Abstract
Predators affect prey demography through direct predation and through the costs of antipredator behavioral responses, or risk effects. Experiments have shown that risk effects can comprise a substantial proportion of a predator's total effect on prey dynamics, but we know little about their strength in wild populations, or the physiological mechanisms that mediate them. When wolves are present, elk alter their grouping patterns, vigilance, foraging behavior, habitat selection, and diet. These responses are associated with decreased progesterone levels, decreased calf production, and reduced population size [Creel S, Christianson D, Liley S, Winnie JA (2007) Science 315:960]. Two general mechanisms for the effect of predation risk on reproduction have been proposed: the predation stress hypothesis and the predator-sensitive-food hypothesis. Here, we used enzyme immunoassay to measure fecal glucocorticoid metabolite concentrations for 1,205 samples collected from 4 elk populations over 4 winters to test the hypothesis that the effect of predation risk on elk reproduction is mediated by chronic stress. Across populations and years, fecal glucocorticoid concentrations were not related to predator-prey ratios, progesterone concentrations or calf-cow ratios. Overall, the effect of wolf presence on elk reproduction is better explained by changes in foraging patterns that carry nutritional costs than by changes in glucocorticoid concentrations.
Keywords: antipredator behavior, nonconsumptive effects, risk effect, wolf
Predators affect prey demography through direct predation and through the costs of antipredator behavioral responses, or “risk effects” (1–4). The benefits of antipredator behavior are manifested as a reduction in the rate of direct predation, and these benefits are automatically included when a study measures the direct predation (killing) rate. Risk effects are more difficult to identify and quantify because they are less direct and can be manifest in many ways, but experiments have shown that risk effects can comprise a large proportion of predation's total effect on prey demography and dynamics (5–8). While there is a growing consensus that risk effects are important for predator-prey and food-web dynamics, we still know little about the mechanisms that produce them because few studies have examined all of the steps in the pathway that must exist if risk effects are important for population dynamics—from predation risk to behavioral responses to physiological costs to changes in demography and population dynamics (for an example, see refs. 9–12).
Two general hypotheses have been proposed for the physiological mechanisms that mediate risk effects on the population dynamics of prey. The predator-sensitive food hypothesis suggests that behavioral responses to predators constrain foraging activity or efficiency, and thus strengthen energetic or nutritional constraints on reproduction or survival (13). Many experimental and observational studies have shown that predation risk affects foraging decisions and constraints (14). Fewer studies have tested whether predator-induced changes in foraging patterns carry energetic/nutritional costs or affect prey dynamics, but these relationships can be strong, for example in wildebeest (Connochaetes taurinus) in Serengeti National Park and snowshoe hares (Lepus americanus) in Kluane National Park (12, 13), but also see ref. 15.
The predation stress hypothesis suggests that exposure to predators causes elevation of glucocorticoid (GC) stress hormones (10, 16–18), which can directly suppress reproduction through effects on the hypothalamic-pituitary-gonadal axis (19, 20), and can indirectly reduce survival and reproduction through effects on the immune and digestive systems (19, 21–23). Experimental studies have shown that exposure to predators or their odors can cause immediate, short-term increases in the circulating GC levels of prey (24–26), but do not always do so (27). Chronic elevation of GCs can interfere with hypothalamic-pituitary-gonadal function, but brief pulses of GC secretion normally do not (19–22). Consequently, experimental studies of short-term responses are important to establish a cause-and-effect relationship between risk and GC responses, but it is also important to test whether natural variation in predation risk is associated with measurable long-term changes in GC levels. Few field studies have examined long-term GC responses to predators, with no clear pattern emerging (10, 16, 28), although 2 recent studies have detected chronic GC responses to predation risk (9, 29).
We have previously shown that mean progesterone concentrations and calf production in Greater Yellowstone Ecosystem (GYE) elk (Cervus elaphus) are negatively correlated with the risk of predation by wolves (Canis lupus) (30). Elk responded to wolf presence by more than doubling the proportion of daylight hours they spent vigilant (31, 32), and consequently decreasing the proportion of time spent feeding by 19% (33). Elk also responded to wolves by moving into the protective cover of wooded areas when wolves were present, reducing their use of preferred grassland foraging habitats (34–36). Elk strongly prefer grazing to browsing, and habitat type is a strong predictor of the balance of grazing and browsing in elk diets (37). Experiments with GYE elk show that changes in the balance of grazing and browsing affect the rate of mass loss over winter (38). For GYE elk in the Gallatin population, changes in habitat selection and feeding behavior were associated with significant changes in diet and nutrition (38, 39), including a shift from grazing to browsing (39) and a reduction in estimated intake rates by 27% of maintenance requirements (40). GYE elk steadily lose body mass and fat through winter (33, 41), and nutritional condition affects pregnancy rates in elk (42). Elk numbers on GYE winter ranges have declined by as much as 60% since wolf reintroduction (34, 43, 44). For example, elk numbers in Yellowstone's Northern Range herd were between 16,791 and 19,045 in the 3 winters up to 1995, then declined through 11 annual counts to between 6,738 and 6,279 in the 3 winters up to 2008 (45). The decline in GYE elk herds occurred while elk populations in Montana as a whole were growing at a geometric mean annual rate (λ) of 1.030 (46). The decline in GYE elk numbers is larger than can be accounted for by direct predation rates, and is associated with significant declines in calf-cow ratios immediately after the birth season, and with significantly decreased progesterone concentrations during late gestation (8, 30).
