Abstract
Implantation is initiated when the embryo attaches to the uterine luminal epithelium during early pregnancy. Following this event, uterine stromal cells undergo steroid hormone-dependent transformation into morphologically and functionally distinct decidual cells in a unique process known as decidualization. An angiogenic network is also formed in the uterine stromal bed, critically supporting the early development of the embryo. The steroid-induced mechanisms that promote stromal differentiation and endothelial proliferation during decidualization are not fully understood. Although the role of ovarian progesterone as a key regulator of decidualization is well established, the requirement of ovarian estrogen (E) during this process remains unresolved. Here we show that the expression of P450 aromatase, a key enzyme that converts androgens to E, is markedly induced in mouse uterine stromal cells undergoing decidualization. The aromatase then acts in conjunction with other steroid biosynthetic enzymes present in the decidual tissue to support de novo synthesis of E. This locally produced E is able to support the advancement of the stromal differentiation program even in the absence ovarian E in an ovariectomized, progesterone-supplemented pregnant mouse model. Administration of letrozole, a specific aromatase inhibitor, to these mice blocked the stromal differentiation process. Gene expression profiling further revealed that the intrauterine E induces the expression of several stromal factors that promote neovascularization in the decidual tissue. Collectively, these studies identified the decidual uterus as a novel site of E biosynthesis and uncovered E-regulated maternal signaling pathways that critically control uterine differentiation and angiogenesis during early pregnancy.
Keywords: aromatase, endometrium, implantation
Implantation involves a series of complex interactions between the developing embryo and the uterus, leading to the establishment of pregnancy (1). Although the details of the implantation process vary among species, the basic features of blastocyst attachment and penetration of the uterine surface epithelium are common to many mammals. In mice, implantation is initiated 4 d after fertilization when the blastocyst reaches the uterus. The concerted actions of the steroid hormones estrogen (E) and progesterone (P) via their cognate receptors orchestrate the changes in the uterine tissue that make it competent to attach to the blastocyst and initiate the process of implantation.
In the mouse, an experimentally induced delayed implantation model provided the evidence that E plays an essential role in triggering the attachment of the embryo to the uterine luminal epithelium (2). In these mice, which have undergone ovariectomy on d 4 of gestation, implantation does not occur in the absence of ovarian E. Continued administration of P allows the blastocysts to remain viable within the uterus, but they fail to attach to the luminal epithelium in the absence of E. Administration of E to these ovariectomized pregnant mice allows attachment of the blastocyst trophectoderm to the luminal epithelium within 12 to 24 h, demonstrating a critical function of E-dependent signaling in initiating the implantation process (2).
When the embryo attaches itself to the uterine epithelium, the underlying stromal cells start to proliferate and then differentiate into unique decidual tissue that forms the implantation chamber. Paracrine factors secreted by the decidual cells are critical regulators of uterine remodeling, maternal immune response, uterine angiogenesis, and early embryonic growth. Decidualization is thus a prerequisite for successful implantation and establishment of pregnancy.
Previous studies established that P, acting via its receptor, plays a central role in regulating decidualization (3). In contrast, a functional requirement of E beyond the embryo attachment step remained ambiguous. An earlier study showed that administration of P alone to ovariectomized mice sustained the decidual response during experimentally induced decidualization, indicating a non-obligatory role of exogenous E in regulating this process (4). Conversely, it was reported that administration of ICI 182780, an E receptor (ER) antagonist, to mice severely impairs the formation of the decidual tissue, hinting that E acting via ER regulates this process (5). These apparently contradictory reports prompted us to further investigate the role of E in the uterus during decidualization.
In this study, we demonstrate that the decidual uterus is a novel site of de novo synthesis of E. The expression of P450 aromatase, a key enzyme that converts testosterone into E, is markedly induced in the pregnant mouse uterus during decidualization and plays an essential role in this process. Even in the absence of ovarian E, this locally synthesized E is able to support the advancement of the stromal differentiation program in an ovariectomized, P-supplemented pregnant mouse model. Our study also reveals that the intrauterine E acts by inducing the expression of several stromal factors critical for the formation of an extensive vascular network that supports the growth and development of the implanting embryo during early pregnancy.
Results
Ovarian E Is not Essential for Decidualization.
