Abstract
Some γδ and αβ T lymphocytes exhibit an “innate” phenotype associated with rapid cytokine responses. The PLZF transcription factor is essential for the innate phenotype of NKT cells. This report shows that PLZF is likewise responsible for the innate, NKT-like phenotype of Vγ1+Vδ6.3/Vδ6.4+ cells. TCR cross-linking induced PLZF expression in all polyclonal immature γδ thymocytes, suggesting that agonist selection might be required for PLZF induction. Transgenic expression of Vγ1Vδ6.4 TCR was sufficient to support the development of large numbers of PLZF+ T cells, further supporting the importance of the TCR for PLZF induction. Interestingly, expression of this TCR transgene led to the development of spontaneous dermatitis.
Keywords: agonist selection, innate T cells, transgenes
Multiple T-cell lineages develop in the thymus from common progenitors. The majority of cells in the adult thymus represent “conventional” naïve CD4 and CD8 T cells or their precursors. Conventional T cells have a highly diverse TCR repertoire, recognize peptides in the context of classical MHC class I or class II molecules, undergo positive and negative selection, and, upon activation in the periphery, require several days to develop effector functions. Once activated, some of the cells can differentiate into memory T cells that rapidly respond to a second stimulus. Several “nonconventional” T-cell subsets break off this mainstream path at different stages of T-cell development. These subsets often have a limited TCR repertoire, recognize nonclassical MHC molecules, exhibit a surface phenotype similar to activated or memory conventional T cells, and rapidly generate effector responses. In this regard, they resemble cells of the innate immune system and thus are often called innate or innate-like lymphocytes (1, 2).
One group of cells that separates from the common stem of T lymphocyte development contains γδ T cells. As a rule, cells that succeed to express TCRγδ on the cell surface, do not coexpress CD4 and CD8 coreceptors, and different subsets egress to different anatomical locations such as epidermis, mucosal surfaces, secondary lymphoid organs, as well as other tissues at different stages of ontogeny (3, 4). Several nonconventional lineages are also generated from pre-TCR-expressing precursors after they progress to the DP stage and rearrange the Tcra locus. For instance, invariant NKT-cells—a subset of T lymphocytes that can recognize lipid antigens in the context of CD1d molecules by a semi-invariant TCR—and intestinal CD8αα TCRαβ intraepithelial lymphocytes (IELs) were shown to break off at the DP stage by fate mapping (5, 6). Other examples of nonconventional T cells that are likely to progress through the DP stage include H2-M3-specific CD8+ T cells (7) and MR1-specific mucosal invariant T (MAIT) cells (8).
Although these subsets diverge from the conventional pathway of T-cell development at different stages and home to different tissues, they exhibit similar innate-like properties (1), suggesting that the common features might depend on a common event during their development. Two mutually nonexclusive hypotheses were proposed. The agonist selection hypothesis suggests that nonconventional T cells are selected by a relatively strong TCR signal resulting from ligation of their TCR by endogenous ligands (9). In fact, the activated phenotype of many nonconventional T-cell subsets suggests that this scenario may apply. A modification of this hypothesis proposes that selection by ligands specifically expressed on hematopoietic cells represents a crucial step for the development of the innate-like properties of NKT and other nonconventional T cells (1). The homotypic interaction between SLAM receptors (10) and downstream signaling via the SAP adaptor (11) was shown to represent an important component during the selection on hematopoietic cells, which is required for NKT cell development and development of other αβ T cells that are selected by classical MHC molecules expressed by thymocytes (12).
Little is known about the transcriptional regulation resulting in the innate-like properties of these cells. Perhaps the best studied cells with regard to transcriptional regulation are NKT cells. Recently the BTB-zinc finger transcription factor PLZF (promyelocytic leukemia zinc finger protein) was shown to be required for the development of functional NKT cells (13, 14). Of note, PLZF was not required for the development of cells with TCRs typical for NKT cells that were present in PLZF-deficient mice, albeit in reduced numbers. Rather, PLZF was required for acquisition of the innate-like properties of these cells such as rapid cytokine production, ability to produce simultaneously Th1 and Th2 cytokines, and exhibiting an activated phenotype (13, 14).
Besides some general similarities between nonconventional T-cell lineages, some of them seem to be more related to each other than the others. For instance, MAIT cells were believed to be very closely related to NKT cells (8), and in fact, MAIT cells exhibit high levels of the PLZF expression (13). Another group of T cells that closely resembles NKT cells in terms of surface phenotype, tissue distribution, and cytokine responses are Vγ1+Vδ6.3+ in B6 or Vγ1+Vδ6.4+ γδ T cell in DBA/2 mice (15–18).
Here we show that Vγ1+Vδ6.3/Vδ6.4+ cells also require PLZF expression to acquire NKT-cell-like properties. Moreover we demonstrate that expression of PLZF can be induced in polyclonal, immature, but not mature γδ thymocytes expressing a diverse TCR repertoire by a TCR cross-linking, suggesting that agonist selection might be a mechanism governing acquisition of “innate” properties in PLZF-expressing T cells. These results reveal a remarkable plasticity in differentiation programs of αβ and γδ T-cell lineages that initially follow different developmental pathways but later can converge in the transcriptional regulation of similar effector function programs.
Results
PLZF Positive Vγ1+Vδ6.3+ γδ T Cells.
