the P14 synapse (7.3 nA) than in the P9 synapse (0.9 nA).
When we plotted the EPSC amplitudes as a function of the presynaptic Ca2+ charge (QCa) on log–log scales (Fig. 1C), the EPSC amplitudes measured in the more mature synapses at P12–P15 (Fig. 1C, filled symbols; n= 7 cell pairs) were consistently leftward-shifted as compared to the data obtained in younger synapses (Fig. 1C, open symbols; n= 6 cell pairs). We fitted the logarithmized data sets with lines, in order to estimate the Ca2+ current–release cooperativity (see Introduction), and to quantify the increased efficiency of brief Ca2+ current in inducing release. We found that the interpolated Ca2+ charge necessary to evoke an EPSC of 2 nA amplitude (Fig. 1C, dashed lines) was 1.97 ± 0.12 pC at P8–P9 (n= 5 cell pairs), and 0.89 ± 0.09 pC at P12–P15 (n= 7 cell pairs); and thus, significantly smaller at the more mature age (P < 0.001; Fig. 1D). The slope in the EPSC versus Ca2+ current charge (QCa) relationship, which indicates the Ca2+ current–release cooperativity, was 3.58 ± 0.26 at P8–P9 (n= 5 cell pairs) and 3.68 ± 0.18 at P12–P15 (n= 8 cell pairs), and thus was not changed significantly (P= 0.75; see Fig. 1E).
We also analysed the amplitude and the activation time constant of the presynaptic whole-cell Ca2+ currents during prolonged (> 6 ms) steps to 0 mV, a voltage which is close to the maximal Ca2+ current in I–V curves. The resulting parameters are summarized in Table 1. The Ca2+ current amplitude and its activation time course were not significantly different between the two age groups (see Table 1). To estimate the Ca2+ current density, we normalized the Ca2+ current measured at 0 mV by the membrane capacitance of each calyx of Held. This revealed a slightly larger Ca2+ current density in more mature calyces, because the membrane capacitance was slightly smaller in the more mature calyces than in the younger ones (Table 1).
Table 1.
Parameters of presynaptic Ca2+ current and Ca2+ current density at two developmental stages
| Parameter | P8–P9 | P12–P15 | P valuea |
|---|---|---|---|
| Ca2+ current at 0 mV (nA)b | 1.69 ± 0.21 | 1.80 ± 0.13 | P= 0.6 |
| (n= 8) | (n= 12) | ||
| Ca2+ current activation time constant (ms)c | 1.16 ± 0.12 | 0.95 ± 0.05 | P= 0.11 |
| (n= 8) | (n= 13) | ||
| Whole-cell membrane capacitance (pF)d | 23 ± 1.5 | 19.1 ± 1.3 | P= 0.035 |
| (n= 9) | (n= 14) | ||
| Ca2+ current density (pA pF−1)e | 73 ± 6.1 | 91 ± 7.4 | P= 0.034 |
| (n= 8) | (n= 12) |
From two-sample unpaired Student's t test.
Measured as the maximal Ca2+ current during the step to 0 mV.
Measured by exponential fits to the rise of Ca2+ currents in response to prolonged (> 6 ms) steps to 0 mV.
Measured by the setting of the slow capacitance cancellation circuit of the patch-clamp amplifier.
Calculated by dividing the Ca2+ current at 0 mV by the membrane capacitance estimated in each cell.
A slight decrease in the Ca2+ current–release cooperativity is revealed by a tail current protocol
The experiments in Fig. 1 suggest that brief Ca2+ currents become significantly more efficient in inducing transmitter release, mainly because of a pronounced leftward-shift in the EPSC–QCa relation, with a largely unchanged slope. Previous experiments with a tail current protocol at the mouse calyx of Held synapse have shown, however, that the slope in the EPSC–QCa relation was significantly reduced with development (Fedchyshyn & Wang, 2005). To resolve this apparent discrepancy, we next used a Ca2+ tail current protocol with brief steps to +80 mV.
Figure 2A and B shows a series of Ca2+ tail currents and the resulting EPSCs for an example cell pair of each age group. During the pulse to +80 mV, there was a transient outward current (Fig. 2A, arrow) that we interpret as gating current caused by the activation of voltage-gated channels. Following the repolarization, there was a transient inward (‘tail’) current flowing through the open Ca2+ channels. We varied the duration of the activating pulse to +80 mV in order to recruit a varying number of Ca2+ channels. In some experiments, we found that it was difficult to obtain a graded Ca2+ tail current amplitude over a reasonably large range with steps to +80 mV, presumably because Ca2+ channels activate very rapidly at positive membrane potentials (Borst & Sakmann, 1998). In the experiments of Fig. 2A and B, it was possible to vary the Ca2+ current integral in the range of about 1 pC and below, and the slopes in the resulting EPSC–QCa plots were 4.6 and 3.6, respectively (Fig. 2B, insets).
When we plotted the absolute data sets from all cells, there was again a clear leftward shift of the EPSC–QCa relation (Fig. 2C). The interpolated QCa value necessary to evoke an EPSC of 2 nA was 0.92 ± 0.06 pC at P8–P9 (n= 8 cell pairs), and 0.63 ± 0.07 pC at P12–P15 (n= 5 cell pairs; P= 0.007; see Fig. 2D). In addition to the leftward shift, the EPSC–QCa relationship also showed a trend towards lower slopes with developmental maturation (Fig. 2C). Specifically, the slopes of line fits to the double-logarithmized data sets were 4.6 ± 0.2 at P8–P9 (n= 8 cell pairs) and 3.7 ± 0.3 at P12–P15 (n= 5 cell pairs; P= 0.04; see Fig. 2E); and thus, significantly lower at the more mature age. This confirms previous findings of a developmental decrease of the Ca2+ current–release cooperativity at the mouse calyx of Held (Fedchyshyn & Wang, 2005). Note, however, that the relative change was larger in the previous study than what we found here.