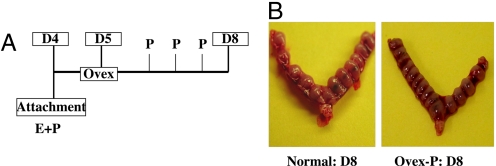
In the mouse, attachment of the embryo to the uterine epithelium occurs on d 4 of pregnancy (at midnight, i.e., 2400 h). This event initiates the process of decidualization, which proceeds through progressive phases during d 5 through 8 of gestation. To assess the role of ovarian E during decidualization, mice were ovariectomized on the morning (6 a.m.) of d 5, approximately 6 h following the attachment of the embryo to the uterine epithelium, and then treated with exogenous P for 3 consecutive days (Fig. 1A). We found that administration of P alone to the ovariectomized pregnant mice maintained the decidualization process, and the growth and development of the implanted embryos (Fig. 1B). In these P-treated ovariectomized uteri, the spatio-temporal expression of known markers of decidualization, such as progesterone receptor (PR) and prolactin-like protein type B (PLP-B), was found to be similar to that seen in normal pregnant uterus, indicating that P is sufficient to sustain decidualization in the absence of exogenous E (Fig. S1). Collectively, these results indicated that ovarian E is not essential for maintenance of decidual response.
Fig. 1.
Administration of exogenous P sustains decidualization in ovariectomized pregnant mice. (A) Experimental scheme. Pregnant mice were ovariectomized on the morning of d 5 (D5) following the attachment of the embryo to the uterine epithelium on d 4 at midnight. Mice were then treated with P (40 mg/kg body weight) for 3 d and uteri were collected on d 8 (D8). (B) Gross morphology indicating the implantation sites in normal D8 uterus (Left) and ovariectomized P-treated pregnant uterus (Right).
Evidence for Biosynthesis of E in the Decidual Uterus.
We next considered the possibility that production of E from an extra-ovarian source, such as the uterus itself, may contribute to the decidualization process. To investigate whether the uterus has the capacity to synthesize E de novo, we monitored the expression of various steroid biosynthetic enzymes in this tissue during early pregnancy. Total RNA was obtained from pregnant uteri on d 4 (morning) preceding implantation and on different days during the decidualization phase. The RNA samples were analyzed by coupled RT-PCR to monitor the expression of various steroidogenic factors. We observed prominent expression of StAR (steroidogenic acute regulatory protein), P450SCC (P450 side chain cleavage enzyme), P450C17 (17α-lyase), 3β-HSD (3β-hydroxysteroid dehydrogenase), and 17β-HSD-1 (17β-hydroxysteroid dehydrogenase type 1) in the pregnant uterus in both preimplantation (d 4 morning) and decidualization (d 6 and 7) stages (Fig. 2A). Interestingly P450 aromatase, the product of the CYP19 gene, which converts testosterone to biologically active E, exhibited markedly altered expression in pregnant uterus. The expression of aromatase mRNA was undetectable in the preimplantation uterus on d 4. However, a robust induction of this mRNA was observed in the decidual uterus on d 6 and 7 of pregnancy. Further analysis by Northern blotting confirmed that the expression of aromatase mRNA is initiated on d 5 of pregnancy and increases further on d 6 as decidualization progresses. It was diminished significantly on d 10 with the cessation of the decidual phase of gestation (Fig. S2).
Fig. 2.
Evidence for local E biosynthesis in decidual uterus. (A) Total uterine RNA obtained on d 4 (D4), d 6 (D6), and d 7 (D7) of pregnancy was analyzed by RT-PCR for the expression of StAR, P450SCC, P450C17, 3β-HSD, 17β-HSD-1, and P450 aromatase. (B) Top: Sections of uteri collected on d 4, 5, and 6 are shown before and after LCM. Bottom: Quantitation of the results of real-time PCR using gene-specific primers for aromatase and alkaline phosphatase. (C) Immunohistochemical analysis using the P450C17 antibody (Santa Cruz Biotechnology); sections of testes from male mice and uteri obtained from d 6 pregnant mice (panels a and b, respectively). Immunohistochemical analysis using the P450 aromatase antibody (Abcam); sections of ovaries collected 48 h after PMSG treatment and 16 h after human chorionic gonadotropin treatment (panels c and d, respectively), sections of uteri collected on d 4 (panel e), d 5 (panels f and g), and d 6 (panels h and i) of pregnancy. Panel j: Aromatase immunostaining in decidual cells when primary stromal cells isolated from uteri of d 4 pregnant mice were subjected to in vitro decidualization for 72 h. LE, S, D, E, LY, ST, G, F, CL, ESC, and AM denote luminal epithelium, stroma, decidua, embryo, Leydig cells, seminiferous tubule, granulosa cells, follicle, corpus luteum, endometrial stromal cells, and anti-mesometrial area, respectively.
