Abstract
Traits that permit successful invasions have often seemed idiosyncratic, and the key biological traits identified vary widely among species. This fundamentally limits our ability to determine the invasion potential of a species. However, ultimately, successful invaders must have positive growth rates that longer term result in higher biomass accumulation than competing established species. In many terrestrial ecosystems nitrogen limits plant growth, and is a key factor determining productivity and the outcome of competition among species. Plant nitrogen use may provide a powerful framework to evaluate the invasive potential of a species in nitrogen-limiting ecosystems. Six mechanisms influence plant nitrogen use or acquisition: photosynthetic tissue allocation, photosynthetic nitrogen use efficiency, nitrogen fixation, nitrogen-leaching losses, gross nitrogen mineralization, and plant nitrogen residence time. Here we show that among these alternatives, the key mechanism allowing invasion for Pinus strobus into nitrogen limited grasslands was its higher nitrogen residence time. This higher nitrogen residence time created a positive feedback that redistributed nitrogen from the soil into the plant. This positive feedback allowed P. strobus to accumulate twice as much nitrogen in its tissues and four times as much nitrogen to photosynthetic tissues, as compared with other plant species. In turn, this larger leaf nitrogen pool increased total plant carbon gain of P. strobus two- to sevenfold as compared with other plant species. Thus our data illustrate that plant species can change internal ecosystem nitrogen cycling feedbacks and this mechanism can allow them to gain a competitive advantage over other plant species.
Keywords: ecosystem feedbacks, plant nitrogen use
The rate of biological plant invasions is accelerating (1). Invasions can have significant impacts on the biodiversity (2), disturbance regimen (3), and water and nutrient cycling (4) of ecosystems. Specific invader success has been attributed to factors such as specific leaf area (5), water use efficiency (6), resistance to disturbance (7), herbivore resistance (8), escape from natural enemies (9), and plant–soil feedbacks (10, 11). However, among invasive species, only a few general plant traits, such as vegetative reproduction, self pollination, phylogeny (i.e., other successful invaders in the family or genus) and native geographic range have been identified (12, 13). This fundamentally limits our ability to determine the invasion potential of a species.
Ultimately, all plants need to produce biomass and successful invaders need to maintain a positive growth rate during the initial establishment phase (14) and eventually many successful invaders accrue significantly more biomass than established competitors (2, 15). Therefore, if high biomass accumulation is key for the success of an invader and if the growing season of the invader corresponds with the native established species, we must understand how it can successfully maintain a positive growth rate and accumulate more biomass than its competitors. In many temperate-zone terrestrial ecosystems plant biomass production is limited by nitrogen (16), and nitrogen is a key factor determining the outcome of interspecific competition in these ecosystems (17). Therefore to elucidate how and why a plant species is a successful invader in a nitrogen-limited environment, we need to understand species-specific patterns of plant nitrogen use and related species-driven changes in nitrogen gains and losses within ecosystems, using a systematic approach.
Plants can use two different nitrogen-based strategies to maximize productivity in nitrogen-limited systems: increased carbon gain per unit plant nitrogen, or increased total plant nitrogen pool (Fig. 1). Given equal pools of plant nitrogen among species, higher plant carbon gain per unit plant nitrogen can be achieved either by allocating proportionally more nitrogen to photosynthetic tissues (18) or by having a higher photosynthetic efficiency through higher carbon gain per unit leaf nitrogen (19). Both mechanisms increase plant carbon gain per unit plant nitrogen because most nitrogen within leaves is used for Rubisco (19), the central enzyme involved in carbon-fixation by plants, and there is a positive linear correlation between photosynthetic rate and leaf nitrogen concentration (20). Therefore, if a given species allocates proportionally more nitrogen to photosynthetic structures relative to other species, it will increase its carbon gain per unit plant nitrogen, as seen in some invasive annual grasses (21). Alternatively, increasing the rate of carbon fixation per unit leaf nitrogen also increases total plant carbon gain per unit plant nitrogen and species differ in this rate (22–24) and successful invasive species can have higher photosynthetic nitrogen use efficiency (22). However, the largest increases in plant carbon per unit leaf nitrogen occur in plants that use the C4 photosynthetic pathway. C4 plants use their Rubisco more efficiently than C3 plants by increasing internal leaf CO2 concentrations (19), allowing several C4 species to be successful invaders (25). This mechanism, however, is taxonomically restricted to some annual and perennial grasses in the Poaceae (19) and to forbs in the family Chenopodiaceae and the genus Euphorbia (26).