The intrinsic Ca2+ cooperativity of transmitter release is unchanged during synapse maturation
The experiments in Figs 1 and 2 showed a pronounced increase in the efficiency of brief presynaptic Ca2+ currents in evoking release, such that a 1.6- to 2-fold smaller Ca2+ current charge was equally efficient in inducing transmitter release in the more mature synapses. The most likely explanation for this effect is that the spatial coupling between readily releasable vesicles and Ca2+ channels becomes tighter with development, as has been suggested before (Fedchyshyn & Wang, 2005). It is also possible, however, that part of the leftward shift in the EPSC–QCa relation (Figs 1 and 2) is caused by an increased intrinsic Ca2+ sensitivity of vesicle fusion. To investigate this possibility, we next performed presynaptic Ca2+ uncaging experiments to measure the intracellular Ca2+ sensitivity of vesicle fusion at two developmental stages, similar to the approach used recently by Wang et al. (2008) at the mouse calyx of Held. Ca2+ uncaging should also allow us to address the question of whether a change in the intrinsic Ca2+ cooperativity of vesicle fusion contributes to the decrease in the Ca2+ current–release cooperativity (Fig. 2; Fedchyshyn & Wang, 2005).
In a first series of Ca2+ uncaging experiments, we used a low concentration of Ca2+-loaded DM-nitrophen in the presynaptic pipette solution (2 mm; see Methods). We applied several Ca2+ uncaging stimuli with different flash light intensities, with the aim to map out the intracellular Ca2+ sensitivity and Ca2+ cooperativity of release in a range of 2 to ∼15 μm[Ca2+]i. At both age groups, [Ca2+]i steps to low micromolar levels induced slowly rising EPSCs (Fig. 3A and B; black traces), whereas [Ca2+]i steps to higher values induced more rapidly rising EPSCs with larger amplitudes (Fig. 3A and B; blue and red traces). We deconvolved the EPSCs to obtain the underlying rates of transmitter release (Fig. 3B, insets; Schneggenburger & Neher 2000; Neher & Sakaba, 2001). The release rates for comparable [Ca2+]i steps were remarkably similar for the young and for the more mature synapse illustrated in Fig. 3A and B.
We next analysed the intrinsic Ca2+ cooperativity of release, by plotting the peak transmitter release rates against the post-flash [Ca2+]i values, and fitting the logarithmized data sets below 10 μm[Ca2+]i with a line (Fig. 3C; black symbols and fit lines). The analysis was restricted to below 10 μm[Ca2+]i because at higher values, the relation between peak release rates and [Ca2+]i starts to become more shallow (see below; Fig. 5A). Linear fits revealed intrinsic Ca2+ cooperativities of 3.5 and 3.4 for the young and for the more mature example cell pairs shown in Fig. 3. For similar experiments in a larger sample of cell pairs in each age group (see Fig. 3C, grey symbols and fit lines), we found average intrinsic Ca2+ cooperativities of release of 3.5 ± 0.3 at P8–P9 (n= 8 cell pairs with 2 mm presynaptic DM-nitrophen) and 3.7 ± 0.2 at P12–P15 (n= 10 cell pairs; P= 0.49; Fig. 3D). Thus, there was no measurable change in the intrinsic Ca2+ cooperativity of transmitter release with development.
As a first estimate for the intrinsic Ca2+ sensitivity of release, we computed, using linear regression of the data points, the post-flash [Ca2+]i value that was necessary to trigger a given value of peak transmitter release rate. We used an arbitrary reference value of 100 vesicles (ves) ms−1 for the age group of P8–P9 (Fig. 3C, left panel). This value was scaled to 160 ves ms−1 for the age group of P12–P15 (Fig. 3C, right panel), since the pool of fast-releasable vesicles, which underlies the peak release rates measured after Ca2+ uncaging, was increased by about 1.6-fold with development (see below; Fig. 4). The corresponding interpolated post-flash [Ca2+]i values were 4.9 ± 0.4 μm at P8–P9 (n= 8 cell pairs) and 6.3 ± 0.4 μm at P12–P15 (n= 10 cell pairs; P= 0.012; Fig. 3E). Thus, a somewhat higher post-flash [Ca2+]i was needed in the more mature synapses to evoke a peak release rate of the same (pool-corrected) amplitude. This difference suggests that the intracellular Ca2+ sensitivity of vesicle fusion might be slightly reduced with development, as found recently in mice (Wang et al. 2008). We will address this possibility in more detail below (see Fig. 5).
Ca2+ uncaging evokes two components of transmitter release at both developmental stages
It has been shown previously in calyces of Held of young rats (P8–P10) that long voltage-clamp depolarizations of 30–50 ms lengths induce two phases of release that are mediated by fast- and slowly releasable vesicle sub-pools (Sakaba & Neher, 2001). Similarly, Ca2+ uncaging stimuli caused two kinetically separate components of transmitter release (Wölfel et al. 2007). This suggested that an intrinsic mechanism, such as different Ca2+ sensitivities, underlies the separation between fast- and slowly releasable vesicles (Wölfel et al. 2007; but see Wadel et al. 2007). It is possible, however, that the slow transmitter release phase could be a hallmark of the relatively young synapses studied previously, and that developmental maturation leads to a suppression of the slow release mechanism (see Price & Trussell, 2007). In order to investigate this possibility, we next analysed the kinetics of transmitter release evoked by Ca2+ uncaging at the two developmental stages, by integrating the transmitter release rate traces obtained from EPSC deconvolution (Fig. 4).
