Abstract
The avian magnetic compass has been well characterized in behavioral tests: it is an “inclination compass” based on the inclination of the field lines rather than on the polarity, and its operation requires short-wavelength light. The “radical pair” model suggests that these properties reflect the use of specialized photopigments in the primary process of magnetoreception; it has recently been supported by experimental evidence indicating a role of magnetically sensitive radical-pair processes in the avian magnetic compass. In a multidisciplinary approach subjecting migratory birds to oscillating fields and using their orientation responses as a criterion for unhindered magnetoreception, we identify key features of the underlying receptor molecules. Our observation of resonance effects at specific frequencies, combined with new theoretical considerations and calculations, indicate that birds use a radical pair with special properties that is optimally designed as a receptor in a biological compass. This radical pair design might be realized by cryptochrome photoreceptors if paired with molecular oxygen as a reaction partner.
Introduction
The ability of many animals to use information from the geomagnetic field for orientation and navigation has fascinated scientists and laymen alike, but progress toward discovering the magnetic sensory mechanism has been slow. This is also true for birds, although the functional characteristics of the avian magnetic compass have been well analyzed in behavioral tests (1), mostly based on the tendency of caged migratory birds to head into their migratory direction during migration season. When magnetic north of the ambient field is experimentally shifted, the birds alter their directional preference so as to maintain their heading with respect to magnetic north, thus demonstrating that they use the magnetic field as a compass (2). Further analysis revealed some unexpected properties of the avian magnetic compass indicating a mechanism fundamentally different from that of a man-made compass based on a magnetic needle: birds do not respond to the polarity of the magnetic field, but instead rely on the axis of the field lines and base their decisions on its inclination or dip angle (3). The avian magnetic compass also depends on the ambient light conditions. Oriented magnetic responses with the features mentioned above have only been observed under either full spectrum “white” light or under monochromatic light with wavelengths ranging from the ultraviolet to the green part of the visual spectrum up to ∼565 nm (4–6). These findings put certain constraints on any model designed to explain magnetoreception in birds.
The radical-pair model (7,8) assumes that these properties of the avian magnetic compass—light-dependence and insensitivity to polarity—directly reflect characteristics of the primary processes of magnetoreception. It postulates a crucial role for specialized photopigments in the retina. A light-induced electron-transfer reaction creates a spin-correlated radical pair with singlet and triplet states. External magnetic fields of the intensity of the geomagnetic field can interfere with the dynamics of the singlet-triplet interconversion and thereby modify the yields of the reaction products (Fig. 1). The magnitude of the response depends on the orientation of the radical pair with respect to the direction of the external magnetic field (9). By comparing the responses of receptor cells with different orientations, as they are found in the eye (7), birds could thus obtain information on the direction of the external field (see the Supporting Material). The predictions of the radical-pair model are in agreement with behavioral findings: the reaction yield is independent of the polarity of the magnetic field (3,9), reception of magnetic information takes place in the eye (10), and reception is strongly affected by the ambient light conditions (4–6), consistent with the postulated role of ocular photoreceptors in creating magnetosensitive radical pairs.
Figure 1.
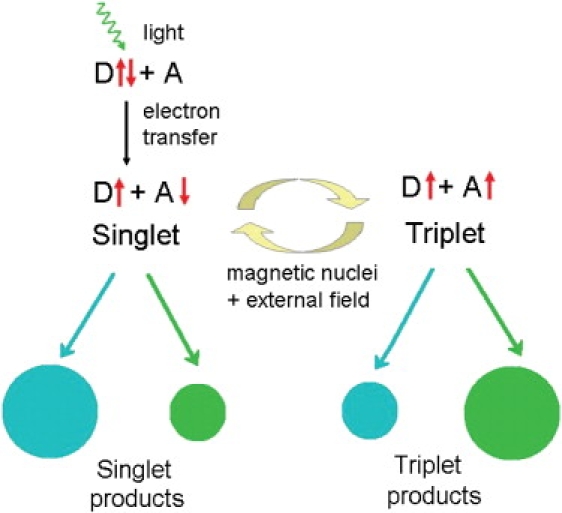
Schematic of the radical-pair mechanism. Light-induced electron transfer from a donor molecule D to an acceptor molecule A creates a radical pair, that is, two molecules each with an unpaired electron spin (up and down arrows next to D and A). Singlet and triplet states, defined by the relative orientation of the electron spins, interconvert due to the combined effects of internal and external magnetic fields. Singlet and triplet radical pairs decay into singlet and triplet products respectively, with relative yields indicated by the sizes of the circles. The relative yields of singlet and triplet products depend on the orientation of the external magnetic field with respect to that of the radicals. The arrows and circles at the bottom of the diagram symbolize pathways of product formation and reaction yields for two different orientations.
Effects of weak magnetic fields on radical-pair reactions in vitro are well established, with directionally sensitive magnetic field effects on radical-pair systems recently demonstrated (11). Modeling shows that they can be amplified beyond the level of stochastic fluctuations in specialized radical-pair receptor systems (12). Thus the molecular substrate for a radical-pair-based magnetic compass in birds may comprise an ordered system of electron donor or acceptor pigments, absorbing in the required wavelength range, in close proximity to electron acceptor or donor reaction partners.