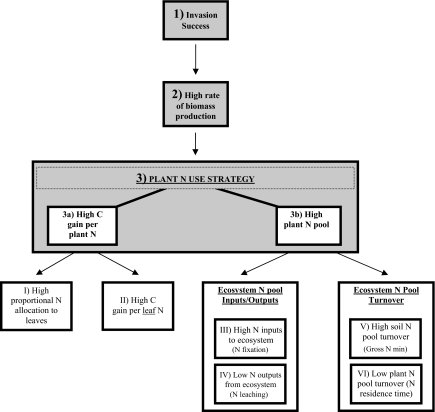
Fig. 1.
General framework for invader success in nitrogen-limited systems (Level 1). Successful invasion of species into these successional grasslands is driven by high invader carbon accrual (Level 2). In these systems, high plant carbon accumulation is dependent on the nitrogen use strategy (Level 3). Plants can maximize productivity through two nitrogen use strategies: increased carbon gain per unit plant nitrogen (Level 3a) or increased nitrogen in plant biomass (Level 3b). A plant can achieve the first strategy through two pathways: I) high proportional nitrogen allocation to leaves, or II) high carbon gain per unit leaf nitrogen. Alternatively, four possible pathways can drive an increased total plant nitrogen pool: III) high ecosystem level nitrogen inputs via nitrogen fixation; IV) low ecosystem-level nitrogen losses via leaching; V) high soil nitrogen pool turnover rate, as measured by gross nitrogen mineralization, resulting in a larger plant-available nitrogen pool; or VI) low plant nitrogen pool turnover via high leaf longevity.
For a plant to increase the total amount of nitrogen in its biomass as compared with other established species, ecosystem nitrogen pools and fluxes have to change. Plant species can change ecosystem nitrogen pools and fluxes through the following: increased ecosystem nitrogen inputs via nitrogen fixation; reduced ecosystem nitrogen losses from nitrogen leaching; increased soil nitrogen turnover through changes in gross nitrogen mineralization; or reduced plant nitrogen turnover via increased plant nitrogen residence time (18) (Fig. 1), which can be caused by longer-lived tissues and/or higher rates of nitrogen retranslocation from senescing tissues (27). All four of these mechanisms have been cited as driving the success of some invaders. Higher ecosystem nitrogen pools caused by nitrogen fixation have been documented for the successful woody invaders Morella faya and Falcataria moluccana (28, 29). For non–nitrogen-fixing plants, there are two main pools of nitrogen in the ecosystem, namely the plant nitrogen pool and soil nitrogen pool, with the soil nitrogen pool being significantly larger than the plant nitrogen pool (27). A plant species that reduces nitrogen-leaching losses or increases soil nitrogen turnover can increase the annual flux of nitrogen from the soil pool to the plant pool. Increases in nitrate leaching have been shown to facilitate the invasion of Bromus tectorum into arid and semiarid ecosystems (30), whereas increased soil nitrogen turnover has been linked to plant species identity (31) and the success of invasive grasses in Hawaii (25). At steady state, plants with low nitrogen residence time rapidly return a large proportion of the nitrogen in their biomass to the soil nitrogen pool through senescence and other annual leaf and root litter losses, allowing the soil nitrogen pool to be replenished annually. Invasion into nitrogen-limited systems by a plant species with a higher nitrogen residence time would reduce the annual flux of nitrogen from the plant pool back to the soil pool but not the nitrogen flux from the soil pool to the plant pool in the short term. Driven by higher plant nitrogen residence time, these changes in nitrogen fluxes would subsequently lead to positive feedback through nitrogen cycling, by annually enhancing the plant nitrogen pool while concurrently depleting the soil nitrogen pool (27). High plant nitrogen residence time via increased tissue longevity is common to successful species in nutrient-poor conditions (32, 33).
Thus, in total there are six mechanisms by which species can either use nitrogen more efficiently or acquire more nitrogen that could explain higher biomass accumulation in nitrogen limited systems of successful invaders (Fig. 1, I–VI). Here we identify key trait differences in plant nitrogen use and nitrogen cycling between Eastern white pine (Pinus strobus), a species that is rapidly invading prairies in central Minnesota (34), two tree species that are encroaching at a much slower rate (35), and four herbaceous grassland plant species that are being replaced.
We conducted a common garden study at the Cedar Creek Ecosystem Science Reserve in central Minnesota, a system in which nitrogen is the primary resource limiting plant productivity (36). To examine species differences in nitrogen use and ecosystem nitrogen cycling, we established replicated closed mesocosms of seven grassland and forest species (and a bare-soil control). By containing the ecosystem nitrogen pool, we were able to evaluate all six potential mechanisms by explicitly quantifying species effects on both plant and soil total nitrogen pools and fluxes, which is essential for determining plant nitrogen use and ecosystem nitrogen gains and losses. We compared P. strobus to the two historically dominant but currently non-invasive oak species (Quercus ellipsoidalis and Q. macrocarpa) (35), two dominant grasses that are being replaced by P. strobus; an introduced C3 species Poa pratensis and a native C4 species Schizachyrium scoparium; and two non-invasive native forbs that locally can attain high abundances in these systems, the nitrogen fixer Lespedeza capitata and the clonal forb Solidago canadensis.