To further establish that the decidual cells are the actual sites of aromatase mRNA expression during pregnancy, we performed laser capture microdissection (LCM) to isolate these cells from uterine sections. Total RNA was prepared from the excised tissue, and the expression of mRNAs corresponding to aromatase and alkaline phosphatase, a well established biomarker of decidual cells, was assessed by real-time PCR. A significant increase in the level of aromatase mRNA expression, relative to its level on d 4 of pregnancy, was observed in the stromal tissue excised from the anti-mesometrial region on d 5 of gestation (Fig. 2B). A dramatic increase (≈12 fold vs. d 4) in the level of both aromatase and alkaline phosphatase mRNAs was seen in the decidual cells collected from the anti-mesometrial area of uterine sections on d 6 of pregnancy. Collectively, these results confirmed that decidual cells are the actual sites of aromatase mRNA expression in the pregnant uterus.
We next examined the spatial expression of the P450C17 and aromatase proteins in normal pregnant mouse uterus using immunohistochemistry (Fig. 2C). The P450C17 antibody, as expected, showed specific immunostaining in the Leydig cells of testis (Fig. 2C, panel a). Probing of uterine sections on d 6 of pregnancy with this antibody revealed prominent expression of P450C17 in decidual cells surrounding the implanted embryo (Fig. 2C, panel b). The authenticity of the aromatase antibody was first confirmed by immunostaining of sections of ovaries obtained from mice treated with pregnant mare serum gonadotropin (PMSG; Fig. 2C, panel c). As expected, intense aromatase expression was observed in the granulosa cells of the ovarian follicles stimulated with PMSG. Sections of ovaries collected from mice treated with human chorionic gonadotropin, which induces follicular rupture, luteinization, and suppression of aromatase expression, showed very little aromatase-specific immunostaining (Fig. 2C, panel d). Immunohistochemical analysis of uterine sections showed no detectable aromatase immunostaining on d 4 (morning) of pregnancy before embryo attachment (Fig. 2C, panel e). However, on d 5 (Fig. 2C, panels f and g) and d 6 (Fig. 2C, panels h and i) of pregnancy, prominent expression of this enzyme was seen in decidualizing stromal cells at both anti-mesometrial and mesometrial regions surrounding the implanted embryo. Additionally, when primary stromal cells were isolated from pregnant uteri (pre-implantation stage, d 4) and subjected to steroid-induced decidualization in vitro, the cytoplasmic staining of aromatase was clearly evident in the decidual cells (Fig. 2C, panel j).
The finding that aromatase is expressed in the decidual uterus raised the possibility that this tissue acquires the ability to produce E locally as it undergoes differentiation. We therefore assessed the enzymatic activity of aromatase in the uterus during decidualization. Mice were ovariectomized on d 5 (morning) of pregnancy, and treated with P in the presence or absence of letrozole, a well known aromatase inhibitor. The uteri were collected on d 6 and tissue extracts were prepared. As shown in Table 1, extracts of d 6 pregnant uteri exhibited significant aromatase activity. Treatment of mice with letrozole resulted in a drastic reduction of this activity. We also determined the intrauterine levels of E (17β-estradiol) by RIA. A significant amount of E (12 pg/mL) was detected in the extracts of decidual uteri on d 6. When the ovariectomized pregnant mice were treated with letrozole, the E (17β-estradiol) level was undetectable in the uterine extracts. Taken together, these results confirmed that a functionally active aromatase enzyme is present in the decidual uterus and it catalyzes the production of E within this tissue.
Table 1.
Measurement of aromatase activity in the uterus during early pregnancy
| Tissue | [3H]water released, fmol/mg net wt/24 h | Estradiol, pg/mL of uterine extract |
|---|---|---|
| Uterus (d 6) | 24 ± 3.2 | 12.12 ± 3.22 |
| Uterus (d 6 + AI) | 8.7 ± 1.5 | Undetectable |
| Ovary | 45 ± 5.4 | Not analyzed |
The tissue homogenates were incubated with [1β-3H]androstenedione for 6 h at 37 °C to estimate the water release per milligram of tissue. The intrauterine levels of estrogen were analyzed by radioimmunoassay.
Uterine Aromatase Activity Is Critical for Successful Implantation.
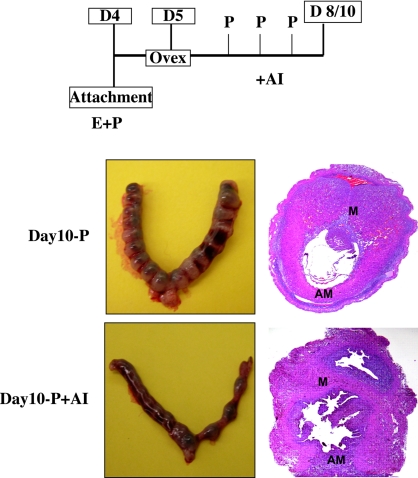
We next investigated whether the intrauterine E produced by aromatase is necessary for the maintenance of pregnancy. Mice were ovariectomized on d 5 (6 a.m.) of pregnancy and given daily injections of P in the presence or absence of letrozole. Uteri were collected on d 8 and 10 of pregnancy and analyzed for the presence of implanted embryos. Morphological and histological analyses of letrozole-treated and untreated uteri revealed that administration of this drug severely impaired embryo implantation. Inhibition of aromatase activity led to a significant reduction in decidual mass, and the majority of the implanted embryos failed to develop properly and were resorbed by d 10 (Fig. 3). This result indicated that the aromatase activity and local E production is essential for endometrial functions that support embryonic development during early pregnancy.