To test for the involvement of a radical-pair mechanism in magnetoreception, one can superimpose weak oscillating electromagnetic fields on a static magnetic field (13). In vitro, this form of electron spin resonance spectroscopy can be used to identify the molecules involved from the spectrum of their responses. By using the birds' behavior, we have adapted this approach for in vivo measurements: orientation in the seasonally appropriate migratory direction serves as a criterion of whether the crucial radical-pair processes are unhindered. Our first experiments showed that oscillating fields in the MHz range disrupted orientation (14–16). Here, to obtain further insight into the nature of the radical pair, we identify the frequencies to which migratory European robins, Erithacus rubecula (Turdidae), respond most sensitively and determine the threshold for the onset of disorientation. The local geomagnetic field in Frankfurt, in the range 46.0–47.4 μT with 66° inclination at the test sites, served as the control condition. The various oscillating test fields were added vertically, thus forming an angle of 24° to the static field vector (see Weil and Bolton (14) and Ritz et al. (15) and Materials and Methods).
Materials and Methods
The orientation tests were performed at the Zoological Institute of the University Frankfurt (geographic coordinates: 50°08′N, 8°40′E) during spring migration from 2003 to 2006.
Test birds
European robins breed all over Europe. The northern and eastern populations are nocturnal migrants and winter in the Mediterranean countries. Each year, 12 robins were mist-netted during September in the Botanical Garden and identified as transmigrants of probably Scandinavian origin by their wing length. They were kept individually in housing cages in the bird room over the winter. The photoperiod simulated the natural one until the beginning of December when it was decreased to light/dark 8:16. Around New Year, the photoperiod was increased in two steps to light/dark 13:11. This induced premature spring migration in early January and allowed us to test the birds from early January to the second half of February.
In 2006, we performed experiments in a static field of 92 μT, 66° inclination, approximately twice the local geomagnetic field. This field is outside the normal functional window of the avian magnetic compass in Frankfurt (1), but robins quickly adjust to that intensity and orient in such a field after 1 h preexposure (17). To not stress birds too much by excessive handling before testing, we moved the individuals to be tested in the strong field that evening into a second set of housing cages within a pair of Helmholtz coils increasing the local field to 92 μT ∼3 h before the tests began. This allowed them to calm down before they were brought into the 92 μT-test field with and without oscillating fields added (see below). In the 92 μT static field, they were well oriented. After the respective tests, they were moved back to their normal housing cages in the bird room.
Test procedure
All birds were tested indoors with the magnetic field providing the only directional cue. Testing took place under 565 nm green light, i.e., in conditions under which robins show excellent orientation using their inclination compass (see, e.g., (1,4,6)) and followed standard procedures: the birds were tested individually once per day in funnel-shaped PVC cages lined with coated paper where they left scratches as they moved (for details, see e.g., (15,16)). Testing began when the lights went off in the birds' housing room and lasted ∼75 min.
Test fields
The test rooms were five wooden buildings where the local geomagnetic field was largely undisturbed. The static magnetic intensity at the testing locations had slightly different values ranging from 46.0 to 47.4 μT. This static field served as a control condition. In 2006, experiments were performed in a static field of 92 μT produced by Helmholtz coils (2 m diameter, 1 m clearance) tilted so that the generated field augmented the local geomagnetic field without affecting its inclination. The inhomogeneity of this field was <5% in the area of the test cages.
For most tests, the geomagnetic field was supplemented by oscillating magnetic fields. As in previous experiments, they were produced by a coil antenna mounted horizontally on a wooden frame surrounding a set of four test cages so that the oscillating field had a vertical axis, forming an angle of 24° with the vector of the local geomagnetic field. A high-frequency generator produced an oscillating signal that was amplified and, for frequencies above 1 MHz, fed through a resistance of 50 Ω or 51 Ω to the coil; for frequencies below 1 MHz, we used a different amplifier and an 8 Ω resistance. The coil consisted of a single coaxial cable, with 2 cm of the screening removed opposite the feed. The oscillating field strength was measured daily before each test session using a spectrum analyzer (Hewlett Packard 89410A). For details on the procedure and the equipment used, see (15,16). We used the same 1.315 MHz field in all test locations based on the median value of the local field at the test sites. There are no indications for a difference between sites.
Data analysis and statistics
For data analysis, the coated paper was removed from each cage, divided into 24 sectors, and the scratches per sector were counted double blind. From the distribution of these scratches, we calculated the heading for that particular test. Each bird was tested three times in each set of conditions, and the respective three headings were added to produce a mean vector with the heading αb and the length rb for each bird. From the mean headings of 12 birds in each test condition, we calculated second-order mean vectors with the heading αN and the length rN. These were tested for significant directional preference using the Rayleigh test (18). The data obtained with oscillating fields added are compared with the control data of the respective year by the Mardia Watson Wheeler test (18) for differences in distribution and by the Mann Whitney test (18) applied to the angular differences of the 12 data points from their own mean for differences in variance.
Results
Lifetime estimate of the radical pair
Birds were first tested at eight frequencies ranging from 0.01 MHz to 7.0 MHz, with an intensity of 470–480 nT. The results are given in Fig. 2; for numerical data, see the Supporting Material, Table S1.
Figure 2.