Collectively, these results support the predation-sensitive food hypothesis, but the stress hypothesis remains untested, and the 2 hypotheses are not mutually exclusive (10, 12). In the GYE, elk are exposed to wolves and mount antipredator responses on a near-daily basis (31, 32). The risk of predation is substantial. Given the rate of detected predation (0.08 elk kills per wolf per day) and the ratio of elk to wolves in the populations in this study (287 ± 81, mean ± SEM, n = 15 site-year combinations) (30), the per capita probability of predation was 0.21 ± 0.03 annually [mean ± SEM, (note that mean of ratios is greater than ratio of means)]. Like other ungulates, the responses of elk to predators are graded (32), and the most overt antipredator behaviors (rapid fleeing, prancing) are only used during close, direct interactions. However, elk engage in less overt responses such as habitat shifts, reduced feeding, and increased vigilance when wolves are up to 3 km away (32). The cognitive and endocrine correlates of these frequent but subtle changes in behavior are not known, but environmental conditions can elicit a glucocorticoid stress response with little change in overt behavior. For example, the behavioral responses of elk to snowmobiles are much weaker than their behavioral responses to wolves, but their fecal glucocorticoid metabolite (fGCM) concentrations nonetheless increase significantly on days with heavier-than-average snowmobile activity (47).
Experiments with rats suggest that unpredictable or uncontrollable stressors provoke the strongest and most persistent glucocorticoid responses (21, 48), and it is plausible to hypothesize that predation risk is perceived as unpredictable or uncontrollable by elk (and other species) that are exposed to intermittent lethal risk. Here, we test whether (in addition to the nutritional effects described above) fGCM concentrations in elk were associated with predation risk or progesterone levels. Because these data come from the same wolf-elk populations and the same time period as previously published data that detected significant changes in foraging behavior, nutrition, reproductive physiology, demography, and population dynamics, they provide a simultaneous test of the predator-sensitive food and predation-stress hypotheses in a single system.
Results
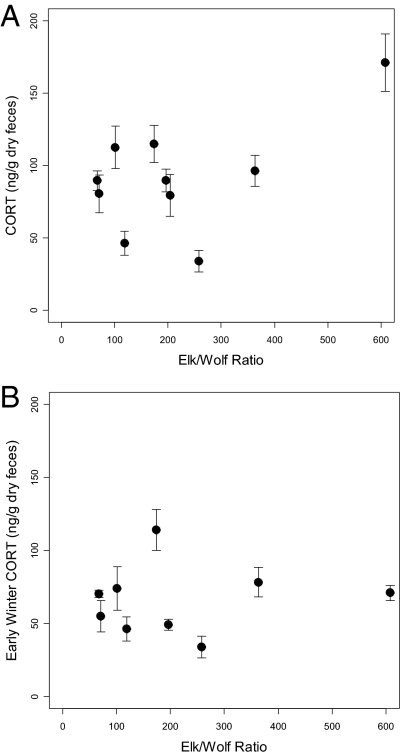
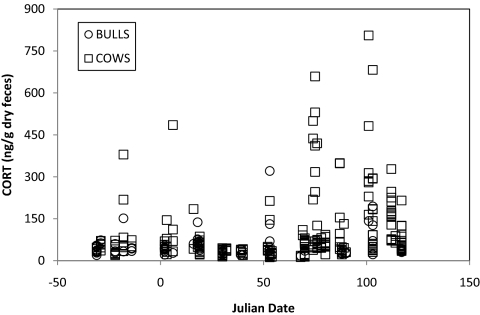
Contrary to the stress hypothesis, the winter-long mean fGCM concentrations of elk were significantly higher in populations with lower predation pressure (i.e., higher elk-wolf ratios: Fig. 1A; b = 0.13 ± SE 0.059, Wald 5.00, P = 0.025). The interpretation of this result is not simple, for 2 reasons. First, the result is highly dependent on data from a single population in a single year (Fig. 1A). If this high-leverage data point is removed there is no detectable relationship between winter-long fGCM concentrations and elk-wolf ratios. Second, plasma steroid binding globulins with a high affinity for progesterone also have high affinity for corticosteroids (49). Consequently, circulating total cortisol concentrations often rise in parallel with progesterone concentrations in the final trimester of gestation, and this increase in circulating cortisol is typically accompanied by an increase in fecal corticosteroid metabolite concentrations (50). The expected late-gestation rise in fGCM concentration has previously been reported for elk (51, 52) and is apparent in our data for all site-years. Fecal GC concentrations notably increased after 15 March (Fig. 2), when elevated progesterone levels can be used to diagnose pregnancy (30). To test whether increased fGCM concentration in late winter could be explained on the basis of increased binding globulin levels that accompany the third trimester rise in progesterone, we examined temporal variation in fGCM concentration for a set of fecal samples from individuals of known sex. For randomly-collected samples not sorted by sex, fGCM concentration increased significantly during the winter [general linear model (GLM) fit by maximum likelihood, b = 0.64 ± 0.14, Wald statistic = 20.97, P < 0.0001]. This seasonal rise was strongly apparent in samples from females (Fig. 2: GLM fit by maximum likelihood, b = 0.70 ± 0.18, Wald statistic = 14.72, P < 0.0001), but was weak or absent in samples from males (Fig. 2: GLM fit by maximum likelihood, b = 0.32 ± 0.19, Wald statistic = 2.90, P = 0.09).