Fig. 3.
Blockade of aromatase function leads to loss of pregnancy. Pregnant mice (d 5 morning) were ovariectomized and treated with exogenous P in combination with or without letrozole, an aromatase inhibitor (AI). The uteri were collected on d 10 of gestation (D10). The sections of d 10 uteri treated with P only (Day10-P) or P and AI (D10-P+AI) were analyzed with eosin and hematoxylin stain. E, AM, and M denote embryo, anti-mesometrial area, and mesometrial area, respectively.
Inhibition of Uterine Aromatase Activity Blocks Stromal Differentiation.
To determine whether the locally produced E controls endometrial functions independent of embryonic development, we subjected mice to experimentally induced decidualization in which a mechanical stimulation of the steroid-primed uteri triggers a decidual response in the absence of the implanting embryo (6). This artificial stimulus mimics the embryonic signal during implantation and sets in motion the decidualization program.
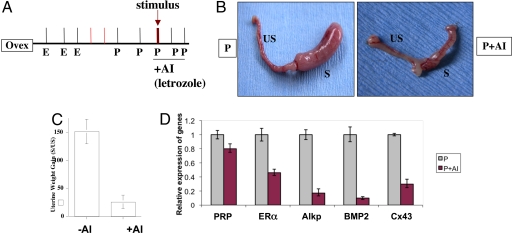
Following this uterine stimulation, the mice were treated with P alone or P plus letrozole for 3 consecutive days (Fig. 4A). Uterine response was assessed at 72 h following the decidual stimulation. As shown in Fig. 4B, the uterine horns of the P-treated animals exhibited a robust decidual response (Fig. 4B Left). In contrast, decidualization was severely compromised in mice that received P plus letrozole (Fig. 4B Right). Treatment with letrozole strongly inhibited the uterine wet weight gain, a classical hallmark of uterine decidual response (Fig. 4C).
Fig. 4.
Inhibition of aromatase activity impairs uterine decidualization. (A) The hormonal regimen used in the artificial decidualization protocol is shown. Mice were treated with or without letrozole (20 mg/kg body weight). Uteri were collected 72 h after the application of stimulus. (B) The extent of decidual response in ovariectomized mice treated with P (Left) and P plus letrozole (P+AI, Right) is shown. s and us denote stimulated and unstimulated uterine horns, respectively. (C) The quantitative analysis of the average weight gain of stimulated relative to unstimulated horns in mice (n = 5) subjected to artificial decidualization with or without letrozole treatment. The data are presented as mean ± SEM. (D) Uterine RNA was isolated 72 h after the initiation of decidualization and subjected to quantitative PCR analysis using gene-specific primers for ERα, alkaline phosphatase (Alk), BMP2, connexin 43 (Cx43), and PRP. P and P+AI represent uterine RNA from ovariectomized mice treated with P and P plus letrozole, respectively.
We further assessed the impact of the loss of aromatase activity on decidual response by monitoring the uterine expression of alkaline phosphatase and decidual prolactin-related protein (PRP) (7), well established biochemical markers of uterine stromal differentiation, in the presence or absence of letrozole. We also examined the expression of 2 additional factors: bone morphogenetic protein 2 (BMP2), a morphogen, and connexin 43 (Cx43), a gap junction protein, which are induced in stromal cells during decidualization and are known to play critical regulatory roles during this process (6, 8, 9). We found that the expression of mRNAs encoding alkaline phosphatase, PRP, BMP2, and Cx43 was markedly reduced in the letrozole-treated uteri (Fig. 4D). Consistent with these results, we observed a drastic reduction in the intensity of Cx43 immunostaining in the uterine sections of letrozole-treated mice (Fig. S3). In contrast, the expression of ERα mRNA was not significantly altered in response to the inhibitor. Taken together, these results indicated that aromatase-driven intrauterine E synthesis plays an important regulatory role in stromal cell differentiation.
Local E Synthesis Is Critical for Uterine Angiogenesis.
