Abstract
Exposure to traffic-related pollution (TRP) and tobacco smoke have been associated with new onset asthma in children. Psychosocial stress-related susceptibility has been proposed to explain social disparities in asthma. We investigated whether low socioeconomic status (SES) or high parental stress modified the effect of TRP and in utero tobacco smoke exposure on new onset asthma. We identified 2,497 children aged 5–9 years with no history of asthma or wheeze at study entry (2002–2003) into the Children's Health Study, a prospective cohort study in southern California. The primary outcome was parental report of doctor-diagnosed new onset asthma during 3 years of follow-up. Residential exposure to TRP was assessed using a line source dispersion model. Information about maternal smoking during pregnancy, parental education (a proxy for SES), and parental stress were collected in the study baseline questionnaire. The risk of asthma attributable to TRP was significantly higher for subjects with high parental stress (HR 1.51 across the interquartile range for TRP; 95% CI 1.16–1.96) than for subjects with low parental stress (HR 1.05, 95% CI 0.74–1.49; interaction P value 0.05). Stress also was associated with larger effects of in utero tobacco smoke. A similar pattern of increased risk of asthma was observed among children from low SES families who also were exposed to either TRP or in utero tobacco smoke. These results suggest that children from stressful households are more susceptible to the effects of TRP and in utero tobacco smoke on the development of asthma.
Keywords: socioeconomic status, tobacco smoke
Asthma is the most common chronic childhood illness in developed countries and a growing concern worldwide (1). It is considered to be a complex disease with a multifactorial etiology as established risk factors have failed to explain trends in the global epidemiology of asthma (2). The incidence of asthma has been associated with environmental factors, including combustion products in tobacco smoke, especially in utero, and in air pollution (3–5). Several studies suggest that increased severity of asthma among low socioeconomic status (SES) children and adults may be explained by stress (6, 7), yet few studies have examined whether these factors modify the risk for asthma attributable to environmental pollution.
It is generally recognized that air pollution exacerbates asthma in children (8), and some studies suggest an effect on induction of asthma (9, 10). We have recently reported associations of residential traffic-related pollution (TRP) with both prevalent and new onset asthma during follow-up in the Southern California Children's Health Study (CHS) (5, 11, 12). Effects of pollution are biologically plausible given emerging evidence from human experimental, animal, and in vitro studies suggesting that ambient particulate matter and gaseous co-pollutants cause oxidative stress and inflammation, which are important features of asthma pathogenesis (13). We have also shown asthma to be associated with another oxidant pollutant, in utero tobacco smoke (4, 14–16), results which are consistent with other studies of in utero and second hand smoke (SHS) exposure (17, 18).
Effects of air pollution on asthma and other respiratory conditions have been found to be greater among individuals of lower SES (19, 20). A possible mechanism by which SES may modify the effects of air pollution is psychological stress (6, 21). Stress has pro-oxidant effects that can increase airway inflammation (22), and high levels of stress in both children and parents predict onset of wheeze and asthma morbidity (e.g., severity, subsequent attacks) in children (23–27). Stress may also increase vulnerability to antigens through direct effects on the endocrine system, autonomic control of airways, and immune function (28, 29). Stress may thus increase vulnerability to environmental factors associated with asthma and may explain the observed susceptibility to asthma attributed to SES. Epidemiological support for this hypothesis is provided by a recent cross-sectional study showing that effects of TRP on lifetime asthma were larger in children who reported exposure to violence, a source of stress (30).
We hypothesized that low SES and high parental stress would increase childhood susceptibility for new onset asthma from 2 sources of oxidant pollution, residential TRP and maternal smoking during pregnancy. Residential exposure to TRP was assessed at study entry based on a line source dispersion model (31). Parental education was used as a proxy for SES, and parental stress was assessed using the Perceived Stress Scale (PSS), which is a widely used measure of the degree to which respondents believed their lives were unpredictable, uncontrollable, or overwhelming (32).
Results
The study population included children enrolled in a prospective cohort study of air pollution and respiratory health and followed for 3 years (11). Age ranged from 5 to 9 years at study entry; 80% of subjects were at least 6 years old (Table 1). There were slightly more girls (52%) than boys. The majority of subjects were of Hispanic ethnicity (55%), and the plurality of the remainder was non-Hispanic white (36%); there were few subjects who were African American (3%) or of other race or ethnicity (6%). The mean score for parental stress using the PSS was 3.85 (standard deviation, 2.79), with a median value of 4. In ascending order, the 4 quartiles of the PSS distribution included values of 0 to 1, 2 to 3, 4 to 5, and 6 to 15 (See Materials and Methods for details). Approximately 21% of children had parents who had not finished high school (“low SES”), while almost 79% had parents with a high school diploma or greater (“high SES”). There were 120 cases of new onset asthma during follow-up (5). Significantly increased risk of new onset asthma was associated with being African American (in comparison to Hispanic children) or underweight, having a history of chest illness or allergy, parental asthma, and musty odor in the home (Table 1). There was no association of asthma with parental stress categorized into quartiles or with a continuous stress index [hazard ratio (HR) 1.02; 95% confidence interval (CI) 0.78–1.33], and children from low SES homes had risk identical to those with a high school education or at least some college.
Table 1.