Results
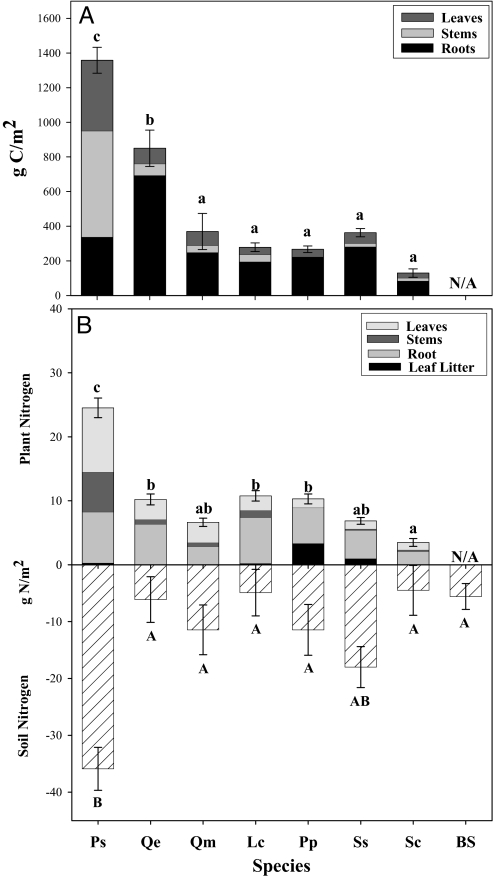
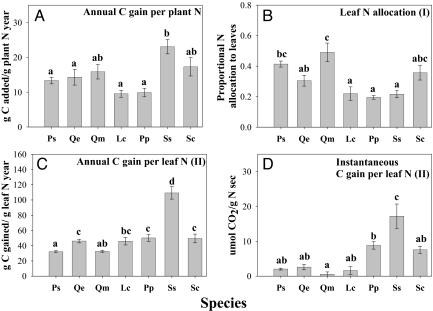
We found that P. strobus accrued nearly twice as much biomass as the next most productive species (Q. ellipsoidalis) and more than three times as much biomass relative to all other species (Fig. 2A). Pinus strobus did not annually accrue more carbon per unit plant nitrogen than the two oak species (Fig. 3A), did not proportionally allocate more nitrogen into photosynthetic tissues (Fig. 3B), and did not have higher carbon gain per unit leaf nitrogen (Figs. 3C, 3D). These results lead us to reject the hypothesis that P. strobus invasiveness reflects a nitrogen use strategy of increased carbon gain per unit plant nitrogen.
Fig. 2.
(A) Plant carbon allocation after six growing seasons. Species differences in plant and soil response variables were examined using one-way ANOVA and letters represent significant differences from post hoc Tukey tests for the total plant carbon pool (P < 0.05). (B) Increase in plant nitrogen and decrease in surface soil nitrogen under each species over 6 years (depth, 0–10 cm), no species differences were found in lower soil depths. Species differences in plant and soil response variables were examined using one-way ANOVA and letters represent significant differences across species from post hoc Tukey tests (P < 0.05) for the change in the total plant nitrogen pool and the top 10 cm of the soil nitrogen pool. No species differences were found in the lower 40 cm of the mesocosm. Ps, Pinus strobus; Qe, Quercus ellipsoidalis; Qm, Q. macrocarpa; Lc, Lespedeza capitata; Pp, Poa pratensis; Ss, Schizachyrium scoparium; Sc, Solidago canadensis; BS, bare soil.
Fig. 3.
Mechanisms that drive annual carbon gained per unit plant nitrogen (A) (g C/g plant N yr): (B) proportional nitrogen allocation to leaves (I); (C) annual carbon gained per unit leaf nitrogen (II); (D) instantaneous carbon gained per unit leaf N at 1500 PAR (II; g C/g N sec). Species differences in plant and soil response variables were examined using one-way ANOVA; letters represent significant differences across species from post hoc Tukey tests (P < 0.05). For species abbreviations, see Fig. 2. Roman numerals refer to the framework in Fig. 1.