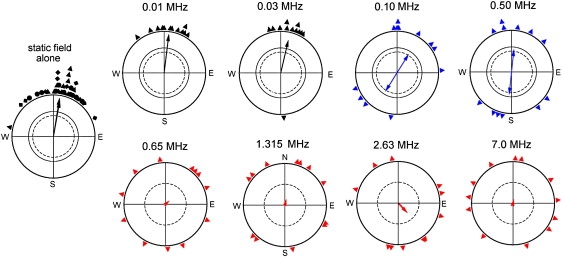
Orientation behavior of European robins in the local geomagnetic field: effects of added 480 nT oscillating fields of various frequencies. The symbols at the periphery of the circles mark the mean headings of the test birds based on three recordings each; the arrows represent the corresponding mean vectors. For the static field, the data from different years are given by different symbols; the three mean vectors almost coincide. The two inner circles are the 5% (dotted) and 1% significance limits of the Rayleigh test (17).
At 0.01 MHz and 0.03 MHz, the birds oriented in the migratory direction, with their response indistinguishable from that found in the local geomagnetic field. At 0.10 MHz and 0.50 MHz, they showed a weak axial response, preferring their migratory direction and the opposite direction, a type of behavior observed before when the magnetic compass is at the limit of its range of operation (5). A 0.658 MHz field, in contrast, caused the birds to be disoriented, and higher frequencies consistently resulted in disorientation as shown here for 2.63 MHz and previously for 1.315 MHz and 7.0 MHz fields (14,15) at this intensity.
Low-frequency oscillating fields with periods that are significantly longer than the lifetime of the radical pair are effectively static and produce effects that are indistinguishable from those of a static field (13). It appears reasonable to assume that the onset of the effect of oscillating fields coincides with the transition from fields that appear static to fields with high enough frequencies to oscillate during the lifetime of the radical pair. With this assumption, one can estimate the lifetime of the radical pair as the reciprocal of the threshold frequency. In the present case of the radical pair crucial for magnetoreception, our data indicate a fairly long lifetime of 2–10 μs.
Resonance at Larmor frequency
To obtain information about the chemical properties of the radical pair, we tested whether a particularly strong response attributable to the electron Zeeman interaction could be detected. When placed in a magnetic field, a magnetic moment responds by precessing around the axis of the magnetic field with a characteristic frequency that is proportional to the intensity, B0, of that field. For an electron spin, this characteristic frequency, the Larmor frequency (14), is given by νL = γe B0/2π, where γe is the gyromagnetic ratio of the electron. In numerical terms, the Larmor frequency for an electron spin is νL (in MHz) = 0.028 B0 (in μT) (see the Supporting Material).
We added magnetic fields oscillating at the Larmor frequency of 1.315 MHz, half the Larmor frequency, 0.658 MHz, and twice the Larmor frequency, 2.63 MHz, to the local geomagnetic field and tested robins at various field intensities to determine the threshold for the onset of disorientation.
The results are given in Fig. 3 (left hand side; see also Table S2, upper part). Although the robins had been disoriented at all three frequencies when the intensity was 480 nT (see Fig. 2, lower right diagrams), they were oriented at 0.658 MHz and 2.63 MHz when the intensity was 150 nT, with their responses indistinguishable from that in the geomagnetic field alone. At 1.315 MHz, however, robins were disoriented at lower intensities, and they continued to be disoriented even when the field at this frequency was as weak as 15 nT. Only at 5 nT was their orientation no longer affected.
Figure 3.
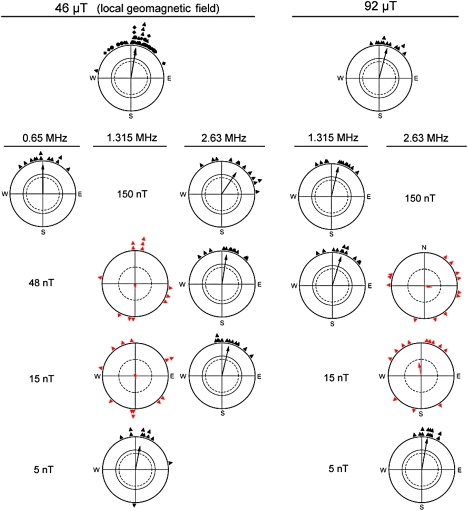
Orientation behavior of European robins: effects of added oscillating fields of various intensities and frequencies. (Left) Responses in the local geomagnetic field. (Right) Responses in a magnetic field of doubled intensity after preexposing birds to this field for 3 h. (Top diagrams) Oriented responses in the expected migratory direction in the static fields alone. (Diagrams below) Responses with oscillating fields added. (In the geomagnetic field, there were no tests at 0.658 MHz, 48, 15, and 5 nT; at 1.315 MHz, 150 nT, and 2.63 MHz, 5 nT due to time constraints; the same applies to the 92 μT static field at 1.315 MHz, 15, and 5 nT and at 2.63 MHz, 150 nT). Oriented responses are observed if the added high-frequency fields are weak, but test birds are disoriented when the intensity of the high-frequency field crosses a threshold. The threshold depends on the intensity of the static field and the frequency of the oscillating field: 1.315 MHz fields have the most pronounced effect on orientation in the geomagnetic field, whereas 2.63 MHz fields have the most pronounced effect in an ambient field of doubled intensity. Symbols as in Fig. 2.