Subject characteristics and associations with new onset asthma
| Risk factor | N (%)* | Hazard ratio (95% confidence interval)† |
|---|---|---|
| Subject characteristics | ||
| Age at baseline | ||
| 5 years | 496 (19.9) | 1.00‡ |
| 6 years | 1,178 (47.1) | 1.12 (0.69–2.83) |
| 7–9 years | 823 (33.0) | 1.11 (0.66–1.87) |
| Male gender | 1,190 (47.7) | 1.07 (0.75–1.53) |
| Race/ethnicity | ||
| Hispanic ethnicity | 1,380 (55.3) | 1.00‡ |
| African-American race | 77 (3.1) | 2.44 (1.05–5.67)§ |
| White non-Hispanic race | 905 (36.2) | 1.08 (0.73–1.59) |
| Other race | 135 (5.4) | 1.67 (0.86–3.27) |
| Spanish language questionnaire | 610 (24.4) | 0.81 (0.49–1.35) |
| Body mass index | ||
| Underweight | 92 (4.0) | 2.50 (1.28–4.89)§ |
| Healthy weight | 1,587 (68.3) | 1.00‡ |
| At risk of overweight | 337 (14.5) | 1.36 (0.79–2.34) |
| Overweight | 308 (13.3) | 1.45 (0.85–2.46) |
| Chest-related illness | ||
| None | 1,932 (85.0) | 1.00‡ |
| Before age 2 | 129 (5.7) | 1.93 (0.96–3.86) |
| After age 2 | 126 (5.6) | 2.76 (1.58–4.84)§ |
| Before and after age 2 | 85 (3.7) | 2.98 (1.48–5.98)§ |
| Allergies | 764 (33.6) | 2.27 (1.54–3.33)§ |
| Child resides in more than one home | 178 (7.3) | 0.60 (0.24–1.46) |
| Parental characteristics | ||
| Parental history of asthma | 389 (17.0) | 2.05 (1.35–3.12)§ |
| Parental stress (PSS) | ||
| Quartile 1 | 563 (23.8) | 1.00‡ |
| Quartile 2 | 605 (25.6) | 1.00 (0.59–1.70) |
| Quartile 3 | 531 (22.5) | 1.21 (0.72–2.04) |
| Quartile 4 | 664 (28.1) | 0.97 (0.57–1.64) |
| Medical care & SES | ||
| Medical insurance coverage | 2,135 (87.7) | 1.36 (0.70–2.63) |
| Type of medical insurance coverage | ||
| HMO/PPO | 1,591 (65.3) | 1.00‡ |
| Social assistance | 547 (22.4) | 1.28 (0.82–2.01) |
| No insurance coverage | 300 (12.3) | 0.79 (0.82–2.01) |
| Parental education | ||
| Did not finish high school | 508 (21.2) | 1.00 (0.61–1.64) |
| High school diploma or some college | 1,318 (55.1) | 1.00‡ |
| College diploma or greater | 567 (23.7) | 0.74 (0.45–1.19) |
| Home characteristics | ||
| Mildew in home | 532 (21.8) | 1.22 (0.80–1.86) |
| Cockroaches in home | 257 (10.8) | 1.53 (0.90–2.63) |
| Water damage/flooding in home | 321 (13.1) | 1.42 (0.88–2.30) |
| Musty odor in home | 84 (3.5) | 2.15 (1.05–4.42)§ |
| Humidifier/vaporizer in home | 484 (20.1) | 1.06 (0.67–1.67) |
| Any daily smoker inside home | 164 (6.7) | 0.88 (0.41–1.90) |
| Carpet in child's bedroom | 2,090 (85.8) | 1.09 (0.63–1.88) |
| Any pets in home | 1,313 (53.9) | 0.84 (0.57–1.23) |
| Dogs in home | 730 (30.0) | 0.79 (0.52–1.21) |
| Cats in home | 462 (19.0) | 0.73 (0.43–1.24) |
| Gas stove in home | 2,042 (84.5) | 1.18 (0.70–2.02) |
| Air conditioning in home | 1,448 (60.0) | 1.16 (0.79–1.72) |
| Type of home | ||
| Single-family house | 1,938 (79.5) | 1.00‡ |
| Apartment (2–10 units) | 328 (13.5) | 1.30 (0.76–2.23) |
| Apartment (>10 units) | 102 (4.2) | 1.71 (0.73–3.96) |
| Mobile home/trailer/other | 69 (2.8) | 1.52 (0.55–4.15) |
*Numbers may not total to 2,497 because of missing values.
†Adjusted for race/ethnicity with baseline strata for age and gender (where appropriate), and community random effects.
‡Denotes reference group.
§Denotes statistically significant finding, i.e., P ≤ 0.05.
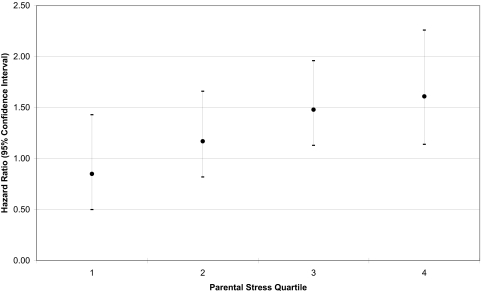
The distribution of traffic-related exposure in this population has been previously described (5, 11). As shown in Table 2, the risk of asthma onset increased with exposure to TRP [HR 1.31, 95% CI 1.07–1.61, across of the interquartile range (IQR) of 21 ppb of nitrogen oxide (NOX), an indicator of the near-source mixture of TRP]. The risk associated with TRP was higher in low SES subjects (HR 1.55, 95% CI 1.09–2.19) than in those of high SES (HR 1.20, 95% CI 0.93–1.55; P value for interaction = 0.25). Traffic-related risk of asthma was also increased in subjects with parental stress above the median (HR 1.51, 95% CI 1.16–.1.96), but there was little risk associated with TRP among subjects with parental stress below the median (HR 1.05, 95% CI 0.74–1.49, P value for interaction = 0.05). The risk of asthma associated with TRP increased monotonically across quartiles of parental stress (Fig. 1). When the interaction between parental stress and TRP was adjusted for the interaction between SES and TRP, the coefficient for the stress-related interaction was unchanged; on the other hand, the coefficient related to the interaction with SES was reduced by 27%. The overall pattern of susceptibility to traffic pollutant exposure based on parental stress was not changed by adjusting for other potential confounding susceptibility factors shown in Table 1, and the interaction P value from the model not adjusted for these factors (see Table 2) remained significant in adjusted models.
Table 2.