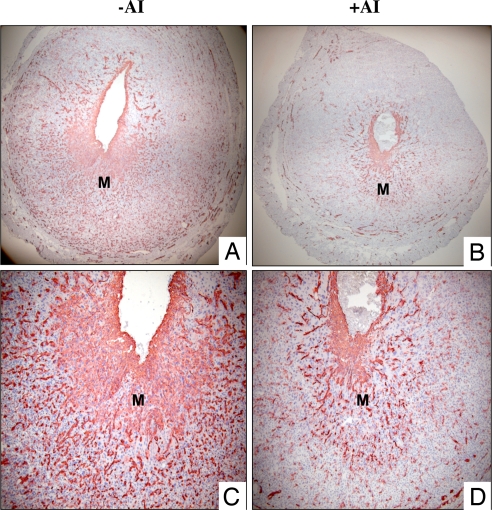
An extensive network of new blood vessels, which support the growth of the implanted embryos, is formed during the decidualization process. Consistent with this scenario, an intense immunostaining of platelet/endothelial cell adhesion molecule (PECAM), an endothelial cell-specific marker, was seen in the stromal compartment of uteri subjected to experimentally induced decidualization. Strikingly, the PECAM staining was markedly diminished in the uteri of letrozole-treated animals, indicating that the expansion of the uterine angiogenic network is impaired in the absence of local E production (Fig. 5).
Fig. 5.
Local production of E regulates uterine neovascularization during decidualization. Mice were subjected to artificial decidual stimulation in the presence or absence of letrozole. Uteri were collected at 72 h following the decidual stimulation and frozen tissue sections were subjected to immunohistochemical analysis using an antibody specific for PECAM-1. (A and C) PECAM-1 expression in uteri of mice without letrozole treatment. (B and D) PECAM-1 expression in uteri of mice treated with letrozole. M, mesometrial area.
To investigate whether the locally produced E controls the expression of factors that promote angiogenesis during decidualization, we performed gene expression profiling of uterine tissue in the presence or absence of letrozole. Uteri were collected from control or letrozole-treated mice at 72 h following administration of the decidual stimulus. Total RNA was isolated from these tissues and subjected to microarray analysis using Affymetrix murine GeneChip arrays. Our study identified many genes whose expression was significantly altered in the uterus in response to letrozole. The expression of 360 genes was up-regulated and that of 440 genes was down-regulated (>1.5-fold) in response to letrozole treatment.
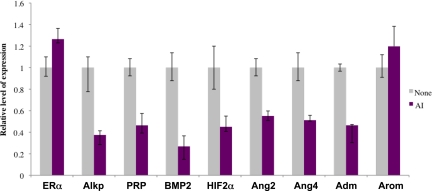
A prominent biological category among the products of the down-regulated genes represented factors known to control endothelial cell function at distinct phases of angiogenesis. These factors included angiopoietin 2, angiopoietin 4, and adrenomedullin, which are known promoters of angiogenesis, and HIF2α, a transcription factor that regulates the expression of the VEGF. To ascertain that the observed letrozole-induced alterations in the expression of these angiogenic factors is indeed caused by the inhibition of aromatase activity in the stromal cells, we used a well established in vitro decidualization system (9). Primary cultures of stromal cells isolated from pregnant uteri (pre-implantation, d 4) were subjected to P-induced decidualization in the absence or presence of letrozole. As shown in Fig. 6, the differentiation of uterine stromal cells to decidual cells was severely inhibited in the presence of letrozole as indicated by the greatly reduced expression of alkaline phosphatase, PRP, and BMP2. We also observed a concomitant decrease in the expression of HIF2α, angiopoietin 2, angiopoietin 4, and adrenomedullin in the letrozole-treated stromal cells. The expression of ERα and aromatase was not altered in response to letrozole. These results are consistent with our hypothesis that the locally produced E is critical for the progression of the stromal differentiation program, which allows the synthesis of factors that are likely mediators of angiogenesis in the decidual uterus.
Fig. 6.
Identification of angiogenic factors regulated by intrauterine E during decidualization. Stromal cells isolated from uteri of d 4 pregnant mice were subjected to in vitro decidualization in the presence or absence of letrozole for 72 h. RNA was prepared from these cells and subjected to real-time PCR analysis using gene-specific primers to assess the expression of ERα, aromatase (arom), alkaline phosphatase (Alkp), PRP, BMP2, and angiogenic regulators: HIF2α, angiopoietin 2 (Ang-2), angiopoietin 4 (Ang-4), and adrenomedullin (adm).