When it was shown that PLZF is required for the acquisition of an innate phenotype by NKT cells (13, 14), we hypothesized that it might play a similar role in other types of nonconventional T cells. We first tested expression in various subsets of T cells with a monoclonal PLZF antibody using PLZF-deficient mice (19) as specificity control. We failed to detect PLZF expression in any subset of intraepithelial T cells of the small intestine including TCRγδ+ and TCRαβ+CD8α+CD8β− populations (Fig. S1). However, a fraction of PLZF+ cells that were not stained by PBS57 loaded CD1d tetramers, specifically binding to TCRs on NKT cells, was readily detected in the thymus. A substantial fraction of PLZF+ non-NKT cells expressed γδ TCRs. In fact 12.9 ± 4.3% of γδ thymocytes, 13.5 ± 6.5% of γδ splenocytes, and 12.7 ± 5.4% of γδ T cells in liver were PLZF+ (Fig. 1A).
Fig. 1.
PLZF is expressed by a subset of γδ T cells. (A) Cells isolated from thymi, spleens, and livers of wt or PLZF-deficient mice were stained for surface expression of B220, TCRγδ, TCRβ, and for intracellular PLZF and analyzed by FACS. TCRγδ versus TCRβ staining on B220− population is shown (Left and Center). PLZF expression in TCRγδ+ population is shown (Right). (B) Wt thymocytes were stained for surface expression of TCRγδ and individual Vγ, and intracellular expression of PLZF was analyzed in Vγ+ and Vγ− populations as indicated. (C) Cells were stained as in A with addition of anti-Vγ1 and anti-Vδ6.3. Plots in the first two columns are gated on TCRγδ+ population, additional gates applied in the other plots as indicated. Representative FACS plots from 1 of 3 independent experiments are shown.
To further characterize PLZF-expressing γδ T cells, we stained thymocytes with a panel of Vγ antibodies before intracellular staining for PLZF. Most of PLZF+ γδ T cells and virtually all γδ T cells expressing high levels of PLZF were contained within the Vγ1+ subset (Fig. 1B). As previously shown, Vγ1+Vδ6.3+ cells in B6 and Vγ1+Vδ6.4+ cells in DBA/2 mice exhibit surface phenotype and cytokine secretion profiles similar to those of NKT cells (15–18). We investigated therefore whether PLZF expression in Vγ1 cells is related to the utilization of the Vδ6.3 chain; most Vγ1+PLZF+ cells were Vδ6.3+ (Fig. 1C). This observation holds true for γδ T cells in the thymus, spleen, or liver (Fig. 1C). Also, some PLZF-expressing Vγ1+Vδ6.3− and Vγ1−Vδ6.3− γδ T cells were detected in these organs. As a rule, they expressed lower levels of PLZF than Vγ1+Vδ6.3+ cells. Thus, a subset of γδ T cells that was previously shown to resemble NKT-cells in several ways express a transcription factor that is required for the development of innate-like properties in NKT cells.
Vγ1+Vδ6.3+ T Cells in PLZF-deficient Mice Are Functionally Impaired.
NKT cells in PLZF-deficient mice were strongly decreased in numbers and frequency in thymus and liver, whereas their frequency was somewhat increased in the lymph nodes (13, 14). However, neither the frequency nor tissue distribution of Vγ1+Vδ6.3+ T cells was dramatically affected by the absence of PLZF (Fig. 1C), since only a relatively small, statistically insignificant decrease in the frequency of Vγ1+Vδ6.3+ cells in the thymus (8.8 ± 3.5% in wt vs. 4.6 ± 0.6% in PLZF−/−), spleen (13.4 ± 2.4% vs. 6.3 ± 0.3%), and liver (28.0 ± 12.0% vs. 11.1 ± 4.3%) (Fig. 1C) as well as a small increase of these cells in lymph nodes was observed.
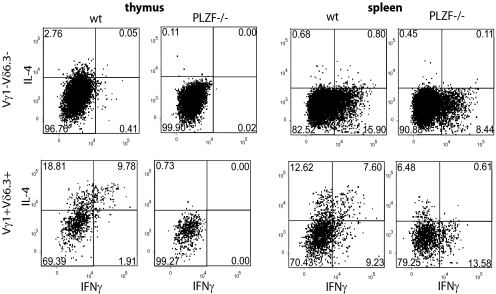
A striking consequence of the PLZF deficiency in NKT cells is their impaired ability to produce various cytokines during short-term responses (13, 14). For instance, their characteristic ability to produce simultaneously IL-4 and IFNγ upon stimulation is completely lost in the absence of PLZF—although some IL-4 or IFNγ “single-producers” could still be detected (14). Multiple subsets of γδ T cells (including Vγ1+Vδ6.3+ cells) were reported to be able to produce IFNγ, but the ability to generate IL-4 was shown to be restricted to the Vγ1+Vδ6.3+ subset, even though the potential to coexpress both cytokines on a single-cell level was not addressed in these studies (15). We therefore investigated whether the production of IL-4 and IFNγ by Vγ1+Vδ6.3+ cells was affected by absence of PLZF. As shown in Fig. 2, both Vγ1+Vδ6.3+ and Vγ1−Vδ6.3− γδ T cells in the spleen were able to produce IFNγ upon stimulation, whereas only Vγ1+Vδ6.3+ cells produced IL-4. In the thymus, Vγ1+Vδ6.3+ cells were the only source of IL-4 and IFNγ among γδ T cells under the used conditions of stimulation. Importantly, IL-4/IFNγ “double-producers” were readily detected among Vγ1+Vδ6.3+ thymocytes and splenocytes. This finding was in marked contrast to PLZF-deficient Vγ1+Vδ6.3+ thymocytes that exhibited a strong reduction of IL-4- and IFNγ-producing cells and a complete absence of “double-producers,” both in spleen and thymus. Lack of PLZF did not affect the ability of Vγ1−Vδ6.3− γδ splenocytes to produce IFNγ. Thus, the PLZF deficiency leads to very similar consequences in NKT-cells and Vγ1+Vδ6.3+ cells.
Fig. 2.