Figure 4A shows the integrated release rate traces for the same cells that are illustrated in Fig. 3A and B (with an identical colour code). These traces of cumulative release were best fitted by functions with at least two exponential terms for the higher [Ca2+]i steps (Fig. 4A, red and blue traces; see Methods for details of determining the best-fit function). On the other hand, cumulative release traces in response to [Ca2+]i steps of lower amplitude were often well fitted by a single exponential term (Fig. 4A, black traces). When the resulting release time constants were plotted as a function of [Ca2+]i (Fig. 4B), both the fast and the slow release time constants were found to be strongly [Ca2+]i dependent (Fig. 4B; Wölfel et al. 2007). The inverse dependence on the [Ca2+]i of both release time constants was highly significant at each developmental stage (Pearson's test; P < 10−8). When the fast release time constants for each developmental stage were overlaid, it became apparent that they were indistinguishable (see Supplemental Fig. 1A).
We next analysed the amounts of fast and slowly released vesicles as a function of [Ca2+]i (Fig. 4C and D). At post-flash [Ca2+]i > 8 μm, a near-maximal number of vesicles was released in the fast release component (Fig. 4C), although there was a slight tendency towards larger cumulative fast release at [Ca2+]i values beyond 10 μm (Fig. 4C, grey fit lines; see also Wölfel et al. 2007). We assumed that flashes that elevated [Ca2+]i to values of 8 μm or higher deplete the pool of fast-releasable vesicles as shown before (Schneggenburger & Neher, 2000). Therefore, we took the amplitude of the fast release component in response to flashes that elevated [Ca2+]i above 8 μm as an estimate of the size of the fast-releasable pool. This number was 1109 ± 153 vesicles at P8–P9 (n= 10 cell pairs) and 1767 ± 194 vesicles at P12–P15 (n= 8 cell pairs; see Fig. 4C, grey average data points). The number of vesicles released in the slow component with [Ca2+]i steps in the range of 7–12 μm was 1542 ± 268 at P8–P9 and 1586 ± 582 at P12–P15 (Fig. 4D, grey data points). With [Ca2+]i steps of larger amplitudes, the number of vesicles released in the slow component showed a tendency to decrease, similar to previous findings in young calyces of Held (Wölfel et al. 2007).
Taken together, the kinetic analysis of transmitter release evoked by Ca2+ uncaging shows clearly separable fast and slow release components after Ca2+ uncaging, as observed before in young rats (Wölfel et al. 2007). This indicates that a mechanism intrinsic to the release machinery is responsible for generating fast and slow release, like the existence of fast- and slowly releasable vesicle sub-pools (FRPs and SRPs) with different effective Ca2+ sensitivities of release (Voets, 2000; Wölfel et al. 2007; but see Wadel et al. 2007). Second, the data also show that with development, the intrinsically slow release component persists, and that the size of the FRP increases with development.
Nearly unchanged intrinsic Ca2+ sensitivity of transmitter release with developmental maturation
We next wished to fit the Ca2+ dependence of transmitter release with a simple model of Ca2+ binding and vesicle fusion, the five-site model (Schneggenburger & Neher, 2000). The parameters of the fit can subsequently be used to back-calculate, for each developmental stage, the ‘local’[Ca2+]i signal that a given readily releasable vesicle might experience during the physiological release response (Schneggenburger & Neher 2005). For fitting the model to the Ca2+ uncaging data, we simultaneously fitted the [Ca2+]i dependencies of the peak release rate, the release delay and the time to peak release rate (Fig. 5Aa, B and C). These three parameters should primarily reflect release from the fast-releasable vesicle sub-pool, since the initial peak in the transmitter release rate after Ca2+ uncaging is carried by fast-releasable vesicles (Wölfel et al. 2007). Thus, fitting these parameters with the five-site model of Ca2+ binding and vesicle fusion will essentially model release from the FRP only. This is justified, because for the back-calculation of the local [Ca2+]i transient that drives the fast phase of transmitter release during a presynaptic AP (see below; Fig. 6), only fast-releasable vesicles are relevant, since AP-evoked release is carried by FRP vesicles (Sakaba, 2006).
Figure 5 shows the [Ca2+]i dependencies of the transmitter release rates (Fig. 5Aa), the release delays (Fig. 5B) and the time to peak release rates (Fig. 5C), as well as the resulting fit predictions. Overall, the data from the two developmental groups overlaid markedly well, indicating that there was no major change in the intrinsic Ca2+ sensitivity of vesicle fusion. The values of the resulting fit parameters (see legend for Fig. 5) indicated a ratio of koff/kon of 54 μm in the young age group and 69 μm in the more mature age group, which might indicate a slight rightward shift of the release rate versus post-flash [Ca2+]i relation. Figure 5Ab shows a plot of pool-normalized peak release rates (normalized by the size of the FRP estimated in each cell), with the pool-normalized fit predictions overlaid (these were normalized to the corresponding average FRP size at each developmental stage; 1109 and 1767 vesicles at P8–P9 and at P12–P15, respectively; see above, Fig. 4C). In this pool-corrected plot, the fit lines illustrate more clearly the slight rightward shift of the more mature age group with respect to the younger age group (Fig. 5Ab; black and grey line, respectively). Note, however, that the scatter of the data within each age group is larger than the difference between the fit predictions for each age group (see also Supplemental Fig. 2). This suggests that the slight, about 1.3-fold rightward shift in the [Ca2+]i sensitivity of release found by fitting the data is not highly significant. It should also be noted that the [Ca2+]i dependency of the release delays was unchanged between the two age groups (Fig. 5B). The [Ca2+]i dependency of the time to peak release was more shallow in the age group of P12–P15 than at P8–P9 especially at low [Ca2+]i (Fig. 5C), but this tendency was not captured by the model fit.