Associations of traffic-related pollution (TRP) with incident asthma, by parental education and parental stress
| Risk factor | Stratum | N (%)* | Mean (SD)† | 25th–75th percentile† | Hazard ratio (95% confidence interval)‡ | Interaction P value§ |
|---|---|---|---|---|---|---|
| TRP | All subjects | 2456 (100) | 18.41 (16.04) | 6.19–27.11 | 1.31 (1.07, 1.61)¶ | |
| Low parental education | 1845 (78.4) | 20.36 (17.15) | 7.53–29.24 | 1.55 (1.09, 2.19)¶ | 0.25 | |
| High parental education | 507 (21.6) | 17.79 (15.58) | 5.71–26.48 | 1.20 (0.93, 1.55) | ||
| High parental stress | 1179 (50.7) | 18.99 (16.42) | 6.85–27.61 | 1.51 (1.16, 1.96)¶ | 0.05 | |
| Low parental stress | 1145 (49.3) | 17.81 (15.79) | 5.76–26.90 | 1.05 (0.74, 1.49) |
*Denominator varies due to missing data about TRP, parental education and parental stress.
†TRP in parts per billion NOx.
‡All models are adjusted for race/ethnicity with baseline strata for age and gender and community random effects. Hazard ratios and 95% confidence intervals are scaled across the interquartile range of exposure to TRP in all subjects (21 ppb).
§P value based on the χ 2 statistic using the likelihood ratio test to compare a model with base terms only to a model also containing the multiplicative interaction term. Interactions involving parental stress are based on a continuous variable describing the PSS.
¶Indicates P value < 0.05.
Fig. 1.
Effect of traffic-related pollution on incident asthma across parental stress quartiles.
In utero tobacco smoke exposure was associated with a modest increased risk of asthma (HR 1.49, 95% CI 0.79–2.80, Table 3). However, maternal smoking during pregnancy was associated with a large increased risk of asthma among subjects with low SES (HR 5.69, 95% CI 1.88–17.3), while there was little indication of an effect among high SES subjects (HR 1.10, 95% CI 0.51–2.41, P value for interaction = 0.03). Subjects with high parental stress had an increased risk of asthma associated with in utero exposure (HR 2.66, 95% CI 1.33–5.33). The HR was less than unity with wide CIs among subjects with low parental stress (HR 0.30, 95% CI 0.04–2.18), but the effect was significantly different from that observed in children with more parental stress (P value for interaction = 0.03). Because there were relatively few participants who reported maternal smoking in utero (156, 6.3%) and small numbers in subgroups based on other potentially relevant confounders (from Table 1), we were not able to co-adjust interactions related to SES and to parental stress. We found a consistent, but weaker, pattern of susceptibility based on SHS in the home at the time of study entry. The HRs for SHS were 1.20 (95% CI 0.51, 2.80) and 0.35 (95% CI 0.05, 2.50) in the high and low stress strata, respectively, and there was little difference in the effect of SHS based on SES.
Table 3.
Associations of in utero tobacco smoke with incident asthma, by parental education and parental stress
| Risk factor | Stratum | N (%)* | Hazard ratio (95% confidence interval)† | Interaction P value‡ |
|---|---|---|---|---|
| Maternal smoking in utero | All subjects | 156 (6.3) | 1.49 (0.79, 2.80) | |
| Low parental education | 16 (3.2) | 5.69 (1.88, 17.3)§ | 0.03 | |
| High parental education | 137 (7.3) | 1.10 (0.51, 2.41) | ||
| High parental stress | 89 (7.5) | 2.66 (1.33, 5.33)§ | 0.03 | |
| Low parental stress | 62 (5.4) | 0.30 (0.04, 2.18) |
*Denominator varies due to missing data about maternal smoking in utero, parental education and parental stress.
†All models are adjusted for race/ethnicity with baseline strata for age and gender and community random effects.
‡P value based on the χ 2 statistic using the likelihood ratio test to compare a model with base terms only to a model also containing the multiplicative interaction term. Interactions involving parental stress are based on a continuous variable describing the PSS.
§Indicates P value < 0.05.
An earlier analysis based on the same study population found that the effect of parental stress on wheeze onset was modified by family history of asthma and gender, where the effect of stress was limited to males with no family history of asthma (26). The effect of regular smoking and of TRP on asthma has also been reported to be modified by personal history of allergy or by gender (4, 11). Therefore, we examined whether stress-related susceptibility to TRP varied by gender, family history of asthma, and personal history of allergy. In separate models, we reparameterized TRP into strata created by combinations of high and low parental stress with either family history of asthma, personal history of allergy and gender (i.e., 3 models, each with 4 strata-specific effects of TRP). The interaction between stress and TRP was similar within strata of family history of asthma and personal history of allergy. Effect modification by stress appeared to be stronger for male subjects. In a model that estimated the effect of TRP in subjects with high versus low parental stress, for males and females separately (i.e., 4 strata total), the HR for TRP among males with high parental stress was 1.60 (95% CI 1.16–2.22) compared to 0.98 (95% CI 0.61–1.59) among males with low parental stress (P value for interaction among males = 0.02). Within the same model, an HR of 1.36 (95% CI .87–2.11) was observed for females with high parental stress compared with 1.14 (95% CI 0.68–1.90) for females with low parental stress (P value for interaction among females = 0.84). The P value for a 3-way “gender-parental stress-TRP” interaction term was 0.10. When parental education was substituted for stress, there was no evidence of a 3-way interaction.
Discussion
Children whose parents perceived their lives as unpredictable, uncontrollable, or overwhelming had increased risk of new onset asthma associated with TRP and maternal smoking during pregnancy. Furthermore, susceptibility to TRP attributable to parental education was markedly attenuated after accounting for the susceptibility attributable to parental stress. While parental stress may influence the development of asthma in a child due to biological and behavioral pathways other than psychological stress in children (26), the observed pattern of susceptibility to air pollution based on stress was not explained by potentially relevant history of illness and a range of behavioral, socioeconomic, and environmental risk factors for asthma. Although there were relatively few children with a history of in utero tobacco smoke exposure, significantly larger effects were observed both among children with low parental education and with high parental stress. Thus, common biological pathways may underlie the relationship of asthma to stress and combustion products common to both air pollution and cigarette smoke. Previous studies have reported effects of stress on lifetime asthma (30), asthma severity (33), and incident wheeze (27, 34).
