Abstract
Anthrax toxin, a major virulence factor of Bacillus anthracis, gains entry into target cells by binding to either of 2 von Willebrand factor A domain-containing proteins, tumor endothelium marker-8 (TEM8) and capillary morphogenesis protein-2 (CMG2). The wide tissue expression of TEM8 and CMG2 suggest that both receptors could play a role in anthrax pathogenesis. To explore the roles of TEM8 and CMG2 in normal physiology, as well as in anthrax pathogenesis, we generated TEM8- and CMG2-null mice and TEM8/CMG2 double-null mice by deleting TEM8 and CMG2 transmembrane domains. TEM8 and CMG2 were found to be dispensable for mouse development and life, but both are essential in female reproduction in mice. We found that the lethality of anthrax toxin for mice is mostly mediated by CMG2 and that TEM8 plays only a minor role. This is likely because anthrax toxin has approximately 11-fold higher affinity for CMG2 than for TEM8. Finally, the CMG2-null mice are also shown to be highly resistant to B. anthracis spore infection, attesting to the importance of both anthrax toxin and CMG2 in anthrax infections.
Keywords: edema toxin, lethal toxin, tumor endothelium marker-8
Bacillus anthracis is a Gram-positive, rod-shaped, spore-forming bacterium, and the causative agent of anthrax. Anthrax toxin is the major virulence factor for this organism and responsible for its lethal effects in the host. Although treatment with antibiotics to eliminate the bacteria can be life-saving at the earlier stages of anthrax disease, once enough toxin has been produced, the disease is often lethal despite treatment. Thus, countermeasures that block toxin or limit its effects are essential at later stages of disease (1). Therefore, a detailed understanding of the interaction between anthrax toxin and the host is needed as a basis for developing improved interventions.
Anthrax toxin is a 3-part toxin consisting of protective antigen (PA, 83 kDa), edema factor (EF, 90 kDa), and lethal factor (LF, 89 kDa) (2–4). These 3 proteins are individually nontoxic, but can assemble on the cell surface to form toxic complexes. To intoxicate host mammalian cells, PA binds to its cellular receptors, tumor endothelium marker-8 [TEM8, also named anthrax toxin receptor 1 (ANTXR1)] and capillary morphogenesis protein-2 [CMG2, also named anthrax toxin receptor 2 (ANTXR2)] (5, 6) with the involvement of a coreceptor LRP6 (7, 8) and is then proteolytically processed to the active form, cell-surface bound PA63. PA63 spontaneously oligomerizes to form a heptamer that binds and delivers LF and EF into the cytosol. EF, which combines with PA to form edema toxin (ET), is a calmodulin-dependent adenylate cyclase that elevates intracellular cAMP levels, thereby causing diverse effects including impairment of phagocytosis and death of experimental animals (9, 10). LF, which combines with PA to form lethal toxin (LT), is a zinc-dependent metalloproteinase that cleaves and inactivates the mitogen-activated protein kinase kinases (MEKs) 1–4, 6, and 7 (11–13), blocking the ERK, p38, and Jun N terminus kinase (JNK) pathways (14). LT causes a range of effects on cellular functions and is lethal in several experimental animal models (15, 16).
TEM8 and CMG2 are the 2 known anthrax toxin receptors. Each is produced having a signal peptide, a single extracellular von Willebrand factor A (VWA) domain, a single-pass transmembrane region (TM) for plasma membrane anchoring and a cytosolic tail that may be involved in cytoskeleton interactions (17). TEM8 and CMG2 share 60% identity in their VWA domains essential for PA binding and a lower degree of similarity in other regions. The physiological functions of these 2 proteins remain unknown. TEM8 was initially identified as a tumor endothelium marker that is up-regulated in human colorectal cancer endothelium (18), suggesting it as a candidate for tumor targeting. Recently, mutations within CMG2 have been identified as causing 2 rare human autosomal recessive conditions, juvenile hyaline fibromatosis (JHF) and infantile systemic hyalinosis (ISH) (19, 20). The wide tissue expression of TEM8 and CMG2 suggest that both receptors could play a role in anthrax pathogenesis (5, 6). A previous study showed that a CMG2-specific PA mutant was able to mediate LT killing of rats, although with lower potency than WT PA (21). However, convincing and definitive information on the roles of CMG2 and TEM8 in anthrax pathogenesis are not available. To explore these issues, we generated TEM8- and CMG2-null mice by deleting TEM8 and CMG2 TM regions. We found that both TEM8 and CMG2-null mice are viable and that CMG2 is the major anthrax toxin receptor in mediating lethality in vivo, whereas TEM8 only plays a minor role in anthrax toxin pathogenesis.
Results
Generation of Transmembrane Domain Deleted-TEM8 and -CMG2 Mice.
