Abstract
Information regarding vagal innervation in the cardiac ventricle is limited and the direct effect of vagal stimulation on ventricular myocardial function is controversial. We have recently provided indirect evidence that the anti-fibrillatory effect of vagus nerve stimulation on the ventricle is mediated by nitric oxide (NO). The aim of this study was to provide direct evidence for the release of nitric oxide in the cardiac ventricle during stimulation of the efferent parasympathetic fibres of the cervical vagus nerve. The isolated innervated rabbit heart was employed with the use of the NO fluorescent indicator 4,5-diaminofluorescein diacetate (DAF-2 DA) during stimulation of the cervical vagus nerves and acetylcholine perfusion in the absence and presence of the non-specific NO synthase inhibitor NG-nito-l-arginine (l-NNA) and the neuronal NO synthase selective inhibitor 1-(2-trifluormethylphenyl)imidazole (TRIM). Using the novel fluorescence method in the beating heart, we have shown that NO-dependent fluorescence is increased by 0.92 ± 0.26, 1.20 ± 0.30 and 1.91 ± 0.27% (during low, medium and high frequency, respectively) in the ventricle in a stimulation frequency-dependent manner during vagus nerve stimulation, with comparable increases seen during separate stimulation of the left and right cervical vagus nerves. Background fluorescence is reduced during perfusion with l-NNA and the increase in fluorescence during high frequency vagal stimulation is inhibited during perfusion with both l-NNA (1.97 ± 0.35% increase before l-NNA, 0.00 ± 0.02% during l-NNA) and TRIM (1.78 ± 0.18% increase before TRIM, −0.11 ± 0.08% during TRIM). Perfusion with 0.1 μm acetylcholine increased NO fluorescence by 0.76 ± 0.09% which was blocked by l-NNA (change of 0.00 ± 0.03%) but not TRIM (increase of 0.82 ± 0.21%). Activation of cardiac parasympathetic efferent nerve fibres by stimulation of the cervical vagus is associated with NO production and release in the ventricle of the rabbit, via the neuronal isoform of nitric oxide synthase.
Abnormal autonomic activity has been shown to be a strong prognostic indicator of mortality in patients with heart failure (Nolan et al. 1998) and those who have had a previous myocardial infarct (La Rovere et al. 1998). Depressed heart rate variability and baroreceptor sensitivity are surrogate markers of impaired vagal tone associated with mortality in these conditions in which most of the deaths are sudden and due to ventricular arrhythmias, especially ventricular fibrillation (VF). We have recently shown that electrical stimulation of the vagus nerve in the neck (Brack et al. 2007) has a strong anti-arrhythmic effect in the rabbit ventricle against VF. These effects are blocked in the presence of a nitric oxide (NO) synthase inhibitor providing indirect evidence that NO is involved in this effect. The implication of NO in mediating vagal activity in the ventricle is novel although there are established data that this occurs at the atrial level (Herring et al. 2002). However, direct evidence of NO release in the ventricle during stimulation of the vagus nerves has never been shown.
A direct effect of vagal stimulation on ventricular function is either absent (Brack et al. 2006) or remains a controversial subject (Zang et al. 2005) despite evidence that vagal nerve fibres are present (Hoover et al. 2004) in the ventricle. We have shown that vagus nerve stimulation affects effective refractory period, VF threshold and electrical restitution (Ng et al. 2007) in the absence of background sympathetic tone. The question of how these effects are brought about is addressed in this study by measuring for the first time, the release of NO in the ventricle during stimulation of the vagus nerves in the neck.
Development of fluorescent indicators specific for NO e.g. derivatives of 4,5-diaminofluorescein (DAF-2) (Kojima et al. 1998) offers a non-invasive, highly selective semiquantitative method to directly visualise and allow analysis of NO activity. Since its emergence, DAF-2 fluorescence has been used in a variety of isolated atrial and ventricular myocyte preparations (LeBuffe et al. 2003; Pott et al. 2003) and was recently applied in the isolated beating heart by our group (Patel et al. 2008). Thus, we set out to discover if stimulation of the cervical vagus nerves causes NO release in the cardiac ventricle of the rabbit using DAF-2 fluorescence recorded from the left ventricle epicardial surface of a novel isolated innervated rabbit heart preparation (Ng et al. 2001).
Methods
Experimental techniques
All procedures were undertaken after local ethics approval at the University of Leicester and were carried out in accordance with the UK Animals (Scientific Procedures) 1986 Act and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1985).
Isolation of heart with intact autonomic nerves
The isolation of this preparation has been described previously (Ng et al. 2001). In brief, rabbits were premedicated with a mixture of Domitor i.m. (0.2 mg kg−1, Pfizer, Sandwich, UK), Ketamine (10 mg kg−1, Fort Lodge, Southampton, UK) and Torbugesic (0.05 mg kg−1, Pharmacia, Corby, UK) and anaesthetised with propofol (1 mg kg−1, i.v., Fresenius, Warrington, UK). After tracheotomy, rabbits were ventilated using a small-animal ventilator (Harvard Apparatus Ltd, Edenbridge, UK; 60 breaths min–1) with an O2–air mixture. The vagus nerves in the neck were isolated and vasculature leading to and from the ribcage were ligated and dissected. The rabbit was killed with 60 mg of Sagital (i.v.) (JM Loveridge, Southampton, UK) with 1000 U heparin (Leo Laboratories, Risborough, UK). The anterior portion of the rib cage was removed and the descending aorta cannulated. The pericardium was cut and ice-cold Tyrode solution applied on the epicardial surface to preserve myocardial function. The preparation extending from the neck to thorax was dissected from surrounding tissues.