If this extraordinarily sensitive response at 1.315 MHz were associated with the Larmor frequency, one would expect that it should shift to twice the original frequency when tested in a static field of twice the intensity. We therefore continued the tests in a static magnetic field of 92 μT, i.e., approximately twice the intensity of the local geomagnetic field. The results of these tests are given in Fig. 3 (right side; see Table S2, lower part). In the 92 μT static field alone, the birds were well oriented in the migratory direction; tests applying oscillating fields of 1.315 MHz and 2.63 MHz at different intensities showed that the birds now responded most sensitively to oscillating fields at 2.63 MHz, with a 15 nT field already disrupting their orientation, whereas the 1.315 MHz field no longer affected their orientation at an intensity of 150 nT or 48 nT.
Thus, the strong resonance at a frequency proportional to the intensity of the static field appears to arise from the Zeeman interaction.
Discussion
Our findings indicate a radical pair with a long lifetime involved in the birds' magnetic compass, with the observation of an intense resonance at the Larmor frequency, significantly stronger than the responses at other frequencies, identifying specific properties of this radical pair.
An optimal radical-pair design
An intense resonance at the Larmor frequency is expected only for a radical pair in which one radical is devoid of hyperfine interactions, that is, a radical whose electron spin is magnetically isolated. In the absence of hyperfine interactions, the unpaired electron interacts with an external magnetic field to produce a unique energy-level splitting that corresponds to the Larmor frequency. Such a radical pair should show a particularly strong response at the Larmor frequency because it is unaffected by the nuclear spin configuration of the counter radical. Superimposed on this dominant resonance would be a forest of much weaker resonances arising from the hyperfine interactions in the counter radical (see the Supporting Material). Simulations of such a radical pair demonstrate that an oscillating magnetic field in the presence of a static magnetic field of 46 μT leads to a resonance close to 1.315 MHz that is 30–50 times stronger than those at other frequencies. This resonance doubles in frequency when the intensity of the static field is increased to 92 μT (Fig. 4), in agreement with our experimental results. Furthermore, the effect of the oscillating field is predicted to vanish when the oscillating and the static fields are parallel (see the Supporting Material), an effect that has been observed in earlier behavioral studies (15,16). The behavior of birds in resonant oscillating fields thus leads to the conclusion that one of the radical partners contains an electron spin that has no magnetic interactions other than with the geomagnetic field.
Figure 4.
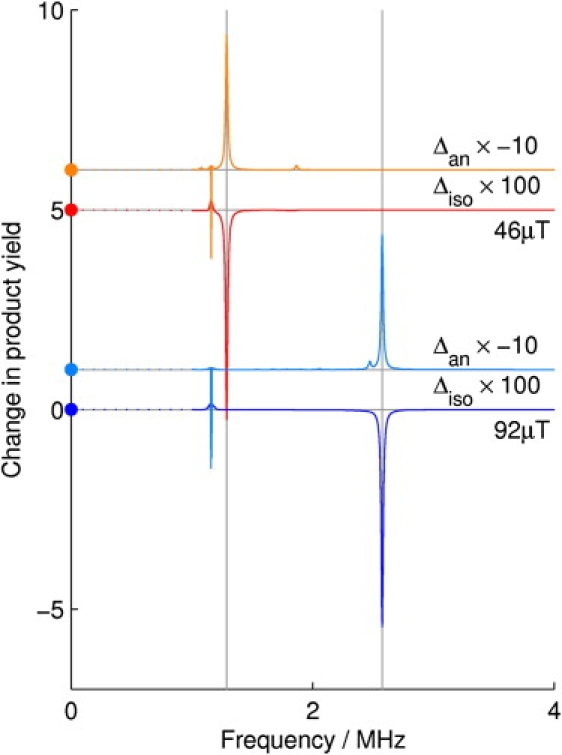
Calculated spectra of a model radical pair in magnetic fields of 46 and 92 μT. Δiso and Δan are, respectively, the isotropic and anisotropic parts of the fractional change in the reaction product yield produced by a 1 μT magnetic field oscillating at frequencies between 1 and 4 MHz. For details of the calculations, including the definitions of Δiso and Δan, see supplementary information. Strong resonances at the Larmor frequencies that correspond to the two applied magnetic field intensities are clearly seen. Much weaker resonances, arising principally from hyperfine interactions, are visible at other frequencies; the vertical scaling factors for each simulation are as indicated. The four traces are offset vertically from zero for clarity. The dots on the vertical axis show the values of Δiso and Δan for a 1 μT additional magnetic field at zero frequency.
Such a radical pair would be particularly suitable as a biological compass sensor for several reasons:
-
1.
Radical pairs of this design are more sensitive to a weak external field than when the nuclear spins are more evenly divided such that there are hyperfine interactions in both radicals (19).
-
2.
The anisotropy of the magnetic field effect, which is crucial for directional sensitivity, is similarly maximized for radical pairs with magnetic nuclei concentrated in one radical (20).
-
3.
The anisotropic effects of the various hyperfine interactions will in general tend to cancel each other, leading to weaker directional sensitivity. With magnetic nuclei only located on one radical, there is a greater chance that the hyperfine interactions are aligned in such a way as to enhance, rather than reduce, directional sensitivity (21).
-
4.
A radical free from internal magnetic interactions is more likely to undergo slow spin relaxation, a prerequisite for sensitivity to weak magnetic fields (9).