CMG2 and TEM8 are large genes, spanning 80 kb and 190 kb, respectively, in the mouse genome, making it difficult to delete the whole genes in gene-targeting. CMG2 and TEM8 are anchored on the cell surface by single-pass TM domains that are essential to their biological functions (22, 23). Furthermore, mutations in the TM region of CMG2 are frequently found in JHF and ISH patients (19, 20). In this report, gene targeting vectors were used to delete the TM domains and thereby inactivate the receptors in mice. The receptors could also be targeted by deletion of an exon in the VWA domains but such an approach could lead to concerns that an altered receptor might still interact with the coreceptor LRP6 or other unidentified cell surface cofactors and thereby exert a dominant negative effect. The CMG2-targeting vector was designed to introduce LoxP sites into introns 11 and 12 of the CMG2 locus, so as to flank exon 12, which encodes the CMG2 TM region (Fig. S1A). One of the resulting ES cell clones was used to generate chimeric mice, and germ line transmission was achieved (termed CMG2flp/+ mice). The CMG2flp/+ mice were crossed with either ACTB-Fple transgenic mice (which contain the Saccharomyces cerevisiae Fple recombinase gene under the direction of the human β-actin gene promoter) to remove the Neo cassette by excisional recombination of Flp/FRT system (CMG2flox/+), or ACTB-Cre transgenic mice to generate heterozygous mice deleted for the CMG2 TM region (CMG2+/−) (Fig. S1 A and B). The CMG2flox/+ mice could be used to generate tissue-specific CMG2 TM deleted mice when needed (Fig. S1C). We used a similar strategy to target the TEM8 gene. In this case, we introduced LoxP sites into TEM8 introns 12 and 13 to flank exon 13, which encodes the TEM8 TM region (Fig. S2).
Both CMG2+/− and TEM8+/− mice were fertile and phenotypically normal. To generate CMG2−/− and TEM8−/− mice, the CMG2+/− and TEM8+/− mice were intercrossed respectively. Genotyping of offspring from these breedings revealed that both CMG2−/− and TEM8−/− mice were viable and could survive to adulthood with genotype frequencies matching the expected Mendelian ratio (Table S1). Surprisingly, CMG2−/− mice did not display any apparent phenotype, which was in contrast to the human JHF and ISH syndromes caused by many different CMG2 mutations. However, we found that most TEM8−/− mice progressively developed misaligned incisor teeth from weaning age (3-weeks-old) to 3-months-old that could be rescued by feeding soft food (Nutra-Gel from Bio-Serv). In the course of breeding the knockout mice over a 5 month period, we found that homozygous matings of TEM8−/− × TEM8−/− and CMG2−/− × CMG2−/− did not yield any pups from 6 and 8 pairs of breeders, respectively (Table S2). To determine which gender was responsible for the fertility problems, both genders of TEM8−/− and CMG2−/− mice were intercrossed with TEM8+/− and CMG2+/− mice, respectively. The male TEM8−/− and CMG2−/− mice reproduced normally when crossed with heterozygous females, whereas both the TEM8−/− and CMG2−/− female mice were infertile (Table S2). Thus, both TEM8 and CMG2 have a nonredundant essential function in female reproduction in mice. The mechanisms underlying the effects of TEM8 and CMG2 on reproduction are subjects of additional study in our lab and will not be discussed here.
To evaluate the tissue expression of CMG2 and TEM8, we performed reverse transcription (RT)-PCR analyses to amplify CMG2 and TEM8 cDNA fragments covering the TM regions from various mouse tissues (Fig. S1C and S2C). CMG2 and TEM8 could be readily detected in all tissues tested by RT-PCR, including brain, heart, spleen, liver, lung, kidney, adrenal gland, and thymus, demonstrating that CMG2 and TEM8 are widely expressed in mice. We further confirmed the ubiquitous expression of CMG2 and TEM8 in mice by real-time RT-PCR analyses (Fig. S1D).
TM-Deleted TEM8 and CMG2 Cannot Function as Anthrax Toxin Receptors.
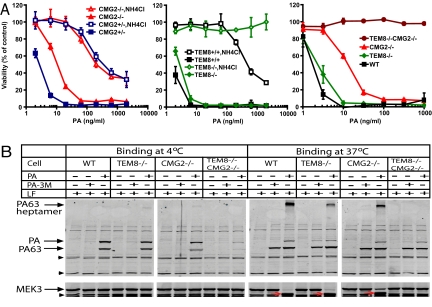
To ascertain that the TM-deleted TEM8 and CMG2 proteins are null mutants and cannot act as anthrax toxin receptors, we generated double heterozygous TEM8+/−CMG2+/− mice, and isolated mouse embryonic fibroblasts (MEFs) with various TEM8 and CMG2 genotypes from the double heterozygous intercrosses. MEFs having the various resulting genotypes, including TEM8−/−, CMG2−/−, CMG2+/−, TEM8−/−CMG2−/−, and WT were treated with varying concentrations of PA in the presence of FP59 (100 ng/mL). FP59 is a fusion protein of LF amino acids 1–254 and the catalytic domain of Pseudomonas aeruginosa exotoxin A (24) that kills cells by ADP-ribosylation and thus inactivation of EF-2 after delivery to cytosol by PA. The EC50s of TEM8−/− MEFs and CMG2−/− MEFs to PA (PA concentrations needed to kill 50% of the cells in the presence of FP59) were 2.7 ng/mL and 12 ng/mL, respectively, compared to 1.8 ng/mL of WT cells (Fig. 1A and Table S3), indicating that CMG2 has higher activity than TEM8 as an anthrax toxin receptor in MEFs. Remarkably, however, TEM8−/−CMG2−/− MEFs were completely resistant to PA + FP59 (Fig. 1A), even when the cells were treated with high concentrations of PA and FP59 (up to 5000 ng/mL PA in the presence of 1000 ng/mL FP59) for 48 h (Fig. S3A).
Fig. 1.
TM-deleted TEM8 and CMG2 cannot function as anthrax toxin receptors. (A) Cytotoxicity of PA plus FP59 to MEFs with various genotypes. MEFs were treated with various concentrations of PA plus FP59 (100 ng/mL) in the absence or presence of 10 mM NH4Cl for 3 h. The toxins were removed and replaced with fresh medium, and the cells were cultured in the presence of 10 mM NH4Cl for 48 h before assessing cell viability. Data are reported as mean viability ± SD. (B) Binding and processing of PA on MEFs with various genotypes. Cells were incubated with 1 μg/mL PA or 1 μg/mL PA-3M in the presence of 1 μg/mL LF at either 4 °C for 4 h or 37 °C for 2 h. Cells were washed, lysed, and cell lysates subjected to Western blotting using either a PA antiserum or a MEK3 antibody. LF cleaved MEK3 is indicted by red arrows. Nonspecific cross-reactive bands indicated by the arrow heads at the left of the images serve as protein loading controls.
