Abstract
Traumatic spinal cord injury is characterized by an immediate, irreversible loss of tissue at the lesion site, as well as a secondary expansion of tissue damage over time. Although secondary injury should, in principle, be preventable, no effective treatment options currently exist for patients with acute spinal cord injury (SCI). Excessive release of ATP by the traumatized tissue, followed by activation of high-affinity P2X7 receptors, has previously been implicated in secondary injury, but no clinically relevant strategy by which to antagonize P2X7 receptors has yet, to the best of our knowledge, been reported. Here we have tested the neuroprotective effects of a systemically administered P2X7R antagonist, Brilliant blue G (BBG), in a weight-drop model of thoracic SCI in rats. Administration of BBG 15 min after injury reduced spinal cord anatomic damage and improved motor recovery without evident toxicity. Moreover, BBG treatment directly reduced local activation of astrocytes and microglia, as well as neutrophil infiltration. These observations suggest that BBG not only protected spinal cord neurons from purinergic excitotoxicity, but also reduced local inflammatory responses. Importantly, BBG is a derivative of a commonly used blue food color (FD&C blue No. 1), which crosses the blood–brain barrier. Systemic administration of BBG may thus comprise a readily feasible approach by which to treat traumatic SCI in humans.
Keywords: astrocytes, inflammation, microglia, motor neurons, purinergic signaling
ATP has been implicated in acute and chronic neuropathic pain and inflammation (1, 2) and it is released in large amounts after tissue injury. In the setting of spinal cord injury (SCI), ATP release is increased in peritraumatic areas for >6 h (3). Because tissue damage is progressively worsened by events that occur after the primary traumatic event, therapeutic interventions that minimize such secondary injury are of considerable clinical interest.
Among the ATP-sensitive purinergic receptors, the P2X7 receptor (P2X7R) is unusual in that it can form a large, macromolecular pore upon repetitive or prolonged exposure to high concentrations of ATP (4). This receptor is particularly important in the context of SCI, because it is abundantly expressed by spinal cord neurons (3). Spinal cord neurons respond to ATP with excessive firing followed by irreversible increases in Ca2+, and ultimately cell death. All of these events may be prevented by P2X7R antagonists (3). To test the idea that the blockade of purinergic receptors might improve the outcome of SCI, we had previously shown that intraspinal injection of the P2X7R antagonist OxATP in the peritraumatic zone reduced spinal cord damage (3). Not only did OxATP-mediated P2X7R inhibition reduce the loss of motor neurons, but it also promoted subsequent functional recovery in the lesioned animals.
Inflammatory responses contribute to the late irreversible loss of peritraumatic tissue after SCI (5). P2X7 receptors were first discovered in leukocytes, and P2X7R activation leads to production and release of interleukins and other cytokines, to the activation of matrix metalloproteinase-9, and to cell death via caspase activation. In addition, microglial cells express P2X7R (6), and P2X7R expression is increased in activated microglia in pathologies as diverse as amyotrophic lateral sclerosis, ischemic cortical injury, kainate-induced seizures, and Alzheimer disease.
These observations suggest that microglial and leukocytic P2X7 is a potential target for limiting inflammatory responses to SCI. In fact, P2X7R antagonists may potentially reduce secondary damage after SCI, both by directly inhibiting excitatory neuronal damage and by reducing both local and systemic inflammatory responses to the traumatic event.
Although our previous data established a proof of principle that P2X7R antagonists can reduce the severity of acute SCI, the inability of OxATP to cross the blood–brain barrier, as well as its cardiovascular toxicity, seriously limited the clinical utility of this compound (7). On investigating potential alternatives to OxATP, we found that a commonly used food additive, FD&C blue dye No. 1 (or brilliant blue FCF), is both structurally and functionally analogous to a highly selective P2X7R antagonist. FD&C blue dye No. 1 is a synthetic dye approved by the Food and Drug Administration as a food additive. FD&C blue dye No. 1 is regarded as one of the safest dyes in current use, with no toxicity at doses up to 12 mg/kg per day in healthy animals (8, 9).