In short, if one were to propose a molecule for a radical-pair-based magnetic-compass sensor, one would be led to the type of radical pair identified via our behavioral resonance spectroscopy technique, one that is optimally designed for detecting magnetic directions.
Does cryptochrome match this optimal design?
Our indirect measurements reported here provide strong constraints that need to be satisfied by any radical pair suggested as an avian magnetoreceptor candidate. We are currently not aware of any observations of radical pairs in biology that immediately match our suggested design. Yet, because much of the current discussion in the literature focuses on the suggestion that cryptochromes form the magnetosensitive radical pairs (7,22–25), it is worth discussing how the unusual radical-pair design identified by our experiments might be realized in these molecules. Our discussion of a possible realization is by necessity speculative, and alternatives cannot be excluded. Cryptochromes, a class of blue-light photoreceptor proteins with flavin adenine dinucleotide (FAD) as the photoactive chromophore (26,27), are the only photoreceptor molecules in birds (22–24,28) known to form radical pairs (24,29). During photoactivation, excitation of the fully oxidized FAD cofactor results in the activated cryptochrome and involves creation of unusually long-lived radical pairs (30). The hyperfine couplings in the FADH• radical are dominated by two strong axially anisotropic 14N hyperfine interactions that have collinear axes. Experiments with the plant Arabidopsis thaliana show that magnetic fields affect a number of blue-light responses that are mediated by cryptochromes (31). However, there is one important caveat: all radicals found so far during photoactivation of cryptochromes have sizeable hyperfine interactions. Yet in addition to the forward photoactivation reaction, cryptochromes undergo a dark reversion to restore oxidized flavin as the resting state of the receptor molecule. This reaction occurs in vitro in the presence of molecular oxygen (30) and involves reoxidation of either the semiquinone (FADH•) or the fully reduced (FADH−) state of the flavin cofactor. The most-likely oxidizing agent is molecular oxygen whose reduced form, the superoxide radical (), is devoid of hyperfine couplings, at least when it is not hydrogen-bonded. So far, little attention has been devoted to this dark reaction of cryptochromes, presumably because the potential for light signaling at this step appears unlikely. Detection of magnetic field effects on the reoxidation of photoreduced FAD could provide a straightforward test of this hypothesis.
The radical pairs found in the forward photoactivation reaction alone cannot be reconciled with the resonance data in this study. However, our suggestion of an important role of a flavin-superoxide radical pair in the dark reaction does not preclude the possibility of magnetic field effects in the forward photoactivation reaction in other organisms as discussed in (32) and observed for photolyase (33). In fact, the coexistence of two magneto-sensitive reactions, both in the forward photoactivation and in the dark reaction of cryptochrome, may explain why cryptochrome can be highly sensitive to weak magnetic fields.
The observation of magnetic effects on cryptochrome-controlled responses in plants, despite the lack of any obvious biological significance, suggests that magnetic sensitivity may be an intrinsic property of cryptochrome signaling (31). Hence, animals that profit from magnetic information for orientation and navigation may have taken the opportunity to evolve this property to serve as the basis for a compass sensor.
Outlook
Hitherto, the radical pair underlying the avian magnetic compass has not been identified. However, by characterizing the effects of oscillating fields on the birds' compass and by relating the observed properties to a potential receptor molecule, we were able to identify a unique type of radical pair as the only one consistent with experimental observations. Our findings mark a breakthrough in pinpointing the molecular mechanism that provides birds with directional information from the geomagnetic field. The crucial analyses were based on the migratory behavior of European robins, but the mechanism described is in no way restricted to them. A magnetic compass has been demonstrated in numerous other species of passerines, in homing pigeons and in a shorebird species. This compass has proved to be an inclination compass in all species thus tested (1). Recently, a magnetic compass has also been demonstrated in domestic chickens (34) and shown to be based on a radical-pair mechanism like that of robins (35). This suggests that the mechanism described here is common to all birds.
Whether the avian molecular mechanism detailed here also plays a role in the magnetic compass of other animals is not yet known. Crustaceans and fish possess a polarity compass (36,37), which is possibly based on magnetite, a permanently magnetic iron oxide (37,39). Some mammals have a polarity compass (40,41), with recent experiments indicating the absence of radical-pair processes (42) and the involvement of a magnetite-based mechanism in mole-rats (43). A magnetite-based structure has also been found in the beak of birds (44) and discussed to play a role in magnetic-field detection (45,46). However, the well-defined magnetic-compass behavior of migratory birds is unaffected by anesthetization or lesion of the magnetite-based structure (47–49), demonstrating that it is not involved in the magnetic-compass mechanism described here. The biological significance of this structure remains unclear, as it has so far been linked only to responses under unnatural light conditions (48–50) or to conditioning responses in strong magnetic anomalies (51), neither of which would occur in nature. Marine turtles also have an inclination compass (50), which, in contrast to that of birds, remains operational in the dark (53), whereas a light-dependent compass, possibly similar to that of birds, was described for amphibians (54,55) and might exist in insects (24,56,57). These mechanisms, however, have not yet been analyzed. We hope that the new understanding of the primary process underlying the magnetic compass of birds presented here will stimulate comparable studies in other animals.
Supporting Material
Materials and methods, numerical results, calculations, two tables, and two figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(09)00468-8.