NH4Cl (ammonium chloride), a lysosomotropic amine that can partially neutralize the low pH of endosomes, completely protected TEM8−/− MEFs (which were using CMG2 as the toxin receptor) but only partially protected CMG2−/− MEFs (which were using TEM8 as the receptor, Fig. 1A). We further performed binding and internalization assays on MEFs incubated with PA + LF at 4 °C and 37 °C. Since receptor-mediated endocytosis does not occur at 4 °C, PA species detected in cell lysates from 4 °C incubations represent cell-surface bound species. We found that the TEM8−/−CMG2−/− MEFs bound much less PA at 4 °C than WT cells and the cells having either the TEM8 or CMG2 mutations (Fig. 1B). The residual PA binding on TEM8−/−CMG2−/− MEFs may be mediated by the TM-deleted forms of TEM8 and CMG2, which may be transiently associated with the cell surface during secretion, possibly by association with LRP6 (7, 8), or by other unidentified VWA proteins that can bind PA but cannot mediate toxin internalization. At 37 °C the WT cells and the cells with either the TEM8 or CMG2 mutations bound and proteolytically processed PA to the PA63 heptamer, which was then internalized into endocytic vesicles to form the characteristic SDS/heat-resistant heptamer, resulting in LF delivery to the cytosol and MEK3 cleavage (Fig. 1B). In contrast, TEM8−/−CMG2−/− MEFs could not mediate heptamer formation and MEK3 cleavage by LF (Fig. 1B and Fig. S3B). Interestingly, although less LF was internalized by CMG2−/− MEFs than by TEM8−/− MEFs (as seen by less MEK3 cleavage), we always observed more SDS-resistant PA heptamer formation in CMG2−/− MEFs than in TEM8−/− MEFs (Fig. 1B). This is likely due to the fact that formation of an SDS-resistant heptamer associated with CMG2 requires lower pH than that associated with TEM8 (23, 25). Therefore, the majority of PA internalized into endocytic vesicles by TEM8 may exist as the SDS-resistant heptamer, while only the small fraction of CMG2-associated PA that reaches the late endosomes formed the SDS-resistant heptamer. This is in agreement with the above differential protection from PA/FP59 treatment conferred by NH4Cl to CMG2−/− MEFs and TEM8−/− MEFs (Fig. 1A). Taken together, the above results demonstrate that the TM-deleted TEM8 and CMG2 are indeed null mutants that cannot function as anthrax toxin receptors. In these experiments, we included the nonbinding mutant PA-3M as a control (26) (Fig. 1B). Following exposure to cells at 37 °C, both PA and PA-3M or their proteolytic products (PA63) were detected in TEM8−/−CMG2−/− and other MEF cell lysates (Fig. 1B), likely due to nonspecific pinocytotic activity of these MEFs at 37 °C.
CMG2 Is the Major Receptor Mediating Lethality of Anthrax Toxin in Vivo.
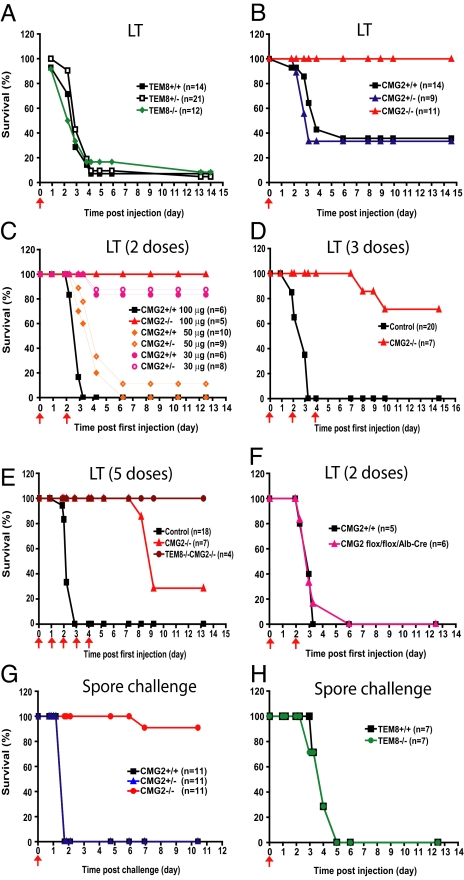
To explore the roles of TEM8 and CMG2 in anthrax toxin pathogenesis, we challenged TEM8−/−, CMG2−/−, their heterozygous littermates, and WT mice i.p. with one dose of 100 μg LT (100 μg LF + 100 μg PA). Surprisingly, the TEM8−/− mice were as susceptible as TEM8+/− and WT mice to LT (Fig. 2A) with about 90% of these mice succumbing within 2 weeks. In contrast, CMG2−/− mice were completely resistant and did not display any sign of disease in the 2 week observation period, whereas about 70% of their littermate CMG2+/− and WT mice died, mostly within 4 days of the challenge (Fig. 2B). We also challenged the mice with 50 μg ET (50 μg EF + 50 μg PA) intravenously and showed that CMG2−/− mice were also totally resistant while TEM8−/− and their littermate control mice succumbed to ET within 2 days (Fig. S4). These results demonstrate that the lethality of anthrax toxin is mostly mediated by CMG2. We next challenged groups of CMG2−/− mice and their littermate control mice i.p. with 2, 3, or 5 doses of 100 μg of LT administered at intervals of 1 or 2 days (Fig. 2 C–E). In these experiments, all control mice died within 3–4 days of the first injection (Fig. 2 C–E). However, 100% of the CMG2−/− mice survived 2 doses of LT, and 70% and 30% of CMG2−/− mice survived 3 and 5 doses, respectively (Fig. 2 C-E). We further generated TEM8−/−CMG2−/− mice by intercrossing TEM8+/−CMG2+/− mice and found these mice were completely resistant to 5 doses of 100 μg LT challenge (Fig. 2E). These results indicate that while the in vivo toxicity caused by anthrax toxin is mostly mediated by CMG2, TEM8 can still play a minor role in anthrax toxin pathogenesis in vivo. The fact that the death of the CMG2−/− mice caused by multiple LT challenge occurred quite late (8–10 days after the first toxin injection vs. 3–4 days for TEM8−/− and the control mice, Fig. 2 C-E) suggests that the in vivo toxicity of anthrax lethal toxin mediated by TEM8 results from cumulative damage that is qualitatively different from that mediated by CMG2.