An analog of FD&C blue dye No. 1, Brilliant Blue G (BBG), is a commonly used selective P2X7R antagonist. The low toxicity (10) and high selectivity of BBG (11) make this compound an ideal candidate for blocking the potential adverse effect of P2X7R activation in peritraumatic regions after SCI. The prospect of administering a neuroprotective drug with no known adverse effects is particularly attractive in the setting of acute traumatic injury. In this article, we explore the therapeutic benefit of BBG in a rat model of SCI. We report that BBG reduces spinal cord damage and improves motor recovery by directly reducing activation of microglia and astrocytes, as well as neutrophil infiltration in the peritraumatic area.
Results
BBG Administered Intravenously Accumulates in the Area of the Spinal Cord Lesion.
To evaluate the neuroprotective action of BBG, we evaluated functional motor recovery as well as histological injury following weight-drop injury of the thoracic spinal cord in rats (Fig. 1). We first assessed the toxicity and blood–brain barrier permeability of BBG. On the basis of prior reports that no adverse effects were observed after oral administration of 1,000 mg/kg per day of FD&C Blue No. 1 (12) and that intestinal absorption is ≈5% (13), we administered 2 doses of BBG, 10 or 50 mg/kg per day, immediately after injury and for 3 consecutive days. To ensure rapid delivery of the P2X7 antagonist to the traumatized tissue, BBG was given intravenously. The albino rats used in the study exhibited noticeable blue coloring of the eyes after receiving either dose of BBG. Rats treated with 50 mg/kg also showed coloring of the skin, which slowly subsided over the course of 1 week (Fig. 2A). Initial studies showed that neither regime of BBG administration had effects on behavior, weight, survival, or other physiological parameters, including body temperature, blood pH, blood gases, or blood pressure.
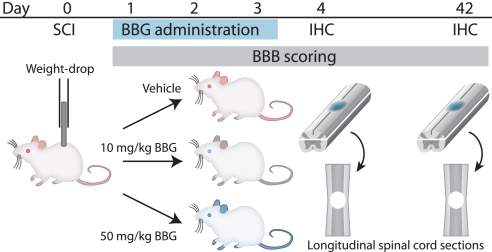
Fig. 1.
Schematic diagram of the experimental design. Spinal cord injury in rats was inflicted by a weight-drop impact (10 g weight dropped from a height of 12.5 mm at T11–T12). Vehicle or BBG at 10 or 50 mg/kg was administered intravenously right after injury and once daily on days 2 and 3. Motor function was evaluated in a first group of rats from days 1–42. Tissue injury was evaluated on longitudinal sections of spinal cord prepared at day 42. Acute inflammatory responses were quantified in a second group of rats processed for immunohistochemistry at day 4.
Fig. 2.
BBG accumulates in the lesion area following spinal cord injury in rats. (A) A rat injected with BBG (50 mg/kg) exhibits blue coloration of the eye and skin 3 days later. The blue color fades slowly and is not noticeable after 1 week. A vehicle-injected rat is shown for comparison. (B) Quantification of tissue BBG concentrations at the injury site and distant area. BBG accumulates in the lesion, but is detectable in the uninjured tissue in rats that received 50 mg/kg. *, P < 0.05; **, P < 0.01; two-way ANOVA with Tukey-Kramer test; n = 9 rats per group. Error bars indicate SEM.
To obtain an objective measure of the blood–brain barrier permeability of BBG in the setting of SCI, BBG was quantified in the lesion area as well as in uninjured tissue located >2 mm from the lesion border. In rats receiving 10 mg/kg, BBG averaged 9.94 ± 8.32 μM within contused spinal cord tissue (Fig. 2B). In rats that received 50 mg/kg of BBG, a mean concentration of 43.59 ± 14.64 μM BBG was achieved in the lesion 3 days later, whereas surrounding tissue contained lower but still detectable levels (0.92 ± 2.21 μM) (Fig. 2B). These values reflect the total tissue content of BBG; it is likely that the concentration of free BBG is lower due to the high binding affinity of BBG for proteins, as is characteristic for all Coomasie dyes (14). Nevertheless, BBG outside the lesion was minimal, indicating that BBG primarily entered the lesion via the disrupted blood–spinal cord barrier.