Langendorff perfusion
The descending aorta was connected to the perfusion apparatus and retrogradely perfused in constant flow Langendorff mode (Gilson Minipulse 3 peristaltic pump, 100 ml min−1; Anachem, Luton, UK) with Tyrode solution containing (mm): Na+: 138.0, K+: 4.0, Ca2+: 1.8, Mg+: 1.0, HCO3−: 24.0, H2PO4−: 0.4, Cl−: 121.0, glucose: 11.0, acetate: 20.0, probenecid 0.3 and was continuously bubbled with 95% O2–5% CO2 (pH 7.4, 37°C). A 3F polypropylene catheter (Portex, Kent, UK) was inserted at the left ventricular apex for venous effluent drainage. Left ventricular pressure was monitored with a fluid-filled latex balloon connected to a pressure transducer (MTL0380, ADInstruments Ltd, Chalgrove, UK). End diastolic pressure was adjusted to zero. Aortic perfusion pressure was monitored with a second pressure transducer connected in series to the aortic cannula.
Fluorescence system and loading
An Optoscan spectrophotometer modular system was used (Cairn Research Ltd, Faversham, UK) to monitor excitation wavelengths at 470 ± 10 nm (F470), 480 ± 10 nm (F480), 490 ± 10 nm (F490) and 500 ± 10 nm (F500) for 20, 20, 8 and 2 ms, respectively, collected at 535 nm through a 50 nm bandpass filter. A bifurcated light guide was positioned midway between the apex and base, perpendicular to the epicardial surface of the left ventricle and routinely repositioned between protocols to prevent epicardial damage. Background ‘auto’ fluorescence was measured whilst fluorescence stabilised. Hearts were subsequently loaded with a bolus injection of DAF-2 DA (150–250 μl, 1 μm; Calbiochem c/o Merck, Nottingham, UK) via a catheter inserted into the right carotid artery. As reported before, the signal obtained at F490 was, among the excitation wavelengths, quantitatively largest and most representative of changes in [NO] (Patel et al. 2008). For the purpose of this report only F490 is shown. The fluorescence signal has a sizable beat-to-beat variation. Analysis of data collected by this method has been described previously (Patel et al. 2008) which revealed that minimum and maximum levels of F490 were altered by manoeuvres which alter [NO] in parallel to the same extent. For clarity, the mean F490 level is presented and analysed in this study.
Experimental protocols
In total, 22 adult New Zealand White rabbits (2.0 to 3.7 kg) were used in this study in three separate experimental protocols:
Vagus nerve stimulation and NO synthase inhibition
A total of seven animals were used. The cervical vagus nerves were supported on separate custom-made bipolar silver electrodes (Advent Research Materials, UK: 0.5 mm O.D.) and stimulated separately using a single channel constant-voltage square-wave pulse stimulator (SD9, Grass Instruments, Astro-Med Inc., Slough, UK). Stimulation frequencies of 5 Hz (low), 10 Hz (medium) and 15 Hz (high) at 8.7 ± 0.6 V, with a square-wave pulse duration of 2 ms, were used. Heart rate changes obtained with the different levels of vagal stimulation were recorded. Following this, protocols were monitored during constant ventricular pacing (200 beats min–1) with a bipolar catheter (EP Technologies, Sunnyvale, CA, USA) inserted into the right ventricular apex. VS was continued for 40 s with 30 s rest in-between stimulations. Left and right VS was carried out first, after which VS was tested before, during and after a 5 min period of perfusion with (1) the non-specific NO synthase inhibitor (NG-nitro-l-arginine, l-NNA, 200 μm; Sigma-Aldrich, Gillingham, UK) and (2) the established and widely used neuronal NO synthase inhibitor 1-(2-trifluormethylphenyl)imidazole (TRIM, 200 μm; Calbiochem).
Effects of acetylcholine
Isolated non-innervated rabbit hearts were perfused in constant flow Langendorff mode and loaded with DAF-2 DA as previously described (Patel et al. 2008). In seven hearts and prior to loading with DAF-2 DA, the chronotropic effects of 5 min perfusion with 0.01 μm, 0.1 μm and 1 μm acetylcholine chloride (ACh, Sigma-Aldrich) were examined followed by effects on perfusion pressure during constant ventricular pacing at 200 beats min–1. In 4 of the 7 hearts and after loading with DAF-2 DA, the effect of 2 min perfusion with 0.1 μm ACh on NO-dependent fluorescence was examined in the presence of both l-NNA (200 μm) and TRIM (200 μm).
Role of endothelially derived NO
In a separate eight isolated non-innervated rabbit hearts (original data presented in Patel et al. 2008), l-NNA (200 μm), sodium nitroprusside (SNP, 100 μm) and bradykinin (BK, 100 μm) were separately perfused for 5 min and the peak effect on aortic perfusion pressure and F490 were analysed.
Signal measurement and analysis
Signals were recorded with a Powerlab 800/s system and digitised at 1 kHz using Chart software (v5, ADInstruments), stored and displayed on a Dell personal computer (Dell, Bracknell, UK). All signals were analysed simultaneously. Data are mean ±s.e.m. and analysed using single- or 2-factor repeated measures ANOVA where appropriate. In both instances data were tested using Bonferroni post tests for comparisons between individual groups of data with significant differences between individual groups of data indicated by the asterisks or horizontal bars on each figure. P < 0.05 was considered significant.