Fig. 2.
CMG2 is the major anthrax toxin receptor in vivo. (A) Susceptibility of TEM8+/+, TEM8+/−, and TEM8−/− mice to 100 μg of LT. The TEM8−/− mice and their littermate WT and TEM8+/− controls were i.p. challenged with a single dose of LT (100 μg), and monitored for survival for 2 weeks. (B) Resistance of CMG2−/− mice to 100 μg of LT. The CMG2−/− mice and their littermate control mice were treated as in (A). (C) Resistance of CMG2−/− mice to 2 doses of LT challenge. CMG2−/− and their control mice were i.p. treated with 2 doses of 100 μg LT at days 0 and 2. Also, CMG2+/+ and CMG2+/− mice were treated with 2 doses of 30 μg LT or 50 μg LT. The mice were monitored for survival for 2 weeks. (D–E) Resistance of CMG2−/− and TEM8−/−CMG2−/− mice to multiple doses of LT. CMG2−/−, TEM8−/−CMG2−/−, and their littermate mice were i.p. treated with either 3 doses of 100 μg LT at days 0, 2, and 4 (D), or 5 doses at days 0, 1, 2, 3, and 4 (E) and monitored for survival. TEM8−/−CMG2−/− mice were completely resistant to 5 doses of LT challenge while CMG2−/− mice were partially resistant. (F) Susceptibility of the liver-specific CMG2−/− mice to LT. Liver-specific CMG2−/− mice (CMG2flox/flox/Alb-Cre) and their littermate WT mice were i.p. challenged with LT (100 μg) on days 0 and 2 and monitored for survival. (G–H) Toxigenic B. anthracis spore infection experiments. CMG2−/− mice (G) and TEM8−/− mice (H) and their littermate control mice were injected with 108 B. anthracis A35 spores s.c. and monitored twice daily for 2 weeks postinfection for signs of malaise or mortality.
Although real-time RT-PCR analyses revealed that TEM8 and CMG2 are ubiquitously expressed, CMG2 was shown to be expressed at a relatively higher level in the liver (Fig. S1D). We previously showed that LT resulted in hypoxia-mediated damage in the mouse liver (15). To determine whether the relatively higher expression of CMG2 in liver accounts for the data presented above identifying CMG2 as the major anthrax toxin receptor, we generated liver-specific CMG2−/− mice by intercrossing CMG2flox/flox and Alb-Cre transgenic mice (having Cre recombinase under the direction of the mouse albumin promoter). The resulting liver-specific CMG2−/− mice (CMG2flox/flox/Alb-Cre) (Fig. S1C) and the WT control mice (from CMG2+/flox/Alb-Cre intercrossing) were then challenged i.p. with 2 doses of 100 μg LT at days 0 and 2. Liver-specific CMG2−/− mice showed similar sensitivity to LT challenge as the WT control mice (Fig. 2F), demonstrating that the greater expression of CMG2 than TEM8 in liver does not contribute to CMG2's role as the major anthrax toxin receptor.
CMG2−/− Mice Are Highly Resistant to B. anthracis Spore Infection.
To assess the role of CMG2 in an anthrax infection model, we used a previously established highly lethal dose of the nonencapsulated toxigenic B. anthracis Ames strain (A35) spores to challenge CMG2−/− and their littermate control mice. The A35 strain relies on the production of toxin for its lethal effects as toxin-deficient mutant variants are highly attenuated, much in the manner described for the very similar Sterne strain (27). We found that while all CMG2+/− and CMG2+/+ mice displayed substantial edema by 12 h after infection and succumbed by 2 days, the CMG2−/− mice were resistant and only a single CMG2−/− mouse (1 of 11) died at a very late time point (Fig. 2G). In contrast to the spore resistance of the CMG2−/− mice, the TEM8−/− mice displayed similar sensitivity as their littermate control mice to the spore infection (Fig. 2H). The high resistance to infection in the CMG2−/− mice verifies the absolute requirement for the anthrax toxins in lethality caused by nonencapsulated toxigenic B. anthracis strains and further establishes the key role of CMG2 in mediating anthrax toxin lethality in vivo. These spore challenge results and the toxin challenge studies described above demonstrating the very limited role of TEM8 in anthrax toxin pathogenesis in vivo are surprising in view of the previous cell-culture based studies showing that TEM8, the first PA receptor to be discovered, functions effectively as a receptor in vitro (5, 22).