BBG Improves Behavioral Recovery and Reduces the Size of the Lesion.
Motor behavior was assessed in open-field testing by using the 21-point Basso, Beattie, and Bresnahan (BBB) locomotor rating scale after traumatic SCI in both vehicle- and BBG-treated animals (15, 3). Functional recovery was evaluated daily for the first 3 days, and twice weekly for the next 6 weeks. All animals developed complete paraplegia after the traumatic SCI injury, corresponding to a BBB score of 0–1, but exhibited modest improvements of motor function as early as days 2–3 after injury. Recovery then proceeded relatively rapidly during the first 13 days, and continued at a slower pace thereafter (Fig. 3A).
Fig. 3.
BBG (10 or 50 mg/kg) significantly improves motor function and reduces tissue loss following traumatic spinal cord damage. (A) The graph depicts the BBB scores for locomotor function, which was evaluated immediately after injury and every 2–3 days thereafter. BBG significantly improved hindlimb motor function from day 13 after injury and onwards. Improvement persisted until 6 weeks postinjury. *, P < 0.05; * *, P < 0.01 compared with vehicle; one-way ANOVA with Dunnett's test. n = 11–17 rats per group. Error bars indicate SEM. (B) Longitudinal spinal cord sections stained with Luxol Fast Blue/Cresyl Violet display how the area of tissue injury and atrophy was quantified. The injury area exhibits less vacuolation and cell death when treated with 10 or 50 mg/kg BBG. (C) The bar histogram compares the loss of tissue following traumatic injury (the sum of the volumes of tissue injury and atrophy) in control vehicle or BBG treated animals. BBG at 10 mg/kg reduced the loss of tissue relatively more than 50 mg/kg. *, P < 0.05; * *, P < 0.01; compared to control volume, one-way ANOVA with Tukey-Kramer test. n = 11–17 rats per group. Error bars indicate SEM. (D) Whole perfusion-fixed spinal cords at 6 weeks. BBG (blue color) was deposited in the injury area in rats treated with BBG.
Throughout the entire recovery period, animals from the 2 BBG-treated groups recovered motor function faster than the vehicle-treated controls. This difference reached statistical significance at day 10, with BBG-treated rats scoring 6.3–6.6 on the BBB scale, whereas nontreated rats were >2 points lower (P < 0.05, ANOVA). At day 42, the BBB scores were 11.7 ± 0.3 and 11.9 ± 0.5 in rats receiving 10 and 50 mg/kg BBG, respectively. The best-performing of the BBG-treated animals exhibited both forelimb–hindlimb coordination and consistent weight-supported plantar steps (Fig. 3A). In contrast, at 42 days after spinal contusion, vehicle-treated animals scored significantly worse (9.4 ± 0.3, P < 0.01, ANOVA). Functionally, essentially none of the injured control rats displayed forelimb–hindlimb coordination, and only occasionally did they manifest weight-supported plantar steps. Thus, BBG significantly improved the recovery of hindlimb motor function, and the differences persisted to the last day of the study.
Lesion volume was then assessed in Luxol Fast Blue/cresyl violet-stained spinal cord sections sampled serially in the longitudinal plane. The lesions often contained vacuoles typical of traumatic injury in rats (Fig. 3B), which exhibited complete loss of myelinated fibers, neurons, oligodendrocytes, and astrocytes within the lesion. Because the posttraumatic spinal cord showed considerable atrophy, we calculated the total lesion as the sum of the lesion volume plus the volume of the atrophic area.
Administration of either 10 or 50 mg/kg BBG caused a significant reduction in posttraumatic spinal cord tissue loss, as shown in Fig. 3 C and D. At 6 weeks BBG was still clearly visible in the spinal cord lesion in rats treated with 50 mg/kg, whereas the blue color was less prominent in rats that received 10 mg/kg BBG. Interestingly, BBG had a more profound suppressive effect on atrophy than on lesion size, consistent with the idea that the primary target of BBG is to limit secondary injury.