Fig. 1.
Relationship of mean fGCM concentration to predation pressure, as measured by elk-wolf ratios. Units of analysis are population-years. Whiskers show standard errors. (A) Winter-long mean fGCM concentration. (B) Early winter mean fGCM concentration [samples collected prior the increase in progesterone after 15 March (see Fig. 2)].
Fig. 2.
fGCM concentrations rise after 15 March (Julian Date = 74), concurrent with significant increases in progesterone concentration at the onset of the third trimester of gestation. This seasonal rise in fGCM concentration is apparent in samples from females (squares) but not males (circles), and can be explained by the late gestational increase in plasma steroid binding globulins in females.
These results suggest that late-gestation samples from pregnant females should be excluded from analyses that test the response of cortisol to potential stressors such as predation risk. This caveat is especially important for comparisons of fGCM concentration levels among groups that vary in their pregnancy rates, as is the case here (30). In particular, the result shown in Fig. 1A was strongly leveraged by the high fGCM concentration for 1 site-year, for which the estimated pregnancy rate was 100%. To avoid the potentially confounding effect of late-gestation changes while testing for a relationship between cortisol and predation pressure, we regressed early winter (before 15 March) fGCM concentration on elk-wolf ratios, and found no relationship (Fig. 1B; b = 0.01 ± SE 0.044, Wald 0.059, P = 0.808). By either test, fGCM did not correlate positively with predation risk as predicted by the stress hypothesis (Fig. 1 A and B).
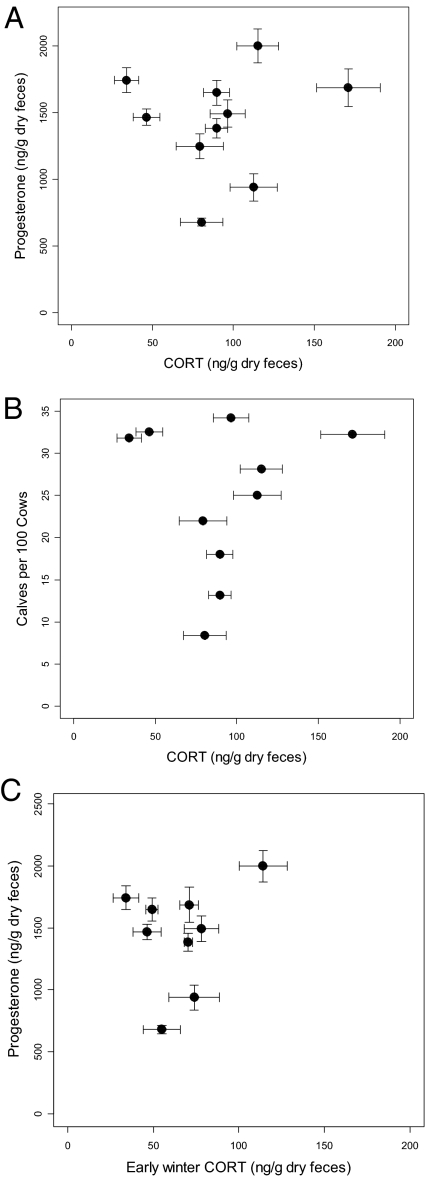
Also contrary to the stress hypothesis, neither progesterone levels nor calf recruitment were detectably related to fGCM concentration (Fig. 3A, regression of progesterone on winter-long mean fGCM concentration: b = 1.10 ± SE 3.24, Wald = 0.114, P = 0.73; Fig. 3B, regression of subsequent calf-cow ratio on winter-long fGCM concentration: b = 0.017 ± SE 0.074, Wald = 0.050, P = 0.82, Fig. 3C, regression of progesterone on early winter fGCM concentration: b = 4.38 ± SE 5.64, Wald = 0.598, P = 0.44; regression of subsequent calf-cow ratio on early winter fGCM concentration: b = 0.041 ± SE 0.013, Wald = 0.095, P = 0.76). Finally, there were no significant negative correlations between progesterone and fGCM concentration for individuals within single populations or population-years.
Fig. 3.