Discussion
In cycling rodents, the circulating E and P are produced and secreted by the concerted actions of ovarian granulosa and theca cells (10). The adrenal glands in rodents are incapable of synthesizing significant levels of these steroid hormones (11). In pregnant mice, the corpora lutea develop fully by d 3 of pregnancy and start to produce P, the level of which increases and remains elevated until mid-gestation (12). In contrast, the level of serum E, which is high on d 1 of pregnancy because of the preovulatory hormonal surge, decreases and reaches approximately 15 pg/mL on d 2 and 3. The serum E level increases transiently to approximately 22 pg/mL on d 4 of pregnancy and plays a critical role in embryo attachment. When the embryo attaches to the uterus, the ovarian E synthesis decreases. During the decidualization phase, which lasts from d 5 through 8 of gestation, the circulating level of E remains low at approximately 15 pg/mL (12). When the ovaries were removed after embryo attachment, administration of exogenous P was found to be sufficient to sustain decidualization in pregnant mice (Fig. 1). The ovarian E, therefore, has no evident role in regulating uterine functions following the embryo attachment step. The present study reveals that the mouse uterus is able to carry out de novo synthesis of E during decidualization, and it is this locally produced hormone, not the ovarian E, that critically supports the stromal differentiation process.
It was previously documented that, during early pregnancy, mouse endometrial stromal cells acquire the ability to express the steroidogenic enzymes required for the synthesis of P starting from cholesterol (13). These factors are StAR, P450scc, and 3β-HSD. The maximal activity of 3β-HSD, which catalyzes the conversion of pregnenolone to P, was detected in decidual tissue on d 6.5 to 7.5 of pregnancy (14). Although these earlier reports provided evidence for the potential de novo production of P in the decidua, the physiological significance of this locally produced hormone remained unclear in the face of high serum levels of P originating from the corpora lutea during early phases of pregnancy. The expression of 17β-HSD in the decidual tissue was also described previously (15). Our present study extends these earlier observations to demonstrate that the decidua expresses additional steroidogenic enzymes P450C17 and P450 aromatase. Therefore, a full complement of steroidogenic enzymes is expressed in the decidual tissue, which allows conversion of P to the androgenic precursors and their eventual aromatization to E.
The P450 aromatase, encoded by the CYP19 gene, is the key enzyme that catalyzes the conversion of C19 steroids to E (16, 17). Previous studies have shown that E is synthesized in a number of extragonadal sites such as breast, brain, and bone (18). This extragonadal E acts locally within the tissue in a paracrine or intracrine fashion. Although only a small amount of E is synthesized at these extragonadal sites, it is possible to attain high local concentrations of the hormone, which then exerts important biological effects within the tissue. It is noteworthy that aromatase expression in the pregnant uterus is initiated in the decidua on d 5 of gestation, immediately following implantation, and continues to increase as the stromal differentiation program advances during d 6 and 7. Our study documents the expression of a functionally active aromatase in the maternal decidua. As the ovarian E secretion decreases to very low levels during decidualization, the local production of this hormone in the decidual tissue at the implantation sites assumes high physiological significance.
Previous studies in aromatase-null mice provided important insights into the role of E in various reproductive tissues. As expected, the homozygous mutant females were infertile (19). Histology of the reproductive tract of these mice demonstrated evidences of follicular depletion and the presence of hemorrhagic follicles in the ovaries, and diminution of uterine weight. Supplementation with E rescued the development of ovarian follicles and allowed the recovery of uterine weight, but did not ameliorate the reproductive failure in the mutant females (20). Interestingly, transplantation of WT ovaries into aromatase-null female mice, which produces a circulating hormonal profile similar to that in WT mice, resulted in only a poor rescue of the pregnancy outcome (21). These results hinted at additional reproductive abnormalities in the mutant females, presumably at the level of the uterus, and are consistent with our current findings demonstrating a critical functional role of local E produced by uterine aromatase during implantation.
Our study revealed that the aromatase-driven intrauterine E plays an important role in decidualization. The expression of alkaline phosphatase and PRP, 2 well characterized bio-markers of decidual response, was compromised when ovariectomized pregnant mice were treated with letrozole (7, 9). Furthermore, the expressions of BMP2 and Cx43, critical regulators of stromal differentiation, were severely reduced in the presence of letrozole, indicating that decidualization is impaired when this inhibitor blocks aromatase activity. Previous studies reported that conditional ablation of BMP2 expression in the mouse uterus leads to infertility resulting from lack of decidualization (8). Recently we have shown that conditional loss of expression of Cx43, a major gap junction component in uterine stromal cells, impairs decidualization and angiogenesis during early pregnancy (6). The uterine expression of Cx43 is regulated by E (22). It is therefore conceivable that the local E produced by the aromatase in the decidua controls Cx43 expression, which in turn contributes to the progression of decidualization by promoting gap junction communication between the stromal cells.