BBG Reduces Microglial Activation and Reactive Gliosis in the Injured Spinal Cord.
Resting microglia are characteristically small cells with long, ramified processes. To evaluate the impact of BBG administration on microglial activation following SCI, we first used immunohistochemistry to quantify Iba1 expression. The Iba1 antigen is involved in motile properties of cells of the macrophage/microglial lineage, and is expressed by quiescent as well as activated microglia (16). Iba1 immunolabeling revealed that microglia in the BBG-treated group exhibited longer processes than in the vehicle group at 4 days and 6 weeks postinjury (Fig. 4B). Moreover, when the number of activated microglial cells was quantified by immunodetection of CD68+ microglia, a clear reduction in the number of CD68+ cells in the peritraumatic area was observed at 4 days after BBG treatment (P < 0.01 for both BBG concentrations, Fig. 4B). The BBG-induced suppression of CD68+ cells persisted throughout the first 6 weeks after injury (P < 0.05 for 10 mg/mL BBG and P < 0.01 for 50 mg/mL BBG).
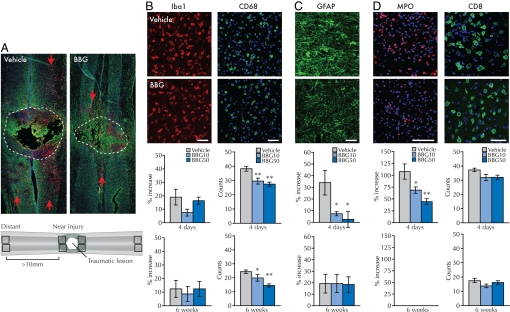
Fig. 4.
BBG reduces microglia cell activation, suppresses reactive gliosis, and decreases the number of infiltrating neutrophils in the spinal cord at 4 days after injury. BBG also reduces activation of microglial cells 6 weeks after injury. (A) Longitudinal sections of spinal cord at the traumatic lesion (injury area outlined). Green, GFAP; red, CD68; blue, DAPI. Red arrows indicate CD68+ cells most distant from the lesion site. (A, Bottom) The scheme used for quantification of immune response. Immunoreactivity in 4 regions close to the lesion was compared with the intensity of the signal in 4 regions located >10 mm from the border of the injury in the same section. (B) BBG did not change the distribution or the intensity of Iba-1 immunoreactivity (Iba-1, red, n = 5), but significantly reduced the number of activated microglial cells (CD68, green, n = 5). (C and D) BBG also reduces the posttraumatic up-regulation of GFAP (green, n = 5) and infiltrating neutrophils (MPO, red, n = 7), but not the number of infiltrating cytotoxic T-lymphocytes (CD8, green, n = 5–6). (B–D, Bottom) Summary of the quantification of immunolabeling at 4 days or 6 weeks postinjury. The data are plotted as the relative ratio of the immunoreactivity near the injury site compared with distant area for Iba1, GFAP, and MPO, and the number of CD68+ or CD8+ cells. Blue, DAPI. Scale bars, 50 μm. *, P < 0.05; **, P < 0.01 by one-way ANOVA with a Newman-Keuls posthoc test. Error bars indicate SEM. Bottom graphs quantify % increase (Iba1, GFAP, and MPO) or number of cells (CD68+ or CD8+) at 6 weeks postinjury.
Analysis of the astrocytic response showed a significant increase in GFAP immunoreactivity in proximity to the injury site compared with astrocytes located further from the damaged area (34 ± 10% increase, P < 0.05, Fig. 4C), consistent with the appearance of SCI-associated local astrogliosis. Morphologically, these regions were characterized by hypertrophic astrocytes with multiple GFAP+ processes (Fig. 4C). In contrast, peritraumatic astrocytes from animals treated with 10 mg/mL BBG were morphologically indistinguishable from astrocytes located distant to the injury site, and exhibited a fluorescence intensity only 7 ± 2% higher than control (P > 0.05) (Fig. 4C). Thus, BBG treatment significantly reduced the severity of initial reactive gliosis after SCI (P = 0.036). This effect was transient, because we did not observe any significant difference in GFAP intensity at 6 weeks postinjury (P = 0.99, Fig. 4C). These data indicate that BBG clearly reduced both astrocytosis and microglial invasion of the peritraumatic zone (Fig. 4 A–C).
