Abstract
DNA damage provokes DNA repair, cell-cycle regulation and apoptosis. This DNA-damage response encompasses gene-expression regulation at the transcriptional and post-translational levels. We show that cellular responses to UV-induced DNA damage are also regulated at the post-transcriptional level by microRNAs. Survival and checkpoint response after UV damage was severely reduced on microRNA-mediated gene-silencing inhibition by knocking down essential components of the microRNA-processing pathway (Dicer and Ago2). UV damage triggered a cell-cycle-dependent relocalization of Ago2 into stress granules and various microRNA-expression changes. Ago2 relocalization required CDK activity, but was independent of ATM/ATR checkpoint signalling, whereas UV-responsive microRNA expression was only partially ATM/ATR independent. Both microRNA-expression changes and stress-granule formation were most pronounced within the first hours after genotoxic stress, suggesting that microRNA-mediated gene regulation operates earlier than most transcriptional responses. The functionality of the microRNA response is illustrated by the UV-inducible miR-16 that downregulates checkpoint-gene CDC25a and regulates cell proliferation. We conclude that microRNA-mediated gene regulation adds a new dimension to the DNA-damage response.
Keywords: cell cycle checkpoints, DNA damage, microRNAs, stress granules
Introduction
Various endogenous and exogenous agents, such as metabolic byproducts and solar UV irradiation, continuously damage cellular DNA. If not repaired properly, this can result in mutations or chromosomal aberrations and might eventually trigger cancer. Alternatively, DNA damage may cause cell death and cellular senescence, which may contribute to aging. Mammalian cells have elaborate systems to counteract the harmful effects of DNA damage, collectively called DNA-damage response (DDR). DNA damage activates repair systems in parallel with complex signal-transduction routes that transiently halt the cell cycle, trigger apoptosis or lead to irreversible growth arrest (replicative senescence). The precise regulation of DDR is critical for cell survival and its abrogation often results in genetic instability and malignant transformation, whereas DDR hyper-activation may trigger excessive cell death or senescence and accelerate aging (Hoeijmakers, 2007).
Many DDR proteins are regulated at the post-translational level through modulation of protein activity or stability (Lukas and Bartek, 2004), for example more than 700 proteins are phosphorylated after DNA-damage induction (Matsuoka et al, 2007). In addition, DDR is controlled at the transcriptional level by the induction of DNA repair and cell-cycle regulatory genes after genotoxic stress (Garinis et al, 2005). A prime example is the transient stabilization of p53 after DNA damage and the subsequent activation of numerous p53 target genes (Colman et al, 2000). Recently, an additional layer of gene regulation has been discovered, which acts at the post-transcriptional level through microRNAs (miRNAs). These small endogenous non-coding RNAs are involved in diverse cellular and developmental processes in plants and animals (Kloosterman and Plasterk, 2006). Mature miRNAs are generated from longer primary transcripts, which are then processed into hairpin RNAs of approximately 70 nucleotides. These precursor miRNAs are exported to the cytoplasm and further processed by Dicer. The RNA-induced silencing complex (RISC), in which argonaute-2 protein (Ago2) represents the catalytic activity, mediates miRNA-induced regulation of mRNAs (Liu et al, 2004; Meister et al, 2004; Jaskiewicz and Filipowicz, 2008). It has been estimated that as many as 1000 miRNA genes are encoded in the mammalian genome, of which >700 have been experimentally verified (Bentwich et al, 2005; Berezikov et al, 2005). Approximately 30% of the protein-coding genes contain potential miRNA binding sites in their 3′ untranslated regions (3′UTR) and may therefore be under miRNA control (Lewis et al, 2003, 2005; John et al, 2004; Krek et al, 2005; Grimson et al, 2007). Aberrantly expressed miRNAs have been linked to a variety of diseases, including cancer, where they can act as tumour suppressors or oncogenes (Calin et al, 2005; Kloosterman and Plasterk, 2006; Zhang et al, 2006). The involvement of miRNAs in cancer prevention has also been confirmed by conditional Dicer inactivation, which led to accelerated tumour development (Kumar et al, 2007).
Most translationally repressed mRNAs are diffusely distributed in the cytoplasm. However, a subset is located in subcellular structures; the Ago2 protein and other components of the RNA-interference machinery accumulate in processing bodies (PBs) in most cell types, suggesting that they are major sites of RNA processing (Eulalio et al, 2007). Various types of cellular stress induce another type of structure, called stress granule (SG) (Kedersha and Anderson, 2007). The function of SGs is not completely understood, but they are generally considered as sites where translationally repressed mRNAs accumulate. Ago2 was found to relocalize to SGs on arsinite treatment (Leung et al, 2006).
In view of the complexity of the DDR pathway, it is expected that miRNAs have a function in this cellular response to environmental insults. However, any function of miRNA-mediated gene silencing in the DDR has not yet been elucidated. Therefore, we exposed cells to UV and investigated SG formation and miRNA-expression patterns. MiRNA-driven RNA silencing influenced survival and cell-cycle progression, indicating that RNA interference mechanisms contribute to the DDR.
Results
Knockdown of Dicer and Ago2 leads to UV hypersensitivity
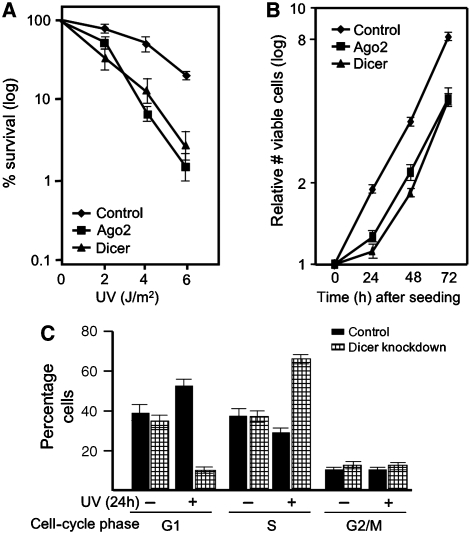
Defects in the DDR usually lead to cellular hypersensitivity to DNA-damaging agents. In order to determine whether miRNA-mediated gene silencing is involved in DDR, we used the natural DNA-damaging agent UV and determined the effect of inhibition of the miRNA response. HeLa cells were transfected with siRNAs that target the crucial components Ago2 and Dicer or control siRNAs. The next day cells were reseeded and 32 h later exposed to UV. We observed a significant decrease in cell survival when miRNA-mediated gene silencing was abrogated. (Figure 1A, Supplementary Figure 1A–D). This was not because of reduced proliferation or cell viability: the cells proliferated at a similar rate between 24 and 72 h after siRNA transfection. (The growth delay observed in the first 24 h after reseeding (Figure 1B) was transient and might be because of a general sensitivity to stresses, such as trypsinization.)