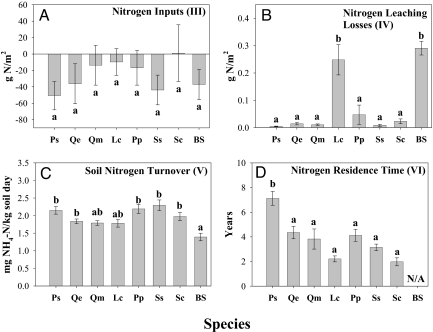
However, P. strobus did accrue significantly more nitrogen in plant biomass as compared with any other species in the study (Fig. 2B), which corresponded with a large decrease in total soil nitrogen (Fig. 2B). This result supports the hypothesis that the higher productivity of P. strobus is caused by an increased biomass nitrogen pool [Supporting Information (SI) Table S1 and Fig. S1]. This increased plant nitrogen pool was not driven by increased ecosystem level nitrogen inputs (i.e., nitrogen fixation), as all plots lost nitrogen during the experiment (Fig. 4A). Nitrogen leaching losses were not significantly different between P. strobus and the two oak species, and the total flux of nitrogen lost was too small to explain the observed productivity differences among species (Fig. 4B). We also did not find any significant differences in soil nitrogen turnover rate among species, as determined by measurements of gross nitrogen mineralization (Fig. 4C), nor in the rate of net nitrogen mineralization (Fig. S2).
Fig. 4.
Mechanisms which drive species differences in plant nitrogen pool size. (A) Ecosystem level nitrogen inputs (III), change in total plot nitrogen over 6 years (g N/m2); (B) Ecosystem level nitrogen losses (IV), nitrogen leaching losses over one growing season (g N/m2); (C) Soil nitrogen turnover rate (V), seasonal average of gross nitrogen mineralization (mg NH4-N/kg soil day); (D) Plant nitrogen turnover rate (VI), nitrogen residence time (Years). Species differences in plant and soil response variables were examined using one-way ANOVA; letters represent significant differences across species from post hoc Tukey tests (P < 0.05). For species abbreviations, see Fig. 2. Roman numerals refer to the framework in Fig. 1.
In contrast, we found that P. strobus had a significantly higher nitrogen residence time than all other species in the study (Fig. 4D). The increased residence time of nitrogen resulted in the accumulation of three times as much nitrogen in photosynthetic tissue in P. strobus as compared with the two oak species (Fig. 2B). We conclude that high nitrogen residence time was the key mechanism driving the significantly higher plant nitrogen pool and the high productivity of P. strobus (Table S2 and Fig. S3). High nitrogen residence time also drove a positive feedback on nitrogen cycling, resulting in the significantly larger depletion of soil nitrogen observed beneath P. strobus than the other species (Fig. 2B). We found that nitrogen retranslocation of P. strobus did not differ significantly from the other species (data not shown), but the woody tissues and evergreen needles of P. strobus have higher tissue longevity. This resulted in higher plant nitrogen residence time and, in turn, higher totals plant nitrogen in P. strobus. In addition, this nitrogen was derived from the soil nitrogen pool and not from nitrogen fixation as has been hypothesized previously (37).
Discussion
By examining these six mechanisms of plant nitrogen use (Fig. 1), our study clearly illustrates that the ability of P. strobus to retain nitrogen in its tissues longer than other species is the key mechanism driving the observed levels of productivity. This longer nitrogen retention leads to lower plant nitrogen losses and a lower return of nitrogen to the soil. In contrast, the nitrogen released from the decomposition of the soil organic matter does not decrease over this time period, resulting in a depletion of the soil organic matter nitrogen pool, which is often observed under trees that are invading into grassland ecosystems (38, 39). Thus the establishment of species into grasslands with high nitrogen residence times, such as P. strobus, can disrupt steady-state ecosystem nitrogen cycling, allowing the accumulation of nitrogen in the biomass. In the case of P. strobus, this accumulation of nitrogen in biomass resulted in more nitrogen in above-ground tissues, particularly leaves. With a larger leaf biomass nitrogen pool, P. strobus can photosynthesize more, accumulate more carbon, and, in turn, develop a canopy above the existing plant community. After this establishment phase, the newly developed woody perennial canopy allows P. strobus to reduce light levels to the herbaceous vegetation and subsequently to become competitively dominant (Fig. S4). It should be noted that other mechanisms also can control plant invasions. In this system, fire can have an impact on the success of invading species because P. strobus and, to a lesser extent, Q. ellipsoidalis are more susceptible to fire than Q. macrocarpa and the other species.