Results
Vagus nerve stimulation
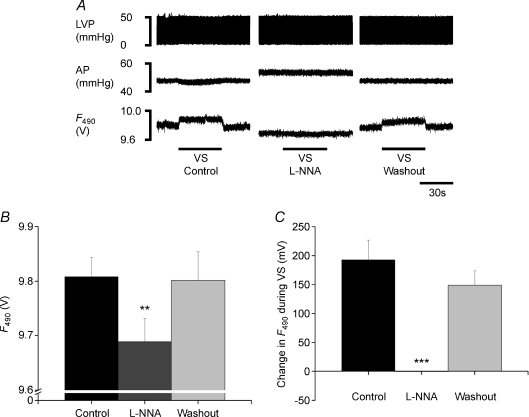
Cervical vagus nerve stimulation (VS) frequencies of 5 Hz (low), 10 Hz (medium) and 15 Hz (high) at 8.7 ± 0.6 V were used which reduced heart rate from a baseline of 154.0 ± 9.2 beats min–1 to 94.0 ± 6.3, 83.4 ± 9.9 and 74.6 ± 12.5 beats min–1, respectively. During constant ventricular pacing at 200 beats min–1, the fluorescence obtained with excitation wavelength of 490 nm (F490) increased in a stimulation frequency-dependent manner with VS without affecting aortic perfusion or left ventricular pressures (Fig. 1A). Two-factor ANOVA revealed a significant frequency-dependent effect during left (P = 0.0012 (difference low vs high 0.1144 V)) and right (P = 0.0126 (difference low vs high 0.1046 V)) vagus nerve stimulation. Figure 1B illustrates that left and right VS produced comparable increases in F490 at each stimulation frequency. Two-factor ANOVA confirms that there is no significant difference (P = 0.28) in the increase in NO fluorescence at low (difference −2.00 mV), medium (difference 25.49 mV) or high (difference 15.22 mV) frequency nerve stimulation between the left and right vagus nerve (Fig. 1C). This is despite the negative chronotropic effect during right VS being stronger than that during left VS and that the effect of left VS being stronger on atrioventricular conduction than right VS in this model (Ng et al. 2001).
Figure 1. Frequency dependent changes in NO fluorescence during VS.
A, raw data illustrating continuous left ventricular pressure (LVP), aortic perfusion pressure (AP) and DAF-2 fluorescence at the excitation wavelength of 490 nm (F490) at varying intensities of vagus nerve stimulation (VS). B, mean data representing F490 at baseline, during steady state VS and post-stimulation for low, medium and high frequency left and right VS. C, mean data representing the actual change in F490 during low, medium and high frequency left and right VS. Data are mean ±s.e.m., n= 7, and analysed using 2-factor repeated measured ANOVA with Bonferroni post test analysis indicating *P < 0.05, **P < 0.01 and ***P < 0.001 steady state vs baseline or the data comparisons indicated by the horizontal bars. For B: 2-factor ANOVA analysis was performed using baseline and steady state values for left and right VS separately.
To further characterise the change in fluorescence during VS, we first repeated high frequency VS (15 Hz) in the presence of the non-specific NO synthase (NOS) inhibitor NG-nitro-l-arginine (l-NNA) and then separately in the presence of the neuronal NOS selective inhibitor 1-(2-trifluormethylphenyl)imidazole (TRIM). Figure 2 illustrates that the vagally mediated increase in fluorescence was abolished during l-NNA perfusion and returns on washout of l-NNA. Compared to control, single-factor ANOVA revealed that l-NNA significantly decreased the basal F490 signal by 1.22 ± 0.24% (F: 11.43, P= 0.0017) whilst significantly increasing aortic perfusion pressure (52.6 ± 4.5 to 55.4 ± 4.0 mmHg, F: 14.69, P= 0.0006). The effect of perfusion of the neuronal NOS inhibitor TRIM is illustrated in Fig. 3. TRIM also abolished the vagally mediated increase in F490 (F: 109.8, P < 0.0001, single-factor ANOVA) that returns on washout. Unlike perfusion with l-NNA, TRIM did not alter the basal F490 signal (F: 0.62, P= 0.55, single-factor ANOVA) or aortic perfusion pressure (52.2 ± 6.2 to 53.6 ± 6.7 mmHg, F: 0.62, P= 0.56, single-factor ANOVA). Left ventricular pressure, however, was decreased during perfusion with TRIM (45.7 ± 2.3 to 38.1 ± 1.1 mmHg, F: 37.91, P < 0.0001, single-factor ANOVA) but not during l-NNA perfusion (45.0 ± 7.4 to 45.4 ± 7.5 mmHg, F: 1.17, P= 0.34, single-factor ANOVA) that returns to baseline on washout.
Figure 2. Inhibition of VS-dependent change in NO fluorescence with L-NNA.
A, raw data illustrating continuous left ventricular pressure (LVP), aortic perfusion pressure (AP) and DAF-2 fluorescence at the excitation wavelength of 490 nm (F490) during high frequency (15 Hz) vagus nerve stimulation (VS) during control, during perfusion with NG-nitro-l-arginine (l-NNA, 200 μm) and after washout. B, basal F490 and C, the change in F490 during VS during control, l-NNA and washout. Data are mean ±s.e.m., n= 7, and analysed using single-factor repeated measures ANOVA with Bonferroni post test indicated by **P < 0.01 and ***P < 0.001 l-NNA vs control and washout.
Figure 3. Inhibition of VS-dependent change in NO fluorescence with TRIM.