Supporting Material
Acknowledgments
We thank S. Denzau, T. Drick, D. Geiss, E. Lange, N. Moldan, C. Nießner, K. Patzolt, T. Pavkovich, M. Stavermann, and A. Wagner for their help with the orientation experiments, and we are grateful to Drs. M. Ahmad and C. Kay for helpful discussions. The experiments with birds were performed in accordance with the rules and regulations of animal welfare in Germany.
Our work was supported by the Human Frontier Science Program (grant to T.R., C.R.T., and R.W.), the Engineering and Physical Sciences Research Council (P.J.H., C.R.T., and C.T.R.), the EMF Biological Research Trust (P.J.H. and C.R.T.), the Royal Society (C.R.T.), the Deutsche Forschungsgemeinschaft (W.W.), and the Freunde und Förderer der Universität Frankfurt (W.W. and R.W.). T. R. is an Alfred P. Sloan Fellow and a Cottrell Scholar of the Research Cooperation. We thank the Oxford Supercomputing Centre for processor time used to calculate Fig. 4.
Footnotes
Katrin Stapput's present address is Department of Biology, University of North Carolina, Chapel Hill, NC.
Contributor Information
Thorsten Ritz, Email: tritz@uci.edu.
Roswitha Wiltschko, Email: wiltschko@bio.uni-frankfurt.de.
P.J. Hore, Email: peter.hore@chem.ox.ac.uk.
References
- 1.Wiltschko W., Wiltschko R. Magnetic orientation and magnetoreception in birds and other animals. J. Comp. Physiol. [A] 2005;191:675–693. doi: 10.1007/s00359-005-0627-7. [DOI] [PubMed] [Google Scholar]
- 2.Wiltschko W. The influence of static magnetic fields on the migratory orientation of European robins (Erithacus rubecula) Z. Tierpsychol. 1968;25:537–558. [PubMed] [Google Scholar]
- 3.Wiltschko W., Wiltschko R. Magnetic compass of European robins. Science. 1972;176:62–64. doi: 10.1126/science.176.4030.62. [DOI] [PubMed] [Google Scholar]
- 4.Wiltschko W., Wiltschko R. Light-dependent magnetoreception in birds: the behaviour of European robins, Erithacus rubecula, under monochromatic light of various wavelengths and intensities. J. Exp. Biol. 2001;204:3295–3302. doi: 10.1242/jeb.204.19.3295. [DOI] [PubMed] [Google Scholar]
- 5.Muheim R., Bäckman J., Åkesson S. Magnetic compass orientation in European robins is dependent on both wavelength and intensity of light. J. Exp. Biol. 2002;205:3845–3856. doi: 10.1242/jeb.205.24.3845. [DOI] [PubMed] [Google Scholar]
- 6.Wiltschko R., Stapput K., Bischof H.J., Wiltschko W. Light-dependent magnetoreception in birds: increasing intensity of monochromatic light changes the nature of the response. Front. Zool. 2007;4(5) doi: 10.1186/1742-9994-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ritz T., Adem S., Schulten K. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 2000;78:707–718. doi: 10.1016/S0006-3495(00)76629-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang K., Mattern E., Ritz T. On the use of magnets to disrupt the physiological compass of birds. Phys. Biol. 2006;3:220–231. doi: 10.1088/1478-3975/3/3/007. [DOI] [PubMed] [Google Scholar]
- 9.Timmel C.R., Till U., Brocklehurst B., McLauchlan K.A., Hore P.J. Effects of weak magnetic fields on free radical recombination reactions. Mol. Phys. 1998;95:71–89. doi: 10.1080/09553000050176270. [DOI] [PubMed] [Google Scholar]
- 10.Wiltschko W., Traudt J., Güntürkün O., Prior H., Wiltschko R. Lateralisation of magnetic compass orientation in a migratory bird. Nature. 2002;419:467–470. doi: 10.1038/nature00958. [DOI] [PubMed] [Google Scholar]
- 11.Maeda K., Henbest K.B., Cintolesi F., Kuprov I., Rodgers C.T. Chemical compass model of avian magnetoreception. Nature. 2008;453:387–390. doi: 10.1038/nature06834. [DOI] [PubMed] [Google Scholar]
- 12.Weaver J., Vaughan T.E., Astumian R.D. Biological sensing of small field differences by magnetically sensitive chemical reactions. Nature. 2000;405:707–709. doi: 10.1038/35015128. [DOI] [PubMed] [Google Scholar]
- 13.Henbest K.B., Kukura P., Rodgers C.T., Hore P.J., Timmel C.R. Radiofrequency magnetic field effects on a radical recombination reaction: a diagnostic test for the radical pair mechanism. J. Am. Chem. Soc. 2004;126:8102–8103. doi: 10.1021/ja048220q. [DOI] [PubMed] [Google Scholar]
- 14.Weil J.A., Bolton J.R. 2nd edition. Wiley & Sons, New York, NY; 2007. Electron Paramagnetic Resonance: Elementary Theory and Practical Applications. [Google Scholar]
- 15.Ritz T., Thalau P., Phillips J.B., Wiltschko R., Wiltschko W. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature. 2004;429:177–180. doi: 10.1038/nature02534. [DOI] [PubMed] [Google Scholar]
- 16.Thalau P., Ritz T., Stapput K., Wiltschko R., Wiltschko W. Magnetic compass orientation of migratory birds in the presence of a 1.315 MHz oscillating field. Naturwissenschaften. 2005;92:86–90. doi: 10.1007/s00114-004-0595-8. [DOI] [PubMed] [Google Scholar]