Another major finding of this study is that de novo production of E plays a central role in the regulation of uterine neovascularization during early pregnancy. Although a functional link between steroid hormone action and uterine angiogenesis in rodents has long been speculated, the precise nature of this regulation remained unclear. The establishment and remodeling of blood vessels during angiogenesis requires the coordinated execution of a series of cellular processes (23, 24). Endothelial cells migrate from the parent vessel and proliferate in response to one or more paracrine signal(s), resulting in the formation of nascent capillary sprouts (25). In the context of the pregnant uterus, critical angiogenic signals are likely to be produced by the decidualizing stromal cells to act on the endothelial cells to promote their proliferation and differentiation. Our study suggested that the local E production in the stromal cells facilitates the decidualization process, which in turn promotes the synthesis and secretion of important angiogenic factors that support the expansion of the endothelial cell network in the stromal bed.
Consistent with this hypothesis, we have identified a number of stromal factors that are induced in response to aromatase-derived E and are likely regulators of neovascularization in the decidua. Our study showed that the intrauterine E controls the stromal expression of HIF2α, a transcription factor that regulates VEGF production (26). VEGF is a potent mitogen for endothelial cells, and is a prime regulator of angiogenesis during implantation and decidualization (27, 28). In many tissues, VEGF acts in concert with angiopoietins to regulate angiogenesis (28, 29). Whereas angiopoietin 2 collaborates with VEGF to advance the invasion by the vascular sprouts and promote vascular remodeling, angiopoietin 4 serves an important function during endothelial cell migration (30). We present evidence that aromatase-driven E controls the expression of both angiopoietin 2 and angiopoietin 4 in uterine stromal cells. The stromal expression of adrenomedullin, a factor involved in angiogenesis and important regulator of uterine function during implantation (31), is also controlled by the E generated in the uterus. The dramatic impairment in the formation of new blood vessels in the pregnant uteri in response to letrozole can be explained by the lack of expression of these angiogenic factors in the stromal cells. Our study therefore uncovers important pathways regulated by local E signaling to promote the establishment of new vascular structures within the decidual tissue. This angiogenic role of intrauterine E is essential for proper embryonic growth and development during critical phases of early pregnancy.
Materials and Methods
Animals and Tissue Collection.
Mice (CD-1) were ovariectomized on d 5 (morning) of pregnancy and injected daily with P or P in combination with letrozole (20 mg/kg body weight) from d 5 through 8. Mice were killed 6 h after the last hormone injection and uteri were isolated. Experimental procedures for decidualization, laser capture microdissection, microarray analysis, real-time PCR, immunohistochemistry, and assay of aromatase activity are provided in SI Methods.
Estradiol Measurement in Uterine Homogenates.
Each uterine tissue (≈25 mg) was homogenized in 1 mL of an extraction buffer. The measurements of estradiol in uterine homogenates were performed by the Center for Research in Reproduction Ligand Assay and Analysis Core of the University of Virginia.
Supplementary Material
Acknowledgments.
We thank Dr. Quanxi Li for help with the microarray analysis. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)/National Institutes of Health (NIH) through grant U54 HD055787 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, and also by NIH grant R01 HD 43381.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901647106/DCSupplemental.
References
- 1.Carson DD, et al. Embryo implantation. Dev Biol. 2000;223:217–237. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- 2.Yoshinaga K, Adams CE. Delayed implantation in the spayed, progesterone treated adult mouse. JReprod Fertil. 1966;12:593–595. doi: 10.1530/jrf.0.0120593. [DOI] [PubMed] [Google Scholar]
- 3.Lydon JP, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9(18):2266–22787. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 4.Paria BC, Tan J, Lubahn DB, Dey SK, Das SK. Uterine decidual response occurs in estrogen receptor-alpha-deficient mice. Endocrinology. 1999;140:2704–2710. doi: 10.1210/endo.140.6.6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtis SW, Clark J, Myers P, Korach KS. Disruption of estrogen signaling does not prevent progesterone action in the estrogen receptor alpha knockout mouse uterus. Proc Natl Acad Sci USA. 1999;96:3646–3651. doi: 10.1073/pnas.96.7.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laws MJ, et al. IC gap junctions between uterine stromal cells play a critical role in pregnancy-associated neovascularization and embryo survival. Development. 2008;135:2659–2668. doi: 10.1242/dev.019810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orwig KE, Ishimura R, Muller H, Liu B, Soares MJ. Decidual/trophoblast prolactin-related protein: characterization of gene structure and cell-specific expression. Endocrinology. 1997;138:5511–5517. doi: 10.1210/endo.138.12.5628. [DOI] [PubMed] [Google Scholar]