A, raw data illustrating continuous left ventricular pressure (LVP), aortic perfusion pressure (AP) and DAF-2 fluorescence at the excitation wavelength of 490 nm (F490) during high frequency (15 Hz) vagus nerve stimulation (VS) during control, during perfusion with 1-(2-trifluormethylphenyl)imidazole (TRIM, 200 μm) and after washout. B, basal F490 and C, the change in F490 with VS during control, TRIM and washout. Data are mean ±s.e.m., n= 7, and analysed using single-factor repeated measures ANOVA with Bonferroni post test indicating ***P < 0.001 TRIM vs control and washout.
Acetylcholine
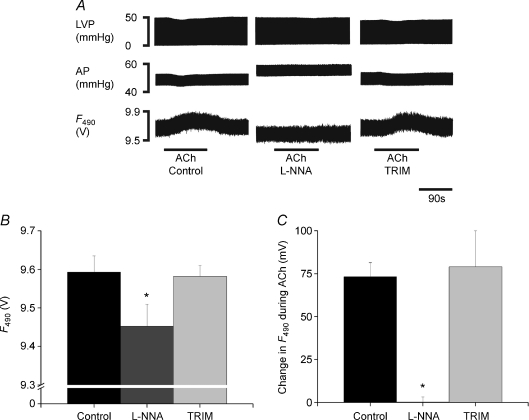
Prior to DAF-2 loading (n= 7), 0.01 μm, 0.1 μm and 1.0 μm ACh reduced heart rate from a baseline of 158.4 ± 13.0 beats min–1 to 150.9 ± 11.8, 127.4 ± 4.1 and 72.4 ± 11.9 beats min–1, respectively. During constant ventricular pacing at 200 beats min–1 the effect of ACh on coronary perfusion pressure was concentration dependent and biphasic in nature. At low concentrations (0.01 μm and 0.1 μm) ACh elicited a monotonic vasodilatation with a decrease in perfusion pressure of −2.79 ± 0.58 and −3.41 ± 0.41%, respectively, whilst the higher dose of 1 μm ACh, which produced a bradycardia comparable to high frequency VS, caused a vasoconstriction with an increase in perfusion pressure of 3.21 ± 1.30%. For this reason we chose 0.1 μm to use as the most suitable dose that would elicit a consistent and monotonic vasodilatation to test after loading hearts with DAF−2 and during NOS inhibition.
After loading with DAF-2 (n= 4) and during control conditions, 0.1 μm ACh reduced perfusion pressure by 1.80 ± 0.30 mmHg (−3.26 ± 0.54%). The effect of ACh on perfusion pressure was blocked during non-specific NOS inhibition with l-NNA (change of +0.46 ± 0.26 mmHg (0.66 ± 0.34%)) but not blocked during nNOS inhibition using TRIM where ACh reduced perfusion pressure by 1.30 ± 0.21 mmHg (−2.41 ± 0.53%). Similar to previous experiments, l-NNA, but not TRIM, significantly reduced basal F490 (Fig. 4A and B) (F: 11.65, P= 0.0086, single-factor ANOVA). Concurrently, ACh produced a significant increase in DAF-2 fluorescence at F490 (Fig. 4C) during control and during perfusion with TRIM, but not during perfusion with l-NNA (Fig. 4C) (F: 8.067, P= 0.0199, single-factor ANOVA).
Figure 4. Change in NO fluorescence with acetylcholine and NOS inhibition.
A, raw data illustrating continuous left ventricular pressure (LVP), aortic perfusion pressure (AP) and DAF-2 fluorescence at the excitation wavelength of 490 nm (F490) with 0.1 μm acetylcholine (ACh) during control, during perfusion with NG-nitro-l-arginine (l-NNA, 200 μm) and 1-(2-trifluormethylphenyl)imidazole (TRIM, 200 μm). Mean data representing B, basal F490 and C, the change in F490 with ACh during control and perfusion with l-NNA and TRIM. Data are mean ±s.e.m.n= 4, and analysed using single-factor repeated measures with Bonferroni post tests for comparisons of individual groups of data as indicated by *P < 0.05 l-NNA vs control and TRIM.
Endothelially derived NO
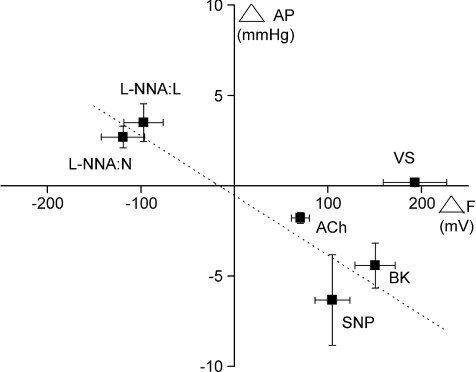
To investigate the involvement of endothelially derived NO in the fluorescence measurement obtained from VS, the change in F490 together with the corresponding change in aortic perfusion pressure during VS and during pharmacological modulation of endothelially dependent changes in vascular tone (Patel et al. 2008) were analysed. As illustrated graphically in the plots of collective group data in Fig. 5, perfusion of l-NNA caused an increase in perfusion pressure and a decrease in fluorescence, whereas sodium nitroprusside, bradykinin and acetylcholine elicited opposite effects. Thus, these vasoactive agents caused concomitant changes in aortic perfusion pressure associated with significant changes in NO-dependent fluorescence. In contrast, the significant increase in NO-dependent fluorescence during VS was not accompanied by any significant change in aortic perfusion pressure.
Figure 5. Correlation of the changes in NO fluorescence with corresponding changes in perfusion pressure.