In conclusion, the drastic leftward shift in the relation between EPSCs and presynaptic Ca2+ current charge (QCa; Figs 1 and 2) cannot be explained by a change in the intrinsic Ca2+ sensitivity of vesicle fusion. On the contrary, there might be a slight, ∼1.3-fold decrease in the intracellular Ca2+ sensitivity of vesicle fusion upon developmental maturation of the presynaptic transmitter release machinery. This agrees with the recent report of Wang et al. (2008), who found an ∼1.5-fold rightward shift of the [Ca2+]i dependency of peak transmitter release, studying developmental maturation in the mouse calyx of Held. Taken together, the pronounced, ∼1.6- to 2-fold leftward shift in the EPSCs–QCa relation that we found here (Figs 1 and 2) is therefore most probably caused by a tighter co-localization of Ca2+ channels and readily releasable vesicles (Fedchyshyn & Wang, 2005).
The local [Ca2+]i signal seen by a readily releasable vesicle is increased during development
A likely consequence of the postulated tighter co-localization of readily releasable vesicles with Ca2+ channels is that vesicles would experience a higher local [Ca2+]i signal. To investigate this possibility, we next measured EPSCs evoked by presynaptic APs at each developmental stage, with the aim to back-calculate the local [Ca2+]i transient at each developmental stage. We recorded EPSCs in response to afferent fibre stimulation at the two age groups, in the presence of CTZ (100 μm). Two example average EPSC traces for one cell of each age group are shown in Fig. 6A. The EPSC amplitudes in these cells were 10.6 nA and 8.4 nA, and among all the cells the average EPSC amplitudes were 11.7 ± 2.0 nA at P8–P9 (n= 6 cells) and 10.3 ± 1.1 nA at P12–P15 (n= 6 cells), not significantly different (P= 0.52). Despite the unchanged average EPSC amplitude, the EPSC rise time was significantly shorter in older animals (199 ± 11 μs at P12–P15, n= 6 cells) than in the younger age group (309 ± 9 μs at P8–P9; n= 6 cells; P < 0.001). Similarly, the release rates obtained by EPSC deconvolution (Fig. 6B) were significantly briefer in the more mature animals, with half-widths of 443 ± 14 μs at P8–P9 and 326 ± 19 μs at P12–P15 (n= 6 cells each; P < 0.001; Fig. 6D, right panel). This indicates that transmitter release occurs over a briefer time window in more mature animals (Taschenberger et al. 2005).
We next back-calculated the ‘local’[Ca2+]i signal that an average readily releasable vesicle might experience at each developmental stage (Fig. 6C). Note that this back-calculation has no free parameters except the timing and the amplitude parameters of the ‘local’[Ca2+]i signal to be found (see Methods); the parameters that describe the Ca2+ sensitivity of vesicle fusion at each developmental stage have been fixed beforehand by fitting the five-site model to the Ca2+ uncaging data (Fig. 5). For the two example synapses shown in Fig. 6A and B, the back-calculated local [Ca2+]i signal had amplitudes of 26 μm and 36 μm with half-widths of 432 μs and 287 μs in a P8 and a P14 synapse, respectively (Fig. 6C). On average, the local [Ca2+]i transient significantly increased with maturation, from a value of 27.5 ± 1.1 μm at P8–P9 (n= 6 cells) to 35.1 ± 2.2 μm at P12–P15 (n= 6 cells; P= 0.009; Fig. 6E, left panel). At the same time, the back-calculated local [Ca2+]i signal was significantly briefer at P12–P15 (half-width, 320 ± 23 μs) as compared to P8–P9 (481 ± 18 μs; P < 0.001; Fig. 6E, right panel). Since the amount of transmitter release depends on the amplitude of the local [Ca2+]i signal (power function with exponent of about 4) as well as on its width (power function with exponent of ∼1.5; Bollmann & Sakmann, 2005), it is likely that the effects on release of a ∼1.3-fold larger, but ∼1.5-fold briefer local [Ca2+]i signal roughly compensate each other. This would explain the similar EPSC amplitudes observed at both developmental stages in rats (see above, Fig. 6A; Taschenberger & von Gersdorff, 2000; Iwasaki & Takahashi, 2001).
The shorter duration of the local [Ca2+]i signal that we derived in Fig. 6 most likely reflects a shortening of the duration of the presynaptic AP that has been observed with developmental maturation at the calyx of Held (Taschenberger & von Gersdorff, 2000; Yang & Wang, 2006). A briefer presynaptic AP would lead to a shorter presynaptic Ca2+ current and thereby to a briefer local [Ca2+]i transient, as found here by the back-calculation approach (Fig. 6). To investigate this possibility, we measured presynaptic APs, and performed a Hodgkin–Huxley simulation of the presynaptic Ca2+ current induced by these APs at P8–P9 and at P12–P15 (see Supplemental Fig. 3). The simulations showed that the Ca2+ current is expected to be about 2-fold faster in the more mature synapses than at the younger age group (Supplemental Fig. 3). This suggests that the changes in the timing of the ‘local’[Ca2+]i signal with development (Fig. 6C and E) can be well explained by the briefer AP in the nerve terminal. On the other hand, the increased amplitude of the local [Ca2+]i signal probably indicates a tighter co-localization of Ca2+ channels and readily releasable vesicles (see Discussion).
Discussion
We have investigated the presynaptic mechanisms underlying the developmental enhancement of Ca2+–secretion coupling at the calyx of Held of rats. We found an about 2-fold leftward shift in the relationship between EPSC amplitudes and the presynaptic Ca2+ current charge (Figs 1 and 2), indicating a significant increase in the efficiency of brief Ca2+ currents in evoking release. Despite this pronounced leftward shift, Ca2+ uncaging experiments revealed a nearly constant, or even slightly rightward-shifted intrinsic Ca2+ sensitivity of transmitter release (Figs 3–5). This strongly suggests that the increased efficiency of brief Ca2+ currents in evoking transmitter release (Taschenberger et al. 2002; Fedchyshyn & Wang, 2005) is caused by a tighter Ca2+ channel–vesicle co-localization. A back-calculation of the ‘local’[Ca2+]i signal based on the intracellular Ca2+ sensitivities of transmitter release at each developmental stage showed that an average readily releasable vesicle experiences a local [Ca2+]i signal with an about 30% increased amplitude upon developmental maturation (Fig. 6). This provides independent evidence for the idea that the Ca2+ channel–vesicle co-localization becomes tighter with development at the calyx of Held (Fedchyshyn & Wang, 2005).