Particulate and gaseous air pollutants can promote inflammatory responses in the airways, which are a central feature of asthma (34–36). The mechanisms linking exposure to inflammation have been intensively studied in recent years. Exposure to ambient particulate matter has been associated with the generation of reactive oxygen species, which are mediators of inflammation (37–40). Air pollution may also have an adjuvant effect with common allergens that favors the development of a T helper cell 2 response, a hallmark of allergic asthma (41, 42). Finally, these pollutants may also directly increase inflammation by enhancing mast cell degranulation and cytokine release (43, 44).
Emerging evidence indicates that individual variation in the inflammatory response to oxidative stress is important in the pathogenesis of asthma associated with oxidant air pollutants (45). Chronic psychological stress may modulate the response to oxidative burden, possibly due to the development of hypothalamic-pituitary-adrenal axis hyporesponsiveness resulting in a shift toward a proinflammatory T helper cell 2 phenotype (22, 29, 46). Therefore, an increase in oxidative stress and associated inflammatory response is 1 possible explanation for the stronger associations of air pollution and tobacco smoke with asthma in children with chronic psychological stress. Chronic psychological stress may also explain the larger effects of air pollution in individuals of lower SES reported elsewhere (19), as low SES is associated with more stressful environments (47).
In utero exposure to tobacco smoke has been found to increase the risk of asthma in several studies including a limited number of prospective studies (4, 18, 48). Although the main effect of this exposure was not statistically significant by itself, our results suggest that oxidant pollutant exposures to tobacco smoke early in life may have increased the susceptibility to later asthma onset due to effects of co-exposure to stress and to factors associated with low parental education. Differences in susceptibility to effects of SHS at the time of study entry were less marked. However, the strong effect of in utero or early life tobacco smoke exposure on subsequent asthma is consistent with previous findings from the CHS and elsewhere (4, 14, 49–52). In utero exposure leads to more direct exposure to, and possibly a higher dose of, combustion products of tobacco than second hand exposure during an especially vulnerable period of lung development.
Although the rates of maternal smoking during pregnancy in our study sample (6.3%) were somewhat lower than in the greater California population (9.5%) (53), this was largely explained by the exclusion by design of participants with wheeze at study entry, who had a higher rate of maternal smoking in utero (11.3%) than our longitudinal study sample. Lower rates of maternal smoking during pregnancy were observed in low (3.2%) than in high SES subjects (7.3%; see Table 3), which is opposite to the relationship reported elsewhere (54). This may be explained by the large proportion of Hispanics in our population who were of low SES (35.0%) compared with non-Hispanic subjects (4.0%), and the low rates of smoking during pregnancy among Hispanic (2.9%) compared with non-Hispanic mothers (10.8%). However, we found no evidence that Hispanic ethnicity explained the increased risk of asthma associated with the joint exposure to stress (or SES) and to in utero tobacco smoke. Although these results are consistent with the robust joint effects of TRP exposure and stress, the effects of in utero tobacco smoke exposure should be interpreted with caution due to small sample size in some strata of exposure and the low smoking rates among the largest ethnic group in our cohort.
Our results may not be generalizable to age groups beyond our primary school age range as risk factors for asthma, including atopy and the effect of variation in genes involved in modulating the response to oxidative stress, depend on age of asthma onset (55, 56). In addition, children's responses to psychological stress are age-dependent. For example, preschool children in a stressful situation are more likely to seek support from a caregiver, intervene by hitting someone, or play as a distracting behavior. School children are more likely to seek support from friends, to have developed cognitive and behavioral intervention strategies based on talking, and to have other problem solving skills less dependent on parents (57). Therefore, the relationship between psychological stress, air pollution exposure, and asthma might not be the same at different ages.
Differences in susceptibility by gender that we observed may reflect differences in the development of asthma, because boys tend to experience asthma onset earlier in childhood than girls (58). On the other hand, boys in our study may have been more likely to be negatively affected by parental stress than girls. In particular, boys may be more sensitive to dysphoria (e.g., mood disorders, sadness) in parents compared to girls (59). Also, there may be differences in behavioral responses to stress between males and females; for example, it has been suggested that girls tend to seek social support when responding to stress, while the reflexive “fight-or-flight” response may be more predominant among boys (60).
Case ascertainment was done by parental report of physician-diagnosed asthma without clinical examination, which is widely used in epidemiological studies (61), is reproducible (62, 63), and is a valid measure of what physicians actually report to patients (64, 65). Examinations of stress and asthma using cross-sectional measurement have limitations, because sick children may cause stress in parents. However, the prospective study design and the restriction at baseline to children with no history of wheeze makes it unlikely that parental stress at study baseline resulted from earlier undiagnosed asthma. Also, in a sensitivity analysis, we excluded cases occurring during the first year of follow-up and the pattern of effects of stress and TRP was not substantially changed. Physician diagnosis of asthma is a relatively specific but somewhat insensitive method of detection of incident asthma and may be subject to bias due to access to care or to differences in assessment between physicians. Therefore, we also examined the effects of stress and TRP on new onset asthma based on either new report of physician diagnosis or first report of severe symptoms suggestive of asthma (4 or more attacks of wheeze, 1 or more nights per week of wheeze, or wheeze with shortness of breath so severe as to interfere with speech). There were 52 new cases of asthma added using this definition. We found a similar pattern of effects, suggesting that diagnostic bias or access to care did not explain our results.
Mean levels of TRP were higher among subjects with higher parental stress and lower parental education, although the ranges of exposure in these strata overlapped. If the main effect of TRP on asthma onset was nonlinear, e.g., quadratic, then the larger effects of TRP observed among subjects with higher parental stress may have reflected exposure to higher levels of TRP. However, a previous analysis found the main effect of TRP on asthma onset was linear over the range of exposure (5), which suggests that the stress-related TRP susceptibility was not simply a reflection of larger effects of TRP at higher exposure levels.