PLZF-deficient Vγ1+Vδ6.3+ cells show impaired cytokine responses. TCRγδ+ thymocytes (Left) or splenocytes (Right) from wt or PLZF-deficient mice were sorted and stimulated with PMA/ionomycin as described in Material and Methods. Surface expression of Vγ1 and Vδ6.3 and intracellular expression of IFNγ and IL-4 were analyzed. Gates applied as indicated. Representative FACS plots from 1 of 3 independent experiments are shown.
PLZF Deficiency Affects Vγ1+Vδ6.3+ Cells Cell-Intrinsically.
To verify that the PLZF deficiency affects Vγ1+Vδ6.3+ cells like NKT cells by cell-intrinsic mechanisms, we generated mixed bone marrow chimeras with wt and PLZF ko bone marrow. This experiment could have been hampered by previously reported evidence that most Thy-1dul γδ thymocytes (a population highly enriched for Vγ1+Vδ6.3+ cells, see ref. 15) might be of fetal origin (20). However, we could detect some Vγ1+Vδ6.3+ cells generated from both wt and PLZF-deficient bone marrow (Fig. S2A). When TCRγδ+ thymocytes were sorted from the thymi of the chimeras and stimulated with PMA/ionomycin, only cells derived from wt bone marrow were able to produce simultaneously IL-4 and IFNγ (Fig. S2B). About one-half of wt bone marrow-derived Vγ1+Vδ6.3+ thymocytes exhibited an activated CD44hiCD62lo phenotype, whereas such a population was virtually absent from cells derived from PLZF-deficient bone marrow (Fig. S2C). Thus, in the development of both Vγ1+Vδ6.3+ and NKT cells, PLZF is essentially required for the acquisition of the innate phenotype by cell-intrinsic mechanisms.
Reduced Numbers of Vγ1+Vδ6.3+ Cells in SAP−/− Mice.
As it was shown that signaling from SLAM receptors (10) via the adaptor molecule SAP (11) is crucial for NKT-cell development, we tested whether Vγ1+Vδ6.3+ cells are affected by the SAP deficiency. Both the relative frequency (Fig. S3A) as well as absolute cell numbers (Fig. S3B) of Vγ1+Vδ6.3+ cells in the thymus, spleen, and liver of SAP knock-out mice were decreased, suggesting that SAP-dependent SLAM signaling contributes to their development.
A Vγ1/Vδ6.4 Transgenic TCR Favors Development of PLZF+ T Cells.
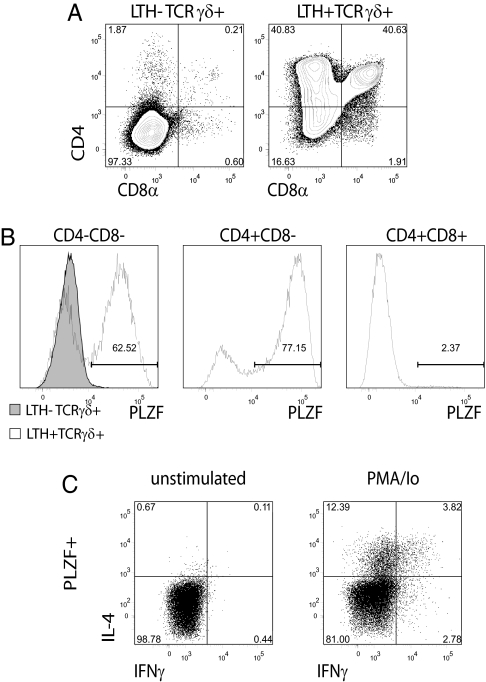
Both with iNKT cells and Vγ1+Vδ6.3+ γδ T cells, expression of PLZF correlates with a particular combination of TCR chains, suggesting that TCR specificity determines PLZF expression. To obtain further insight into the role of the TCR in development of PLZF+ T cells, we analyzed a transgenic mouse (21) expressing Vγ1/Vδ6.4 TCR transgenes that were cloned from the DTN40 T-cell hybridoma (16). Expression was controlled by a tetracycline-regulatable promoter. These mice were crossed to the LTH-1 strain, in which the TetR-VP16 transactivator is expressed under control of the lck proximal promoter allowing for T-cell-specific expression of the TCR transgene (22). In this system, the TCR transgenes are only expressed when the transactivator is present. Mice were maintained on a Rag1-deficient background. Vδ6.4 in DBA/2 mice is homologous to Vδ6.3 in B6 mice, and the Vγ1+Vδ6.4+ population in DBA/2 mice is likewise highly enriched for PLZF+ cells. In the absence of the LTH-1 transgene, thymi of Vγ1/Vδ6.4 transgenic mice looked like thymi of Rag1 knock-out mice (Fig. 3A), and no PLZF expression was detected (Fig. 3B). The presence of the LTH transgene allowed thymocytes to acquire CD4 and CD8 expression (Fig. 3A). A substantial fraction of CD4+CD8- cells was present in the double-transgenic thymi. This observation is in agreement with the described phenotype of Vγ1+Vδ6.3/Vδ6.4+ cells from wt mice, of which about half express CD4 (15). CD4−CD8− (64.3 ± 6.3%) and 78.8 ± 3.9% CD4+CD8−, but only 0.9 ± 0.4% of CD4+CD8+, thymocytes in double-transgenic mice expressed PLZF (Fig. 3B). Although the TCR transgene was expressed at low levels preventing clear gating on a TCR+ population, it is apparent that progression to the CD4+CD8− and CD4+CD8+ stages and expression of PLZF were dependent on TCR expression, as it was not observed in LTH negative Vγ1/Vδ6.4+ thymi. Cells from double-transgenic animals were able to produce IL-4 and IFNγ upon stimulation (Fig. 3C), similar to their wild-type counterparts. Thus, the transgenic Vγ1Vδ6.4 TCR was sufficient to support the development of large numbers of PLZF+ T cells.