CMG2 Has a Higher Affinity for PA than TEM8 Does.
To further explore why CMG2 is the major anthrax toxin receptor in vivo, we measured the apparent functional affinities (Kd) of PA for CMG2−/− and TEM8−/− MEFs using competitive Schild Plot analyses (23, 28). We determined that the apparent affinities of PA-U7 (and, by implication, WT PA) for CMG2-expressing MEFs (TEM8−/−) and TEM8-expressing MEFs (CMG2−/−) were 0.9 nM and 10.9 nM, respectively (Fig. S5), revealing that the apparent affinity of PA for CMG2 is 11-fold higher than that for TEM8. The apparent Kd of PA binding to WT MEFs was 3.0 nM (Fig. S5), intermediate to the values for TEM8−/− and CMG2−/− MEFs, reflecting the fact that WT cells use both TEM8 and CMG2 as receptors. Thus, the greater importance of CMG2 as a toxin receptor in vivo is at least partially due to its higher affinity for PA.
The LT Resistance of CMG2−/− Mice Is Not Due to the Decoy Action of the TM-Deleted CMG2.
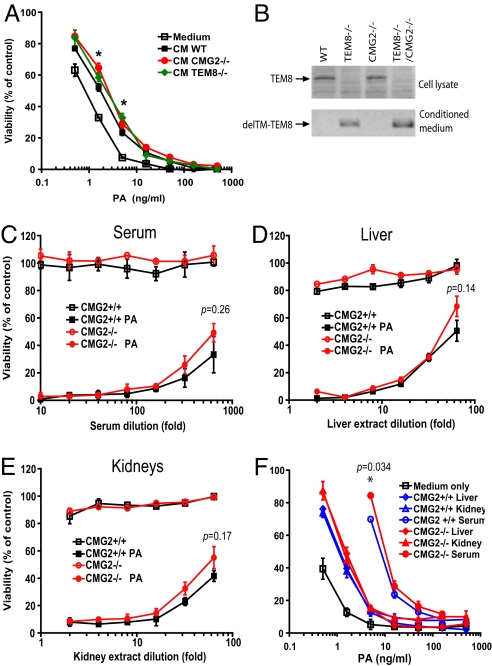
To determine whether TM-deleted CMG2 is secreted and might be acting as a decoy receptor, we collected 24 h conditioned media (CM) from WT, TEM8−/−, and CMG2−/− MEFs. The CM from WT, TEM8−/−, and CMG2−/− MEFs could significantly shift the killing curves of WT MEFs rightward when compared to the nonconditioned medium, suggesting that these cells including WT MEFs did secret soluble isoforms of toxin receptors (Fig. 3A). In addition, the CM from TEM8−/− and CMG2−/− MEFs displayed slightly more protection than that from WT MEFs (only significant when PA was lower than approximately 5 ng/mL) (Fig. 3A), suggesting that TM-deleted CMG2 and TEM8 might be secreted but at levels too low to confer significant protection to the cells from toxin treatment. Because anthrax toxin can reach very high levels in blood during anthrax infection in animals and humans (29), the TM-deleted TEM8 and CMG2 are unlikely to be effective as decoy receptors in this context. We next performed Western blotting using a TEM8 antibody and found that TEM8 could not be detected in cell lysates of TEM8−/− and TEM8−/−CMG2−/− MEFs but was detected in WT and CMG2−/− cells (Fig. 3B). Furthermore, TM-deleted TEM8 could only be detected in the conditioned media from TEM8−/− and TEM8−/−CMG2−/− MEFs but not WT and CMG2−/− MEFs. (Anti-CMG2 specific antibody suitable for similar experiments with CMG2 was unavailable.) These results indicate that the mutated TEM8 and possibly also the mutated CMG2 are secreted as a result of their TM region deletion. Because data presented above show that TEM8−/− mice are fully sensitive to anthrax toxin (Fig. 2A and Fig. S4), it can be concluded that the small amounts of TM-deleted TEM8 secreted in TEM8−/− mice are not sufficient to have a measurable effect as a decoy in our toxin challenge model.
Fig. 3.
TM-deleted CMG2 in the sera and tissue extracts of CMG2−/− mice does not act as an effective decoy in cytotoxicity assays. (A) Competition cytotoxicity assays using CM from WT, TEM8−/−, and CMG2−/− MEFs. The WT MEFs were incubated with PA (0–500 ng/mL) plus FP59 (100 ng/mL) in the presence of medium only or the 24 h CM from WT, TEM8−/−, and CMG2−/− MEFs at 37 °C for 24 h before cell viability was determined by MTT assay. CM from TEM8−/− and CMG2−/− MEFs displayed slightly more protection than that from WT MEFs only when PA was lower than approximately 5 ng/mL. *, P < 0.05. (B) TEM8 expression in MEFs. The MEF lysates and the 10-fold concentrated CM from the MEFs were analyzed by Western blotting using an anti-TEM8 monoclonal antibody (SB5). TM-deleted TEM8 was detected in the medium of TEM8−/− and TEM8−/−CMG2−/− MEFs but not in their cell lysates. (C–E) Cytotoxicity assays of the sera (C), liver (D), and kidney (E) extracts from CMG2+/+ and CMG2−/− mice after 100 μg PA injection (i.p.). The sera and the tissue extracts from CMG2+/+ and CMG2−/− mice 1 h after 100 μg PA injection (i.p.) were serially diluted as indicated and applied to the WT MEFs in the presence of 1 μg/mL of FP59 at 37 °C for 4 h. The cells were then changed into fresh medium and cultured at 37 °C for 24 h before assessing cell viability using MTT. Sera and tissues extracts from mice that received no PA were tested in parallel. The sera and tissue extracts from CMG2+/+ and CMG2−/− mice treated with PA showed comparable potency to WT MEFs. In no case did the small differences observed reach statistical significance (i.e., all p values were > 0.1). (F) Competition cytotoxicity assays of the sera, liver, and kidney extracts from CMG2+/+ and CMG2−/− mice. The MEFs were treated with PA (0–500 ng/mL) and FP59 (100 ng/mL) in the presence of the sera and tissue extracts from CMG2+/+ and CMG2−/− mice at 37 °C for 4 h. The cells were then changed into fresh medium and cultured at 37 °C for 24 h before assessing cell viability using MTT. The sera and tissue extracts from CMG2−/− mice could not provide significantly higher protection to WT MEFs against PA + FP59 treatment than those from CMG2+/+ mice.