Figure 1.
MiRNA-mediated gene silencing is important for cell survival and checkpoint response after UV treatment. (A) Clonal survival of HeLa cells transfected with Dicer, Ago2 or control siRNAs (transfected twice with 50 nM final concentration). Results are the aggregate of four different siRNAs per gene. (B) Growth characteristics of HeLa cells that were either transfected with Ago2, Dicer or control siRNAs (transfected twice with 50 nM final concentration). (C) Cell-cycle analysis using HeLa cells transfected twice with Dicer and control siRNAs (50 nM final concentration) 24 h after UVC treatment.
Dicer-depleted cells had a similar cell-cycle distribution compared with normal cells after reseeding (Figure 1C). However, when both Dicer-depleted and control cells were exposed to UV 48 h after reseeding, the cell-cycle distribution was markedly different: we observed a dramatic depletion of G1-phase cells and enormous increase in S-phase cells, which suggests that miRNAs have a role in cell-cycle checkpoints and/or DNA repair and replication. Thus, miRNA-mediated gene regulation has physiological relevance for DDR.
UV irradiation leads to Ago2 relocalization in stress granules
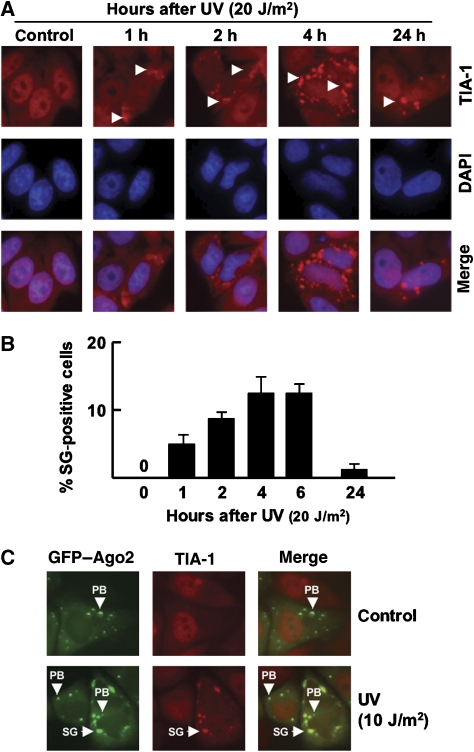
To address the role of miRNA-mediated gene-expression regulation in DDR in more detail, we used two different readouts: (I) changes in miRNA-expression levels, and (II) UV-induced SG formation. Stress stimuli have been shown to cause the relocalization of RISC-complex components into SGs, suggesting that both SGs and miRNA-mediated gene silencing have a role in stress-induced gene-expression changes (Leung et al, 2006; Leung and Sharp, 2007). Therefore, we determined whether UV damage also led to SG formation in HeLa cells and primary human fibroblasts. As described previously, UV irradiation triggered the accumulation of SGs, as judged from TIA-1 staining patterns (Figure 2A; Anderson and Kedersha, 2002). SG formation was dose- and time-dependent and became apparent within 1 h after UV exposure. The SG size and the number of cells carrying SGs increased until they reached their maximum at approximately 4 h after UV irradiation, whereas most cells lost SGs after 24 h (Figure 2A and B; data not shown). Next, we tested whether Ago2 translocates to SGs after UV damage as shown for arsenite treatment. We transfected HeLa cells with a previously described GFP–Ago2 transgene, which mimics the behavior of endogenous Ago2 (Leung et al, 2006), and monitored its localization on UV stress. The untreated cells showed diffuse cytoplasmic localization of GFP–Ago2 with accumulation in a small number of P-bodies (Figure 2C). UV irradiation changed Ago2–GFP localization: it accumulated in several large, newly formed structures in the perinuclear region (Figure 2C). These newly formed structures were identified as SGs by TIA-1 co-staining and all SGs had acquired GFP–Ago2. Thus, SG formation and Ago2 relocalization are part of the cellular response to UV stress.
Figure 2.
SG formation after UV irradiation. (A) Time-course analysis of TIA-1 accumulation after UVC irradiation. HeLa cells were subjected to 20 J/m2 UV and SGs were visualized through TIA-1 staining. (B) Quantification of cells containing stress granules (SGs) after UVC irradiation. For un-irradiated cells >104 cells have been counted per culture. For irradiated cell cultures approximately 500 cells have been counted and SG-positive cells are presented as a percentage of the total population (n=4). (C) At 4 h after UVC irradiation (10 J/m2), GFP–Ago2 is redistributed to stress granules. SG, stress granule; PB, processing body.
SG formation is cell-cycle dependent
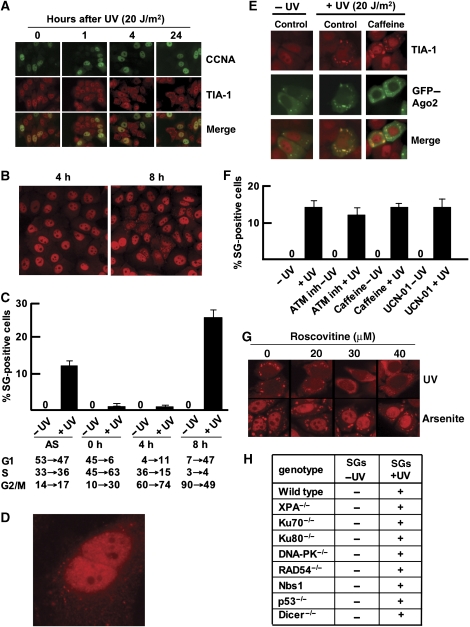
SGs were only present in a subset of cells, suggesting that this might be a cell-cycle-dependent phenomenon. We indeed found, that SGs were never detected in cyclin-A-positive S-phase cells (Figure 3A). This was confirmed by the absence of SGs in cells that showed bright γH2AX staining (Supplementary Figure 1E), which only forms when replication forks encounter UV-induced DNA damage (Marti et al, 2006). Furthermore, only 15–20% of the cells formed SGs in asynchronously growing cells (Figure 2B and C), suggesting that only a subset of cells in G1 and/or G2 phase form SGs. Importantly, we noticed that the large majority of SG-positive cells appeared in pairs, suggesting that these cells have just passed through mitosis and entered the next G1 phase. To investigate the cell-cycle dependence in more detail, we synchronized HeLa cells at the G1–S boundary and UV irradiated them at different time points after release from the cell-cycle block (Supplementary Figure 1F). SG accumulation was monitored by TIA-1 staining 4 h after UV treatment. Again, only the cells irradiated in the G2 phase (8 h after release of the cell-cycle block) accumulated SGs in a high percentage of the cells (Figure 3B and C). These cells apparently passed through mitosis, as judged from the fact that they always appeared in pairs. Interestingly, we occasionally observed cells that had not yet fully stretched after mitosis, which contained many tiny SGs as judged from the TIA-1 staining pattern (Figure 3D). Thus, SG formation is triggered by UV irradiation during the G2 phase, but SGs were present only in the G1 phase after mitosis.