Fig. 3.
T cells from Vγ1Vδ6.4 TCR transgenic mice express PLZF. (A) CD4/CD8a expression profiles of thymocytes from Vγ1Vδ6.4 TCR transgenic mice with (Right) or without (Left) LTH-1 transgene. (B) Intracellular expression of PLZF in thymocytes of these mice. Gates applied as indicated. Representative FACS plots from 1 of 8 independent experiments are shown (A and B). (C) Thymocytes from LTH-1/TCR double-transgenic mouse were stimulated with PMA/ionomycin (Right) or left unstimulated (Left) and stained for intracellular expression of PLZF, IL-4, and IFNγ; gated on PLZF+ cells. Representative FACS plots from 1 of 2 independent experiments are shown (C).
Strong TCR Signaling Induces PLZF in Polyclonal γδ Thymocytes.
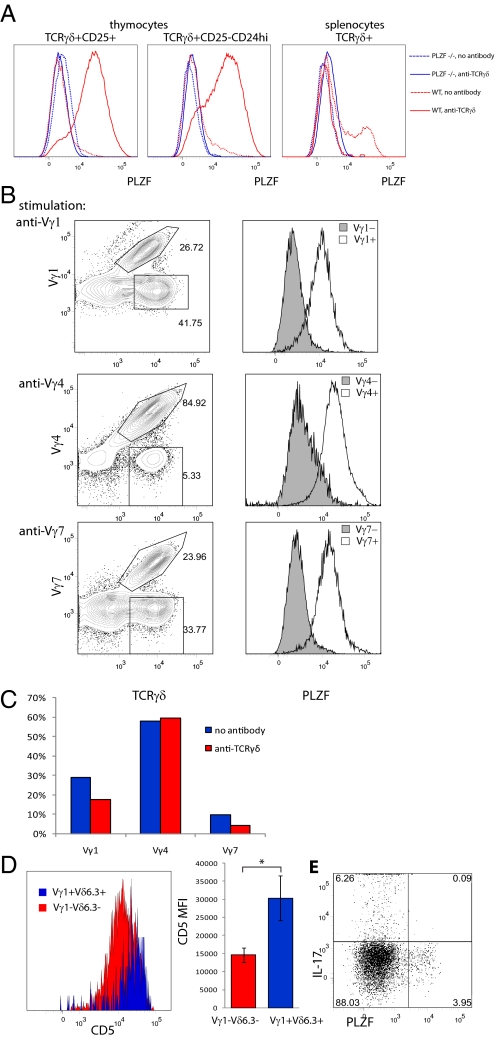
It has been suggested that at least some γδ T cells might be instructed by self-ligands present in the thymus to develop along certain pathways (23, 24). In one particular scenario, ligands were not required for the generation of cells expressing a certain TCR but were required to modify the TCR signaling-dependent effector functions, thereby enabling cells to produce IFNγ instead of IL-17 (24). It was also reported that a strong signal from the γδ TCR favors γδ lineage development, whereas weak signaling is compatible with the development of αβ lineage cells (25–27). We previously reported that some immature TCRγδ+ thymocytes can progress to CD4+CD8+ double-positive stage (a hallmark of αβ lineage differentiation) in OP9-DL1 cocultures and that this progression can be irreversibly blocked by TCR cross-linking that promotes γδ lineage differentiation (27). Interestingly, cells cultured in the presence of TCR antibodies acquired a surface phenotype (27) resembling the surface phenotype of NKT cells as well as that described for the so-called “cluster B” γδ thymocytes—a population highly enriched for Vδ6.3+ cells (28). It was therefore investigated whether generally strong TCR signaling can induce PLZF expression in polyclonal TCRγδ+ cells expressing a diverse TCR repertoire. To this end, wt and PLZF-deficient TCRγδ+ thymocytes, as well as splenocytes, were cocultured on OP9-DL1 monolayers in the presence or absence of anti-TCRγδ antibodies. Both the most immature CD25+ and the more mature CD25-CD24hi TCRγδ+ thymocytes up-regulated PLZF after 5 days of coculture in presence of cross-linking antibodies (Fig. 4A). The PLZF staining was specific, as no shift in fluorescence was observed in PLZF-deficient cells cultured under the same conditions (Fig. 4A). Ex vivo CD25-CD24lo TCRγδ+ thymocytes were already enriched for PLZF+ cells. Presumably, these cells had already received a strong TCR signal before their cultivation. On the other hand, TCRγδ+ splenocytes were unable to up-regulate PLZF upon culture with TCRγδ antibodies (Fig. 4A). In fact, the PLZF+ population disappeared from the cultures conducted in presence of antibody. These results suggest that PLZF can be induced only at immature stages of T-cell development.
Fig. 4.
PLZF can be induced in γδ thymocytes by strong TCR signal. (A) Wt and PLZF−/− TCRγδ thymocytes (Left and Center) of indicated phenotype or splenocytes (Right) were sorted and cocultured with irradiated OP9-DL1 cells in plates precoated with 0.1 μg/mL anti-TCRγδ or left uncoated. PLZF expression was analyzed on day 5 of culture. Representative FACS plots from 1 of 4 independent experiments are shown. (B) CD25+ TCRγδ+ thymocytes were sorted and cultured with irradiated OP9-DL1 cells in plates precoated with 0.01 μg/mL anti-Vγ1 (Top), 0.1 μg/mL anti-Vγ4 (Middle), or 0.01 μg/mL anti-Vγ7 (Bottom). PLZF expression was analyzed on day 5 of culture. (C) CD25+ TCRγδ+ thymocytes were sorted and cultured as in A and frequency of Vγ1, Vγ4, and Vγ7 positive cells among total TCRγδ+ cells was analyzed by FACS. (D) Ex vivo CD5 expression on Vγ1+Vδ6.3+ and Vγ1−Vδ6.3− γδ thymocytes of individual mouse (Left) or average of median fluorescent intensity for 3 individual mice (±SD). *, indicates statistical significance (P < 0.05) with a paired t-test. (E) Sorted TCRγδ+ wt thymocytes were stimulated with PMA/ionomycin and analyzed for expression of PLZF and IL-17.