Parental stress measured with the PSS was 1 of the few indicators available for assessment of psychological stress in a large population-based survey of young school children. Although we did not directly measure stress in the children, previous research has demonstrated a relationship between parental, especially maternal, stress and psychological stress in children (66, 67). When we limited the analysis to children whose biological mother responded to the baseline questionnaire (81% of subjects), the interaction between TRP and parental stress grew stronger (interaction term HR 1.41 versus 1.36 in the total sample). The effect of TRP in high maternal stress children was 2.04 (95% CI 1.31–3.16) and was 0.84 (95% CI 0.50–1.40) in children of low maternal stress. These results are consistent with the intensive caregiving role that mothers traditionally play during childhood (68). Parental PSS in other studies predicted asthma-related outcomes in children prospectively. For example, high parental stress measured in the months immediately following birth predicted increased severity of asthma and onset of wheeze among children (27, 69). The joint effects of stress and traffic or tobacco smoke exposure were not examined in these studies. We observed little effect of stress in the absence of exposure to oxidant pollutants, so it is possible that the children in these studies were in high pollution environments or that the effect of stress varies by age, requiring co-exposure to oxidant pollutants in children of school age, but not in younger children.
This study provides evidence that parental stress increases susceptibility to new onset childhood asthma associated with traffic-related air pollution. The similarity in the pattern of susceptibility to maternal smoking in utero suggests that biological pathways common to the response to combustion products may explain this susceptibility. Further study is warranted to evaluate the role of stress induced by characteristics of life in low SES environments as a potential explanation for disparities in the health impact of air pollution observed in low SES populations. More broadly, understanding the role of air pollution in the causation of complex diseases like asthma requires consideration of how social factors may modify the effects of environmental exposures.
Materials and Methods
Study Population.
The CHS cohort enrolled students in kindergarten and first grade (ages 5–9) from participating schools in 13 southern California communities in 2002 and 2003 (11). All students in kindergarten and first grade at selected schools in the 13 study communities were invited to participate, and 5,349 (65%) returned valid questionnaires. In order to remove subjects with previously undiagnosed asthma from follow-up, children were excluded if they had a history of physician diagnosed asthma at study entry (715), a history of wheezing episodes (1,505), and missing or “don't know” responses about history of asthma (397) or wheeze (261). Of the 3,372 children classified as “disease free” at baseline, 340 children had no information about residential TRP because their home address could not be geo-coded, and another 535 children were lost before 1 year of follow-up. Therefore, the study population for this analysis included 2,497 children with no history of asthma or wheeze at study entry. Informed consent was obtained from parents, and the study was approved by the University of Southern California Institutional Review Board.
Assessment of New Onset Asthma and Covariates.
Assessment of new onset asthma and covariates was based on questionnaires at study entry and annually during follow-up by parents of children enrolled in the study. Children with new onset asthma were identified by parental report of physician-diagnosed asthma on annual questionnaires during 3 years of follow-up. Household exposure to TRP was assessed based on a line source dispersion model of total NOX (see below), and information was collected at study entry from responses given by parents on a baseline questionnaire about in utero exposure to tobacco smoke. Variables describing potential effect modifiers were also measured from responses on the baseline questionnaire. Educational attainment in parents was used as a measure of SES. The PSS, which was used to measure parental stress, has been validated as a measure of negative affective states and physical symptoms of stress (70, 71). We used a 4-item version of the scale that has been previously used to predict incidence of wheeze in children (26, 27). Items included: “In the last month, how often have you felt”: (i) “that you were unable to control the important things in your life;” (ii) “confident about your ability to handle your personal problems;” (iii) “that things were going your way;” and (iv) “your difficulties were piling up so high that you could not overcome them.” Each item is scored on a scale of 0–4, and the PSS gives equal weight to each item, resulting in scores ranging from 0 to 16. A representative U.S. sample found an overall mean and standard deviation of 4.49 and 2.96, respectively (32).
Potential confounders in this study were defined as variables that could plausibly explain increased effects of TRP or in utero exposure to tobacco smoke on new onset asthma in subjects with lower SES or with higher parental stress. Covariates considered as potential confounders in this study were measured from responses given by parents on the baseline questionnaire at study entry. In addition to race and ethnicity, English- or Spanish-language questionnaire response was recorded for each subject. Characteristics of the child's current residence included mold or mildew on household surfaces, history of water damage or flooding, presence of a musty odor, history of cockroaches and other pests, use of a gas stove, air conditioner, humidifier or vaporizer, carpet in the child's bedroom, type of dwelling, and whether the child lived at another dwelling for more than 50 days per year. Exposure to SHS was assessed by asking whether anyone currently living in the child's home smoked cigarettes, cigars, or pipes inside the home on a daily basis. Type of medical insurance coverage, history of chest-related illness and allergies, and family history of asthma were reported, and body mass index was calculated based on measurements of height and weight at study baseline using the Centers for Disease Control and Prevention gender-specific body mass index-for-age reference values for the year 2000. Accordingly, subjects with a body mass index below the 5th percentile of the reference values were classified as underweight, while those between 85th and 95th percentile were at risk for becoming overweight, and those above the 95th percentile were overweight.
Air Pollution Exposure Assessment.
Methods to estimate exposure to local TRP in this cohort have been described elsewhere (11). Briefly, household exposure to total NOX from traffic on local roads was estimated as a marker for pollutants from traffic exhaust using the CALINE4 dispersion model (31). Estimates of TRP represented annual average incremental increases due to primary emissions from local vehicular traffic independent of background ambient levels (11). Because there was a high correlation between measures of TRP and other pollutants generated using the same model (e.g., carbon monoxide, nitrogen dioxide, elemental and organic carbon, and particulate matter with aerodynamic diameter less than 10 and less than 2.5 μg/m3) (R >0.90), measures of TRP represented not only primary local NOX from vehicular traffic, but a mixture of other pollutants related to near-source traffic exposure (11).
Statistical Methods.