Relationships of mean fecal progesterone concentration and calf recruitment in the subsequent year to mean fGCM concentration. (A) Relationship of fecal progesterone concentration to winter-long fGCM concentration. (B) Relationship of subsequent calf-cow ratios to winter-long fGCM concentration. (C) Relationship of fecal progesterone concentration to early-winter fGCM concentration
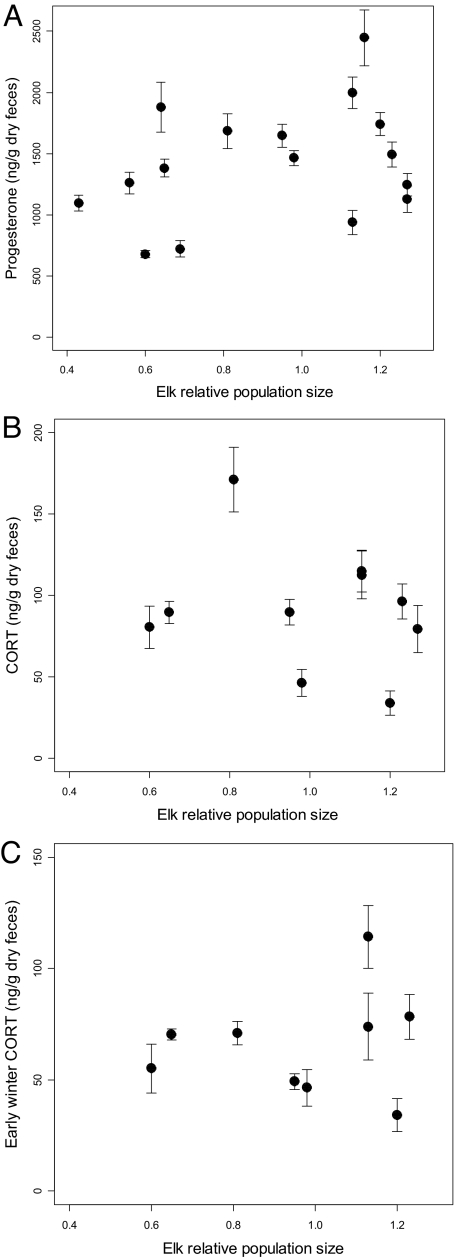
Density dependence is not a plausible explanation for low progesterone and calf recruitment in the populations with high predation risk because reproduction declined as these populations declined in size following wolf recolonization (30, 43). As expected, given this general pattern, population density was not a strong driver of fecal progesterone or fGCM concentrations (progesterone slope is of incorrect sign, b = 854.7 ± SE 512.0, Wald = 2.79, P = 0.10) (fGCM slope is of incorrect sign, b = −9.14 ± SE 45.98, Wald = 0.039, P = 0.84) (Fig. 4).
Fig. 4.
Mean fecal progesterone and glucocorticoid metabolite concentrations were not detectably related to changes in population density. Relative population density was measured as a population's current size divided by the long-term mean size of that population, so that a value of one indicates a population at its long-term mean density. (A) Fecal progesterone concentration. (B) Winter-long mean fGCM concentration. (C) Early winter mean fGCM concentration.
Discussion
Risk effects can be an important part of the total impact of predation on prey dynamics (5, 6, 53). Risk effects can also cascade to affect community composition and ecosystem function (2, 7, 54, 55). For elk, exposure to wolf predation is associated with changes in grouping patterns (56, 57), habitat selection (34, 35), vigilance (31–33), foraging (33), diet (39, 58), nutrition (38–40, 58), reproductive physiology (30), and demography (8, 30). Elk populations in the GYE (and elsewhere e.g., Banff National Park) have decreased in size since wolf recolonization, while elk populations in neighboring areas with low wolf density grew steadily as a result of weak limitation by winter snowfall (43, 45, 46, 59). Measured rates of direct predation are too low to explain the observed changes in elk calf production and population size (8), and radio-tagging of newborn calves reveals little direct predation by wolves in the first several months of life (30, 60). Collectively, these results strongly suggest a nonconsumptive risk effect of wolves on elk dynamics.
Our data suggest that the nonconsumptive effect of wolves on elk reproductive physiology and calf recruitment is not mediated by chronic activation of the GC stress response. Prior research shows that the effect of predation risk on elk reproduction is associated with changes in nutrition. Elk in Yellowstone steadily lose body mass and body fat throughout winter (33, 41), and when the body fat of elk drops below 12%, the probability of maintaining pregnancy declines rapidly (42). In the presence of wolves, we found a decrease in the proportion of time spent foraging, a shift from grazing to browsing, and a substantial reduction in the rate of intake (33, 24, 39). Together with the results presented here, the data suggest that the effect of wolf presence on elk reproduction is mediated by nutritional effects, and is not mediated by a chronic GC response.
Chronic elevation of GCs can have many pathological consequences (19, 21, 22), including shifts in metabolic pathways that mobilize energy in the short term, but reduce metabolic efficiency. Consequently, it might be expected that prey would not mount GC responses to predators, if encounters with predators are an everyday event, as they are for Yellowstone elk and many other species in intact ecosystems. Because elk have consistently negative energy budgets and steadily lose body mass during winter plant dormancy (37), any physiological response to wolves that reduced winter-long metabolic efficiency should be disfavored by natural selection. We still know little about the psycho-neuro-endocrinology of antipredator responses (24, 25), but psychological stressors generally cause strong and persistent glucocorticoid responses when they involve unpredictable or uncontrollable upsets to homeostasis (19, 21). Elk typically detect wolves and take action to reduce their risk; perhaps these behavioral responses avoid or diminish hypothalamic-pituitary-adrenal responses so that there is no detectable effect of risk on GC concentrations at the population level, even though mean progesterone levels declined by a factor of 2.5 in the same set of fecal samples (30). We did not test for short-term GC responses, and our data are compatible with the hypothesis that immediate encounters with predators produce GC responses that are small enough, or brief enough, that they do not detectably alter mean GC concentrations, but it is unlikely that such GC responses would interfere with progesterone secretion and the maintenance of pregnancy.