Plant nitrogen use and the resulting species-driven changes in ecosystem nitrogen gains and losses can provide a framework to evaluate the invasive potential during the establishment phase of a species in nitrogen-limited ecosystems. This resource based framework cannot be applied to invasions in which community interactions, such as preemptive resource uptake (40) or allelopathy (11), directly impact competition among species or in the case in which an invading species alters the ecosystem fire regimen (41). However, this framework can be used to examine a number of important community interactions and plant traits which have been suggested as potential mechanisms driving invasion success. These include release from natural enemies (8), plant-herbivore interactions (8), plant-pathogen interactions (10), high growth rate (5), water use efficiency (6), nitrogen use efficiency (22), nitrogen additions (42), fire (43), and resistance to disturbance (7). All of these potential mechanisms can be expressed and evaluated precisely in terms of plant nitrogen use, gain, and loss. This is because each of these interactions or traits differentially affect plant species nitrogen use and therefore can change competitive outcomes between invasive species and the established plant community. Finally, this resource-based predictive framework provides a quantitative, functional basis to evaluate management options for invasive species control. Evaluation of plant nitrogen use dynamics can be used to determine whether management practices such as burning, introduction of natural enemies, and resource availability manipulations (44) can sufficiently change plant nitrogen use, loss, or gain to alter the competitive advantage held by an invasive species.
Materials and Methods
Experimental mesocosms were established at Cedar Creek Ecosystem Science Reserve in central Minnesota in late 2000. Soils are sandy and derived from glacial outwash (45), with nitrogen being the primary resource limiting plant productivity (36). Mesocosms consisted of large plastic pots (60 cm in diameter and 50 cm in depth) that were dug into the ground so that the top of the pot was flush with the soil surface. Each pot drained into a hose, and there was an access tube next to the pot that collected all water draining from a pot. Each pot was filled with locally collected representative field soil, with the lower 40 cm being filled with subsurface soil and the top 10 cm being filled with topsoil. Grasses and forbs were seeded in at the time of initial setup; Quercus acorns were planted in the fall of 2000; and 1-year-old seedlings of Pinus were planted in the early spring of 2001. In 2006, the species in the experimental mesocosms were well established. Lespedeza capitata mesocosms had an average density of 110.6 individuals/m2, Solidago altissima had an average density of 130 individuals/m2, Quercus ellipsoidalis had an average density of 82.5 individuals/m2, Quercus macrocarpa had an average density of 79 individuals/m2, Pinus strobus had average density of 49.5 individuals/m2, and the grass species, Poa pratensis and Schizichyrium scoparium had a percent cover greater than 90% of the soil surface.
To determine species-mediated changes on total soil nitrogen, we sampled soil at four depths in early spring 2001 and early fall 2006 (0–5 cm, 5–10 cm, 10–25 cm, and 25–50 cm). Soil percent nitrogen was determined using combustion analysis from a Costech Analytical ECS 4010 at the University of Nebraska Ecosystem Analysis Laboratory (Lincoln, NE). Soil percent nitrogen was converted to g nitrogen/m2 following an equation developed for soil bulk density at Cedar Creek (46). Measured values of soil bulk density at 0–10 cm (≈1.40 g/cm3) and 10–25 cm (≈1.48 g/cm3) were comparable to those calculated following the equation (≈1.45 g/cm3). Change in the total soil nitrogen pool was the difference between soil nitrogen content in 2001 and 2006. Extractable inorganic soil nitrogen and water leachate samples were collected seven times between June 2006 and September 2006 and were analyzed colorimetrically for NH4+ and NO3− concentration using a Bran Luebbe AA3 at the University of Nebraska Ecosystem Analysis Laboratory (Lincoln, NE). The total flux of NH4+ and NO3− in the leachate was calculated as the product of the water volume collected at each sampling date and the NH4+ and NO3− concentrations of the measured leachate sample. The data presented is a cumulative total of both NH4+ and NO3− across all seven sampling periods. Soil nitrogen turnover was measured as rates of gross nitrogen mineralization in late 2005 and summer 2006. Measurements were taken in September 2005, May 2006, June, 2006, and July 2006 to encompass possible temporal variability in gross nitrogen mineralization rates. The data presented are a seasonal average across the four sampling dates. Gross nitrogen mineralization rates were determined using 15N isotopic-pool dilution of homogenized sieved soil samples from the top 10 cm of the mesocosm (47). Approximately 250 g soil (fw) was amended with 10 ml of 0.1309 mmol/l solution of (15NH4)2SO4 (99% atom) in a resealable plastic bag and homogenized by hand to ensure even distribution of the solution throughout the bag. Soil samples were extracted immediately after 15N addition and 24 hours after 15N addition in 75 ml of 2 mol/l KCl. Isotopic samples were processed at the University of California–Davis Stable Isotope Facility.