BBG Treatment Diminishes Neutrophil Infiltration After SCI.
Both neutrophils and lymphocytes express high levels of P2X7R and infiltrate lesioned tissue as part of the systemic immune response to SCI (17). Because BBG was administered intravenously, we asked whether BBG decreased secondary tissue loss by counteracting extravasation of leukocytes after SCI. To this end, neutrophils were stained by using an antibody against myeloperoxidase (MPO), and T-lymphocytes were identified by using an antibody against CD8. At 4 days after injury, control animals showed strong increase in the intensity of MPO immunosignal in areas near the injury (107 ± 16% increase). This increase was markedly reduced by treatment with 10 mg/kg BBG (68 ± 7% intensity increase, P < 0.05, Fig. 4D). At 6 weeks postinjury, the MPO immunosignal was below detection in both vehicle- and BBG-treated animals (Fig. 4D). Thus, neutrophil invasion was significantly attenuated by BBG. On the other hand, although a trend toward fewer CD8+ T cells was observed in the BBG-treated groups, it did not reach statistical significance (Fig. 4D).
Discussion
No effective treatment is currently available to treat acute spinal cord injury, apart from the use of steroids, which provide at best modest protection to a subset of patients (18). In this study, we propose the use of a systematically administered P2X7R inhibitor as a clinically feasible strategy for the treatment of SCI.
We found that i.v. administration of the P2X7R inhibitor BBG significantly reduced the severity of spinal cord damage without any evident toxicity. Remarkably, BBG is a derivative of the widely used food additive FD&C Blue number 1 (BB-FCF). Currently, more than 1 million pounds of FD&C blue dye No. 1 are consumed yearly in the United States, corresponding to a daily intake of 16 mg per person; it has no known toxicity, except for the potentiation of metabolic acidosis in septic patients. This favorable toxicity profile, paired with its potent P2X7R inhibition, prompted us to test the neuroprotective actions of its close structural relative, BBG, in SCI. Our analysis showed that BBG significantly reduced spinal cord injury; its only notable side effect was the transient acquisition of a blue tint to the skin.
P2X7 receptors are unique among purine receptors in that they posses a low affinity for binding to ATP. Whereas the binding constant of other members of the P2X and P2Y receptor families is in the low μM range, P2X7 receptors bind with an affinity of ≈140 μM. Elevation of extracellular ATP concentration in the high μM range is most likely only attained in the setting of acute injury. It is not surprising then that P2X7 receptors were first discovered in macrophages, in which injury-associated receptor activation triggers the opening of a large membrane pore, followed by the release of inflammatory mediators. P2X7Rs are more broadly recognized for their proinflammatory effects, in that they trigger the release of interleukins, including IL-1β (19), activation of superoxide release and caspase activation (20), as well as cycloxygenase-2 and tumor necrosis factor-α (TNF-α) (21, 22). Blockade of P2X7R has accordingly been shown to attenuate microglial activation and inflammation (23).
Spinal cord trauma is associated with a striking increase in ATP release that lasts for at least 6 h after the initial injury (3). We have previously found that P2X7 receptors are expressed by spinal neurons, and that both ATP and BzATP (a P2X7 receptor agonist) triggered irreversible increases in cytosolic Ca2+ and neuronal death. Moreover, direct intraspinal injection of a P2X7 receptor antagonist, oxATP, reduced neuronal death and improved motor recovery when delivered shortly after the traumatic event. Although these experiments were important as a proof-of-principle, they offered no immediate avenue for clinical translation because intraspinal injection is rarely clinically feasible in the setting of an already injured cord. In this study, we capitalized on the availability and nontoxicity of systemically administered BBG to establish a means of systemic P2X7R inhibition. We found that BBG indeed acted as a potent CNS anti-inflammatory compound that suppressed activation of astroglial and microglial cells. Moreover, BBG suppressed neutrophil infiltration, suggesting that BBG not only reduced the local inflammatory response, but also reduced leukocyte infiltration from the periphery. As neutrophils release reactive oxygen species, chemokines and other key determinants of secondary tissue damage (24), the invasion of neutrophils after SCI is closely linked to the severity of secondary spinal cord injury. In addition, leukocyte infiltration may be important for the progressive collapse of the microvasculature observed after SCI (25). That being said, the i.v. route of delivery also expands the number of cell types potentially affected by the P2X7 receptor blockade, hence introducing the possibility of off-target effects.