The cognitive and emotional aspects of avoiding predation remain unknown in this case, as in virtually all studies of “the ecology of fear” (58, 61). Many authors implicitly or explicitly assume that risk effects are mediated by stress or fear, and the term “predation stress” is becoming a synonym for “predation risk” (62, 63), but it should be remembered that risk effects can logically arise through mechanisms that do not involve the stress response. Our failure to detect a long-term GC response does not prove that elk do not fear wolves. By the same token, a demonstration that elk avoid wolves need not imply that fear drives the response. Females of many species avoid mates of low quality, but do they fear them? Vegetarians avoid meat, but do they fear it?
Materials and Methods
We collected data from elk on 4 winter ranges within the GYE, for 1–4 winters (2002–2006) at each site. The 4 sites are at similar elevation, with mixtures of coniferous forest and sage-steppe. Elk migrate from these winter ranges to higher elevation sites in the summer, but studies using radio-telemetry and ear-tagging have detected little movement between populations during the winter (34, 64); movement between sites would require an elk to cross one or more alpine passes with deep snow.
Elk were counted and herd compositions were recorded in aerial total counts conducted by the Montana Department of Fish, Wildlife, and Parks during winter, when elk aggregate at predictable low-elevation locations and are more easily detected due to high contrast with the snowy environment. Methods for these aerial surveys are described in detail elsewhere (46). Wolf numbers come from distribution maps and pack size tallies in US Fish and Wildlife Service annual reports, based on aerial radiotracking to locate packs that were then counted by direct observation. Methods for quantifying wolf numbers are described in Fish and Wildlife Service annual reports for the Northern Rocky Mountains Wolf Recovery Project (available at http://www.fws.gov/mountain-prairie/species/mammals/wolf/). For 4 years on the Gallatin site, we had independent data from near-daily observations, which confirmed the above numbers.
For fGCM determinations, we collected fresh elk fecal pellets from the snow and stored them at −40 °C in screw top vials. For each winter range, we collected 3–10 samples per sampling occasion, stratified at 2 week intervals throughout the winter (December 1–May 15). We analyzed 1,205 samples from 10 site-year combinations. When resting elk stand up, they frequently defecate in a discrete pile of pellets within the snow crater they have created, and these individual defecation sites are easily distinguished by snow tracking. We collected 10 pellets from each individual defecation site that we sampled. The fecal pellets of calves and adults differ significantly in size (39), and we avoided collecting samples from calves. The adult sex ratio of elk populations is consistently female-biased, and adult females comprised 73% of these populations (including calves), so the samples primarily represent the endocrine responses of adult females. Nonetheless, we tested for effects of variation in the adult sex ratio on hormone concentrations by calculating the proportion of the population that was composed of adult females for each site in each year, using aerial count data. This proportion ranged from 0.67–0.83, and none of the inferences we report were altered by the inclusion of sex-ratio as a covariate.
We homogenized each sample, dried ≈1g of homogenate with a rotary evaporator, and extracted steroids from ≈200 μg of dried feces (weighed to 0.01 mg) by boiling in ethanol. We dried extracts under forced air and reconstituted them with 1 mL 95% methanol for storage at −80 °C. With these extracts, we assayed cortisol at 36-fold methanol dilution with a monoclonal double-antibody enzyme immunoassay (R&D Systems KGE008). This cortisol antibody has <4.4% cross reactivity with other steroids (1.7% for progesterone). Binding curves for serial dilution of extracts and cortisol standards were parallel from 156–10,000 pg/mL. Recovery of cortisol added to extracts was 1.02 ± 0.03 (mean ± SEM). Intra-assay and interassay coefficients of variation were 5.26% and 7.31%. Sensitivity was 2 orders of magnitude below the minimum concentration of diluted fecal extracts. For biological validation, we compared concentrations to those we obtained with an RIA previously validated for elk feces by corticotropin challenge (65) for 232 samples, and found a close correlation [r2 = 0.90, RIA = 184 + 3.8 enzyme immunoassay (EIA) −0.011EIA2]. Because this EIA employs a monoclonal cortisol antibody, our fecal fGCM concentrations are measured as ng CORT immunoreactivity/g dry feces, although circulating glucocorticoids are enzymatically modified during biliary excretion, with 11-17-dioxoandrostanes predominating in ruminant feces (50, 51).