To address whether a putative secreted TM-deleted CMG2 contributes to, or accounts for, the resistance of CMG2−/− mice to LT, we isolated sera, liver, and kidney extracts from CMG2−/− and the WT control mice 1 h after 100 μg PA injection (i.p.) or without injection. The supernatants of these tissues homogenized in serum-free DMEM were assayed for the presence of PA and a CMG2 decoy. Extracts from the mice treated with PA could efficiently kill WT MEFs (in the presence of added FP59), with comparable potency seen in extracts from CMG2+/+ and CMG2−/− mice, suggesting that the TM-deleted CMG2 in blood and tissues from CMG2−/− mice was not sufficiently abundant to neutralize PA and is therefore unlikely to account for the LT resistance of CMG2−/− mice (Fig. 3 C-E).
We also evaluated the PA neutralizing activities of sera, liver, and kidney extracts from CMG2+/+ and CMG2−/− mice in competition cytotoxicity assays using WT MEFs. Similar to the results obtained using MEF conditioned media shown above in Fig. 3A, the sera and tissue extracts from both CMG2+/+ and CMG2−/− mice had some toxin inhibitory activity, shifting the dose-response curves rightward (Fig. 3F). However, the sera and tissue extracts from CMG2−/− mice did not confer more protection than those from CMG2+/+ mice to MEFs treated with PA/FP59, except when PA was at very low concentrations of about 5 ng/mL (Fig. 3F).
We also performed LT dose-dependent challenges to CMG2+/+ and CMG2+/− mice to evaluate the inhibiting effects of a putative TM-deleted CMG2 in CMG2+/− mice, which would be predicted to equal 50% of that in CMG2−/− mice. CMG2+/+ and CMG2+/− mice showed similar sensitivity to 2 doses (2×) of 30 μg or 50 μg challenges (Fig. 2C). CMG2+/+ and CMG2+/− mice were equally resistant to 2 × 30-μg LT challenge (only one-sixth and one-eighth died, respectively). However, both types of mice largely succumbed to a 2 × 50 μg LT challenge (10/10 and 8/9 died, respectively, Fig. 2C). Since these mice differ little in response to toxin, one would conclude that the 50% equivalent of a putative CMG2 decoy receptor made by the CMG2+/− mice had no measurable impact in protecting against 2 × 30 μg LT. It then becomes difficult to attribute to a CMG2 decoy the complete resistance of the CMG2−/− mice to 2 × 100 μg of LT, since these mice would have only twice as much decoy as the amount present in CMG2+/− mice. These data strongly argue that the high resistance of CMG2−/− mice to LT is not due to the decoy action of a TM-deleted CMG2.
Discussion
The first step of anthrax toxin action is to gain entry into target cells through either of 2 VWA domain-containing proteins, TEM8 and CMG2. Although considerable data on these toxin receptors has been reported in recent years, questions remain as to their normal physiological roles and their relative roles as toxin receptors in vivo. To address these questions, we generated TEM8- and CMG2-null mice by deleting the TM regions for these receptors, which are essential for their anchoring on the cell surface. We confirmed that the TM-deleted TEM8 and CMG2 are null mutants and cannot function as anthrax toxin receptors. We found, interestingly, that both TEM8−/− and CMG2−/− mice are viable. This allowed us to further investigate the roles of these 2 receptors in anthrax toxin pathogenesis in vivo. We showed that even though TEM8 and CMG2 are both ubiquitously expressed in various tissues, only CMG2−/− mice are resistant to LT challenge, whereas TEM8−/− mice are as susceptible to a single dose of LT as the WT control mice. Importantly, CMG2−/− mice are also highly resistant to nonencapsulated toxigenic B. anthracis spore infection, while TEM8−/− mice remain sensitive. These results show that the in vivo toxicity of anthrax toxin is mostly mediated by CMG2. These data are in sharp contrast to previous in vitro studies that demonstrated the robust efficacy of TEM8 as an anthrax toxin receptor (5, 22) and show that the behavior of TEM8 in cell culture models does not recapitulate in the whole animal model.
Injection of CMG2−/− mice with multiple doses of LT did lead to some deaths at later times whereas no deaths occurred in similarly challenged TEM8−/−CMG2−/− mice, demonstrating that TEM8 functions as only a minor anthrax toxin receptor in vivo. This is likely because anthrax toxin has approximately 11-fold higher affinity for CMG2 than for TEM8 as judged by Schild plot analyses using WT, TEM8−/−, and CMG2−/− MEFs.