- 17.Wiltschko W., Stapput K., Thalau P., Wiltschko R. Avian magnetic compass: fast adjustment to intensities outside the functional window. Naturwissenschaften. 2006;93:300–304. doi: 10.1007/s00114-006-0102-5. [DOI] [PubMed] [Google Scholar]
- 18.Batschelet E. Academic Press; London: 1981. Circular Statistics in Biology. [Google Scholar]
- 19.Rodgers C.T., Norman S.A., Henbest K.B., Timmel C.R., Hore P.J. Determination of radical re-encounter probability distributions from magnetic field effects on reaction yields. J. Am. Chem. Soc. 2007;129:6746–6755. doi: 10.1021/ja068209l. [DOI] [PubMed] [Google Scholar]
- 20.Efimova O., Hore P.J. The role of exchange and dipolar interactions in the radical pair model of the avian magnetic compass. Biophys. J. 2008;94:1565–1574. doi: 10.1529/biophysj.107.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cintolesi F., Ritz T., Kay C.W.M., Timmel C.R., Hore P.J. Anisotropic recombination of an immobilized photoinduced radical pair in a 50-μT magnetic field: a model avian photomagnetoreceptor. Chem. Phys. 2003;294:385–399. [Google Scholar]
- 22.Möller A., Sagasser W., Wiltschko W., Schierwater B. Retinal cryptochrome in a migratory passerine bird: a possible transducer for the avian magnetic compass. Naturwissenschaften. 2004;91:585–588. doi: 10.1007/s00114-004-0578-9. [DOI] [PubMed] [Google Scholar]
- 23.Mouritsen H., Janssen-Bienhold U., Liedvogel M., Feenders G., Stalleicken J. Cryptochromes and neuronal-activity markers colocalize in the retina of migratory birds during magnetic orientation. Proc. Natl. Acad. Sci. USA. 2004;101:14294–14299. doi: 10.1073/pnas.0405968101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liedvogel M., Maeda M., Henbest K., Schleicher E., Simon T. Chemical magnetoreception: bird cryptochrome 1a is excited by blue light and forms long-lived radical-pairs. PLoS ONE. 2007;2(10):e1106. doi: 10.1371/journal.pone.0001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gegear R.J., Casselman A., Waddell S., Reppert S.M. Cryptochrome mediates light-dependent magnetosensitivity in Drosophila. Nature. 2008;454:1014–1018. doi: 10.1038/nature07183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad M., Cashmore A.R. HY4 gene of Arabidopsis thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993;366:162–166. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- 27.Sancar A. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem. Rev. 2003;103:2203–2237. doi: 10.1021/cr0204348. [DOI] [PubMed] [Google Scholar]
- 28.Haque R., Charausia S.S., Wessel J.H., Iuvone P.M. Dual regulation of cryptochrome I mRNA expression in chicken retina by light and circadian oscillators. Neuroreport. 2002;13:2247–2251. doi: 10.1097/00001756-200212030-00016. [DOI] [PubMed] [Google Scholar]
- 29.Giovani B., Byrdin M., Ahmad M., Brettel K. Light-induced electron transfer in a cryptochrome blue-light photoreceptor. Nat. Struct. Biol. 2003;6:489–490. doi: 10.1038/nsb933. [DOI] [PubMed] [Google Scholar]
- 30.Bouly J.P., Schleicher E., Dionisio-Sese M., Vandenbussche F., Van Der Straeten D. Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J. Biol. Chem. 2007;282:9383–9391. doi: 10.1074/jbc.M609842200. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad M., Galland P., Ritz T., Wiltschko R., Wiltschko W. Magnetic intensity affects cryptochrome-dependent responses in Arabidopsis thaliana. Planta. 2007;225:615–624. doi: 10.1007/s00425-006-0383-0. [DOI] [PubMed] [Google Scholar]
- 32.Solov'yov I.A., Chandler D.E., Schulten K. Magnetic field effects in Arabidopsis thaliana cryptochrome-1. Biophys. J. 2007;92:2711–2726. doi: 10.1529/biophysj.106.097139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henbest K.B., Maeda K., Hore P.J., Joshi M., Bacher A. Magnetic field effect on the photoactivation reaction of Escherichia coli DNA photolyase. Proc. Natl. Acad. Sci. USA. 2008;105:14395–14399. doi: 10.1073/pnas.0803620105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freire R., Munro U.H., Rogers L.J., Wiltschko R., Wiltschko W. Chicken orient using a magnetic compass. Curr. Biol. 2005;15:R620–R621. doi: 10.1016/j.cub.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Wiltschko W., Freire R., Munro U., Ritz T., Rogers L. The magnetic compass of domestic chickens, Gallus gallus. J. Exp. Biol. 2007;210:2300–2310. doi: 10.1242/jeb.004853. [DOI] [PubMed] [Google Scholar]