The experiments reported so far do not directly show that PLZF can be induced by a cell-intrinsic mechanism that depends on strong TCR signaling, as the enrichment of PLZF+ cells in cultures containing TCR antibodies could be due to outgrowth of small numbers of PLZF+ cells with a restricted TCR repertoire and present at the beginning of the culture. Another possibility is that PLZF is induced by a cell-extrinsic mechanism, for instance by TCR cross-linking, leading to the production of soluble factors that in turn induce PLZF. To exclude these possibilities, we coated plates with antibodies specific for individual Vγ subtypes and analyzed whether PLZF can be induced in populations expressing different Vγ chains and whether PLZF up-regulation is restricted to the specific population that binds a particular antibody. Vγ1, Vγ4, and Vγ7 antibodies induced PLZF, specifically in subsets that express corresponding TCRγ chains, excluding a cell-extrinsic mechanism (Fig. 4B). In other experiments, cells cultured in the presence or absence of pan-TCRγδ antibody were analyzed with regard to their Vγ repertoire. In these experiments, the Vγ distribution on cells cultured in the presence or absence of TCRγδ antibody was similar, with Vγ1+ cells being a minority in both cases (Fig. 4C) strongly arguing against the outgrowth of 2–5% of preexisting PLZF+ cells with immature phenotype (not shown), as the majority of preexisting PLZF+ cells are Vγ1+ (Fig. 1B). Altogether, these data indicate that strong TCR signaling can induce PLZF in all immature γδ T cells by a cell-intrinsic mechanism.
It was then studied whether Vγ1+Vδ6.3+ cells exhibit a surface phenotype that is usually associated with strong TCR signaling. Here, levels of CD5 are widely used as measure of TCR signal strength (29, 30). Vγ1+Vδ6.3+ thymocytes on average expressed twice as much CD5 on their surface when compared with Vγ1−Vδ6.3− γδ thymocytes (Fig. 4D), suggesting that Vγ1+Vδ6.3+ cells received a stronger TCR signal in vivo. Previous results had indicated that agonist-selected γδ T cells do not produce IL-17 but do secrete IFNγ (24). Indeed, PLZF+ cells produced IFNγ and IL-4 upon stimulation (Fig. 2 and Fig. S2B), but all γδ T cells that were able to produce IL-17 were PLZF− (Fig. 4E). Thus, all data are consistent with the notion that TCR ligand-induced signaling plays a direct role in PLZF expression in developing γδ T cells.
Vγ1/Vδ6.4 TCR Transgenic Rag1−/− Mice Develop Spontaneous Dermatitis.
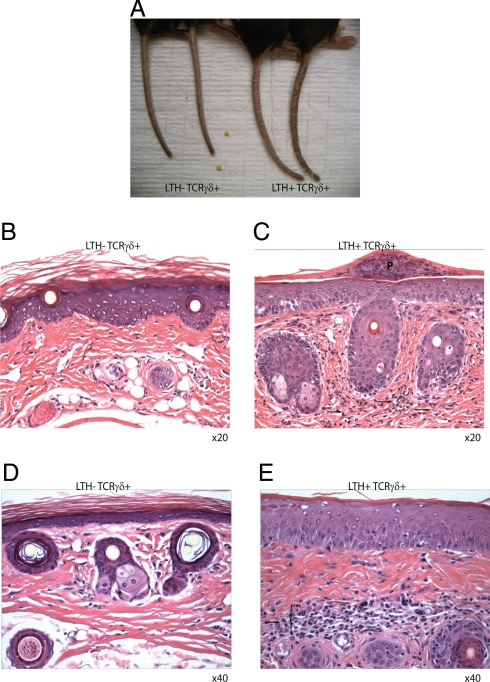
All Vγ1/Vδ6.4 TCR transgenic mice in our colony developed spontaneous dermatitis of the tail with increasing age (Fig. 5). Dermatitis developed only in animals doubly transgenic for Vγ1/Vδ6.4 and the LTH-1 transactivator, but not in mice lacking any one of the transgenes. The disease was characterized by severe tail scaling (Fig. 5A) and massive infiltrates of lymphocytes and granulocytes into the dermis of tail skin (Fig. 5 B–E). Inflammation was also obvious in the epidermis of double-transgenic animals—as evidenced by the presence of pustules (Fig. 5C). Ongoing crossing of the TCR transgenic mice onto a PLZF-deficient background will determine whether the development of the disease requires properties conferred to this subtype of T-cells by PLZF.
Fig. 5.
Vγ1Vδ6.4 TCR transgenic mice develop spontaneous dermatitis. (A) Tails of 4-month-old LTH-1/TCR double-transgenic (Right) or TCR single-transgenic (Left) littermates are shown. (B–E) HE staining of tails from single-transgenic (B and D) or double-transgenic (C and E) mice are shown. Arrows and brace indicate infiltrate, P indicate a pustule.
Discussion
Here, we report that transcriptional factor PLZF that was shown to be important for acquisition of the effector program in NKT-cell development plays a similar role in a subset of γδ T cells. These Vγ1+Vδ6.3/Vδ6.4+ cells were previously reported to share phenotypic and functional properties with NKT cells (15–18). A recent study reported that their numbers are increased in Itk−/− mice and that total γδ T cells from these mice express high levels of PLZF mRNA (31). Our results demonstrate that at least some of the NKT-like properties of these cells are a part of a common program that requires the transcription factor PLZF for its execution.