Above- and below-ground biomass was harvested in 2006 in all experimental mesocosms. Above-ground biomass was sampled in a 10 × 60-cm strip through the center of the pot and was separated into leaves, stems (for Quercus species, P. strobus, S. altissima, and L. capitata), and leaf litter. For the woody species, the clipped area contained two to three individual trees that were all harvested; this is equivalent to 33–66 individuals/m2. Below-ground biomass was sampled at three points within the clipped area at three depths (0–10 cm, 10–25 cm, and 25–50 cm) using a 2-inch-diameter core. For all species, below-ground biomass was assumed to be at steady state in 2006. Assuming that root biomass was at steady state, root ingrowth cores were put in place to determine annual root growth. These cores were placed down to a depth of 20 cm in May 2006 and harvested in late August 2006. Plant photosynthetic light response curves were generated using a Li-COR 6400 Portable Photosynthesis system (Lincoln, NE). For all non-woody species, above-ground tissue longevity (non-woody stems and leaves) was assumed to be 1 year. For the two deciduous oak species, leaf longevity was assumed to be 1 year, whereas for P. strobus leaf longevity was estimated to be 2 years based on observations that two cohorts of needles were present on the plant at the peak of the growing season.
Each pool of plant biomass (leaves, stems, litter, roots, and root ingrowth) was dried to a constant weight and analyzed for carbon and nitrogen content using combustion analysis from a Costech analytical ECS 4010 at the University of Nebraska Ecosystem Analysis Laboratory (Lincoln, NE). Plant biomass was converted to carbon mass using the measured carbon concentrations. For all non-woody species, annual carbon gain was determined as the sum of standing leaf carbon, stem carbon (for S. altissima and L. capitata), and root ingrowth carbon. For the two oak species, annual carbon gain was the sum of standing leaf carbon, 1/6 of stem carbon, and root ingrowth carbon. For P. strobus, annual carbon gain was the sum of 1/2 standing leaf carbon, 1/6 of stem carbon, and root ingrowth carbon. Only 1/2 of standing leaf carbon was used for P. strobus because leaf longevity was estimated at 2 years. Instantaneous carbon gain per unit leaf nitrogen was calculated using the leaf nitrogen content and measured photosynthetic rates at 1500 PAR. Plant nitrogen residence time was calculated as the inverse of annual plant nitrogen losses per unit standing plant nitrogen (48). Annual plant nitrogen losses were measured as the sum of leaf litter nitrogen losses and root ingrowth nitrogen.
Data were analyzed in SPSS version 17 software (SPSS Inc., Chicago, IL). Species differences in plant and soil response variables were examined using one-way analysis of variance (ANOVA), with species as a fixed factor. Differences between individual species were examined using post hoc Tukey tests (P < 0.05).
Supplementary Material
Acknowledgments.
We thank Chad Brassil, Nick Haddad, Stan Harpole, Amy Kochsiek, Svata Louda, Joe Mascaro, Cathleen McFadden, Shahid Naeem, Diana Pilson, Sabrina Russo, and Dave Tilman for comments and Wendy Bengston, Jenny Goth, Troy Mielke, Kally Worm and the Cedar Creek interns for analytical and field help and the University of Nebraska–Lincoln Ecosystem Analysis Laboratory and the University of California–Davis Stable Isotope Facility for sample analysis. This research was supported by the Center for Invasive Plant Management, the National Science Foundation, and the University of Nebraska.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900921106/DCSupplemental.