Developmental changes in the intrinsic Ca2+ sensitivity of vesicle fusion
We used presynaptic Ca2+ uncaging to study possible developmental changes in the intracellular Ca2+ sensitivity of vesicle fusion during development. We found an overall very similar [Ca2+]i-dependent regulation of transmitter release at both developmental stages studied here. Thus, the peak release rates were steeply dependent on the post-flash [Ca2+]i with identical slopes (Figs 3C and 5A), indicating no change in the intrinsic (‘biochemical’) Ca2+ cooperativity of release. In addition, the [Ca2+]i-dependent release delays were unchanged (Fig. 5B). Furthermore, we found that at both developmental stages, release evoked by Ca2+ uncaging showed two distinct kinetic phases (Fig. 4), confirming earlier findings made in young calyces of Held (Wölfel et al. 2007). Since Ca2+ uncaging induces a spatially homogeneous [Ca2+]i signal (Naraghi et al. 1998; Wölfel et al. 2007), the observation of two kinetically distinct release components indicates that the separation between the fast and the slow release components is caused by a mechanism intrinsic to the release machinery. As a working hypothesis for this intrinsic heterogeneity, we assume that the total readily releasable pool is sub-divided into a fast- and a more slowly releasable sub-pool (FRP and SRP, respectively), which have distinct intrinsic Ca2+ sensitivities (Voets, 2000; Wölfel et al. 2007). Adaptation of the vesicle fusion machinery to Ca2+ (Hsu et al. 1996) has also been considered as a mechanism of intrinsic heterogeneity of release kinetics (Wölfel et al. 2007). Regardless of the exact explanation, the persistence of a slow release component after Ca2+ uncaging in more mature synapses indicates that the intrinsic propensity for slow transmitter release is maintained during development.
The number of vesicles released in the fast release component with [Ca2+]i steps above 8 μm increased by ∼1.6-fold, indicating an increase of the FRP with development (Fig. 4C). This increase in the fast-releasable pool probably corresponds to the developmental pool size increase that was found previously by analysing cumulative peak EPSC amplitudes during 100 Hz trains (Iwasaki et al. 2000; Taschenberger & von Gersdorff, 2000), since the phasic release during AP-evoked EPSCs is carried by fast-releasable vesicles (Sakaba, 2006). When we normalized the measured absolute peak release rates by the FRP size determined in each synapse, a slight, ∼1.3-fold rightward shift of the peak release rate became apparent (Fig. 5Ab). Similarly, a (pool-corrected) reference release rate was only reached at an ∼1.3-fold higher post-flash [Ca2+]i in the more mature synapses (Fig. 3C). Both findings are consistent with the recent report of an about 1.5-fold rightward shift of the intrinsic Ca2+ sensitivity of transmitter release in mice (Wang et al. 2008); the more pronounced rightward shift in the previous study is probably explained by the use of older animals in the more mature age group (P16–P19 mice). Together, the present study, and the recently published work by Wang et al. (2008) show that changes in the intrinsic Ca2+ sensitivity of vesicle fusion cannot explain the increased efficiency of brief Ca2+ currents in evoking release (Figs 1 and 2; and see Taschenberger et al. 2002; Fedchyshyn & Wang, 2005). These findings from Ca2+ uncaging experiments present significant new evidence in support of the hypothesis that the spatial coupling between Ca2+ channels and vesicles is enhanced during developmental maturation (Fedchyshyn & Wang, 2005).
Increased release efficiency of brief presynaptic Ca2+ currents with development
Previous work has shown a developmental decrease in the Ca2+ current–release cooperativity at the mouse calyx of Held (Fedchyshyn & Wang, 2005), but there was little emphasis on the release efficiency of brief Ca2+ currents on an absolute scale. Here, we found that brief presynaptic Ca2+ currents become significantly more efficient in triggering transmitter release, as manifested by a clear, 1.6- to 2-fold leftward shift in double-logarithmic plots of the EPSC amplitude versus presynaptic Ca2+ charge (QCa; Figs 1 and 2). When release was evoked by brief presynaptic Ca2+ tail currents, we found a slight decrease in the Ca2+ current–release cooperativity with development, in addition to the clear, ∼1.6-fold leftward shift on the QCa axis (Fig. 2). Although the change in Ca2+ current–release cooperativity was smaller than what was previously found in mice, the trend in our data confirms the previous study (Fedchyshyn & Wang, 2005), and indicates that fewer Ca2+ channels are involved in the release control of a given readily releasable vesicle (see Discussion below). With depolarizing steps to 0 mV there was no measurable change in the steepness of the EPSC–QCa relationship (Fig. 1). This might have been caused by the confounding effects of the Ca2+ charge that enters the nerve terminal during the pulse to 0 mV, which is probably less efficient in triggering release as compared to the larger Ca2+ tail currents.