Methods for progesterone enzyme immunoassay and procedural validation data have been published previously (30). We restricted progesterone determinations to samples collected after 15 March, when elevated progesterone can be used to detect pregnancy. Overall, the mean pregnancy rate [determined by fecal progesterone >830 ng/g dry feces (30)] averaged 85% for these populations. For biological validation, we compared pregnancy detection via fecal EIA with detection via serum pregnancy-specific protein B for 30 samples collected from the same individuals on the same day. This comparison yielded 93.3% sample-concordance, with identical population pregnancy rates. We also compared pregnancy detection by EIA to results from a previously validated progesterone RIA (66), with 94.3% sample-concordance and r2 = 0.95 for 35 samples. We further tested the EIA's repeatability by drying, extracting and assaying 38 samples twice (in a blind manner), yielding 95% concordance in one-sample pregnancy diagnoses and r2 = 0.74 for concentrations. Finally, we tested repeatability by determining the pregnancy rate in 2 independent sets of fecal samples (n = 38 and 50) from the same population and year. These rates agreed to <1%.
Circulating cortisol levels commonly increase during gestation because the plasma binding globulin for progesterone also has strong affinity for cortisol (49). Consequently, we determined whether our tests might be affected by late gestational increases in steroid binding globulins of females, but not males. To do this, we used a set of 177 fecal samples from individuals of known gender (see Results, Fig. 2).
We used maximum likelihood to fit general linear models with normal errors and an identity link, unless stated otherwise with results. Assumptions were tested by visually checking residual distributions and Q-Q plots. After log-transformation, elk-wolf ratios met the assumption of normality of residuals. The analyses we report use unweighted means, but no inferences changed when we fit models weighted by sample size or the inverse of variance. Finally, no reported inferences changed when we fit more complex mixed models that included variation among populations as a random effect.
Acknowledgments.
We thank Stewart Liley for his excellent work in the field and laboratory; Yellowstone National Park and the Montana Department of Fish, Wildlife, and Parks for permission to conduct the research and logistical assistance. This work was funded by grants from Animal Behavior program of the National Science Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.S.B. is a guest editor invited by the Editorial Board.
References
- 1.Peckarsky BL, Cowan CA, Penton MA, Anderson C. Sublethal consequences of stream-dwelling predatory stoneflies on mayfly growth and fecundity. Ecology. 1993;74:1836–1846. [Google Scholar]
- 2.Schmitz OJ, et al. From individuals to ecosystem function: Toward an integration of evolutionary and ecosystem ecology. Ecology. 2008;89:2436–2445. doi: 10.1890/07-1030.1. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz OJ, Krivan V, Ovadia O. Trophic cascades: The primacy of trait-mediated indirect interactions. Ecol Lett. 2004;7:153–163. [Google Scholar]
- 4.Sih A. Prey uncertainty and the balancing of antipredator and feeding needs. Amer Nat. 1992;139:1052–1069. [Google Scholar]
- 5.Pangle KL, Peacor SD, Johannsson OE. Large nonlethal effects of an invasive invertebrate predator on zooplankton population growth rate. Ecology. 2007;88:402–412. doi: 10.1890/06-0768. [DOI] [PubMed] [Google Scholar]
- 6.Preisser EL, Bolnick DI, Blumstein DT. Scared to death? The effects of intimidation and consumption in predator prey interactions. Ecology. 2005;86:501–509. [Google Scholar]
- 7.Schmitz OJ, Beckerman AP, O'Brien KM. Behaviorally mediated trophic cascades: Effects of predation risk on food web interactions. Ecology. 1997;78:1388–1399. [Google Scholar]
- 8.Creel S, Christianson D. Relationships between direct predation and risk effects. Trends Ecol Evol. 2008;23:194–201. doi: 10.1016/j.tree.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Sherriff MJ, Krebs CJ, Boonstra R. The sensitive hare: Sublethal effects of predator stress on reproduction in snowshoe hares. J Anim Ecol. 2009 doi: 10.1111/j.1365-2656.2009.01552.x. advanced online doi: 10.1111/j.1365–2656.2009.01552.x. [DOI] [PubMed] [Google Scholar]
- 10.Boonstra R, Hik D, Singleton GR, Tinnikov A. The impact of predator-induced stress on the snowshoe hare cycle. Ecol Monogr. 1998;68:371–394. [Google Scholar]
- 11.Boonstra R, Singleton G. Population declines in the snowshoe hare and the role of stress. Gen Comp Endocrinol. 1993;91:126–143. doi: 10.1006/gcen.1993.1113. [DOI] [PubMed] [Google Scholar]