Data illustrating the change in aortic perfusion pressure (ΔAP) with the change in DAF-2 fluorescence at the excitation of 490 nm (ΔF) during high frequency vagus nerve stimulation (VS, protocol I, n= 7), 0.1 μm acetylcholine (ACh, protocol II, n= 4) and perfusion with NG-nitro-l-arginine (l-NNA, 200 μm, protocol I (n= 7) + protocol III (n= 8)), sodium nitroprusside (SNP, 100 μm, protocol III, n= 8) and bradykinin (BK, 100 μm, protocol III, n= 8). l-NNA:L, non-innervated Langendorff-perfused isolated heart. l-NNA:N, innervated isolated heart preparation. Data are mean ±s.e.m. Dotted line represents a linear fitted line for data representing l-NNA:L, l-NNA:N, SNP, BK and ACh.
Discussion
To the best of our knowledge, this study demonstrates for the first time that direct stimulation of the cervical vagus nerves in the rabbit stimulates the release of nitric oxide via a neuronal NOS mechanism in the cardiac ventricle. The most compelling evidence herein, suggesting that NO is released during vagus nerve stimulation, is that the rise in the fluorescence signal seen during VS was abolished in the presence of NO synthase inhibition. Interestingly the basal signal was also reduced in the presence of LNA as reported previously (Patel et al. 2008), suggesting there is background NO release in the isolated heart preparation. Studies monitoring stable NO metabolites, chemiluminescence of NO and studies using an NO-sensitive probe to test for NO within coronary effluent support this observation (Pabla & Curtis, 1994; Kupatt et al. 1997; Paz et al. 2003; Tsukada et al. 2003).
Origin of NO release
NO is synthesised by three isoforms of NOS: endothelial (eNOS/NOS III), neuronal (nNOS/NOS I) and inducible NOS (iNOS/NOS II). All three isoforms are present within the heart and are subcellularly localised. In normal circumstances, however, receptor-mediated NOS-dependent mechanisms involve nNOS or eNOS, whilst iNOS is stress activated (Paz et al. 2003). Abolition of the vagally mediated increase in fluorescence following the nNOS selective inhibitor, TRIM, suggests that nNOS mediates the VS-induced NO release although an eNOS-dependent mechanism cannot be ruled out. However, our evidence that vagally induced increase in fluorescence was not associated with any change in perfusion pressure, suggests that an eNOS or endothelially dependent mechanism is unlikely to contribute significantly to the increase in ventricular NO seen during vagal stimulation. This may not seem surprising since nNOS is present in ventricular intracardiac nerve fibres (Sosunov et al. 1995) although it is not clear if these fibres are of parasympathetic origin. Additional to this, nNOS is found in sarcoplasmic reticulum and mitochondria (Xu et al. 1999; Kanai et al. 2001) which raises the possibility that NO from myocardial nNOS could be responsible for the increase in DAF-2 fluorescence seen during vagal stimulation. TRIM did not alter basal DAF-2 fluorescence, suggesting that nNOS, regardless of localisation, does not participate significantly in the background DAF-2 fluorescence signal and basal release of NO. This suggests that the basal NO signal originates from an eNOS-dependent source such as plasmalemmal and T-tubule caveolae and within coronary endothelium (Miethke et al. 2003; Massion et al. 2004) where the majority of cardiac eNOS is considered to be located. Left ventricular pressure was decreased during perfusion of TRIM but perfusion pressure was not. The mechanism underlying this effect on LVP is unknown but is likely to be due to direct effects on processes involved in excitation–contraction coupling. This is supported by reports that TRIM can affect muscle tension and calcium homeostasis in anococcygeus muscle (Che et al. 2007).
In an attempt to elucidate if myocardial nNOS may be responsible for the increase in NO-dependent fluorescence seen during vagus nerve stimulation, DAF-2 fluorescence was measured during perfusion with the muscarinic receptor agonist acetylcholine. It was shown that there was a dose-dependent biphasic effect of ACh on perfusion pressure, with low doses of ACh causing vasodilatation and high doses of ACh causing vasoconstriction. This biphasic ACh-dependent vascular action has been reported in a range of species (Knight et al. 1991; Motterlini & Macdonald, 1993; Takahashi et al. 2003; Gwozdz et al. 2007). In our experiments, 0.1 μm ACh produced a consistent vasodilatation that was accompanied by an increase in the DAF-2 NO fluorescence signal. These data are supported by previous studies illustrating an increase in NO production following ACh infusion using chemiluminescence in isolated rabbit hearts (Amezcua et al. 1988). In the current study, the increase in F490 and decrease in perfusion pressure were inhibited during non-specific NOS inhibition with l-NNA, as observed by Amezcua et al. (1989), but not during nNOS selective inhibition using TRIM. These data demonstrate significant differences between vagus nerve stimulation and acetylcholine infusion as has been shown in the past in historical studies (Levy & Zieske, 1969) and in recent studies from our group (Mantravadi et al. 2007). The increase in NO fluorescence with acetylcholine infusion associated with vasodilatation suggests an endothelial effect and origin of NO (not blocked by TRIM) whilst that with vagus nerve stimulation suggests a non-endothelial nNOS effect and a neural origin (blocked by both l-NNA and TRIM).
Pharmacological manipulation of NO fluorescence using l-NNA (with increase in vascular tone), sodium nitroprusside, bradykinin and ACh (with decrease in vascular tone) are all associated with alterations in aortic perfusion pressure (Patel et al. 2008), which is a surrogate for vascular resistance and is intimately controlled by the endothelium/eNOS-mediated NO. Since all endothelial-dependent changes in aortic perfusion pressure that we have tested are associated with changes in DAF-2 fluorescence, our data suggest that endothelial-derived NOS is unlikely to be involved in the VS-mediated increase in NO fluorescence. If this was the case, we should have observed some decrease in perfusion pressure during VS associated with the increase in NO fluorescence, which we did not, unless VS promoted a simultaneous vasoconstriction to counterbalance the effect of endothelial eNOS-mediated NO.