BBG targeted resident microglial cells because they express high levels of P2X7 receptors. BBG thus strongly inhibited microglial cell activation at both 4 and 48 days after injury. As such, the suppression of reactive gliosis in rats treated with BBG might be secondary to the blockade of P2X7R in microglia. Astrocytes normally express low levels of P2X7 receptors (26–28), but IL-1β promotes increased levels of glial P2X7R expression (29) so that astrocytes too may be direct targets of the P2X7R blockade in the setting of inflammation. Together, these data suggest that the neuroprotective actions of BBG result from the blockade of P2X7 receptor activity in multiple cell types, including neurons, microglia, astrocytes, and inflammatory cells.
The hypothesis that inflammation contributes to the pathogenesis of neurodegenerative diseases has led to the idea that anti-inflammatory drugs can halt disease progression (30). The neuroprotective potential of the small BBB-permeable tetracycline antibiotic, minocycline, has recently been explored in a number of neurodegenerative diseases, as well as in stroke and spinal cord injury. In animal models of SCI, minocycline reduced microglial activation, cell death, and lesion size with significant motor recovery (31). Nevertheless, Pinzon et al. (32) did not find functional motor improvement in a similar model of contusive SCI, questioning the effectiveness of minocycline in SCI treatment.
Another more potent anti-inflammatory, the steroid methylprednisolone, has been used as a treatment for SCI, and can significantly improve motor and sensory function in SCI patients (18). However, criticisms have been raised regarding the window of administration and overall effectiveness of the compound (33). Nonetheless, it remains the only drug in clinical use that may provide some benefit in some cases of SCI.
A critical difference in the actions of BBG and both minocycline and methylprednisolone is that P2X7 receptors are activated within seconds when cytosolic ATP flows out of traumatized cells, whereas both steroids and minocycline target inflammatory processes far downstream to P2X7 receptor activation (34). Another important difference is that P2X7 receptor expression is widespread, so that BBG thus has multiple cellular targets, including neurons and astrocytes, as well as microglia. BBG may thus have a unique therapeutic profile, capably suppressing the earliest steps in posttraumatic inflammatory cascades.
These data provide a strategy by which to limit secondary damage after SCI, using a broadly available, inexpensive, well-tolerated, and systemically potent P2X7R inhibitor. In our rat model of spinal cord injury, early treatment with BBG significantly improved functional recovery, and reduced posttraumatic inflammatory responses and tissue loss. Although our analysis was focused on BBG as monotherapy for SCI, its low toxicity suggests that BBG may be added as adjunctive therapy to methylprednisone, minocycline, or other anti-inflammatories as well. Such combination therapy may well enhance the possibility of optimal recovery of function following traumatic injury to the human spinal cord.
Materials and Methods
Surgery, SCI, and Drug Administration.
Adult Sprague–Dawley rats were anesthetized, a laminectomy was performed, and a catheter inserted for delivery of drugs (see details SI Materials and Methods). Spinal cord injury was applied with the weight-drop paradigm. BBG (10 or 50 mg/kg) or vehicle was given intravenously 10–15 min after injury and once daily for the next 2 days (3 times total) and the animals were divided either for immunohistochemistry or for quantification of tissue injury. See SI Materials and Methods for details.
Preparation of BBG and Quantification of Its Concentration.