Figure 3.
SG formation is cell-cycle dependent. (A) SGs are not present in S-phase cells. Immunofluorescence imaging of cyclin A (CCNA) and TIA-1 in HeLa cells after irradiation with 20 J/m2 UVC. (B) SG accumulation after release from the double-thymidine block. Cells were UV irradiated at 4 and 8 h after release and stained for TIA-1 4 h later. (C) Quantification of SG-positive cells subjected to UV irradiation at different cell-cycle stages. Cells were UV irradiated at 0, 4 and 8 h after release and stained for TIA-1 4 h later. Cell-cycle profiling was carried out before UV exposure and 4 h later for each irradiation experiment. Cell-cycle profiles before and after irradiation are listed (separated by an arrow) (n=4). (D) Cells just after mitosis that start to develop TIA-1 positive SGs. (E) GFP–Ago2 translocation to SGs after genotoxic stress is independent of ATM and ATR kinase activities. HeLa cells were treated with caffeine and Ago2 relocalization to SGs was monitored 4 h after UVC irradiation (20 J/m2). (F) Quantification of SG-positive HeLa cells 4 h after UV irradiation (20 J/m2) in combination with various kinase inhibitors. Approximately 500 cells have been counted for UV-treated cells and >104 cells for non-irradiated cells. SG-positive cells are presented as a percentage of the total population (n=4). (G) HeLa cells were treated with roscovitine (5, 10 and 20 μM) 4 h before either UVC (20 J/m2) or sodium-arsenite treatment (0.5 mM). At 4 h after UVC or 1 h after sodium-arsenite treatment, cells were stained for TIA-1. (H) SG formation in various genetic backgrounds after UV irradiation (20 J/m2). AS, asynchronous cells.
Subsequently, we investigated which cell-cycle control factors are involved in SG formation. The ATM and ATR DNA-damage checkpoint kinases are quickly activated in response to DNA damage and their recruitment to the DNA lesions has a pivotal role in inducing cell-cycle arrest (Shiloh, 2003). Therefore, we investigated whether ATM and/or ATR influenced SG formation. We treated GFP–Ago2-expressing HeLa cells with caffeine, in a concentration that inhibits both ATR- and ATM-kinase activity (Supplementary Figure 1G). Surprisingly, caffeine had no apparent effect on SG formation and Ago2 accumulation after UV damage (Figure 3E and F). The number of SG-positive cells and co-localization with Ago2 remained unaltered, indicating that the ATM- and ATR-kinase activities were not required for SG assembly or Ago2 translocation. As expected, specific inhibition of the ATR effector kinase Chk1 by UCN-01 or of ATM by the KU55933 inhibitor did not affect SG formation either (Figure 3F).
As SG formation requires mitotic transition, we investigated whether CDK activity influenced this process. Cells were treated with the CDK inhibitor roscovitine under conditions that allowed normal passage through M-phase and did not induce apoptosis (data not shown). We observed a clear change in the SG appearance: much smaller SGs were scattered throughout the cytoplasm (Figure 3G). Remarkably, arsenite-induced SGs were not affected by this roscovitine treatment, showing that CDK inhibition did not influence the general ability of cells to form SGs. Apparently, CDK signalling is specifically required for efficient SG assembly after UV-damage induction.
In an attempt to identify the genetic factors that could sense DNA damage leading to SG formation, we stained for TIA-1 in various MEF and ES cell cultures after UV irradiation (Figure 3H). We found normal SG formation for cells deficient in various DNA-repair genes involved in UV-damage repair and DNA double-strand break repair (UV damage causes double-stranded DNA breaks when cells are in S-phase Marti et al (2006)) and checkpoint signalling. In conclusion, CDK activity influences SG formation, but the main repair and checkpoint pathways do not. The actual damage sensor remains unknown.
miRNA expression is altered after UV irradiation
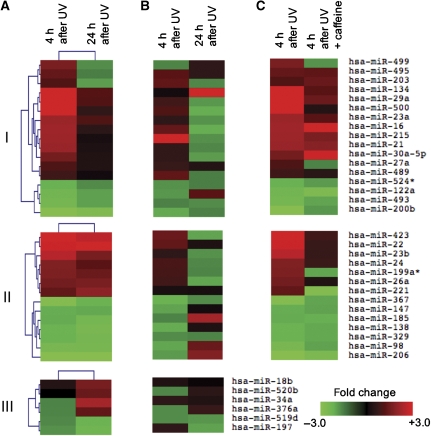
In addition to UV-induced Ago2 relocalization, we addressed which miRNAs are regulated on UV stress. Therefore, we carried out miRNA profiling of primary human fibroblasts, which have an intact DDR. The expression of 328 different miRNAs was tested at 4 and 24 h after UV exposure using locked nucleic-acid-based miRNA arrays (see Supplementary data for details). Three expression patterns became evident (Figure 4A): (I) a short-term early regulation (panel I), (II) a long-lasting regulation that started early (panel II) and (III) miRNAs that were regulated late after UV (panel III).
Figure 4.
MiRNA expression changes in response to UV treatment. (A) Heatmap of differentially expressed miRNAs (P<0.01; −1.5>fold change>1.5) 4 and 24 h after UVC irradiation (8 J/m2) in primary human fibroblasts. (B) Many differentially regulated miRNAs in primary human fibroblasts are also regulated in HeLa cells after UVC treatment (8 J/m2). (C) Dependence of miRNA regulation on ATM/ATR activity. Cells were treated with 8 mM caffeine 4 h before UVC exposure. After UV treatment cells were recovered in medium containing caffeine for 4 h.
The miRNA-expression profile at 4 h after UV exposure, when SG formation was most prominent, showed significant upregulation of 20 miRNAs of which 15 had a more than two-fold change, whereas 11 miRNAs were significantly downregulated. The miRNA regulation was predominantly temporary: expression levels of 21 out of 31 miRNAs were not significantly different from miRNA levels in untreated cells after 24 h (Figure 4A, panel I). Interestingly, most of these miRNAs (17 out of 21) were upregulated, indicating that repression of gene expression by upregulation of UV-responsive miRNAs, rather than upregulation of gene expression by miRNA repression, is the main function of miRNA-mediated gene silencing during the first hours after UV damage.