Risk factors for asthma onset were assessed using multilevel Cox proportional hazards models (72). All models contained age and gender stratifications of the baseline hazard, adjustment for race and ethnicity, and random effects for community of residence, which allowed for clustering and assessment of residual community variation in time to asthma onset. Analyses were conducted using R software (73) and software designed to run within R for implementing random effects Cox proportional hazards models (72, 74). The multilevel Cox proportional hazards model took the following form:
hij (t) = h0s(t) ηj exp(βXij + δTZij);
hij(t): hazard function for the ith subject in jth community;
h0s(t) : the baseline hazard function for stratum s (i.e., age at study entry and gender);
ηj : positive random effects for community j with expectation 1 and variance σ2;
Zij : risk factors (e.g., race and ethnicity) for individual i in community j; and
Xij : TRP or maternal smoking in utero for individual i in community j.
Modification of the effect of TRP and maternal smoking during pregnancy by SES and parental stress was assessed by modeling multiplicative interaction terms along with base terms. We evaluated confounding of pollutant interactions with parental stress using a 2-step process. For example, for TRP, all relevant covariates were first screened for 2-way interactions with TRP on asthma onset using an alpha level of 0.20. Second, the 2-way interaction models for parental stress with TRP were co-adjusted for relevant interactions from the first step (i.e., P < 0.20), and confounding was identified where the coefficient for the stress-specific interaction term was changed by more than 10%.
Acknowledgments.
This work was supported by National Institute of Environmental Health Sciences Grants 5R03ES014046, 1R01 ES016535, 5P01ES009581, 5P01ES011627, and 5P30ES007048; U.S. Environmental Protection Agency Grants R831845, RD831861, and R826708; National Cancer Institute Grant 1U54CA116848- 01; the Hastings Foundation; and the Canadian Institutes of Health Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Centers for Disease Control and Prevention. National Center for Environmental Health. 2003 [Google Scholar]
- 2.Pearce N, Douwes J. The global epidemiology of asthma in children. Int J Tuberc Lung Dis. 2006;10:125–132. [PubMed] [Google Scholar]
- 3.King ME, Mannino DM, Holguin F. Risk factors for asthma incidence. A review of recent prospective evidence. Panminerva Med. 2004;46:97–110. [PubMed] [Google Scholar]
- 4.Gilliland FD, et al. Regular smoking and asthma incidence in adolescents. Am J Respir Crit Care Med. 2006;174:1094–1100. doi: 10.1164/rccm.200605-722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McConnell R, et al. Childhood incident asthma and traffic-related pollution in a longitudinal cohort study. Am J Respir Crit Care Med. 2007;175:A304. [Google Scholar]
- 6.Chen E, et al. Socioeconomic status and inflammatory processes in childhood asthma: The role of psychological stress. J Allergy Clin Immunol. 2006;117:1014–1020. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Chen E, Fisher EB, Bacharier LB, Strunk RC. Socioeconomic status, stress, and immune markers in adolescents with asthma. Psychosom Med. 2003;65:984–992. doi: 10.1097/01.psy.0000097340.54195.3c. [DOI] [PubMed] [Google Scholar]
- 8.Schildcrout JS, et al. Ambient air pollution and asthma exacerbations in children: An eight-city analysis. Am J Epidemiol. 2006;164:505–517. doi: 10.1093/aje/kwj225. [DOI] [PubMed] [Google Scholar]
- 9.McConnell R, et al. Asthma in exercising children exposed to ozone: A cohort study. Lancet. 2002;359:386–391. doi: 10.1016/S0140-6736(02)07597-9. [DOI] [PubMed] [Google Scholar]
- 10.Sarnat JA, Holguin F. Asthma and air quality. Curr Opin Pulm Med. 2007;13:63–66. doi: 10.1097/MCP.0b013e3280117d25. [DOI] [PubMed] [Google Scholar]
- 11.McConnell R, et al. Traffic, susceptibility, and childhood asthma. Environ Health Perspect. 2006;114:766–772. doi: 10.1289/ehp.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jerrett M, et al. Traffic-related air pollution and asthma onset in children: A prospective cohort study with individual exposure measurement. Environ Health Perspect. 2008;116:1433–1438. doi: 10.1289/ehp.10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li N, Hao M, Phalen RF, Hinds WC, Nel AE. Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in pm-induced adverse health effects. Clin Immunol. 2003;109:250–265. doi: 10.1016/j.clim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med. 2001;163:429–436. doi: 10.1164/ajrccm.163.2.2006009. [DOI] [PubMed] [Google Scholar]
- 15.Salam MT, Li YF, Langholz B, Gilliland FD. Early-life environmental risk factors for asthma: Findings from the children's health study. Environ Health Perspect. 2004;112:760–765. doi: 10.1289/ehp.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li YF, Langholz B, Salam MT, Gilliland FD. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest. 2005;127:1232–1241. doi: 10.1378/chest.127.4.1232. [DOI] [PubMed] [Google Scholar]
- 17.Boulet LP, et al. Smoking and asthma: Clinical and radiologic features, lung function, and airway inflammation. Chest. 2006;129:661–668. doi: 10.1378/chest.129.3.661. [DOI] [PubMed] [Google Scholar]
- 18.Pattenden S, et al. Parental smoking and children's respiratory health: Independent effects of prenatal and postnatal exposure. Tob Control. 2006;15:294–301. doi: 10.1136/tc.2005.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wheeler BW, Ben-Shlomo Y. Environmental equity, air quality, socioeconomic status, and respiratory health: A linkage analysis of routine data from the health survey for england. J Epidemiol Community Health. 2005;59:948–954. doi: 10.1136/jech.2005.036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burra TA, Moineddin R, Agha MM, Glazier RH. Social disadvantage, airpollution, and asthma physician visits in Toronto, Canada. Environ Res. 2009;109:567–574. doi: 10.1016/j.envres.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Gold DR, Wright R. Population disparities in asthma. Annu Rev Public Health. 2005;26:89–113. doi: 10.1146/annurev.publhealth.26.021304.144528. [DOI] [PubMed] [Google Scholar]