References
- 1.Sax DF, Gaines SD. Species diversity: From global decreases to local increases. Trends Ecol Evolut. 2003;18:561–566. [Google Scholar]
- 2.Mack RN, et al. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol Appl. 2000;10:689–710. [Google Scholar]
- 3.Mack MC, D'Antonio CM. Impacts of biological invasions on disturbance regimes. Trends Ecol Evolut. 1998;13:195–198. doi: 10.1016/S0169-5347(97)01286-X. [DOI] [PubMed] [Google Scholar]
- 4.Ehrenfeld JG. Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems. 2003;6:503–523. [Google Scholar]
- 5.Grotkopp E, Rejmanek M, Rost TL. Toward a causal explanation of plant invasiveness: Seedling growth and life-history strategies of 29 pine (Pinus) species. Am Naturalist. 2002;159:396–419. doi: 10.1086/338995. [DOI] [PubMed] [Google Scholar]
- 6.Eggemeyer KD, Awada T, Wedin DA, Harvey FE, Zhou XH. Ecophysiology of two native invasive woody species and two dominant warm-season grasses in the semiarid grasslands of the Nebraska sandhills. Int J Plant Sci. 2006;167:991–999. [Google Scholar]
- 7.Richardson DM, Bond WJ. Determinants of plant distribution—evidence from pine invasions. Am Naturalist. 1991;137:639–668. [Google Scholar]
- 8.Fagan WF, et al. When can herbivores slow or reverse the spread of an invading plant? A test case from Mount St. Helens. Am Naturalist. 2005;166:669–685. doi: 10.1086/497621. [DOI] [PubMed] [Google Scholar]
- 9.Callaway R, Thelen G, Rodriguez A, Holben W. Soil biota and exotic plant invasion. Nature. 2004;427:731–733. doi: 10.1038/nature02322. [DOI] [PubMed] [Google Scholar]
- 10.Klironomos JN. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature. 2002;417:67–70. doi: 10.1038/417067a. [DOI] [PubMed] [Google Scholar]
- 11.Callaway RM, Aschehoug ET. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science. 2000;290:521–523. doi: 10.1126/science.290.5491.521. [DOI] [PubMed] [Google Scholar]
- 12.Kolar CS, Lodge DM. Progress in invasion biology: Predicting invaders. Trends Ecol Evolut. 2001;16:199–204. doi: 10.1016/s0169-5347(01)02101-2. [DOI] [PubMed] [Google Scholar]
- 13.Gravuer K, Sullivan JJ, Williams PA, Duncan RP. Strong human association with plant invasion success for Trifolium introductions to New Zealand. Proc Natl Acad Sci USA. 2008;105:6344–6349. doi: 10.1073/pnas.0712026105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pastor J. Mathematical Ecology of Populations and Ecosystems. Oxford, UK: Blackwell; 2008. [Google Scholar]
- 15.McKinley DC, Blair JM. Woody plant encroachment by Juniperus virginiana in a mesic native grassland promotes rapid carbon and nitrogen accrual. Ecosystems. 2008;11:454–468. [Google Scholar]
- 16.Vitousek PM, Howarth RW. Nitrogen limitation on land and in the sea— how can it occur? Biogeochemistry. 1991;13:87–115. [Google Scholar]
- 17.Wedin DA, Tilman D. Competition among grasses along a nitrogen gradient: Initial conditions and mechanisms of competition. Ecol Monogr. 1993;63:199–229. [Google Scholar]
- 18.Berendse F, Oudhof H, Bol J. A comparative study on nutrient cycling in wet heathland ecosystems. Oecologia. 1987;74:174–184. doi: 10.1007/BF00379357. [DOI] [PubMed] [Google Scholar]
- 19.Wedin D. C4 grasses: Resource use, ecology, and global change. In: Moser LE, Burson BL, Sollenberger LE, editors. Warm Season (C4) Grasses. Madison: ASA, CSSA, SSSA; 2004. pp. 15–50. [Google Scholar]
- 20.Reich PB, Walters MB, Ellsworth DS. From tropics to tundra: Global convergence in plant functioning. Proc Natl Acad Sci USA. 1997;94:13730–13734. doi: 10.1073/pnas.94.25.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.James JJ. Leaf nitrogen productivity as a mechanism driving the success of invasive annual grasses under low and high nitrogen supply. J Arid Environ. 2008;72:1775–1784. [Google Scholar]
- 22.Funk JL, Vitousek PM. Resource-use efficiency and plant invasion in low-resource systems. Nature. 2007;446:1079–1081. doi: 10.1038/nature05719. [DOI] [PubMed] [Google Scholar]
- 23.Onoda Y, Hikosaka K, Hirose T. Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct Ecol. 2004;18:419–425. [Google Scholar]
- 24.Pons TL, Westbeek MHM. Analysis of differences in photosynthetic nitrogen-use efficiency between four contrasting species. Physiol Plant. 2004;122:68–78. [Google Scholar]
- 25.Mack MC, D'Antonio CM. Exotic grasses alter controls over soil nitrogen dynamics in a Hawaiian woodland. Ecol Appl. 2003;13:154–166. [Google Scholar]