In addition to miRNAs that were regulated only during the first hours after UV exposure, some miRNAs were differentially expressed early, but had a long-lasting expression regulation (Figure 4A, panel II), which includes the upregulation of miR-221 that regulates the UV-responsive cell-cycle control gene p27(kip1) (Poon et al, 1995; Galardi et al, 2007; le Sage et al, 2007). Finally, there is a class of miRNAs that was regulated late after UV exposure (Figure 4A, panel III). Most notably, we found upregulation of miR-34a, which is a direct p53 target (Chang et al, 2007; He et al, 2007; Raver-Shapira et al, 2007).
Next, we determined whether observed miRNA-expression changes are conserved in other cell types. As various experiments were carried out in HeLa cells, we determined miRNA-expression profiles of these cells 4 and 24 h after UV exposure (Figure 4B). Many miRNAs are regulated in a similar manner in HeLa cells and primary human fibroblasts at 4 h after UV exposure (Figure 4B, panel I) although there are some differences: in general, the fold change after UV is lower than in primary human fibroblasts. The long-lasting response that starts early is virtually absent (Figure 4B, panel II) as well as the miRNAs that are regulated late after UV exposure, such as the p53-dependent miR-34a (Figure 4B, panel III). This indicates that the fast miRNA response is largely present in HeLa cells, but late responses are aberrant. Differences in miRNA expression changes between primary human fibroblasts and HeLa cells are probably due to the transformed status of HeLa cells as well as the difference in cell type (fibroblast versus epithelial cell).
As SG formation and Ago2 translocation were not dependent on ATM- and ATR-kinase activity, we investigated whether this was also the case for miRNA-expression regulation. The caffeine-treated primary human fibroblasts were UV-irradiated and subjected to miRNA profiling. Here, a considerable fraction of the miRNA was regulated similarly in the presence of caffeine (Figure 4C). However, the regulation of several other miRNAs was considerably less pronounced or even absent, indicating that both ATM/ATR-dependent and independent pathways are involved in miRNA-expression regulation after UV damage.
miRNAs contribute to gene silencing after UV exposure
The fast regulation of miRNAs after UV treatment indicates that miRNA-mediated gene silencing acts later than the fast protein modifications that occur minutes after UV damage, but acts earlier than most gene transcriptional responses of which the p53 response is best characterized. Indeed, p21 upregulation after UV exposure (a p53 target gene) was slower than many miRNAs (Supplementary Figure 1H). To provide more evidence for this hypothesis, we sought to identify a miRNA target gene that has an important role in the DDR, is preferentially regulated at the post-translational level and mRNA level, which could serve as an example. Previously, we reported a study on gene-expression profile for genes that are regulated after UV-induced DNA damage (Garinis et al, 2005). We noticed that the mRNA of a central mediator of the G1–S cell-cycle checkpoint CDC25a was downregulated already 1 h after UV, whereas it has been reported the CDC25a protein is degraded minutes after UV (Mailand et al, 2000) and that p53-induced p21-dependent downregulation of the CDC25a promoter occurred only after 9 h following DNA-damage treatment (Vigneron et al, 2006; Rother et al, 2007). We reasoned that UV-responsive miRNA could in principle regulate CDC25a mRNA in the hours before promoter silencing.
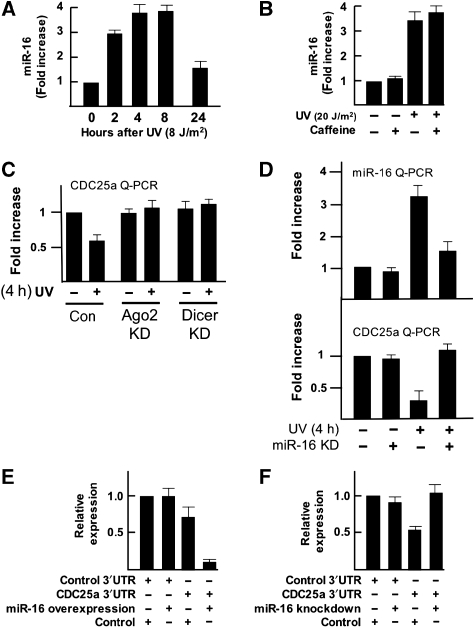
First, we used the miRNA target-prediction program Targetscan (Lewis et al, 2003, 2005; Grimson et al, 2007) to determine whether the CDC25a 3′UTR had the predicted target sites for miRNAs that were upregulated after UV exposure, preferentially those miRNAs that were only regulated early after UV damage. Indeed, the CDC25a 3′UTR has two predicted miRNA target sites against miR-16, which is upregulated early after UV damage in both human fibroblasts and HeLa cells in an ATM/ATR independent manner. Then, we confirmed the UV-dependent upregulation of miR-16 using quantitative PCR (Figure 5A) that verifies the rapid induction of miR-16 expression on UV damage. This induction was ATM/ATR-kinase independent (Figure 5B).
Figure 5.
Analysis of CDC25a as a miR-16 target gene. (A) miR-16 quantitative PCR in mock-treated HeLa cells and HeLa cells at various time points after UVC exposure (8 J/m2) shows upregulation of mature miR-16 after UV irradiation. The ribosomal RNA RPL21 was used as control. Similar results were obtained when the nucleolar RNA RNU43 was used (data not shown). Results are described as the fold increase when compared with mock-treated cells. (B) miR-16 quantitative PCR in mock-treated HeLa cells and HeLa cells after UV exposure (8 J/m2) with and without caffeine treatment (8 mM) 4 h before irradiation. The ribosomal RNA RPL21 was used as control. Similar results were obtained when the nucleolar RNA RNU43 was used (data not shown). Results are described as the fold increase when compared with mock-treated cells. (C) Quantitative RT–PCR analysis of CDC25a expression. HeLa cells were transfected twice either with control, Dicer- or Ago2-specific siRNAs, reseeded and 48 h later CDC25a mRNA was analyzed 4 h after UV irradiation using quantitative RT–PCR. GAPDH was used as control. Fold differences in expression levels are shown when compared with GAPDH levels. (D) HeLa cells were transfected with either control inhibitor or miR-16 inhibitor. At 48 h later miR-16 and CDC25a mRNA was analyzed by quantitative RT–PCR 4 h after UV irradiation. Fold differences are shown. (E) The full-length CDC25a 3′UTR was cloned downstream a Renilla luciferase (RLuc) gene (Psi-CHECK2 vector). HEK293T cells were transiently transfected with either the RLuc–CDC25a-3′UTR vector or RLuc with a control 3′UTR in combination with a specific miR-16 overexpression (50 nM) or control overexpression (50 nM) oligonucleotide. A ubiquitously expressed firefly luciferase gene, which is also present in the vector, was used as a transfection control. Luciferase activity was measured 24 h after transfection. (F) HEK293T cells were transiently transfected with either the RLuc–CDC25a-3′UTR vector or RLuc with a control 3′UTR in combination with a specific miR-16 inhibitor (50 nM) or control inhibitor (50 nM). A ubiquitously expressed firefly luciferase gene, which is also present in the vector, was used as a transfection control. Luciferase activity was measured 24 h after transfection.