- 22.Wright RJ, Cohen RT, Cohen S. The impact of stress on the development and expression of atopy. Curr Opin Allergy Clin Immunol. 2005;5:23–29. doi: 10.1097/00130832-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Kilpelainen M, Koskenvuo M, Helenius H, Terho EO. Stressful life events promote the manifestation of asthma and atopic diseases. Clin Exp Allergy. 2002;32:256–263. doi: 10.1046/j.1365-2222.2002.01282.x. [DOI] [PubMed] [Google Scholar]
- 24.Sandberg S, et al. The role of acute and chronic stress in asthma attacks in children. Lancet. 2000;356:982–987. doi: 10.1016/S0140-6736(00)02715-X. [DOI] [PubMed] [Google Scholar]
- 25.Shalowitz MU, Berry CA, Quinn KA, Wolf RL. The relationship of life stressors and maternal depression to pediatric asthma morbidity in a subspecialty practice. Ambul Pediatr. 2001;1:185–193. doi: 10.1367/1539-4409(2001)001<0185:trolsa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Milam J, et al. Parental stress and childhood wheeze in a prospective cohort study. J Asthma. 2008;45:319–323. doi: 10.1080/02770900801930277. [DOI] [PubMed] [Google Scholar]
- 27.Wright RJ, Cohen S, Carey V, Weiss ST, Gold DR. Parental stress as a predictor of wheezing in infancy: A prospective birth-cohort study. Am J Respir Crit Care Med. 2002;165:358–365. doi: 10.1164/ajrccm.165.3.2102016. [DOI] [PubMed] [Google Scholar]
- 28.Marshall GD, Jr, Agarwal SK. Stress, immune regulation, and immunity: Applications for asthma. Allergy Asthma Proc. 2000;21:241–246. doi: 10.2500/108854100778248917. [DOI] [PubMed] [Google Scholar]
- 29.Wright RJ, Rodriguez M, Cohen S. Review of psychosocial stress and asthma: An integrated biopsychosocial approach. Thorax. 1998;53:1066–1074. doi: 10.1136/thx.53.12.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clougherty JE, et al. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ Health Perspect. 2007;115:1140–1146. doi: 10.1289/ehp.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benson P. Sacramento, CA: Division of New Technology and Research; 1989. State of California Department of Transportation. [Google Scholar]
- 32.Cohen S, Williamson G. In: The Social Psychology of Health. Spacapan S, Oskamp S, editors. Newbury Park, CA: Sage; 1988. pp. 31–67. [Google Scholar]
- 33.Liu LY, et al. School examinations enhance airway inflammation to antigen challenge. Am J Respir Crit Care Med. 2002;165:1062–1067. doi: 10.1164/ajrccm.165.8.2109065. [DOI] [PubMed] [Google Scholar]
- 34.McConnell R, Milam J, Jerrett M, Yao L, Richardson J. Parental stress and incident wheeze in a cohort of children from southern California. Am J Respir Crit Care Med. 2005;2:A605. [Google Scholar]
- 35.Tatum AJ, Shapiro GG. The effects of outdoor air pollution and tobacco smoke on asthma. Immunol Allergy Clin North Am. 2005;25:15–30. doi: 10.1016/j.iac.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Nel AE, Diaz-Sanchez D, Li N. The role of particulate pollutants in pulmonary inflammation and asthma: Evidence for the involvement of organic chemicals and oxidative stress. Curr Opin Pulm Med. 2001;7:20–26. doi: 10.1097/00063198-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Li XY, Gilmour PS, Donaldson K, MacNee W. Free radical activity and pro-inflammatory effects of particulate air pollution (pm10) in vivo and in vitro. Thorax. 1996;51:1216–1222. doi: 10.1136/thx.51.12.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumagai Y, et al. Generation of reactive oxygen species during interaction of diesel exhaust particle components with nadph-cytochrome p450 reductase and involvement of the bioactivation in the DNA damage. Free Radic Biol Med. 1997;22:479–487. doi: 10.1016/s0891-5849(96)00341-3. [DOI] [PubMed] [Google Scholar]
- 39.Blomberg A, et al. Nasal cavity lining fluid ascorbic acid concentration increases in healthy human volunteers following short term exposure to diesel exhaust. Free Radic Res. 1998;28:59–67. doi: 10.3109/10715769809097876. [DOI] [PubMed] [Google Scholar]
- 40.Pourazar J, et al. Diesel exhaust activates redox-sensitive transcription factors and kinases in human airways. Am J Physiol Lung Cell Mol Physiol. 2005;289:L724–L730. doi: 10.1152/ajplung.00055.2005. [DOI] [PubMed] [Google Scholar]
- 41.Fujieda S, Diaz-Sanchez D, Saxon A. Combined nasal challenge with diesel exhaust particles and allergen induces in vivo IgE isotype switching. Am J Respir cell Mol Biol. 1998;19:507–512. doi: 10.1165/ajrcmb.19.3.3143. [DOI] [PubMed] [Google Scholar]
- 42.Diaz-Sanchez D, Tsien A, Fleming J, Saxon A. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human in vivo nasal ragweed-specific IgE and skews cytokine production to a T helper cell 2-type pattern. J Immunol. 1997;158:2406–2413. [PubMed] [Google Scholar]
- 43.Nel A. Atmosphere. Air pollution-related illness: Effects of particles. Science. 2005;308:804–806. doi: 10.1126/science.1108752. [DOI] [PubMed] [Google Scholar]
- 44.Diaz-Sanchez D, Penichet-Garcia M, Saxon A. Diesel exhaust particles directly induce activated mast cells to degranulate and increase histamine levels and symptom severity. J Allergy Clin Immunol. 2000;106:1140–1146. doi: 10.1067/mai.2000.111144. [DOI] [PubMed] [Google Scholar]
- 45.Salam MT, Islam T, Gilliland FD. Recent evidence for adverse effects of residential proximity to traffic sources on asthma. Curr Opin Pulm Med. 2008;14:3–8. doi: 10.1097/MCP.0b013e3282f1987a. [DOI] [PubMed] [Google Scholar]