- 26.Sage RF, Wedin DA, Li M. The biogeography of C4 photosynthesis: Patterns and controlling factors. In: Sage RF, Monson RK, editors. C4 Plant Biology. San Diego: Academic; 1999. pp. 313–373. [Google Scholar]
- 27.Knops JMH, Bradley KL, Wedin DA. Mechanisms of plant species impacts on ecosystem nitrogen cycling. Ecol Lett. 2002;5:454–466. [Google Scholar]
- 28.Hughes RF, Denslow JS. Invasion by a N2-fixing tree alters function and structure in wet lowland forests of Hawaii. Ecol Applic. 2005;15:1615–1628. [Google Scholar]
- 29.Vitousek PM, Walker L, Whiteaker L, Mueller-Dombois D, Matson P. Biological invasion by Myrica faya alters ecosystem development in Hawaii. Science. 1987;238:802–804. doi: 10.1126/science.238.4828.802. [DOI] [PubMed] [Google Scholar]
- 30.Sperry LJ, Belnap J, Evans RD. Bromus tectorum invasion alters nitrogen dynamics in an undisturbed arid grassland ecosystem. Ecology. 2006;87:603–615. doi: 10.1890/05-0836. [DOI] [PubMed] [Google Scholar]
- 31.Hawkes CV, Wren IF, Herman DJ, Firestone MK. Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecol Lett. 2005;8:976–985. doi: 10.1111/j.1461-0248.2005.00802.x. [DOI] [PubMed] [Google Scholar]
- 32.Aerts R. Interspecific competition in natural plant communities: Mechanisms, trade-offs and plant-soil feedbacks. J Exp Bot. 1999;50:29–37. [Google Scholar]
- 33.Chapin F, Matson P, Mooney HA. Terrestrial plant nutrient use. In: Chapin F, Matson P, Mooney HA, editors. Principles of Terrestrial Ecosystem Ecology. New York: Springer-Verlag; 2002. pp. 176–196. [Google Scholar]
- 34.Dovciak M, Frelich LE, Reich PB. Pathways in old-field succession to white pine: Seed rain, shade, and climate effects. Ecol Monogr. 2005;75:363–378. [Google Scholar]
- 35.Inouye RS, Allison TD, Johnson NC. Old field succession on a Minnesota sand plain—effects of deer and other factors on invasion by trees. Bull Torrey Botanical Club. 1994;121:266–276. [Google Scholar]
- 36.Tilman D. Plant dominance along an experimental nutrient gradient. Ecology. 1984;65:1445–1453. [Google Scholar]
- 37.Bormann BT, et al. Rapid N2 fixation in pines, alder, and locust—evidence from the sandbox ecosystem study. Ecology. 1993;74:583–598. [Google Scholar]
- 38.de Koning GHJ, Veldkamp E, Lopez-Ulloa M. Qualification of carbon sequestration in soils following pasture to forest conversion in northwestern Ecuador. Global Biogeochem Cycles. 2003;17:1098–1110. [Google Scholar]
- 39.Jackson RB, Banner JL, Jobbagy EG, Pockman WT, Wall DH. Ecosystem carbon loss with woody plant invasion of grasslands. Nature. 2002;418:623–626. doi: 10.1038/nature00910. [DOI] [PubMed] [Google Scholar]
- 40.Craine JM, Fargione J, Sugita S. Supply pre-emption, not concentration, is the mechanism of competition for nutrients. New Phytol. 2005;166:933–940. doi: 10.1111/j.1469-8137.2005.01386.x. [DOI] [PubMed] [Google Scholar]
- 41.D'Antonio CM, Hughes RF, Vitousek PM. Factors influencing dynamics of two invasive C4 grasses in seasonally dry Hawaiian woodlands. Ecology. 2001;82:89–104. [Google Scholar]
- 42.Leishman MR, Thomson VP. Experimental evidence for the effects of additional water, nutrients and physical disturbance on invasive plants in low fertility Hawkesbury Sandstone soils, Sydney, Australia. J Ecol. 2005;93:38–49. [Google Scholar]
- 43.Reed HE, Seastedt TM, Blair JM. Ecological consequences of C4 grass invasion of a C4 grassland: A dilemma for management. Ecol Appl. 2005;15:1560–1569. [Google Scholar]
- 44.Blumenthal D. Ecology—interrelated causes of plant invasion. Science. 2005;310:243–244. doi: 10.1126/science.1114851. [DOI] [PubMed] [Google Scholar]
- 45.Grigal DF, Chamberlain LM, Finney HR, Wroblewski DW, Gross ER. Soils of the Cedar Creek Natural History Area. St. Paul: University of Minnesota Agriculture Experiment Station; 1974. [Google Scholar]
- 46.Wedin DA, Tilman D. Species effects on nitrogen cycling: A test with perennial grasses. Oecologia. 1990;84:433–441. doi: 10.1007/BF00328157. [DOI] [PubMed] [Google Scholar]
- 47.Hart SC, Stark JM, Davidson EA, Firestone MK. Nitrogen mineralization, immobilization, and nitrification. In: Weaver R, Angle J, Bottomley P, editors. Methods of Soil Analysis Part 2— Microbiological and Biochemical Properties. Vol 2. Madison: Soil Sci Society of America; 1994. pp. 985–1018. [Google Scholar]
- 48.Berendse F, Aerts R. Nitrogen-use-efficiency: A biological meaningful definition. Funct Ecol. 1987;1:293–296. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.