- 12.Krebs CJ, et al. Impact of food and predation on the snowshoe hare cycle. Science. 1995;269:1112–1115. doi: 10.1126/science.269.5227.1112. [DOI] [PubMed] [Google Scholar]
- 13.Sinclair ARE, Arcese P. Population consequences of predation-sensitive foraging: The Serengeti wildebeest. Ecology. 1995;76:882–891. [Google Scholar]
- 14.Brown JS, Kotler BP. Hazardous duty pay and the foraging cost of predation. Ecol Lett. 2004;7:999–1014. [Google Scholar]
- 15.Mduma SAR, Sinclair ARE, Hilborn R. Food regulates the Serengeti wildebeest: A 40-year record. J Anim Ecol. 1999;68:1101–1122. [Google Scholar]
- 16.Clinchy M, Zanette L, Boonstra R, Wingfield JC, Smith JNM. Balancing food and predator pressure induces chronic stress in songbirds. Proc R Soc London Ser B. 2004;271:2473–2479. doi: 10.1098/rspb.2004.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frid A, Dill LM. Human-caused disturbance stimuli as a form of predation risk. Cons Ecol. 2002;6:11. [Google Scholar]
- 18.Lima SL. Stress and decision-making under the risk of predation: Recent developments from behavioral, reproductive, and ecological perspectives. Adv Stud Behav. 1998;27:215–290. [Google Scholar]
- 19.Romero LM. Physiological stress in ecology: Lessons from biomedical research. Trends Ecol Evol. 2004;19:249–255. doi: 10.1016/j.tree.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Moberg GP. How behavioral stress disrupts the endocrine control of reproduction in domestic animals. J Dairy Sci. 1991;74:304–311. doi: 10.3168/jds.S0022-0302(91)78174-5. [DOI] [PubMed] [Google Scholar]
- 21.Sapolsky RM. In: Behavioral Endocrinology. Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Cambridge: MIT Press; 2002. pp. 409–450. [Google Scholar]
- 22.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 23.Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocrinol Rev. 1984;5:25. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 24.Cockrem JF, Silverin B. Sight of a predator can stimulate a corticosterone response in the great tit (Parus major) Gen Comp Endocrinol. 2002;125:248–255. doi: 10.1006/gcen.2001.7749. [DOI] [PubMed] [Google Scholar]
- 25.Monclus R. Behavioural and physiological responses of naive European rabbits to predator odour. Anim Behav. 2005;70:753–761. [Google Scholar]
- 26.Thaker M, Lima SL, Hews DK. Alternative antipredator tactics in tree lizard morphs: Hormonal and behavioural responses to a predator encounter. Anim Behav. 2009;77:395–401. [Google Scholar]
- 27.Ylönen H, Eccard JA, Jokinen I, Sundell J. Is the antipredatory response in behaviour reflected in stress measured in faecal corticosteroids in a small rodent? Behav Ecol Sociobiol. 2006;60:350–358. [Google Scholar]
- 28.Scheuerlein A. Predators as stressors? Physiological and reproductive consequences of predation risk in tropical stonechats (Saxicola torquata axillaris) Proc R Soc London Ser B. 2001;268:1575–1582. doi: 10.1098/rspb.2001.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCormick MI. Indirect effects of heterospecific interactions on progeny size through maternal stress. Oikos. 2009;118:744–752. [Google Scholar]
- 30.Creel S, Christianson D, Liley S, Winnie JA. Predation risk affects reproductive physiology and demography of elk. Science. 2007;315:960. doi: 10.1126/science.1135918. [DOI] [PubMed] [Google Scholar]
- 31.Creel S, Winnie J, Jr, Christianson D, Liley S. Time and space in general models of antipredator response: Tests with wolves and elk. Anim Behav. 2008;76:1139–1146. [Google Scholar]
- 32.Liley S, Creel S. What best explains vigilance in elk: Characteristics of prey, predators, or the environment? Behav Ecol. 2008;19:245–254. [Google Scholar]
- 33.Winnie J, Creel S. Sex-specific behavioural responses of elk to spatial and temporal variation in the threat of wolf predation. Anim Behav. 2007;73:215–225. [Google Scholar]
- 34.Creel S, Winnie J, Maxwell B, Hamlin KL, Creel M. Elk alter habitat selection as an antipredator response to wolves. Ecology. 2005;86:3387–3397. [Google Scholar]
- 35.Fortin D, et al. Wolves influence elk movements: Behavior shapes a trophic cascade in Yellowstone National Park. Ecology. 2005;86:1320–1330. [Google Scholar]
- 36.Kauffman MJ, et al. Landscape heterogeneity shapes predation in a newly restored predatorprey system. Ecol Lett. 2007;10:690–700. doi: 10.1111/j.1461-0248.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 37.Christianson D, Creel S. A review of environmental factors affecting winter elk diets. J Wildl Manage. 2007;71:164–176. [Google Scholar]
- 38.Christianson D, Creel S. Effects of grass and browse consumption on winter mass dynamics of elk. Oecologia. 2009;158:603–613. doi: 10.1007/s00442-008-1200-1. [DOI] [PubMed] [Google Scholar]
- 39.Christianson D, Creel S. Risk effects in elk: Sex-specific response in grazing and browsing due to predation risk from wolves. Behav Ecol. 2008;19:1258–1266. [Google Scholar]