- 46.Umetsu DT, Dekruyff RH. Immune dysregulation in asthma. Curr Opin Immunol. 2006;18:727–732. doi: 10.1016/j.coi.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Steptoe A, Feldman PJ. Neighborhood problems as sources of chronic stress: Development of a measure of neighborhood problems, and associations with socioeconomic status and health. Ann Behav Med. 2001;23:177–185. doi: 10.1207/S15324796ABM2303_5. [DOI] [PubMed] [Google Scholar]
- 48.Strachan DP, Cook DG. Health effects of passive smoking. 6. Parental smoking and childhood asthma: Longitudinal and case-control studies. Thorax. 1998;53:204–212. doi: 10.1136/thx.53.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilliland FD, Berhane K, Li YF, Rappaport EB, Peters JM. Effects of early onset asthma and in utero exposure to maternal smoking on childhood lung function. Am J Respir Crit Care Med. 2003;167:917–924. doi: 10.1164/rccm.200206-616OC. [DOI] [PubMed] [Google Scholar]
- 50.Gilliland FD, et al. Maternal smoking during pregnancy, environmental tobacco smoke exposure and childhood lung function. Thorax. 2000;55:271–276. doi: 10.1136/thorax.55.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilliland FD, et al. Effects of glutathione s-transferase m1, maternal smoking during pregnancy, and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med. 2002;166:457–463. doi: 10.1164/rccm.2112064. [DOI] [PubMed] [Google Scholar]
- 52.Li YF, et al. Effects of in utero and environmental tobacco smoke exposure on lung function in boys and girls with and without asthma. Am J Respir Crit Care Med. 2000;162:2097–2104. doi: 10.1164/ajrccm.162.6.2004178. [DOI] [PubMed] [Google Scholar]
- 53.Schumacher J. In: Women's Health: Findings from the California Women's Health Survey, 1997–2003. Weinbaum Z, Thorfinnson T, editors. Sacramento, California: California Department of Health Services, Office of Women's Health; 2006. [Google Scholar]
- 54.Lu Y, Tong S, Oldenburg B. Determinants of smoking and cessation during and after pregnancy. Health Promot Int. 2001;16:355–365. doi: 10.1093/heapro/16.4.355. [DOI] [PubMed] [Google Scholar]
- 55.Pearce N, Pekkanen J, Beasley R. How much asthma is really attributable to atopy? Thorax. 1999;54:268–272. doi: 10.1136/thx.54.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li YF, et al. Glutathione s-transferase p1, maternal smoking, and asthma in children: A haplotype-based analysis. Environ Health Perspect. 2008;116:409–415. doi: 10.1289/ehp.10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skinner EA, Zimmer-Gembeck MJ. The development of coping. Annu Rev Psychol. 2007;58:119–144. doi: 10.1146/annurev.psych.58.110405.085705. [DOI] [PubMed] [Google Scholar]
- 58.De Marco R, et al. Incidence and remission of asthma: A retrospective study on the natural history of asthma in Italy. J Allergy Clin Immunol. 2002;110:228–235. doi: 10.1067/mai.2002.125600. [DOI] [PubMed] [Google Scholar]
- 59.Cummings E, Davies PT. Children and Marital Conflict: The Impact of Family Dispute and Resolution. New York: Guilford; 1994. [Google Scholar]
- 60.Taylor SE, et al. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- 61.Burr ML. Diagnosing asthma by questionnaire in epidemiological surveys. Clin Exp Allergy. 1992;22:509–510. doi: 10.1111/j.1365-2222.1992.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 62.Ehrlich RI, et al. Prevalence and reliability of asthma symptoms in primary school children in cape town. Int J Epidemiol. 1995;24:1138–1145. doi: 10.1093/ije/24.6.1138. [DOI] [PubMed] [Google Scholar]
- 63.Peat JK, Salome CM, Toelle BG, Bauman A, Woolcock AJ. Reliability of a respiratory history questionnaire and effect of mode of administration on classification of asthma in children. Chest. 1992;102:153–157. doi: 10.1378/chest.102.1.153. [DOI] [PubMed] [Google Scholar]
- 64.Greer JR, Abbey DE, Burchette RJ. Asthma related to occupational and ambient air pollutants in nonsmokers. J Occup Med. 1993;35:909–915. doi: 10.1097/00043764-199309000-00014. [DOI] [PubMed] [Google Scholar]
- 65.Burney PG, et al. Validity and repeatability of the IUATLD (1984) Bronchial Symptoms Questionnaire: An international comparison. Eur Respir J. 1989;2:940–945. [PubMed] [Google Scholar]
- 66.Hodges WF, London J, Colwell JB. Stress in parents and late elementary age children in divorced and intact families and child adjustment. J Divorce Remarriage. 1990;14:63–80. [Google Scholar]
- 67.Alpern L, Lyons-Ruth K. Preschool children at social risk: Chronicity and timing of maternal depressive symptoms and child behavior problems at school and at home. Dev Psychopathol. 1993;5:371–387. [Google Scholar]
- 68.Russell G, Russell A. Mother-child and father-child relationships in middle childhood. Child Dev. 1987;58:1573–1585. [Google Scholar]
- 69.Wright RJ, et al. Community violence and asthma morbidity: The inner-city asthma study. Am J Public Health. 2004;94:625–632. doi: 10.2105/ajph.94.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hewitt PL, Flett GL, Mosher SW. The perceived stress scale: Factor structure and relation to depression symptoms in a psychiatric sample. J Psychopathol Behav Assess. 1992;14:247–257. [Google Scholar]
- 71.Pbert L, Doerfler LA, DeCosimo D. An evaluation of the perceived stress scale in two clinical populations. J Psychopathol Behavior Assess. 1992;14:363–375. [Google Scholar]
- 72.Ma R, Krewski D, Burnett RT. Random effects cox models: A poisson modelling approach. Biometrika. 2003;90:157–169. [Google Scholar]
- 73.R Development Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2005. [Google Scholar]
- 74.Jerrett M, et al. Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology. 2005;16:727–736. doi: 10.1097/01.ede.0000181630.15826.7d. [DOI] [PubMed] [Google Scholar]