APPLIED PHYSICAL SCIENCES, BIOPHYSICS AND COMPUTATIONAL BIOLOGY Correction for “Global distribution of conformational states derived from redundant models in the PDB points to non-uniqueness of the protein structure,” by Prasad V. Burra, Ying Zhang, Adam Godzik, and Boguslaw Stec, which appeared in issue 26, June 30, 2009, of Proc Natl Acad Sci USA (106:10505–10510; first published June 24, 2009; 10.1073/pnas.0812152106).
The authors note that due to a printer's error, the legends for Figs. 5 and 6 appeared incorrectly. The figures and their corrected legends appear below. In addition, the author name Boguslaw Stec appeared without an affiliation designation. The corrected author line, the affiliation line, and the related footnote appear below.
Fig. 5.
An example of the 25 amino acids sliding window RMSD distribution in cluster 633 (diphtheria toxin). (A) Dots represent all individual 25 aa RMSDs. (B) Three examples of models with conformational states showing small (blue), intermediate (yellow), and large (red) divergence.
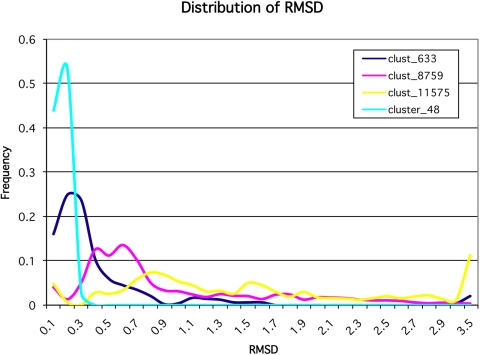
Fig. 6.
Distribution of normalized frequency for 25 aa sliding window RMSDs with all proteins from Fig. S2. The shift in the maximum represents a variable level of rigidity among the proteins with transhydroxylase (light blue) the most rigid, diphtheria toxin (dark blue) and calmodulin (purple) intermediate, and apolipoprotein A (yellow) the most flexible. Change in the distribution shows varied contribution between rigid (solid) and mobile (liquid) components to the individual structure.
Prasad V. Burraaa, Ying Zhangbb, Adam Godzikbb, and Boguslaw Stecb,1
aDepartment of Bioengineering, University of California at San Diego, La Jolla, CA 92037; and bBurnham Institute for Medical Research, La Jolla, CA 92037
Footnotes
To whom correspondence should be addressed. E-mail: bstec@burnham.org.