Effect of VS on cardiac physiology
The importance of the vagus nerve in cardiac ventricles of most mammalian species is still largely overlooked. Part of this may be explained by concentration on the cholinergic effect of the vagus nerve on heart rate and muscarinic receptor activity (Casadei et al. 1993). Neuronally derived NO can account for a number of the effects of vagus nerve stimulation in the heart including heart rate control (Jumrussirikul et al. 1998), atrio-ventricular conduction (Conlon & Kidd, 1999), and inhibition of the sympathetic mediated increase in left ventricular force (Hare et al. 1995). However, the present study and our recent evidence (Brack et al. 2007) demonstrate that vagus nerve stimulation has an NO-dependent direct effect on the cardiac ventricle.
There are concerns that the cervical vagus nerve may contain some sympathetic nerve fibres since sympathetic and parasympathetic nerve fibres are known to form a vago-sympatho plexus/bundle low in the neck of many species (Daly & Mount, 1951; Randall et al. 1971; Armour & Randall, 1975). However, in the rabbit, it is known that the cervical vagus is unequivocally free of sympathetic nerve fibres at this level (Evans & Murray, 1954) suggesting that the release of NO during stimulation of the vagus nerve is purely parasympathetic in origin.
In summary, these results suggest a physiological and putative parasympathetic-NO system within the rabbit cardiac ventricle. Using DAF-2 DA in the whole heart is a significant development over early and indirect measurements of NO (Pabla & Curtis, 1994; Zweier et al. 1995; Kupatt et al. 1997; Pinsky et al. 1997; Tsukada et al. 2003; Paz et al. 2003) and enables a more practical and straightforward method to monitor NO in the beating heart that can be simultaneously correlated with electrophysiological and mechanical events. The data from this study support a growing body of evidence from a range of species (Akiyama & Yamazaki, 2001; Kawano et al. 2003; Zang et al. 2005) that vagal innervation of the cardiac ventricle is more prevalent than originally thought. A recent study illustrating that action potential duration prolongation during vagus nerve stimulation occurred across the heart in the rabbit (Mantravadi et al. 2007) provides physiological evidence of diffuse vagal innervation in the ventricle although more detailed histological description in this species and in others is warranted. The results presented in this paper suggest that stimulation of the efferent vagus nerves in the neck produces NO release in the rabbit cardiac ventricle as a result of nNOS activation. We have previously shown that direct vagus nerve stimulation reduces the susceptibility of the heart toward VF which appears to be mediated via changes in electrical restitution (Ng et al. 2007). Furthermore, we have shown the vagal-mediated effect on electrical restitution and cardioprotective effect against VF are blocked during NOS inhibition and reversed on substrate supplementation with arginine (Brack et al. 2007), suggesting that NO is intimately involved in the vagal modulation of ventricular electrophysiology. More detailed information on the vagal/NO pathway on ventricular electrophysiology and ion channel activity is, however, lacking. These findings have important implications for the development of pharmacological manipulations of the vagal-NO pathway in the treatment of cardiac diseases.
Acknowledgments
This work was supported by a British Heart Foundation (PG/02/088) and Garfield Weston Trust Project Grant. We thank Mrs Susan Rafelt for help with statistical analysis on some of the experimental data.
Glossary
Abbreviations
- ACh
acetylcholine chloride
- AP
aotric perfusion pressure
- BK
bradykinin
- DAF-2 DA
4,5-diaminofluorescein diacetate
- l-NNA
NG-nitro-l-arginine
- LVP
left ventricular pressure
- eNOS/NOS III
endothelial nitric oxide synthase
- nNOS/NOS I
neuronal nitric oxide synthase
- iNOS/NOS II
inducible nitric oxide synthase
- NO
nitric oxide
- SNP
sodium nitroprusside
- TRIM
1-(2-trifluormethylphenyl)imidazole
- VF
ventricular fibrillation
- VS
vagus nerve stimulation
Author contributions
All authors have contributed to: (1) the conception, design, or analysis and interpretation of data, (2) drafting or revising the manuscript for important intellectual content, and (3) final approval of the version to be published.