- 36.Lohmann K.J., Pentcheff N.D., Nevitt G.A., Stetten G., Zimmer-Faust R.K. Magnetic orientation of spiny lobsters in the ocean: experiments with undersea coil systems. J. Exp. Biol. 1995;198:2041–2048. doi: 10.1242/jeb.198.10.2041. [DOI] [PubMed] [Google Scholar]
- 37.Quinn T.P., Brannon E.L. The use of celestial and magnetic cues by orienting sockeye salmon fry. J. Comp. Physiol. 1982;147:547–552. [Google Scholar]
- 38.Walker M.M., Diebel C.E., Haugh C.V., Pankhurst P.M., Montgomery J.V. Structure and function of the vertebrate magnetic sense. Nature. 1997;390:371–376. doi: 10.1038/37057. [DOI] [PubMed] [Google Scholar]
- 39.Diebel C.E., Proksch R., Green C.R., Neilson P., Walker M.M. Magnetite defines a vertebrate magnetoreceptor. Nature. 2000;406:299–302. doi: 10.1038/35018561. [DOI] [PubMed] [Google Scholar]
- 40.Marhold S., Wiltschko W., Burda H. A magnetic polarity compass for direction finding in a subterranean mammal. Narurwissenschaften. 1997;84:421–423. [Google Scholar]
- 41.Wang Y., Pan Y., Parsons S., Walker M., Zhang S. Bats respond to polarity of a magnetic field. Proc. R. Soc. Lond. B. Biol. Sci. 2007;274:2901–2905. doi: 10.1098/rspb.2007.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thalau P., Ritz T., Burda H., Wegner R.E., Wiltschko R. The magnetic compass mechanisms of birds and rodents are based on different physical principles. J.R. Soc. Interface. 2006;3:583–587. doi: 10.1098/rsif.2006.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wegner R.E., Begall S., Burda H. Magnetic compass in the cornea: local anaesthesia impairs orientation in a mammal. J. Exp. Biol. 2006;209:4747–4750. doi: 10.1242/jeb.02573. [DOI] [PubMed] [Google Scholar]
- 44.Fleissner G., Holtkamp-Rötzler E., Hanzlik M., Winklhofer M., Fleissner G. Ultrastructural analysis of a putative magnetoreceptor in the beak of homing pigeons. J. Comp. Neurol. 2003;458:350–360. doi: 10.1002/cne.10579. [DOI] [PubMed] [Google Scholar]
- 45.Fleissner G., Stahl B., Thalau P., Falkenberg G., Fleissner G. A novel concept of Fe- mineral based magnetoreception: Histological and physicochemical data from the upper beak of homing pigeons. Naturwissenschaften. 2007;94:631–642. doi: 10.1007/s00114-007-0236-0. [DOI] [PubMed] [Google Scholar]
- 46.Solov'yov I.A., Greiner W. Theoretical analysis of an iron mineral-based magnetoreceptor model in birds. Biophys. J. 2007;93:1493–1509. doi: 10.1529/biophysj.107.105098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beason R.C., Semm P. Does the avian ophthalmic nervecarry magnetic navigational information? J. Exp. Biol. 1996;199:1241–1244. doi: 10.1242/jeb.199.5.1241. [DOI] [PubMed] [Google Scholar]
- 48.Wiltschko R., Stapput K., Ritz T., Thalau P., Wiltschko W. Magnetoreception in birds: different physical processes for two types of directional responses. HFSP Journal. 2007;1:41–48. doi: 10.2976/1.2714294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiltschko R., Munro U., Ford H., Stapput K., Wiltschko W. Light-dependent magnetoreception: orientation behaviour of migratory birds under dim red light. J. Exp. Biol. 2008;211:3344–3350. doi: 10.1242/jeb.020313. [DOI] [PubMed] [Google Scholar]
- 50.Wiltschko R., Ritz T., Stapput K., Thalau P., Wiltschko W. Two different types of light-dependent responses to magnetic fields in birds. Curr. Biol. 2005;15:1518–1523. doi: 10.1016/j.cub.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 51.Mora C.V., Davison M., Wild J.M., Walker M.M. Magnetoreception and its trigeminal mediation in the homing pigeon. Nature. 2004;432:508–511. doi: 10.1038/nature03077. [DOI] [PubMed] [Google Scholar]
- 52.Light P., Salmon M., Lohmann K.J. Geomagnetic orientation of loggerhead sea turtles: evidence for an inclination compass. J. Exp. Biol. 1993;182:1–9. [Google Scholar]
- 53.Lohmann K.J. Magnetic orientation by hatchling loggerhead turtles (Caretta caretta) J. Exp. Biol. 1993;155:37–49. doi: 10.1242/jeb.155.1.37. [DOI] [PubMed] [Google Scholar]
- 54.Phillips J.B. Two magnetoreception pathways in a migratory salamander. Science. 1986;233:765–767. doi: 10.1126/science.3738508. [DOI] [PubMed] [Google Scholar]
- 55.Phillips J.B., Borland S.C. Behavioral evidence for the use of light-dependent magnetoreception mechanism by a vertebrate. Nature. 1992;359:142–144. [Google Scholar]
- 56.Phillips J.B., Sayeed O. Wavelength-dependent effects of light on magnetic compass orientation in Drosophila melanogaster. J. Comp. Physiol. [A] 1993;172:303–308. doi: 10.1007/BF00216612. [DOI] [PubMed] [Google Scholar]
- 57.Vácha M., Soukopova H. Magnetic orientation in the mealworm beetle Tenebrio and the effect of light. J. Exp. Biol. 2004;207:1241–1248. doi: 10.1242/jeb.00874. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