As miRNA-mediated mRNA degradation depends on Dicer and Ago2, we first evaluated whether UV-induced CDC25a downregulation depended on these factors. The CDC25a-transcript levels decreased significantly after UV exposure in an Ago2- and Dicer-dependent manner (Figure 5C), indicating that CDC25a mRNA is targeted by miRNAs. The downregulation of CDC25a-transcript levels was solely dependent on miR-16, as shown by the abrogation of this response in a miR-16 knockdown experiment (Figure 5D). Further evidence that miR-16 was indeed able to regulate CDC25a expression was obtained using a luciferase reporter construct containing the CDC25a 3′UTR. As shown in Figure 5E and F, miR-16 knockdown led to increased luciferase activity, whereas ectopic overexpression of miR-16 resulted in a downregulation of luciferase activity in HEK293T cells, showing that CDC25a mRNA is directly downregulated by miR-16. This example provides more evidence that miRNA-mediated gene silencing contributes to the cellular responses to UV stress in a timed manner.
MiR-16 regulates cell proliferation and the G1–S checkpoint
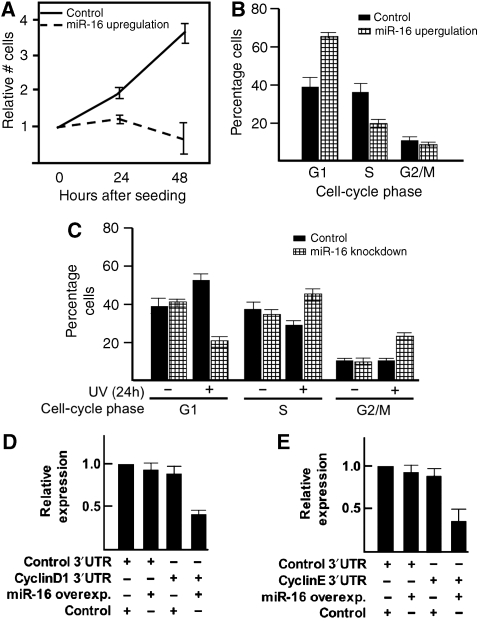
CDC25a has an essential role in the cell cycle and it's downregulation leads to an immediate cell-cycle stop (Mailand et al, 2000). Therefore, we investigated whether miR-16 has a role in the cell cycle as well. Ectopic overexpression of miR-16 halted cell proliferation already 24 h after transfection (Figure 6A). Moreover, 24 h after transfection, clearly more cells were in the G1 phase of the cell cycle as compared with control cells (Figure 6B). Next, we determined cell-cycle phase distribution in miR-16 knocked-down cells with and without UV treatment (Figure 6C). The MiR-16-depleted cells had a similar cell-cycle distribution as control cells when unexposed. After UV treatment, however, miR-16-depleted cells accumulated in the S phase, similar to the cell-cycle profile seen after Dicer knock-down (Figure 1C).
Figure 6.
miR-16 regulates the cell cycle. (A) Control overexpression or miR-16 overexpression oligonucleotides were transfected in HeLa cells (50 nM final concentration). The next day, cells were reseeded and at given time points the number of cells was counted. (B) Control overexpression or miR-16 overexpression oligonucleotides were transfected in HeLa cells (50 nM final concentration). After 24 h, cells were reseeded and the next day harvested and used for cell-cycle analysis by FACS. (C) Cell-cycle analysis using HeLa cells transfected twice either with control knockdown or miR-16-specific knockdown oligonucleotides (50 nM final concentration) and 24 h later reseeded. After 32 h, cells were treated with UVC and 24 h later harvested for cell-cycle analysis by FACS. (D) The full-length cyclin D1 3′UTR was cloned downstream a Renilla luciferase (RLuc) gene (Psi-CHECK2 vector). HEK293T cells were transiently transfected with either the RLuc–cyclin D1-3′UTR vector or RLuc with a control 3′UTR in combination with a specific miR-16 (50 nM) or control (50 nM) oligonucleotide. A ubiquitously expressed firefly luciferase gene, which is also present in the vector, was used as a transfection control. Luciferase activity was measured 24 h after transfection. (E) The full-length cyclin E1 3′UTR was cloned downstream a Renilla luciferase (RLuc) gene (Psi-CHECK2 vector). HEK293T cells were transiently transfected with either the RLuc–cyclin E1-3′UTR vector or RLuc with a control 3′UTR in combination with a specific miR-16 (50 nM) or control (50 nM) oligonucleotide. A ubiquitously expressed firefly luciferase gene, which is also present in the vector, was used as a transfection control. Luciferase activity was measured 24 h after transfection.
While assessing the role of miR-16-dependent CDC25a regulation in the cell cycle after DNA damage, we observed that additional genes that regulate the G1–S transition were potential targets for miR-16, such as cyclin D1 and cyclin E. Indeed, luciferase experiments indicate that miR-16 is able to regulate these genes (Figure 6D and E). More G1-related cell-cycle genes have been indicated as miR-16 target genes (Linsley et al, 2007), which together point towards a regulating role for miR-16 at multiple steps in the G1–S checkpoint.
Discussion
We provide evidence for a new level of gene-expression regulation in DDR. Using a combination of fluorescence microscopy, miRNA profiling and reverse genetics, we showed that Ago2 and miRNA expression regulate several aspects of this response, eventually leading to increased survival after UV irradiation. The intracellular relocalization of Ago2 to SGs and miRNA-expression changes imply that microRNA-mediated gene silencing is an integral part of DDR.
The exact role of SGs in the miRNA-mediated regulation of gene expression after UV irradiation (and stress in general) remains to be elucidated. We found a similar temporal regulation of miRNA expression and SG formation. However, UV irradiation leads to SG formation in only a subset of cells, in contrast to the 100% of SG-positive cells after arsenite treatment (Figure 3G and (Anderson and Kedersha, 2002, 2006)), suggesting that miRNA-mediated gene silencing is only partially associated with SGs on UV damage. In addition, SG formation after UV-irradiation did not depend on miRNA biogenesis (Figure 3H), suggesting that SGs have functions in addition to miRNA-mediated gene silencing or is an upstream event. The appearance of SGs in cells that have just divided indicates that SGs are important for events that occur during G2–M transition or after exit from mitosis. The aberrant appearance of SGs after roscovitine treatment indicates that Cdk activity has a role in SG formation after UV irradiation. However, the relocalization of TIA-1 from nucleus to cytoplasm suggests that other factors are probably responsible for transmission of the UV-damage signal. The nature of this signal is not yet clear, but it does not seem to involve signalling by ATM/ATR or the major DNA-repair pathways.