- 8.Lee KY, et al. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27:5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, et al. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem. 2007;282:31725–31732. doi: 10.1074/jbc.M704723200. [DOI] [PubMed] [Google Scholar]
- 10.Gibori G, Rodway R, Rothchild I. The luteotrophic effect of estrogen in the rat: prevention by estradiol of the luteolytic effect of an antiserum to luteinizing hormone in the pregnant rat. Endocrinology. 1977;101:1683–1689. doi: 10.1210/endo-101-6-1683. [DOI] [PubMed] [Google Scholar]
- 11.Gibori G, Sridaran R. Sites of androgen and estradiol production in the second half of pregnancy in the rat. Biol Reprod. 1981;24:249–256. doi: 10.1095/biolreprod24.2.249. [DOI] [PubMed] [Google Scholar]
- 12.McCormack JT, Greenwald GS. Progesterone and oestradiol-17beta concentrations in the peripheral plasma during pregnancy in the mouse. J Endocrinol. 1974;62:101–107. doi: 10.1677/joe.0.0620101. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Zimra M, et al. Uterine and placental expression of steroidogenic genes during rodent pregnancy. Mol Cell Endocrinol. 2002;187:223–231. doi: 10.1016/s0303-7207(01)00713-4. [DOI] [PubMed] [Google Scholar]
- 14.Peng L, Arensburg J, Orly J, Payne AH. The murine 3beta-hydroxysteroid dehydrogenase (3beta-HSD) gene family: a postulated role for 3beta-HSD VI during early pregnancy. Mol Cell Endocrinol. 2002;187:213–221. doi: 10.1016/s0303-7207(01)00689-x. [DOI] [PubMed] [Google Scholar]
- 15.Nokelainen P, Peltoketo H, Mustonen M, Vihko P. Expression of mouse 17beta-hydroxysteroid dehydrogenase/17-ketosteroid reductase type 7 in the ovary, uterus, and placenta: localization from implantation to late pregnancy. Endocrinology. 2000;141:772–778. doi: 10.1210/endo.141.2.7309. [DOI] [PubMed] [Google Scholar]
- 16.Bulun SE, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57:359–383. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- 17.Simpson ER, et al. Estrogen-the good, the bad, and the unexpected. Endocr Rev. 2005;26:322–330. doi: 10.1210/er.2004-0020. [DOI] [PubMed] [Google Scholar]
- 18.Simpson ER, et al. Aromatase–a brief overview. Annu Rev Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- 19.Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toda K, et al. Targeted disruption of the aromatase P450 gene (Cyp19) in mice and their ovarian and uterine responses to 17beta-oestradiol. J Endocrinol. 2001;170:99–111. doi: 10.1677/joe.0.1700099. [DOI] [PubMed] [Google Scholar]
- 21.Toda K, et al. Aromatase-knockout mouse carrying an estrogen-inducible enhanced green fluorescent protein gene facilitates detection of estrogen actions in vivo. Endocrinology. 2004;145:1880–1888. doi: 10.1210/en.2003-0952. [DOI] [PubMed] [Google Scholar]
- 22.Grummer R, Hewitt SW, Traub O, Korach KS, Winterhager E. Different regulatory pathways of endometrial connexin expression: preimplantation hormonal-mediated pathway versus embryo implantation-initiated pathway. Biol Reprod. 2004;71:273–281. doi: 10.1095/biolreprod.103.024067. [DOI] [PubMed] [Google Scholar]
- 23.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 24.Bryan BA, D'Amore PA. What tangled webs they weave: Rho-GTPase control of angiogenesis. Cell Mol Life Sci. 2007;64:2053–2065. doi: 10.1007/s00018-007-7008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hellstrom M, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:76–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 26.Ema M, et al. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakraborty I, Das SK, Dey SK. Differential expression of vascular endothelial growth factor and its receptor mRNAs in the mouse uterus around the time of implantation. J Endocrinol. 1995;147:339–352. doi: 10.1677/joe.0.1470339. [DOI] [PubMed] [Google Scholar]
- 28.Hess AP, et al. Angiopoietin-1 and −2 mRNA and protein expression in mouse preimplantation embryos and uteri suggests a role in angiogenesis during implantation. Reprod Fertil Dev. 2006;18:509–516. doi: 10.1071/rd05110. [DOI] [PubMed] [Google Scholar]
- 29.Gale NW, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 30.Lee HJ, et al. Biological characterization of angiopoietin-3 and angiopoietin-4. FASEB J. 2004;18:1200–1208. doi: 10.1096/fj.03-1466com. [DOI] [PubMed] [Google Scholar]
- 31.Li M, Yee D, Magnuson TR, Smithies O, Caron KM. Reduced maternal expression of adrenomedullin disrupts fertility, placentation, and fetal growth in mice. J Clin Invest. 2006;116:2653–2662. doi: 10.1172/JCI28462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.