It was previously shown that PLZF is also expressed by MAIT cells (13). Although the function of PLZF in this cell type was not assessed, the similarities between the NKT and MAIT lineages (8) suggest that PLZF might play similar role in both subsets. However both NKT and MAIT cells belong to the lineage of αβ T cells and progress through the same developmental path up to the DP stage of development. Vγ1+Vδ6.3/Vδ6.4+ cells as γδ T cells diverge from the αβ lineage after TCR expression at the DN3 stage. Although fate-mapping experiments were not performed with this population, it is very unlikely that they share further steps with αβ cells as the αβ lineage program involves silencing of Tcrg genes (32) and deletion of Tcrd genes during rearrangement of the Tcra locus. Thus, a subset of αβ (NKT) and γδ (Vγ1+Vδ6.3+/Vδ6.4) T cells that progress through distinct developmental pathways can acquire expression of the same transcription factor PLZF that is required for the same “innate” differentiation program. Even though global gene expression analysis of these different subsets still needs to be performed, the remarkable similarities in surface phenotype, cytokine profiles, and tissue distribution suggests that these cells converge in their differentiation program. In this regard, it is of interest to note that comparison of gene expression profiles from various populations of intestinal IELs revealed similarities between CD8αα TCRαβ and TCRγδ IELs (33), suggesting that certain convergence of molecular programs between αβ and γδ T-cell sublineages is not an uncommon event.
In all 3 subsets of cells expressing PLZF [NKT (13, 14), MAIT (13), and Vγ1+Vδ6.3/Vδ6.4+ cells], the presence of PLZF was associated with the expression of a particular TCR, suggesting that TCR specificity regulates PLZF expression during T-cell development. In fact, we report here that the expression of Vγ1/Vδ6.4 transgenes was sufficient to support the development of large numbers of PLZF+ T cells. In the context of hypotheses postulating that “agonist selection” and “selection on hematopoietic cells” is required for acquisition of innate-like properties, it is of interest to determine whether one or both of these mechanisms play a role in PLZF induction. Here we demonstrated that PLZF can be induced in developing γδ T cells by TCR cross-linking and that ex vivo Vγ1+Vδ6.3+ cells show some evidence for having received a strong signal from their TCR. These findings suggest the presence of an endogenous ligand for the Vγ1Vδ6.3 TCR. It was also suggested that NKT cells undergo agonist selection (9).
NKT-cell development is influenced by SAP-dependent SLAM signaling (10, 11). Likewise, we found the development of Vγ1+Vδ6.3+ T cells to be perturbed in SAP-deficient mice; the frequency and absolute number of Vγ1+Vδ6.3+ cells in SAP−/− animals was severely reduced. However, in contrast to NKT cells (11), the dependence on SAP was not absolute. Nevertheless, the results might suggest that Vγ1+Vδ6.3+ cells could likewise be selected by TCR ligands on other thymocytes. However, the true nature of selecting cells can only be addressed when the ligand(s) for Vγ1+Vδ6.3+ TCR are identified.
PLZF deficiency leads to dramatic changes in surface phenotype and cytokine production in both NKT (13, 14) and Vγ1+Vδ6.3+ cells. However, some “NKT traits” were not affected by lack of PLZF; even though IL-4/IFNγ “double producers” were completely absent among PLZF-deficient NKT (14) and Vγ1+Vδ6.3+ cells, cells rapidly producing either IL-4 or IFNγ were readily detectable. Also, the surface phenotype of PLZF-deficient NKT cells was not entirely identical to that of naïve CD4 cells (14). Moreover, PLZF is not expressed by other innate-like αβ and γδ T cells (such as TCRαβ CD8αα and TCRγδ IELs). These observations suggest that factors other than PLZF are required for the acquisition of innate-like features of developing T cells.
Finally, it is worth considering that Vγ1/Vδ6.4 transgenic mice on the Rag1−/− background developed spontaneous dermatitis. The epidermis represents a tissue to which normally a large population of γδ T cells, the Vγ5+ dendritic epidermal T cells (DETC) home. These cells have regulatory properties as TCRδ−/− mice on certain backgrounds develop spontaneous dermatitis that can be rescued by the transfer of Vγ5+ fetal thymocytes (34). It was previously demonstrated that in the absence of canonical Vγ5+ DETCs, other γδ T cells can take over their niche (23). However, this replacement leads to abnormalities in skin physiology such as increased baseline ear thickness and exaggerated irritant contact dermatitis (23). It is tempting to speculate that under the conditions when both αβ and γδ T cells with regulatory properties are absent, transgenic Vγ1Vδ6.4 T cells take over their niche in the skin and initiate uncontrolled inflammation. Whether or not the disease is related to PLZF-dependent effector program of these cells is currently under investigation.
Materials and Methods
Mice.
PLZF−/− (19) and Rag1−/− Vγ1/Vδ6.4 (21) transgenic mice described previously were bred and maintained in the animal facilities at Center for Life Science, Boston, and at the Dana-Farber Cancer Institute (DFCI), respectively. SAP−/− (35) mice were bred and maintained in the animal facility of the University of Chicago. C57BL/6 and CD45.1 C57BL/6 mice were obtained from The Jackson Laboratory and Taconic, respectively. Mice were maintained in the specific pathogen-free animal facilities of the DFCI and Center for Life Science. All animal procedures were done in compliance with the guidelines of the DFCI Animal Resources Facility, which operates under regulatory requirements of the U.S. Department of Agriculture and Association for Assessment and Accreditation of Laboratory Animal Care.