BBG was dissolved in 0.9% NaCl for i.v. injection. For quantification of BBG concentrations in the spinal cord, tissue was collected 3 days after SCI. After isolation, pieces that contained the lesion site or that were 2 mm away from the tissue were weighted and homogenized. The BBG concentration in the tissue was quantified with a spectrophotometer at the maximal absorbance of BBG (576 nm) by using 280 nm for reference. See SI Materials and Methods for details.
Tissue Preparation and Immunohistochemistry.
The rats were anesthetized, perfused with 4% paraformaldehyde, and the spinal cords were dissected and postfixed overnight. Twenty-micrometer coronal cryosections were obtained for staining with Luxol Fast Blue and cresyl violet or immunohistochemistry. The primary antibodies used in this study were anti-GFAP (1:500, Sigma), anti-Iba1 (1:500, Wako), anti-MPO (1:500, DAKO), anti-CD8a (1:50, BD), or anti-MAP2 (1:5000, Abcam). See SI Materials and Methods for details.
Quantification of Lesion Volume.
For quantification of spinal cord damage, a group of cryosections was stained with the combined Klüver–Barrera procedure to detect myelin. Areas in individual sections were measured by using imageJ software. Total lesion volume was defined as the sum of the lesion volume inside the spinal cord and the atrophy volume outside. The total lesion volume was obtained by the sum of the total lesion area multiplied by distance between the sections (200 μm). The different groups were compared by using ANOVA with Tukey-Kramer posthoc test (probability values <0.05 were considered to be statistically significant). See SI Materials and Methods for details.
Quantification of BBB Scores.
The BBB 21-point open-field locomotor rating scale was used for evaluating hindlimb movement (15, 3). In our experiments, the rats were evaluated every day for the first 3 days and every 3.5 days for the remaining 6 weeks after injury. See SI Materials and Methods for details.
Quantification of Immunoreactivity and Statistical Analysis.
After immunohistochemistry, spinal cord slides were subject to quantification by using the Image J software. For GFAP, Iba1, and MPO, immunostaining intensities in 4 areas (640 μm × 640 μm) adjacent to the lesion were measured and normalized to fluorescence intensities detected in areas at least 10 mm from the injury (far injury area). For CD8+ and CD68+ cells, the number of nuclei in 4 near-injury areas (640 μm × 640 μm) was counted. See SI Materials and Methods for details.
Acknowledgments.
We thank Dr. Glenn Rechtine for helpful conversations. This work was supported by the NY State Spinal Cord Injury program, the Miriam and Sheldon Adelson Medical Research Foundation, and National Institutes of Health grants.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902531106/DCSupplemental.
References
- 1.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 2.Gourine AV, et al. Release of ATP in the central nervous system during systemic inflammation: Real-time measurement in the hypothalamus of conscious rabbits. J Physiol. 2007;585:305–316. doi: 10.1113/jphysiol.2007.143933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, et al. P2X7 receptor inhibition improves recovery after spinal cord injury. Nature Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 4.North A. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 5.Gao H, Hong J. Why neurodegenerative diseases are progressive: Uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collo G, et al. Tissue distribution of the P2X7 receptor. Neuropharmacol. 1997;36:1277–1283. doi: 10.1016/s0028-3908(97)00140-8. [DOI] [PubMed] [Google Scholar]
- 7.Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4:1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hess SM, Fitzhugh OG. Absorption and excretion of certain triphenylmethane colors in rats and dogs. J Pharmacol Exp Ther. 1955;114:38–42. [PubMed] [Google Scholar]
- 9.Borzelleca JF, Depukat K, Hallagan JB. Lifetime toxicity/carcinogenicity studies of FD&C Blue No. 1 (brilliant blue FCF) in rats and mice. Food Chem Toxicol. 1990;28:221–234. doi: 10.1016/0278-6915(90)90034-k. [DOI] [PubMed] [Google Scholar]
- 10.Remy M, et al. An in vivo evaluation of Brilliant Blue G in animals and humans. Br J Ophtalmol. 2008;92:1142–1147. doi: 10.1136/bjo.2008.138164. [DOI] [PubMed] [Google Scholar]
- 11.Jiang LH, Mackenzie AB, North RA. Surprenant A. Brilliant Blue G selectively blocks ATP-gated rat P2X7 receptors. Mol Pharmacol. 2000;58:82–88. [PubMed] [Google Scholar]
- 12. [Accessed June 30, 2009];71 Federal Register 70. 2006 available at http://edocket.access.gpo.gov/2006/06–3307.htm.