The leftward shift in the EPSC–QCa relationship probably represents a tighter spatial coupling between Ca2+ channels and vesicles localized within each active zone. Two alternative explanations need to be considered, though. First, the increased size of the FRP (∼1.6-fold; Fig. 4C) could contribute to an apparent leftward shift of the EPSC–QCa relationship. However, given the steep relationship between EPSC amplitudes and QCa (slopes of ∼3.5 in the double-logarithmized data sets), a 2-fold leftward shift of the EPSC–QCa relation corresponds to an about 10-fold increased transmitter release at any given QCa value (see also Figs 1C and 2C; left vertical dashed line). Therefore, the ∼1.6-fold increased FRP size should contribute only by a small degree to the observed leftward shift of the EPSC–QCa data. Second, a reduced endogenous Ca2+ buffering strength could, in principle, also contribute to a higher efficiency of presynaptic Ca2+ entry in evoking release. However, the available evidence indicates that the Ca2+ buffering strength of the calyx of Held increases with development (Chuhma et al. 2001; Felmy & Schneggenburger, 2004), making decreased Ca2+ buffering an unlikely explanation.
It is currently not known which fraction of the measured presynaptic Ca2+ current corresponds to Ca2+ channels located at the active zone. For many CNS synapses, including the calyx of Held, immunogold EM analysis of the presynaptic localization of P/Q-type Ca2+ channels is not yet available (but see Bucurenciu et al. 2008). At the calyx of Held, there is also a developmental down-regulation of presynaptic N- and R-type Ca2+ channels (Wu et al. 1999), such that at ∼P12–P14 a majority of the presynaptic Ca2+ channels are of the P/Q subtype (Iwasaki et al. 2000). This re-organization of voltage-gated Ca2+ channel subtypes might go along with a change in the fraction of Ca2+ channels located at active zones. Despite this possibility, the simplest explanation for the pronounced leftward shift in the EPSC–QCa relationship is to assume that a significantly smaller Ca2+ current at each active zone is sufficient to cause a given rate of transmitter release in more mature calyces of Held.
Implications for Ca2+ signalling at single active zones
What are the consequences of these developmental changes for Ca2+ signalling at single active zones? If one assumes, for the purpose of this argument, that during development the total number of Ca2+ channels stays constant at each active zone, then the integral Ca2+ influx at each active zone would be reduced by about 50%, because of the significant developmental shortening of the AP duration (Supplemental Fig. 3; Yang & Wang, 2006). Part of the reduced Ca2+ entry is probably caused by the opening of fewer Ca2+ channels, and another part by the shorter duration of current flow through individual channels (see Supplemental Fig. 3). Without a tighter co-localization, as suggested previously by the decreased efficiency of EGTA in suppressing release (Fedchyshyn & Wang, 2005), a 2-fold reduction of the Ca2+ influx at an active zone would lead to a strong reduction of release probability, which is not observed. On the contrary, we found that the back-calculated ‘local’[Ca2+]i became significantly higher by ∼30% with development; at the same time, however, the width of the local [Ca2+]i signal was decreased, reflecting a briefer Ca2+ current in response to briefer APs (Fig. 6; Supplemental Fig. 3). Very similar findings were reported recently for the developing mouse calyx of Held (Wang et al. 2008). This increase in the peak amplitude of the local [Ca2+]i signal is consistent with the idea that the Ca2+ channel–vesicle co-localization becomes tighter in development (Fedchyshyn & Wang, 2005). The alternative explanation of an increased Ca2+ channel density around a docked vesicle is unlikely because the decreased Ca2+ current–release cooperativity suggests that fewer Ca2+ channels contribute to the release control of a given vesicle.
Taken together, the developmental changes in Ca2+ signalling during a presynaptic AP at the calyx of Held are dictated by the shorter presynaptic AP and, as a consequence, by a briefer Ca2+ current. Nevertheless, the ‘local’[Ca2+]i signal is enhanced because of a tighter co-localization of probably fewer (open) Ca2+ channels with a given readily releasable vesicle. An active zone contains 3–5 docked vesicles and it is likely that each docked vesicle is quite tightly coupled to several Ca2+ channels. Thus, the co-localization of docked vesicles and Ca2+ channels in the active zone is probably not random, but a higher local density of Ca2+ channels must exist around docked vesicles. How this process of docking and positioning close to Ca2+ channels is molecularly coordinated is currently unknown. A tight molecular interaction between Ca2+ channels and vesicles can be mediated by SNARE proteins and Ca2+ channels via their ‘Synprint’ site (Sheng et al. 1994; Rettig et al. 1996), but such a tight molecular coupling may not explain the entire complexity of docked vesicle–Ca2+ channel co-localization. Alternatively or additionally, presynaptic scaffolding- and organizing-molecules such as the Rab-interacting molecules (Rims) could be involved in mediating co-localization, since these molecules probably integrate functions in vesicle docking and priming (Kaeser & Sudhof, 2005) with direct or indirect interaction with Ca2+ channels at the active zone (Hibino et al. 2002; Kiyonaka et al. 2007).
Acknowledgments
We thank Martin Müller for helpful discussions and for providing part of the data shown in Supplemental Fig. 3, and Holger Taschenberger for providing the mEPSC analysis routine. This work was supported by the Swiss National Science Foundation (3100A0-114069), by the Synapsis Foundation, and by the European Commission Coordination Action ENINET (contract number LSHM-CT-2005-19063).
Author contributions
O.K., Y.H. and R.S. designed the research; O.K. and Y.H. performed the experiments and analysed the data; O.K., Y.H. and R.S. contributed to manuscript drafts; R.S. wrote the paper. All the experiments were done at EPFL, Lausanne, Switzerland.