Through the study of TEM8−/− and CMG2−/− MEFs, we verified by Western blotting that the TM-deleted TEM8 is secreted into culture media, and this might also be true of TM-deleted CMG2 because the conditioned media from TEM8−/− and CMG2−/− MEFs provided slightly higher protection than that from WT MEFs to cells against cytotoxicity mediated by low concentrations of PA (<5 ng/mL). Through analyzing the sera and tissue extracts from CMG2+/+ and CMG2−/− mice, we demonstrated that the secreted TM-deleted CMG2 could not account for the LT resistance of CMG2−/− mice. We also performed LT dose-dependent challenges to CMG2+/+ and CMG2+/− mice to detect any inhibitory effects of the putative TM-deleted CMG2 in CMG2+/− mice, which is assumed to be 50% of that in CMG2−/− mice. These experiments demonstrated that if there was any decoy action of a TM-deleted CMG2 in CMG2+/− mice, it could only neutralize the effects of <2 × 20 μg of LT. Based on this, the TM-deleted CMG2 in CMG2−/− mice should not block the toxicity mediated by 2 × 100 μg of LT to which CMG2−/− mice were completely resistant. Therefore, the LT resistance of CMG2−/− mice is not due to the decoy action of a TM-deleted CMG2.
Although CMG2−/− mice lack easily observable phenotypic changes, we did find that the female CMG2−/− mice are infertile; the mice became pregnant but failed to support normal embryonic development. Surprisingly, similar to CMG2−/− mice, TEM8−/− female mice are also infertile, perhaps with similar processes being affected. Thus it appears that both TEM8 and CMG2 have certain nonredundant functions involved in embryonic development.
In summary, through generation and study of TEM8−/− and CMG2−/− mice, we found that the in vivo toxicity of anthrax toxin is mostly mediated by CMG2 and modestly by TEM8. This is at least partially because PA has higher affinity for CMG2 than for TEM8. Interestingly, both TEM8 and CMG2 have a nonredundant function in female reproduction. The results reported here offer a sound basis from which to further explore the mechanisms of TEM8 and CMG2 in normal physiology and pathogenesis.
Materials and Methods
MEF Isolation, Culture, and Cytotoxicity Assay.
MEFs were isolated from E13.5 embryos as described previously (30) and cultured in DMEM with 10% FBS. For cytotoxicity assays, cells were grown in 96-well plates and treated with serial dilutions of PA combined with FP59 for 48 h. Cell viability was then assayed by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] as described previously (22). In the NH4Cl protection experiments, cells were incubated with toxin for 3 h in the presence of 10 mM NH4Cl, then the toxins were removed and the cells were cultured in the presence of 10 mM NH4Cl for 48 h before cell viability analyses.
PA Binding, LF Translocation, and MEK Cleavage Assays.
MEFs grown to confluence in 12-well plates were incubated with growth medium containing PA/LF (1 μg/mL for each) for 4 h at 4 °C or 0–8 h at 37 °C, then washed 5 times with Hanks's Balanced Salt Solution (Biofluids) to remove unbound toxins. Cells were then lysed in modified RIPA lysis buffer containing protease inhibitors (22) and lysates were subjected to SDS/PAGE and Western blotting to detect cell-associated PA and MEK cleavage. Anti-PA polyclonal rabbit antiserum (#5308) was made in our laboratory. Anti-MEK3 (catalog no. sc-961) was obtained from Santa Cruz Biotechnology.
Mouse Toxin and B. anthracis Spore Challenge.
In toxin challenge experiments, 8–10 week old mice with various genotypes were injected with different doses of toxin (1.0 mL i.p. or 0.2 mL i.v.). Mice were observed for signs of malaise twice daily for 2 weeks following injection.
B. anthracis spores were prepared from the nonencapsulated toxigenic B. anthracis Ames 35 (A35) strain (31). The bacteria were grown on nutrient sporulation agar at 37 °C for 3 days. Spores were removed from plates by washing with sterile water and further purified by centrifugation (32). Spore counts were assessed by dilution plating and microscopy. For spore infections, mice were injected with 108 spores s.c. and monitored twice daily for 2 weeks postinfection for signs of malaise or mortality. In this study, we used the littermates as controls in all toxin and spore challenge experiments.
More information about materials and methods can be found in SI Methods.
Supplementary Material
Acknowledgments.
We thank Rasem Fattah for assistance in protein purification, Andrew Griffin for assistance in genotyping, Thomas Bugge, Pradeep Gupta, and Yogendra Singh for helpful discussion, and Brad St. Croix for TEM8 monoclonal antibodies and for sharing his unpublished work before publication. This research was supported by the intramural research program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905409106/DCSupplemental.