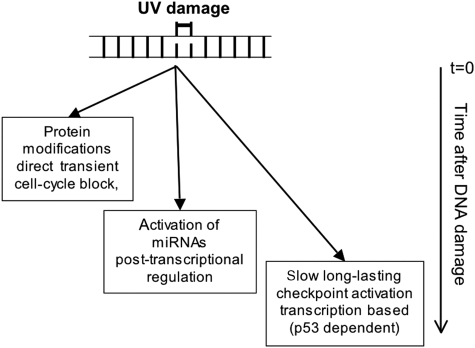
The timing of SG formation and miRNA regulation is different from the previously identified processes of protein modification and transcriptional regulation. A variety of ATM/ATR-dependent protein modifications result in a direct, but transient, cell-cycle block within minutes after DNA damage (see model in Figure 7; Lukas and Bartek, 2004). In addition, a p53-dependent transcription-based response is initiated that needs many hours to establish a stable and prolonged cell-cycle arrest (Lukas and Bartek, 2004). On the basis of the timeframe of SG formation and miRNA expression after UV irradiation, we propose that miRNA-mediated gene silencing acts at the intermediate time points in the DDR. This concept is further supported by the study of CDC25a as a miRNA target gene after UV irradiation, which is required for proper cell-cycle checkpoint control. In minutes after UV irradiation, the CDC25a protein is phosphorylated by ATR, which results in rapid protein degradation and immediate cell-cycle block (Mailand et al, 2000). At least 9 h later CDC25a transcription is downregulated in a p53-dependent manner by p21 (Vigneron et al, 2006; Rother et al, 2007). However, a previous study showed that the CDC25a mRNA is already downregulated as early as 1 h after UV treatment (Garinis et al, 2005). Our finding that miR-16-mediated CDC25a-mRNA degradation functions early after UV exposure supports the idea that miRNA-mediated gene silencing operates indeed in the first few hours after UV-damage induction, earlier than most gene transcriptional responses. A similar kinetics has been observed in the downregulation of various members of the let-7 miRNA family on being subjected to ionizing radiation (Weidhaas et al, 2007).
Figure 7.
Model of the miRNA-mediated gene-silencing function in the DDR. MiRNA mediated gene-expression regulation appears in time after the fast protein modification response that happens within minutes after genotoxic insult, but earlier than the transcriptional reprogramming that takes many hours to develop (see Discussion for details).
In addition to CDC25a, miR-16 also regulates the cell-cycle genes cyclin D1 and cyclin E. Moreover, we showed that miR-16 has an active role in the cell-cycle checkpoint after UV damage. Although we did not study the regulation of other miRNA targets in similar detail, we argue that such a division of DDR in a fast, intermediate and slow component is probably a general phenomenon. A quick response of protein degradation or activity regulation is necessary to react to the genotoxic stress and prevent direct adverse effects of for example, cell-cycle progression. Subsequently, miRNA-mediated silencing prevents de novo protein synthesis, which would otherwise initiate a futile cycle of protein synthesis and degradation or inactivation. Finally, gene transcriptional regulation takes over the gene-expression regulation function at later time points, in order to sustain the changed expression pattern when a persistent alteration of gene expression is required.
In addition to survival defects, inefficiency of the DDR is also strongly correlated with cancer. All cancers have at least one defect in the DDR, such as inactivation of p53. Furthermore, many hereditary cancer syndromes originate from germline mutations in genes that have a role in DDR (Hoeijmakers, 2001; Vogelstein and Kinzler, 2004). Interestingly, many human tumours had lower overall miRNA levels (Lu et al, 2005) and Dicer deficiency accelerated the malignant transformation (Kumar et al, 2007). This paper shows that lower or absent miRNA levels in cells can have a profound influence on the DDR (Figure 1). Therefore, we hypothesize that lower miRNA levels in cancer cause defects in DDR that might contribute to the acceleration of the malignant phenotype. This hypothesis is also consistent with the observation that some miRNAs that are regulated after DNA damage have been identified as tumour-suppressor genes or oncogenes, such as miR-16, which was the first miRNA shown to be causally involved in human cancer (chronic lymphoid leukemia) (Calin et al, 2004). In general, UV-responsive miRNAs are regulated in opposite direction compared with their mis-regulation in cancer, indicating that these cancers have an abrogated DDR. The other frequently deregulated miRNAs across solid tumours originated from six different tissues (that are also UV-responsive) are miR-29b, miR-21, miR-221 and the miR–23a–27a–24 cluster (Volinia et al, 2006). Taken together the function of miRNA-mediated gene silencing in DDR could be an essential component in cancer etiology.
Materials and methods
Cell culture and UV irradiation
HeLa cells were grown in Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL) supplemented with 10% fetal calf serum (FCS) and human fibroblasts in a 1:1 mixture of DMEM and Ham F10 medium. ES cells were cultured on gelatin-coated dishes in a 1:1 mixture of DMEM and Buffalo Rat Liver (BRL)-conditioned medium supplemented with 10% FCS, penicillin (100 U/ml)–streptomycin (100 μg/ml), 0.1 mM non-essential amino acids, 50 μM β-mercaptoethanol and 500 U/ml leukemia inhibitory factor. For UV irradiation, cells were washed twice with phosphate buffered saline (PBS) without Mg2+ and Ca2+, pH 7.5. Cells were irradiated at 50–70% confluency. Where indicated, HeLa cells or human fibroblasts were treated with caffeine (ATM/ATR inhibitor, 8 mM) (Sigma), the ATM inhibitor Ku55933 (20 μM; Kudos Pharmaceuticals), Chk1 inhibitor UCN-01 (300 nM; Drug Synthesis and Chemistry Branch, NIH, Bethesda, MD) or roscovitine (Sigma, 5, 10 and 20 μM).