Flow Cytometry and Cell Sorting.
mAbs specific for CD4 (RM4-5), CD8a (53-6.7), CD25 (PC61), CD44 (IM7), TCRβ (H57-597), TCRγδ (GL3), NK1.1 (PK136), CD45.1 (A20), CD45.2 (104), CD24 (M1/69), CD5 (53-7.3), CD62L (MEL-14), IFNγ (XMG1.2), and IL-4 (BVD6-24G2) were purchased from BD Biosciences or eBioscience and were used as biotin, FITC, phycoerythrin (PE), peridinin chlorophyll protein (PerCP), PerCP-Cy5.5, PE-Cy7, allophycocyanin (APC), APC-Cy7, or Pacific Blue conjugates. PLZF antibody (D-9) was purchased from Santa Cruz Biotechnology and conjugated to Alexa-Fluor 647 with a kit from Invitrogen. Anti-Vγ1 (2.11), anti-Vγ4 (UC3–10A6), anti-Vγ7 (F2.67), and anti-V6.4/6.3 (clone 9D3) were prepared and used as described (36). Fluorochrome-conjugated streptavidin was used to reveal staining with biotinylated mAb. Intracellular staining for PLZF and cytokines was performed using Foxp3 staining buffer set (eBioscience). Flow cytometry and cell sorting was performed on FACSAria (BD Biosciences) cell sorter. Data were analyzed with FlowJo software (Treestar). Frequencies of some cell populations are mentioned in the text as mean ± SD.
Generation of Mixed Bone Marrow Chimeras.
Bone marrow cells from CD45.1 wt and CD45.2 PLZF−/− mice were stained with CD4, CD8a, TCRβ, TCRγδ, and NK1.1 biotinilated antibodies followed by incubation with streptavidin-conjugated magnetic beads (Dynal) and magnetic bead depletion of T cells and NK cells. Six- to 8-week-old CD45.1/CD45.2 heterozygous recipients were a subject of a split-dose irradiation (500 rads, twice) with a γ-cell 40 irradiator with a cesium source. Mixed (1:1) T-cell- and NK-cell-depleted wt and PLZF−/− bone marrow cells (4–8 × 106) were intra-orbitally injected into the recipient mice. Mice were analyzed 5 weeks after the transfer by flow cytometry.
OP9 Cocultures.
OP9 bone marrow stromal cells expressing the Notch ligand DL-1 (OP9-DL1) provided by Juan Carlos Zúñiga-Pflücker (University of Toronto, Toronto) were maintained as described previously (37). Plates (24-well) were coated with mAbs specific for TCRγδ (clone UC7–13D5, final concentration 0.1 μg/mL; BD Biosciences), Vγ1, Vγ4, or Vγ7 (at indicated concentrations) or left uncoated. γ-Irradiated (1,500 rads) OP9-DL1 cells were plated at 2 × 104 cells/well. Sorted γδ thymocytes or splenocytes were plated onto OP9-DL1 monolayers, harvested 5 days later, and analyzed by FACS. All cocultures were performed in the presence of 1 ng/mL IL-7 and 5 ng/mL Flt3L.
In Vitro T-Cell Activation.
Thymocytes or splenocytes from wt, PLZF−/− mice, or from mixed bone marrow chimeras were stained with biotinylated antibodies to TCRβ, CD8a, and CD19 molecules, followed by incubation with streptavidin-conjugated magnetic beads (Dynal) and magnetic bead depletion of αβ T cells and B cells. Enriched cell suspensions were surface stained with fluorochrome-conjugated streptavidin and TCRγδ antibody. TCRγδ+ cells were sorted using a FACSAria (BD Biosciences) and stimulated with 50 ng/mL phorbol 12-myristate 13-acetate and 500 ng/mL ionomycin for 6–8 h with brefeldin A added after 1 h of incubation or left unstimulated. Expression of IL-4, IFNγ, TCRγδ, Vγ1, Vδ6.3 (and, for experiments with mixed bone marrow chimeras, CD45.1 and CD45.2) were analyzed by FACS as described above.
Histology.
Tails of mice were fixed with Bouin's fixative and stained with hematoxylin eosin. Bright-filed images were collected with 20× or 40× objective lenses.
Supplementary Material
Acknowledgments.
We thank J.C. Zúñiga-Pflücker (University of Toronto, Toronto) for providing reagents and G. Turchinovich, A. Krueger, M. Gleimer, and C. Daniel for helpful discussions. We are grateful to V. Schmidt for technical assistance. We thank the National Institutes of Health tetramer core facility for preparation of CD1d tetramer. These studies were supported by National Institutes of Health Grants R01 A145846 and R01 A151378.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903895106/DCSupplemental.