References
- 1.Cui X, et al. Late treatment with a protective antigen-directed monoclonal antibody improves hemodynamic function and survival in a lethal toxin-infused rat model of anthrax sepsis. J Infect Dis. 2005;191:422–434. doi: 10.1086/427189. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, Schubert RL, Bugge TH, Leppla SH. Anthrax toxin: Structures, functions and tumour targeting. Expert Opin Biol Ther. 2003;3:843–853. doi: 10.1517/14712598.3.5.843. [DOI] [PubMed] [Google Scholar]
- 3.Leppla SH. Bacillus anthracis toxins. In: Alouf JE, Popoff MR, editors. The Comprehensive Sourcebook of Bacterial Protein Toxins. Burlington, MA: Academic; 2006. pp. 323–347. [Google Scholar]
- 4.Young JA, Collier RJ. Anthrax toxin: Receptor-binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
- 5.Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414:225–229. doi: 10.1038/n35101999. [DOI] [PubMed] [Google Scholar]
- 6.Scobie HM, Rainey GJ, Bradley KA, Young JA. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci USA. 2003;100:5170–5174. doi: 10.1073/pnas.0431098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei W, Lu Q, Chaudry GJ, Leppla SH, Cohen SN. The LDL receptor-related protein LRP6 mediates internalization and lethality of anthrax toxin. Cell. 2006;124:1141–1154. doi: 10.1016/j.cell.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 8.Abrami L, et al. Functional interactions between anthrax toxin receptors and the WNT signalling protein LRP6. Cell Microbiol. 2008;10:2509–2519. doi: 10.1111/j.1462-5822.2008.01226.x. [DOI] [PubMed] [Google Scholar]
- 9.Leppla SH. Anthrax toxin edema factor: A bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci USA. 1982;79:3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Firoved AM, et al. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am J Pathol. 2005;167:1309–1320. doi: 10.1016/S0002-9440(10)61218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duesbery NS, et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 12.Vitale G, et al. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem Biophys Res Commun. 1998;248:706–711. doi: 10.1006/bbrc.1998.9040. [DOI] [PubMed] [Google Scholar]
- 13.Vitale G, Bernardi L, Napolitani G, Mock M, Montecucco C. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem J. 2000;352(Pt 3):739–745. [PMC free article] [PubMed] [Google Scholar]
- 14.Baldari CT, Tonello F, Paccani SR, Montecucco C. Anthrax toxins: A paradigm of bacterial immune suppression. Trends Immunol. 2006;27:434–440. doi: 10.1016/j.it.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Moayeri M, Haines D, Young HA, Leppla SH. Bacillus anthracis lethal toxin induces TNF-á-independent hypoxia-mediated toxicity in mice. J Clin Invest. 2003;112:670–682. doi: 10.1172/JCI17991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui X, et al. Lethality during continuous anthrax lethal toxin infusion is associated with circulatory shock but not inflammatory cytokine or nitric oxide release in rats. Am J Physiol. 2004;286:R699–R709. doi: 10.1152/ajpregu.00593.2003. [DOI] [PubMed] [Google Scholar]
- 17.Abrami L, Leppla SH, van der Goot FG. Receptor palmitoylation and ubiquitination regulate anthrax toxin endocytosis. J Cell Biol. 2006;172:309–320. doi: 10.1083/jcb.200507067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St Croix B, et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 19.Dowling O, et al. Mutations in capillary morphogenesis gene-2 result in the allelic disorders juvenile hyaline fibromatosis and infantile systemic hyalinosis. Am J Hum Genet. 2003;73:957–966. doi: 10.1086/378781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanks S, et al. Mutations in the gene encoding capillary morphogenesis protein 2 cause juvenile hyaline fibromatosis and infantile systemic hyalinosis. Am J Hum Genet. 2003;73:791–800. doi: 10.1086/378418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scobie HM, et al. Anthrax toxin receptor 2-dependent lethal toxin killing in vivo. PLoS Pathog. 2006;2:e111. doi: 10.1371/journal.ppat.0020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu S, Leppla SH. Cell surface tumor endothelium marker 8 cytoplasmic tail-independent anthrax toxin binding, proteolytic processing, oligomer formation, and internalization. J Biol Chem. 2003;278:5227–5234. doi: 10.1074/jbc.M210321200. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Leung HJ, Leppla SH. Characterization of the interaction between anthrax toxin and its cellular receptors. Cell Microbiol. 2007;9:977–987. doi: 10.1111/j.1462-5822.2006.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arora N, Leppla SH. Residues 1–254 of anthrax toxin lethal factor are sufficient to cause cellular uptake of fused polypeptides. J Biol Chem. 1993;268:3334–3341. [PubMed] [Google Scholar]
- 25.Rainey GJ, et al. Receptor-specific requirements for anthrax toxin delivery into cells. Proc Natl Acad Sci USA. 2005;102:13278–13283. doi: 10.1073/pnas.0505865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosovitz MJ, et al. Alanine scanning mutations in domain 4 of anthrax toxin protective antigen reveal residues important for binding to the cellular receptor and to a neutralizing monoclonal antibody. J Biol Chem. 2003;278:30936–30944. doi: 10.1074/jbc.M301154200. [DOI] [PubMed] [Google Scholar]
- 27.Pezard C, Berche P, Mock M. Contribution of individual toxin components to virulence of Bacillus anthracis. Infect Immun. 1991;59:3472–3477. doi: 10.1128/iai.59.10.3472-3477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varughese M, Teixeira AV, Liu S, Leppla SH. Identification of a receptor-binding region within domain 4 of the protective antigen component of anthrax toxin. Infect Immun. 1999;67:1860–1865. doi: 10.1128/iai.67.4.1860-1865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molin FD, et al. Ratio of lethal and edema factors in rabbit systemic anthrax. Toxicon. 2008;52:824–828. doi: 10.1016/j.toxicon.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Nagy A, Gertsenstein M, Vintersten K, Behringer RR. Manipulating the Mouse Embryo: A Laboratory Manual. NY: Cold Spring Harbor Lab Press. Cold Spring Harbor; 2003. [Google Scholar]
- 31.Pomerantsev AP, Sitaraman R, Galloway CR, Kivovich V, Leppla SH. Genome engineering in Bacillus anthracis using Cre recombinase. Infect Immun. 2006;74:682–693. doi: 10.1128/IAI.74.1.682-693.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu H, Sa Q, Koehler TM, Aronson AI, Zhou D. Inactivation of Bacillus anthracis spores in murine primary macrophages. Cell Microbiol. 2006;8:1634–1642. doi: 10.1111/j.1462-5822.2006.00738.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.