RNA and protein analysis
Total RNA was prepared from cultured cell lines using Trizol (Invitrogen). The CDC25a, GAPDH, Dicer and Ago2 levels were monitored by RT–QPCR (Invitrogen) according to manufacturer's protocol and/or RT–PCR for which quantification of real-time PCR products was carried out by image analysis software after scanning of ethidium-bromide-stained gels. The CDC25a primers: 5′-GAAGACTTCTTATTGAAGAAGCCC-3′ and 5′-CTCAGAGCAGCTTGACACGGTG-3′. Dicer primers: 5′-CATGGATAGTGGGATGTCAC-3′ and 5′-CTACTTCCACAGTGACTCTG-3′. Ago2 primers: 5′-CGCGTCCGAAGGCTGCTCTA-3′ and 5′-TGGCTGTGCCTTGTAAAACGCT-3′. GAPDH primers: 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′. The quantification of mature miR-16 and RPL21 expression levels was carried out by real-time PCR assay (Applied Biosystems). Immunoblot assays were carried out using polyclonal antibodies that specifically recognized Ago2 (Upstate), anti-phospho Chk1S317 (Bethyl Laboratories, Inc.), GRB2 (Abcam) and Dicer (Abcam).
siRNA experiments
Knockdown experiments were carried out using four different siRNAs against Dicer, Ago2 and non-specific controls (Dharmacon). HeLa cells were seeded and at ∼70% confluency transfected twice (6 h apart) with siRNAs at 50 nM final concentrations using Lipofectamine 2000 (Invitrogen). The next day, cells were reseeded. The UV treatments were carried out as indicated. Dicer and Ago2 knockdowns were confirmed by RT–PCR and western blotting.
Clonal survival
The siRNA-transfected HeLa cells were reseeded (±150 cells per plate) and 30 h later treated with UVC. After 7 days colony-formation capacity was assayed. Dicer and Ago2 siRNA-transfected cells were reseeded and live-cell number was counted at 24, 48 and 72 h after reseeding (using trypan-blue exclusion).
Cell synchronization and cell-cycle profiling
Transfected HeLa cells were reseeded at 50% confluency and 48 h later treated with UVC. At 15 min before collection, BrdU was added. After 24 h cells were collected through trypsinization, fixed in 75% ethanol. Fixed cells were stained with anti-BrdU–FITC and propidium iodide and used for FACS analysis.
Cells were arrested on the G1–S boundary by incubation in medium containing 7.5 mM thymidine for 24 h. Cells were released from the thymidine for 18 h before a second incubation with 7.5 mM thymidine for 24 h. Cells were released from the thymidine block and subjected to UVC (20 J/m2) irradiation at different time points. Cell-cycle profiling and UVC irradiation were carried out at 0, 4 and 8 h after release. TIA-1 immunofluorescence and cell-cycle analysis for SG formation was carried out 4 h after UV treatment.
Immunofluorescence analysis
For immunofluorescence analysis, cells were fixed with 2% paraformaldehyde for 15 min at room temperature and permeabilized and blocked with PBS containing 5% BSA and 0.1% Triton X-100 for 30 min. The anti-TIA-1 (Santa Cruz Biotechnology) antiserum was used at 1:100 dilution and γH2AX (Upstate Biotechnology) antibody at 1:500 dilution. Secondary anti-goat and anti-rabbit antibodies labelled with Alexa488 and Alexa594 fluorochromes (Molecular Probes) were used at 1:1000 dilutions.
Microarray analysis
A detailed overview of miRNA profiling can be found in Supplementary data. Total RNA was labelled (Cy3 only) using the ULS aRNA labelling kit (Kreatech). The LNA-based capture probeset (Exiqon) was spotted on Nexterion E slides using a Virtek Chipwriter Pro. The labelled total RNA was hybridized in a salt-based hybridization buffer (Ocimum Biosolutions) overnight at 60°C in a Tecan HS4800 pro hybridization station. Slides were scanned in a Tecan LS Reloaded scanner. Data extraction was carried out by Imagene software. The raw data were normalized using quantile normalization and used for statistical analysis. Heatmaps were generated using TM4 microarray software suite (Saeed et al, 2003).
Luciferase assay
The full-length CDC25a, cyclin D1 or cyclin E 3′UTR was cloned downstream Renilla luciferase in a Psi-check2 vector (Promega). The control and miR-16 inhibitor and overexpression oligonucleotides were obtained from Dharmacon. HeLa cells were co-transfected using Lullaby (Boca Scientific) at 50 nM final concentrations with the vector and miR-16 overexpression or knockdown oligonucleotide. Luciferase activity was measured 24 h later by Dual Glow luciferase kit (Promega).
Supplementary Material
Supplementary Figure S1
Supplementary Figure Legend
Supplementary Material and Methods
Review Process File
Acknowledgments
We thank P Sharp and A Leung for providing the GFP–Ago2 expression vector and G Hannon and E Murchison for dicer-deficient ES cells. We thank T Boersma, P Kuijk and K Kockx for technical assistance. We thank the Hoeijmakers lab for critical discussions. This study was supported by the Association for International Cancer Research (AICR grant 05-135), the Dutch Cancer Foundation KWF (EMCR 2007-3794), the Netherlands Scientific Organization for Biomedical Research (ZonMW, Veni 016-076-069), Netherlands Genomics Initiative grant nr 050-060-510 and the European Commission (projects DNA Repair (LSHG-CT-2005-512113) and RIBOREG (LSHG-CT-2003-503022).
Footnotes
JHJH is the Chief Scientific Officer of DNage/Pharming. The other authors declare that they have no conflict of interest.