References
- Akiyama T, Yamazaki T. Effects of right and left vagal stimulation on left ventricular acetylcholine levels in the cat. Acta Physiol Scand. 2001;172:11–16. doi: 10.1046/j.1365-201X.2001.00812.x. [DOI] [PubMed] [Google Scholar]
- Amezcua JL, Dusting GJ, Palmer RMJ, Moncada S. Acetylcholine induces vasodilatation in the rabbit heart through the release of nitric oxide, the endogenous nitrovasodilator. Br J Pharmacol. 1988;95:830–834. doi: 10.1111/j.1476-5381.1988.tb11711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amezcua JL, Palmer RMJ, de Souza BM, Moncada S. Nitric oxide synthesized from l-arginine regulates vascular tone in the coronary circulation of the rabbit. Br J Pharmacol. 1989;97:1119–1124. doi: 10.1111/j.1476-5381.1989.tb12569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armour JA, Randall WC. Functional anatomy of the canine cardiac nerves. Acta Anat. 1975;91:510–528. doi: 10.1159/000144411. [DOI] [PubMed] [Google Scholar]
- Brack KE, Coote JH, Ng GA. The effect of direct autonomic nerve stimulation on left ventricular force in the isolated innervated Langendorff perfused rabbit heart. Auton Neurosci. 2006;124:69–80. doi: 10.1016/j.autneu.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Brack KE, Patel VH, Coote JH, Ng GA. Nitric oxide mediates the vagal protective effect in ventricular fibrillation – studies in the isolated rabbit heart with intact autonomic innervation. J Physiol. 2007;583:695–704. doi: 10.1113/jphysiol.2007.138461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadei B, Pipilis A, Sessa F, Conway J, Sleight P. Low doses of scopolamine increase vagal tone in the acute phase of myocardial infarction. Circulation. 1993;88:353–357. doi: 10.1161/01.cir.88.2.353. [DOI] [PubMed] [Google Scholar]
- Che Y, Potocnik S, Ellis A, Li CG. Effects of TRIM on tension, intracellular calcium and nitrergic transmission in the rat annococcygeneus muscle. Nitric Oxide. 2007;16:29–35. doi: 10.1016/j.niox.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Conlon K, Kidd C. Neuronal nitric oxide facilitates vagal chronotropic and dromotropic action on the heart. J Auton Nerv Syst. 1999;75:136–146. doi: 10.1016/s0165-1838(98)00185-4. [DOI] [PubMed] [Google Scholar]
- Daly MB, Mount LE. The origin, course and nature of bronchomotor fibres in teh cervical sympathetic nerve of the cat. J Physiol. 1951;113:43–62. doi: 10.1113/jphysiol.1951.sp004555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CH, Murray JG. Histological and functional studies on the fibre composition of the vagus nerve in the rabbit. J Anat. 1954;88:320–337. [PMC free article] [PubMed] [Google Scholar]
- Gwozdz P, Drelicharz L, Kozlovski VI, Chlopicki S. Prostacyclin, but not nitric oxide, is the major mediator of acetylcholine-induced vasodilatation in the isolated mouse heart. Pharmacol Rep. 2007;59:545–552. [PubMed] [Google Scholar]
- Hare JM, Keaney JF, Jr, Balligand JL, Loscalzo J, Smith TW, Colucci WS. Role of nitric oxide in parasympathetic modulation of beta-adrenergic myocardial contractility in normal dogs. J Clin Invest. 1995;95:360–366. doi: 10.1172/JCI117664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring N, Danson EJ, Paterson DJ. Cholinergic control of heart rate by nitric oxide is site specific. News Physiol Sci. 2002;17:202–206. doi: 10.1152/nips.01386.2002. [DOI] [PubMed] [Google Scholar]
- Hoover DB, Ganote CE, Ferguson SM, Blakely RD, Parsons RL. Localization of cholinergic innervation in guinea pig heart by immunohistochemistry for high-affinity choline transporters. Card Res. 2004;62:112–121. doi: 10.1016/j.cardiores.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Jumrussirikul P, Dinerman J, Dawson TM, Dawson VL, Ekelund U, Georgakopoulos D, Schramm LP, Calkins H, Snyder SH, Hare JM, Berger RD. Interaction between neuronal nitric oxide synthase and inhibitory G protein activity in heart rate regulation in conscious mice. J Clin Invest. 1998;102:1279–1285. doi: 10.1172/JCI2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai AJ, Pearce LL, Clemens PR, Birder LA, VanBibber MM, Choi SY, de Groat WC, Pererson J. Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc Natl Acad Sci U S A. 2001;98:14126–14131. doi: 10.1073/pnas.241380298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. 2003;18:32–39. doi: 10.1007/s003800300005. [DOI] [PubMed] [Google Scholar]
- Knight DR, Shen YT, Young MA, Vatner SF. Acetylcholine-induced coronary vasoconstriction in tranquilised baboons. Circ Res. 1991;69:706–713. doi: 10.1161/01.res.69.3.706. [DOI] [PubMed] [Google Scholar]
- Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- Kupatt C, Habazettl H, Zahler S, Weber C, Becker BF, Messmer K, Gerlach E. ACE-inhibition prevents postischemic coronary leukocyte adhesion and leukocyte-dependent reperfusion injury. Card Res. 1997;36:386–395. doi: 10.1016/s0008-6363(97)00191-0. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- LeBuffe G, Schumacker PT, Shao ZH, Andeson T, Iwasa H, Vanden Hoek TL. ROS and NO trigger early preconditioning: relationship to mitochondrial KATP channel. Am J Physiol Heart Circ Physiol. 2003;284:H299–H308. doi: 10.1152/ajpheart.00706.2002. [DOI] [PubMed] [Google Scholar]
- Levy MN, Zieske H. Comparison of the cardiac effects of vagus nerve stimulation and of acetylcholine infusions. Am J Physiol. 1969;216:890–897. doi: 10.1152/ajplegacy.1969.216.4.890. [DOI] [PubMed] [Google Scholar]