Supplemental material
References
- Augustine GJ, Adler EM, Charlton MP. The calcium signal for transmitter secretion from presynaptic nerve terminals. Ann N Y Acad Sci. 1991;635:365–381. doi: 10.1111/j.1749-6632.1991.tb36505.x. [DOI] [PubMed] [Google Scholar]
- Bollmann JH, Sakmann B. Control of synaptic strength and timing by the release-site Ca2+ signal. Nat Neurosci. 2005;8:426–434. doi: 10.1038/nn1417. [DOI] [PubMed] [Google Scholar]
- Bollmann JH, Sakmann B, Borst JG. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- Borst JG, Sakmann B. Calcium current during a single action potential in a large presynaptic terminal of the rat brainstem. J Physiol. 1998;506:143–157. doi: 10.1111/j.1469-7793.1998.143bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst JG, Sakmann B. Effect of changes in action potential shape on calcium currents and transmitter release in a calyx-type synapse of the rat auditory brainstem. Philos Trans R Soc Lond B Biol Sci. 1999;354:347–355. doi: 10.1098/rstb.1999.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A, Khimich D, Moser T. Few CaV1.3 channels regulate the exocytosis of a synaptic vesicle at the hair cell ribbon synapse. J Neurosci. 2005;25:11577–11585. doi: 10.1523/JNEUROSCI.3411-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucurenciu I, Kulik A, Schwaller B, Frotscher M, Jonas P. Nanodomain coupling between Ca2+ channels and Ca2+ sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron. 2008;57:536–545. doi: 10.1016/j.neuron.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Chuhma N, Koyano K, Ohmori H. Synchronisation of neurotransmitter release during postnatal development in a calyceal presynaptic terminal of rat. J Physiol. 2001;530:93–104. doi: 10.1111/j.1469-7793.2001.0093m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedchyshyn MJ, Wang LY. Developmental transformation of the release modality at the calyx of Held synapse. J Neurosci. 2005;25:4131–4140. doi: 10.1523/JNEUROSCI.0350-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmy F, Neher E, Schneggenburger R. The timing of phasic transmitter release is Ca2+ dependent and lacks a direct influence of presynaptic membrane potential. Proc Natl Acad Sci U S A. 2003;100:15200–15205. doi: 10.1073/pnas.2433276100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmy F, Schneggenburger R. Developmental expression of the Ca2+-binding proteins calretinin and parvalbumin at the calyx of Held of rats and mice. Eur J Neurosci. 2004;20:1473–1482. doi: 10.1111/j.1460-9568.2004.03604.x. [DOI] [PubMed] [Google Scholar]
- Futai K, Okada M, Matsuyama K, Takahashi T. High-fidelity transmission acquired via a developmental decrease in NMDA receptor expression at an auditory synapse. J Neurosci. 2001;21:3342–3349. doi: 10.1523/JNEUROSCI.21-10-03342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geal-Dor M, Freeman S, Li G, Sohmer H. Development of hearing in neonatal rats: air and bone conducted ABR thresholds. Hear Res. 1993;69:236–242. doi: 10.1016/0378-5955(93)90113-f. [DOI] [PubMed] [Google Scholar]
- Gentile L, Stanley EF. A unified model of presynaptic release site gating by calcium channel domains. Eur J Neurosci. 2005;21:278–282. doi: 10.1111/j.1460-9568.2004.03841.x. [DOI] [PubMed] [Google Scholar]
- Hibino H, Pironkova R, Onwumere O, Vologodskaia M, Hudspeth AJ, Lesage F. RIM binding proteins (RBPs) couple Rab3-interacting molecules (RIMs) to voltage-gated Ca2+ channels. Neuron. 2002;34:411–423. doi: 10.1016/s0896-6273(02)00667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffpauir BK, Grimes JL, Mathers PH, Spirou GA. Synaptogenesis of the calyx of Held: Rapid onset of function and one-to-one morphological innervation. J Neurosci. 2006;26:5511–5523. doi: 10.1523/JNEUROSCI.5525-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S-F, Augustine GJ, Jackson MB. Adaptation of Ca2+-triggered exocytosis in presynaptic terminals. Neuron. 1996;17:501–512. doi: 10.1016/s0896-6273(00)80182-8. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Momiyama A, Uchitel OD, Takahashi T. Developmental changes in calcium channel types mediating central synaptic transmission. J Neurosci. 2000;20:59–65. doi: 10.1523/JNEUROSCI.20-01-00059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Takahashi T. Developmental regulation of transmitter release at the calyx of Held in rat auditory brainstem. J Physiol. 2001;534:861–871. doi: 10.1111/j.1469-7793.2001.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett DL, Romano MN. Neonatal development of auditory system potentials averaged from the scalp of rat and cat. Brain Res. 1972;36:101–115. doi: 10.1016/0006-8993(72)90769-x. [DOI] [PubMed] [Google Scholar]
- Joshi I, Wang L-Y. Developmental profiles of glutamate receptors and synaptic transmission at a single synapse in the mouse auditory brainstem. J Physiol. 2002;540:861–873. doi: 10.1113/jphysiol.2001.013506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Sudhof TC. RIM function in short- and long-term synaptic plasticity. Biochem Soc Trans. 2005;33:1345–1349. doi: 10.1042/BST0331345. [DOI] [PubMed] [Google Scholar]
- Kandler K, Friauf E. Pre- and postnatal development of efferent connections of the cochlear nucleus in the rat. J Comp Neurol. 1993;328:161–184. doi: 10.1002/cne.903280202. [DOI] [PubMed] [Google Scholar]
- Kiyonaka S, Wakamori M, Miki T, Uriu Y, Nonaka M, Bito H, Beedle AM, Mori E, Hara Y, De Waard M, Kanagawa M, Itakura M, Takahashi M, Campbell KP, Mori Y. RIM1 confers sustained activity and neurotransmitter vesicle anchoring to presynaptic Ca2+ channels. Nat Neurosci. 2007;10:691–701. doi: 10.1038/nn1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou X, Scheuss V, Schneggenburger R. Allosteric modulation of the presynaptic Ca2+ sensor for vesicle fusion. Nature. 2005;435:497–501. doi: 10.1038/nature03568. [DOI] [PubMed] [Google Scholar]