References
- Anderson P, Kedersha N (2002) Stressful initiations. J Cell Sci 115: 3227–3234 [DOI] [PubMed] [Google Scholar]
- Anderson P, Kedersha N (2006) RNA granules. J Cell Biol 172: 803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, Sharon E, Spector Y, Bentwich Z (2005) Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet 37: 766–770 [DOI] [PubMed] [Google Scholar]
- Berezikov E, Guryev V, van de Belt J, Wienholds E, Plasterk RH, Cuppen E (2005) Phylogenetic shadowing and computational identification of human microRNA genes. Cell 120: 21–24 [DOI] [PubMed] [Google Scholar]
- Calin GA, Liu CG, Sevignani C, Ferracin M, Felli N, Dumitru CD, Shimizu M, Cimmino A, Zupo S, Dono M, Dell'Aquila ML, Alder H, Rassenti L, Kipps TJ, Bullrich F, Negrini M, Croce CM (2004) MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc Natl Acad Sci USA 101: 11755–11760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, Iuliano R, Palumbo T, Pichiorri F, Roldo C, Garzon R, Sevignani C, Rassenti L, Alder H, Volinia S, Liu CG et al. (2005) A microRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med 353: 1793–1801 [DOI] [PubMed] [Google Scholar]
- Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ, Arking DE, Beer MA, Maitra A, Mendell JT (2007) Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell 26: 745–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman MS, Afshari CA, Barrett JC (2000) Regulation of p53 stability and activity in response to genotoxic stress. Mutat Res 462: 179–188 [DOI] [PubMed] [Google Scholar]
- Eulalio A, Behm-Ansmant I, Izaurralde EP (2007) bodies: at the crossroads of post-transcriptional pathways. Nat Rev Mol Cell Biol 8: 9–22 [DOI] [PubMed] [Google Scholar]
- Galardi S, Mercatelli N, Giorda E, Massalini S, Frajese GV, Ciafrè SA, Farace MG (2007) miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J Biol Chem 282: 23716–23724 [DOI] [PubMed] [Google Scholar]
- Garinis GA, Mitchell JR, Moorhouse MJ, Hanada K, de Waard H, Vandeputte D, Jans J, Brand K, Smid M, van der Spek PJ, Hoeijmakers JH, Kanaar R, van der Horst GT (2005) Transcriptome analysis reveals cyclobutane pyrimidine dimers as a major source of UV-induced DNA breaks. EMBO J 24: 3952–3962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP (2007) MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 27: 91–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ (2007) A microRNA component of the p53 tumour suppressor network. Nature 447: 1130–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers JH (2001) Genome maintenance mechanisms for preventing cancer. Nature 411: 366–374 [DOI] [PubMed] [Google Scholar]
- Hoeijmakers JH (2007) Genome maintenance mechanisms are critical for preventing cancer as well as other aging-associated diseases. Mech Ageing Dev 128: 460–462 [DOI] [PubMed] [Google Scholar]
- Jaskiewicz L, Filipowicz W (2008) Role of Dicer in posttranscriptional RNA silencing. Curr Top Microbiol Immunol 320: 77–97 [DOI] [PubMed] [Google Scholar]
- John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS (2004) Human MicroRNA targets. PLoS Biol 2: e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Anderson P (2007) Mammalian stress granules and processing bodies. Methods Enzymol 431: 61–81 [DOI] [PubMed] [Google Scholar]
- Kloosterman WP, Plasterk RH (2006) The diverse functions of microRNAs in animal development and disease. Dev Cell 11: 441–450 [DOI] [PubMed] [Google Scholar]
- Krek A, Grün D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N (2005) Combinatorial microRNA target predictions. Nat Genet 37: 495–500 [DOI] [PubMed] [Google Scholar]
- Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T (2007) Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet 39: 673–677 [DOI] [PubMed] [Google Scholar]
- Leung AK, Calabrese JM, Sharp PA (2006) Quantitative analysis of Argonaute protein reveals microRNA-dependent localization to stress granules. Proc Natl Acad Sci USA 103: 18125–18130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AK, Sharp PA (2007) microRNAs: A safeguard against turmoil? Cell 130: 581–585 [DOI] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB (2003) Prediction of mammalian microRNA targets. Cell 115: 787–798 [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20 [DOI] [PubMed] [Google Scholar]
- Linsley PS, Schelter J, Burchard J, Kibukawa M, Martin MM, Bartz SR, Johnson JM, Cummins JM, Raymond CK, Dai H, Chau N, Cleary M, Jackson AL, Carleton M, Lim L (2007) transcripts targeted by the microRNA-16 family cooperatively regulate cell cycle progression. Mol Cell Biol 27: 2240–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ (2004) Argonaute2 is the catalytic engine of mammalian RNAi. Science 305: 1437–1441 [DOI] [PubMed] [Google Scholar]
- Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR (2005) MicroRNA expression profiles classify human cancers. Nature 435: 834–838 [DOI] [PubMed] [Google Scholar]
- Lukas J, Bartek J (2004) Cell division: the heart of the cycle. Nature 432: 564–567 [DOI] [PubMed] [Google Scholar]
- Mailand N, Falck J, Lukas C, Syljuâsen RG, Welcker M, Bartek J, Lukas J (2000) Rapid destruction of human CDC25a in response to DNA damage. Science 288: 1425–1429 [DOI] [PubMed] [Google Scholar]
- Marti TM, Hefner E, Feeney L, Natale V, Cleaver JE (2006) H2AX phosphorylation within the G1 phase after UV irradiation depends on nucleotide excision repair and not DNA double-strand breaks. Proc Natl Acad Sci USA 103: 9891–9896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER III, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, Elledge SJ (2007) ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316: 1160–1166 [DOI] [PubMed] [Google Scholar]
- Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T (2004) Human argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell 15: 185–197 [DOI] [PubMed] [Google Scholar]
- Poon RY, Toyoshima H, Hunter T (1995) Redistribution of the CDK inhibitor p27 between different cyclin.CDK complexes in the mouse fibroblast cell cycle and in cells arrested with lovastatin or ultraviolet irradiation. Mol Biol Cell 6: 1197–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, Moskovits N, Bentwich Z, Oren M (2007) Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell 26: 731–743 [DOI] [PubMed] [Google Scholar]
- Rother K, Kirschner R, Sänger K, Böhlig L, Mössner J, Engeland K (2007) p53 downregulates expression of the G1/S cell cycle phosphatase CDC25a. Oncogene 26: 1949–1953 [DOI] [PubMed] [Google Scholar]
- Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V et al. (2003) TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34: 374–378 [DOI] [PubMed] [Google Scholar]
- le Sage C, Nagel R, Egan DA, Schrier M, Mesman E, Mangiola A, Anile C, Maira G, Mercatelli N, Ciafrè SA, Farace MG, Agami R (2007) Regulation of the p27(Kip1) tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J 26: 3699–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh Y (2003) ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 3: 155–168 [DOI] [PubMed] [Google Scholar]
- Vigneron A, Cherier J, Barre B, Gamelin E, Coqueret O (2006) The cell cycle inhibitor p21waf1 binds to the myc and CDC25a promoters upon DNA damage and induces transcriptional repression. J Biol Chem 281: 34742–34750 [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW (2004) Cancer genes and the pathways they control. Nat Med 10: 789–799 [DOI] [PubMed] [Google Scholar]
- Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM (2006) A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA 103: 2257–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidhaas JB, Babar I, Nallur SM, Trang P, Roush S, Boehm M, Gillespie E, Slack FJ (2007) MicroRNAs as potential agents to alter resistance to cytotoxic anticancer therapy. Cancer Res 67: 11111–11116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Huang J, Yang N, Greshock J, Megraw MS, Giannakakis A, Liang S, Naylor TL, Barchetti A, Ward MR, Yao G, Medina A, O'brien-Jenkins A, Katsaros D, Hatzigeorgiou A, Gimotty PA, Weber BL, Coukos G (2006) MicroRNAs exhibit high frequency genomic alterations in human cancer. Proc Natl Acad Sci USA 103: 9136–9141 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure Legend
Supplementary Material and Methods
Review Process File