- Mantravadi R, Gabris B, Liu T, Choi BR, de Groat WC, Ng GA, Samala G. Autonomic nerve stimulation reverses ventricular repolarisation sequence in rabbit hearts. Circ Res. 2007;100:e72–e80. doi: 10.1161/01.RES.0000264101.06417.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massion PB, Dessy C, Desjardins F, Pellat M, Havaux X, Belge C, Moulin P, Guiot Y, Feron O, Janssens S, Balligand JL. Cardiomyocyte-restricted overexpression of endothelial nitric oxide synthase (NOS3) attenuates beta-adrenergic stimulation and reinforces vagal inhibition of cardiac contraction. Circulation. 2004;110:2666–2672. doi: 10.1161/01.CIR.0000145608.80855.BC. [DOI] [PubMed] [Google Scholar]
- Miethke A, Feussner M, Planitzer G, Richter H, Gutsmann M, Gossrau R. Localization of NOS-1 in the sarcolemma region of a subpopulation of atrial cardiomyocytes including myoendocrine cells and NOS-3 in vascular and endocardial endothelial cells of the rat heart. Acta Histochem. 2003;105:43–55. doi: 10.1078/0065-1281-00692. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Macdonald VW. Cell-free haemoglobin potentiates acetylcholine-induced coronary vasoconstriction in rabbit hearts. J App Physiol. 1993;75:2224–2233. doi: 10.1152/jappl.1993.75.5.2224. [DOI] [PubMed] [Google Scholar]
- Ng GA, Brack KE, Coote JH. Effects of direct sympathetic and vagus nerve stimulation on the physiology of the whole heart – a novel model of isolated Langendorff perfused rabbit heart with intact dual autonomic innervation. Exp Physiol. 2001;86:319–329. doi: 10.1113/eph8602146. [DOI] [PubMed] [Google Scholar]
- Ng GA, Brack KE, Patel VH, Coote JH. Autonomic modulation of electrical restitution, alternans and ventricular fibrillation initiation in the isolated heart. Card Res. 2007;73:750–760. doi: 10.1016/j.cardiores.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Nolan J, Batin PD, Andrews R, Lindsay SJ, Brooksby P, Mullen M, Baig W, Flapan AD, Cowley A, Prescott RJ, Neilson JM, Fox KA. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart) Circulation. 1998;98:1510–1516. doi: 10.1161/01.cir.98.15.1510. [DOI] [PubMed] [Google Scholar]
- Pabla R, Curtis MJ. Endogenous protection against reperfusion-induced ventricular fibrillation: role of neuronal versus non-neuronal sources of nitric oxide and species dependence in the rat versus rabbit isolated heart. J Mol Cell Cardiol. 1994;28:2097–2110. doi: 10.1006/jmcc.1996.0202. [DOI] [PubMed] [Google Scholar]
- Patel VH, Brack KE, Coote JH, Ng GA. A novel method of measuring nitric-oxide-dependent fluorescence using 4,5-diaminofluorescein (DAF-2) in the isolated Langendorff-perfused rabbit heart. Pflugers Archiv. 2008;456:635–645. doi: 10.1007/s00424-007-0440-y. [DOI] [PubMed] [Google Scholar]
- Paz Y, Frolkis I, Pevni D, Shapira I, Yuhas Y, Iaina A, Wollman Y, Chernichovski T, Nesher N, Locker C, Mohr R, Uretzky G. Effect of tumor necrosis factor-alpha on endothelium and inducible nitric oxide synthase messenger ribonucleic acid expression and nitric oxide synthesis in ischemic and nonischemic isolated rat heart. J Am Coll Cardiol. 2003;42:1299–1305. doi: 10.1016/s0735-1097(03)00992-6. [DOI] [PubMed] [Google Scholar]
- Pinsky DJ, Patton S, Mesaros S, Broykovych V, Kubaszewski E, Grunfeld S, Malinski T. Mechanical transduction of nitric oxide synthesis in the beating heart. Circ Res. 1997;81:372–379. doi: 10.1161/01.res.81.3.372. [DOI] [PubMed] [Google Scholar]
- Pott C, Brixius K, Bundkirchen A, Bolck B, Bloch W, Steinritz D, Mehlhorn U, Schwinger RH. The preferential β3-adrenoceptor agonist BRL 37344 increases force via β1-/β2-adrenoceptors and induces endothelial nitric oxide synthase via β3-adrenceptors in human atrial myocardium. Br J Pharmacol. 2003;138:521–529. doi: 10.1038/sj.bjp.0705065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall WC, Armour JA, Randall DC, Smith OA. Functional anatomy of the cardiac nerves in the baboon. Anat Rec. 1971;170:183–198. doi: 10.1002/ar.1091700205. [DOI] [PubMed] [Google Scholar]
- Sosunov AA, Hassall CJ, Loesch A, Turmaine M, Burnstock G. Ultrastructural investigation of nitric oxide synthase-immunoreactive nerves associated with coronary blood vessels of rat and guinea-pig. Cell Tissue Res. 1995;280:575–582. doi: 10.1007/BF00318361. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Maehara K, Onuki N, Saito T, Maruyama Y. Decreases in contractility of the left ventricle is induced by the neurotransmitter acetylcholine, but not vagal simulation in rats. Jpn Heart J. 2003;44:257–270. doi: 10.1536/jhj.44.257. [DOI] [PubMed] [Google Scholar]
- Tsukada Y, Yasutake M, Jia D, Kusama Y, Kishida H, Takano T, Tsukada S. Real-time measurement of nitric oxide by luminol-hydrogen peroxide reaction in crystalloid perfused rat heart. Life Sci. 2003;72:989–1000. doi: 10.1016/s0024-3205(02)02353-6. [DOI] [PubMed] [Google Scholar]
- Xu KY, Huso DL, Dawson TM, Bredt DS, Becker LC. Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1999;96:657–662. doi: 10.1073/pnas.96.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang WJ, Chen LN, Yu XJ. Progress in the study of vagal control of cardiac ventricles. Acta Physiol Sinica. 2005;57:659–672. [PubMed] [Google Scholar]
- Zweier JL, Wang P, Kuppusamy P. Direct measurement of nitric oxide generation in the ischemic heart using electron paramagnetic resonance spectroscopy. J Biol Chem. 1995;270:304–307. doi: 10.1074/jbc.270.1.304. [DOI] [PubMed] [Google Scholar]