References
- 1.Berg LJ. Signalling through TEC kinases regulates conventional versus innate CD8(+) T-cell development. Nat Rev Immunol. 2007;7:479–485. doi: 10.1038/nri2091. [DOI] [PubMed] [Google Scholar]
- 2.Jameson J, Witherden D, Havran WL. T-cell effector mechanisms: Gammadelta and CD1d-restricted subsets. Curr Opin Immunol. 2003;15:349–353. doi: 10.1016/s0952-7915(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 3.Hayday AC. γδ cells: A right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 4.Hayday A, Tigelaar R. Immunoregulation in the tissues by γδ T cells. Nat Rev Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 5.Eberl G, Littman DR. Thymic origin of intestinal αβ T cells revealed by fate mapping of RORγt+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 6.Egawa T, et al. Genetic evidence supporting selection of the Vα14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22:705–716. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Urdahl KB, Sun JC, Bevan MJ. Positive selection of MHC class Ib-restricted CD8(+) T cells on hematopoietic cells. Nat Immunol. 2002;3:772–779. doi: 10.1038/ni814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treiner E, Lantz O. CD1d- and MR1-restricted invariant T cells: Of mice and men. Curr Opin Immunol. 2006;18:519–526. doi: 10.1016/j.coi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Baldwin TA, Hogquist KA, Jameson SC. The fourth way? Harnessing aggressive tendencies in the thymus. J Immunol. 2004;173:6515–6520. doi: 10.4049/jimmunol.173.11.6515. [DOI] [PubMed] [Google Scholar]
- 10.Griewank K, et al. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasquier B, et al. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, et al. The SLAM-associated protein signaling pathway is required for development of CD4+ T cells selected by homotypic thymocyte interaction. Immunity. 2007;27:763–774. doi: 10.1016/j.immuni.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savage AK, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovalovsky D, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azuara V, Levraud JP, Lembezat MP, Pereira P. A novel subset of adult gamma delta thymocytes that secretes a distinct pattern of cytokines and expresses a very restricted T cell receptor repertoire. Eur J Immunol. 1997;27:544–553. doi: 10.1002/eji.1830270228. [DOI] [PubMed] [Google Scholar]
- 16.Azuara V, Lembezat MP, Pereira P. The homogeneity of the TCRδ repertoire expressed by the Thy-1dull γδ T cell population is due to cellular selection. Eur J Immunol. 1998;28:3456–3467. doi: 10.1002/(SICI)1521-4141(199811)28:11<3456::AID-IMMU3456>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 17.Gerber DJ, et al. IL-4-producing gamma delta T cells that express a very restricted TCR repertoire are preferentially localized in liver and spleen. J Immunol. 1999;163:3076–3082. [PubMed] [Google Scholar]
- 18.Azuara V, Grigoriadou K, Lembezat MP, Nagler-Anderson C, Pereira P. Strain-specific TCR repertoire selection of IL-4-producing Thy-1 dull gamma delta thymocytes. Eur J Immunol. 2001;31:205–214. doi: 10.1002/1521-4141(200101)31:1<205::AID-IMMU205>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Barna M, Hawe N, Niswander L, Pandolfi PP. Plzf regulates limb and axial skeletal patterning. Nat Genet. 2000;25:166–172. doi: 10.1038/76014. [DOI] [PubMed] [Google Scholar]
- 20.Grigoriadou K, Boucontet L, Pereira P. Most IL-4-producing gamma delta thymocytes of adult mice originate from fetal precursors. J Immunol. 2003;171:2413–2420. doi: 10.4049/jimmunol.171.5.2413. [DOI] [PubMed] [Google Scholar]
- 21.Garbe AI, Krueger A, Gounari F, Zuniga-Pflucker JC, von Boehmer H. Differential synergy of Notch and T cell receptor signaling determines αβ versus γδ lineage fate. J Exp Med. 2006;203:1579–1590. doi: 10.1084/jem.20060474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labrecque N, et al. How much TCR does a T cell need? Immunity. 2001;15:71–82. doi: 10.1016/s1074-7613(01)00170-4. [DOI] [PubMed] [Google Scholar]
- 23.Lewis JM, et al. Selection of the cutaneous intraepithelial γδ+ T cell repertoire by a thymic stromal determinant. Nat Immunol. 2006;7:843–850. doi: 10.1038/ni1363. [DOI] [PubMed] [Google Scholar]
- 24.Jensen KD, et al. Thymic selection determines γδ T cell effector fate: Antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haks MC, et al. Attenuation of γδTCR signaling efficiently diverts thymocytes to the αβ lineage. Immunity. 2005;22:595–606. doi: 10.1016/j.immuni.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Hayes SM, Li L, Love PE. TCR signal strength influences αβ/γδ lineage fate. Immunity. 2005;22(5):583–593. doi: 10.1016/j.immuni.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Kreslavsky T, Garbe AI, Krueger A, von Boehmer H. T cell receptor-instructed αβ versus γδ lineage commitment revealed by single-cell analysis. J Exp Med. 2008;205:1173–1186. doi: 10.1084/jem.20072425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prinz I, et al. Visualization of the earliest steps of γδ T cell development in the adult thymus. Nat Immunol. 2006;7:995–1003. doi: 10.1038/ni1371. [DOI] [PubMed] [Google Scholar]
- 29.Azzam HS, et al. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarakhovsky A, et al. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science. 1995;269:535–537. doi: 10.1126/science.7542801. [DOI] [PubMed] [Google Scholar]
- 31.Felices M, Yin CC, Kosaka Y, Kang J, Berg LJ. Tec kinase Itk in γδ T cells is pivotal for controlling IgE production in vivo. Proc Natl Acad Sci USA. 2009;106:8308–8313. doi: 10.1073/pnas.0808459106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishida I, et al. T-cell receptor gamma delta and gamma transgenic mice suggest a role of a gamma gene silencer in the generation of alpha beta T cells. Proc Natl Acad Sci USA. 1990;87:3067–3071. doi: 10.1073/pnas.87.8.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denning TL, et al. Mouse TCRαβ+CD8αα intraepithelial lymphocytes express genes that down-regulate their antigen reactivity and suppress immune responses. J Immunol. 2007;178:4230–4239. doi: 10.4049/jimmunol.178.7.4230. [DOI] [PubMed] [Google Scholar]
- 34.Girardi M, et al. Resident skin-specific γδ T cells provide local, nonredundant regulation of cutaneous inflammation. J Exp Med. 2002;195:855–867. doi: 10.1084/jem.20012000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu C, et al. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nat Immunol. 2001;2:410–414. doi: 10.1038/87713. [DOI] [PubMed] [Google Scholar]
- 36.Pereira P, et al. Developmentally regulated and lineage-specific rearrangement of T cell receptor Vα/δ gene segments. Eur J Immunol. 2000;30:1988–1997. doi: 10.1002/1521-4141(200007)30:7<1988::AID-IMMU1988>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 37.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.