- 40.Christianson D, Creel S. A nutritionally-mediated risk effect of wolves on elk. Ecology. 2009 doi: 10.1890/09-0221.1. in press. [DOI] [PubMed] [Google Scholar]
- 41.Cook RC, Cook JG, Mech LD. Nutritional condition of northern Yellowstone elk. J Mammal. 2004;85:714–722. [Google Scholar]
- 42.Cook RC, Murray DL, Cook JG, Zager P, Monfort SL. Nutritional influences on breeding dynamics in elk. Can J Zool. 2001;79:845–853. [Google Scholar]
- 43.White PJ, Garrott RA. Yellowstone's ungulates after wolves—Expectations, realizations, and predictions. Biol Cons. 2005;125:141–152. [Google Scholar]
- 44.Beyer HL, Merrill EH, Varley N, Boyce MS. Willow on Yellowstone's northern range: Evidence for a trophic cascade? Ecol Appl. 2007;17:1563–1571. doi: 10.1890/06-1254.1. [DOI] [PubMed] [Google Scholar]
- 45.White PJ. 2007–2008 Annual Winter Trend Count of Northern Yellowstone Elk. Yellowstone National Park, WY: Yellowstone Center for Resources; 2008. [Google Scholar]
- 46.Hamlin KL. Montana Elk Management Plan. Helena, MT: Montana Fish, Wildlife & Parks; 2004. [Google Scholar]
- 47.Creel S, et al. Snowmobile activity and glucocorticoid stress responses in wolves and elk. Cons Biol. 2002;16:809–814. [Google Scholar]
- 48.Weiss JM. Somatic effects of predictable and unpredictable shock. Psychosom Med. 1970;32:397–408. doi: 10.1097/00006842-197007000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Breuner CW, Orchinik M. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J Endocrinol. 2002;175:99–112. doi: 10.1677/joe.0.1750099. [DOI] [PubMed] [Google Scholar]
- 50.Touma C, Palme R. Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann New York Acad Sci. 2005;1046:54–74. doi: 10.1196/annals.1343.006. [DOI] [PubMed] [Google Scholar]
- 51.Huber S, Palme R, Arnold W. Effects of season, sex, and sample collection on concentrations of fecal cortisol metabolites in red deer (Cervus elaphus) Gen Comp Endocrinol. 2003;130:48–54. doi: 10.1016/s0016-6480(02)00535-x. [DOI] [PubMed] [Google Scholar]
- 52.Millspaugh JJ, et al. Fecal glucocorticoid assays and the physiological stress response in elk. Wildl Soc Bull. 2001;29:899–907. [Google Scholar]
- 53.Schmitz OJ. Effects of predator hunting mode on grassland ecosystem function. Science. 2008;319:952–954. doi: 10.1126/science.1152355. [DOI] [PubMed] [Google Scholar]
- 54.Frank DA. The interactive effects of grazing ungulates and aboveground production on grassland diversity. Oecologia. 2005;143:629–634. doi: 10.1007/s00442-005-0019-2. [DOI] [PubMed] [Google Scholar]
- 55.Frank DA. Evidence for top predator control of a grazing ecosystem. Oikos. 2008;117:1718–1724. [Google Scholar]
- 56.Creel S, Winnie JA. Responses of elk herd size to fine-scale spatial and temporal variation in the risk of predation by wolves. Anim Behav. 2005;69:1181–1189. [Google Scholar]
- 57.Hebblewhite M, Pletscher DH. Effects of elk group size on predation by wolves. Can J Zool. 2002;80:800–809. [Google Scholar]
- 58.Hernandez L, Laundre JW. Foraging in the “landscape of fear” and its implications for habitat use and diet quality of elk Cervus elaphus and bison Bison bison. Wildl Biol. 2005;11:215–220. [Google Scholar]
- 59.Hebblewhite M, Pletscher D, Paquet PC. Elk population dynamics in areas with and without predation by recolonizing wolves in Banff National Park, Alberta. Can J Zool. 2002;80:789–799. [Google Scholar]
- 60.Barber SM, Mech LD, White PJ. Yellowstone elk calf mortality following wolf restoration: Bears remain top summer predators. Yellowstone Sci. 2005;13:37–44. [Google Scholar]
- 61.Brown JS, Laundre JW, Gurung M. The ecology of fear: Optimal foraging, game theory, and trophic interactions. J Mammal. 1999;80:385–399. [Google Scholar]
- 62.Relyea RA, Mills N. Predator-induced stress makes the pesticide carbaryl more deadly to gray treefrog tadpoles (Hyla versicolor) Proc Natl Acad Sci. 2001;98:2491–2496. doi: 10.1073/pnas.031076198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stoks R. Food stress and predator-induced stress shape developmental performance in a damselfly. Oecologia. 2001;127:222–229. doi: 10.1007/s004420000595. [DOI] [PubMed] [Google Scholar]
- 64.Brazda AR. Elk migration patterns, and some of the factors affecting movements in the Gallatin River drainage, Montana. J Wildl Manage. 1953;17:9–23. [Google Scholar]
- 65.Wasser SK, et al. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen Comp Endocrinol. 2000;120:260–275. doi: 10.1006/gcen.2000.7557. [DOI] [PubMed] [Google Scholar]
- 66.Garrott RA, Monfort SL, White PJ, Mashburn KL, Cook JG. One-sample pregnancy diagnosis in elk using fecal steroid metabolites. J Wildl Dis. 1998;34:126–131. doi: 10.7589/0090-3558-34.1.126. [DOI] [PubMed] [Google Scholar]