- 13.Brown JP, et al. Synthesis of C14-labelled FD&C Blue No. 1 (Brilliant Blue FCF) and its intestinal absorption and metabolic fate in rats. Fd Cosmet Toxicol. 1980;18:1–5. doi: 10.1016/0015-6264(80)90002-4. [DOI] [PubMed] [Google Scholar]
- 14.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 15.Basso DM, Beattle MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 16.Ito D, et al. Microglia-specific localization of a novel calcium binding protein, Iba1. Mol Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 17.Fleming JC, et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- 18.Baptiste DC, Fehlings MG. Update on the treatment of spinal cord injury. Prog Brain Res. 2007;161:217–233. doi: 10.1016/S0079-6123(06)61015-7. [DOI] [PubMed] [Google Scholar]
- 19.Solle M, et al. Altered cytokine production in mice lacking P2X7 receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 20.Kahlenberg J, Dubyak GW. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol. 2004;286:C1100–fC1108. doi: 10.1152/ajpcell.00494.2003. [DOI] [PubMed] [Google Scholar]
- 21.Samad TA, et al. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki T, et al. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chessell IP, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Trivedi A, Olivas AD, Noble-Haeusslein LJ. Inflammation and spinal cord injury: Infiltrating leukocytes as determinants of injury and repair processes. Clin Neurosci Res. 2006;6:283–292. doi: 10.1016/j.cnr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simard JM, et al. Endothelial sulfonylurea receptor 1-regulated NCCa-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J Clin Inv. 2007;117:2105–21013. doi: 10.1172/JCI32041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kukley M, Barden JA, Steinhauser C, Jabs R. Distribution of P2X receptors on astrocytes in juvenile rat hippocampus. Glia. 2001;36:11–21. doi: 10.1002/glia.1091. [DOI] [PubMed] [Google Scholar]
- 27.Jabs R, Grote A, Grauer M, Seifert G, Steinhauser C. Lack of P2X Receptor mediated currents in astrocytes and GluR type glial cells of the hippocampal CA1 region. Glia. 2007;55:1648–1655. doi: 10.1002/glia.20580. [DOI] [PubMed] [Google Scholar]
- 28.Yu Y, et al. Cellular localization of P2X7 receptor mRNA in the rat brain. Brain Res. 2008;1194:45–55. doi: 10.1016/j.brainres.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 29.Narcisse L, Scemes E, Zhao Y, Lee SC, Brosnan CF. The cytokine IL-1beta transiently enhances P2X7 receptor expression and function in human astrocytes. Glia. 2005;49:245–258. doi: 10.1002/glia.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klegeris A, McGeer EG, McGeer PL. Therapeutic approaches to inflammation in neurodegenerative disease. Curr Opin Neurol. 2007;20:351–357. doi: 10.1097/WCO.0b013e3280adc943. [DOI] [PubMed] [Google Scholar]
- 31.Wells JE, Hurlbert RJ, Fehlings MG, Yong VW. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126:1628–1637. doi: 10.1093/brain/awg178. [DOI] [PubMed] [Google Scholar]
- 32.Pinzon A, et al. A re-assessment of minocycline as a neuroprotective agent in a rat spinal cord contusion model. Brain Res. 2008;1243:146–151. doi: 10.1016/j.brainres.2008.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JM, et al. Methylprednisolone protects oligodendrocytes but not neurons after spinal cord injury. J Neurosci. 2008;28:3141–3149. doi: 10.1523/JNEUROSCI.5547-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seabrook TJ, Jiang L, Maier M, Lemere CA. Minocycline affects microglia activation, Aβ deposition and behavior in APP-tg Mice. Glia. 2006;53:776–782. doi: 10.1002/glia.20338. [DOI] [PubMed] [Google Scholar]