- Meinrenken C, Borst JGG, Sakmann B. Calcium secretion coupling at calyx of Held governed by nonuniform channel-vesicle topography. J Neurosci. 2002;22:1648–1667. doi: 10.1523/JNEUROSCI.22-05-01648.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Felmy F, Schneggenburger R. A limited contribution of Ca2+-current facilitation to paired-pulse facilitation of transmitter release at the rat calyx of Held. J Physiol. 2008;586:5503–5520. doi: 10.1113/jphysiol.2008.155838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naraghi M, Müller TH, Neher E. Two-dimensional determination of the cellular Ca2+ binding in bovine chromaffin cells. Biophys J. 1998;75:1635–1647. doi: 10.1016/S0006-3495(98)77606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Usefulness and limitations of linear approximations to the understanding of Ca++ signals. Cell Calcium. 1998;24:345–357. doi: 10.1016/s0143-4160(98)90058-6. [DOI] [PubMed] [Google Scholar]
- Neher E, Sakaba T. Combining deconvolution and noise analysis for the estimation of transmitter release rates at the calyx of Held. J Neurosci. 2001;21:444–461. doi: 10.1523/JNEUROSCI.21-02-00444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GD, Trussell LO. Good players left on the sidelines: why some synaptic vesicles don't get in the game. Neuron. 2007;53:471–473. doi: 10.1016/j.neuron.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Rettig J, Sheng ZH, Kim DK, Hodson CD, Snutch TP, Catterall WA. Isoform-specific interaction of theα1A subunits of brain Ca2+ channels with the presynaptic proteins syntaxin and SNAP-25. Proc Natl Acad Sci U S A. 1996;93:7363–7368. doi: 10.1073/pnas.93.14.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Contreras A, van Hoeve JS, Habets RL, Locher H, Borst JG. Dynamic development of the calyx of Held synapse. Proc Natl Acad Sci U S A. 2008;105:5603–5608. doi: 10.1073/pnas.0801395105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T. Roles of the fast-releasing and the slowly releasing vesicles in synaptic transmission at the calyx of Held. J Neurosci. 2006;26:5863–5871. doi: 10.1523/JNEUROSCI.0182-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Quantitative relationship between transmitter release and calcium current at the calyx of Held synapse. J Neurosci. 2001;21:462–476. doi: 10.1523/JNEUROSCI.21-02-00462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Presynaptic calcium and control of vesicle fusion. Curr Opin Neurobiol. 2005;15:266–274. doi: 10.1016/j.conb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Ann Statist. 1978;6:461–464. [Google Scholar]
- Sheng ZH, Rettig J, Takahashi M, Catterall WA. Identification of a syntaxin-binding site on N-type calcium channels. Neuron. 1994;13:1303–1313. doi: 10.1016/0896-6273(94)90417-0. [DOI] [PubMed] [Google Scholar]
- Steinert JR, Kopp-Scheinpflug C, Baker C, Challiss RA, Mistry R, Haustein MD, Griffin SJ, Tong H, Graham BP, Forsythe ID. Nitric oxide is a volume transmitter regulating postsynaptic excitability at a glutamatergic synapse. Neuron. 2008;60:642–656. doi: 10.1016/j.neuron.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Sun J, Pang ZP, Qin D, Fahim AT, Adachi R, Sudhof TC. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, Leao RM, Rowland KC, Spirou GA, von Gersdorff H. Optimizing synaptic architecture and efficiency for high-frequency transmission. Neuron. 2002;36:1127–1143. doi: 10.1016/s0896-6273(02)01137-6. [DOI] [PubMed] [Google Scholar]
- Taschenberger H, Scheuss V, Neher E. Release kinetics, quantal parameters and their modulation during short-term depression at a developing synapse in the rat CNS. J Physiol. 2005;568:513–537. doi: 10.1113/jphysiol.2005.093468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci. 2000;20:9162–9173. doi: 10.1523/JNEUROSCI.20-24-09162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO. Synaptic mechanisms for coding timing in auditory neurons. Annu Rev Physiol. 1999;61:477–496. doi: 10.1146/annurev.physiol.61.1.477. [DOI] [PubMed] [Google Scholar]
- Voets T. Dissection of three Ca2+-dependent steps leading to secretion in chromaffin cells from mouse adrenal slices. Neuron. 2000;28:537–545. doi: 10.1016/s0896-6273(00)00131-8. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Schneggenburger R, Weis S, Neher E. Presynaptic depression at a calyx synapse: The small contribution of metabotropic glutamate receptors. J Neurosci. 1997;17:8137–8146. doi: 10.1523/JNEUROSCI.17-21-08137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadel C, Neher E, Sakaba T. The coupling between synaptic vesicles and Ca2+ channels determines fast neurotransmitter release. Neuron. 2007;53:563–575. doi: 10.1016/j.neuron.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Wang L-Y, Neher E, Taschenberger H. Synaptic vesicles in mature calyx of Held synapses sense higher nanodomain calcium concentrations during action potential-evoked glutamate release. J Neurosci. 2008;28:14450–14458. doi: 10.1523/JNEUROSCI.4245-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel M, Lou X, Schneggenburger R. A mechanism intrinsic to the vesicle fusion machinery determines fast and slow transmitter release at a large CNS synapse. J Neurosci. 2007;27:3198–3210. doi: 10.1523/JNEUROSCI.4471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Westenbroek RE, Borst JGG, Catterall WA, Sakmann B. Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. J Neurosci. 1999;19:726–736. doi: 10.1523/JNEUROSCI.19-02-00726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Wang LY. Amplitude and kinetics of action potential-evoked Ca2+ current and its efficacy in triggering transmitter release at the developing calyx of Held synapse. J Neurosci. 2006;26:5698–5708. doi: 10.1523/JNEUROSCI.4889-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikami D, Bagabaldo Z, Olivera BM. The inhibitory effects of omega-conotoxins on Ca channels and synapses. Ann N Y Acad Sci. 1989;560:230–248. doi: 10.1111/j.1749-6632.1989.tb24100.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.