Abstract
Diffuse infiltration of glioma cells into normal brain tissue is considered to be a main reason for the unfavorable outcomes of patients with malignant gliomas. Invasion of glioma cells into the brain parenchyma is facilitated by metalloprotease-mediated degradation of the extracellular matrix. Metalloproteases are released as inactive pro-forms and get activated upon cleavage by membrane bound metalloproteases. Here, we show that membrane type 1 metalloprotease (MT1-MMP) is up-regulated in glioma-associated microglia, but not in the glioma cells. Overexpression of MT1-MMP is even lethal for glioma cells. Glioma-released factors trigger the expression and activity of MT1-MMP via microglial toll-like receptors and the p38 MAPK pathway, as deletion of the toll-like receptor adapter protein MyD88 or p38 inhibition prevented MT1-MMP expression and activity in cultured microglial cells. Microglial MT1-MMP in turn activates glioma-derived pro-MMP-2 and promotes glioma expansion, as shown in an ex vivo model using MT1-MMP-deficient brain tissue and a microglia depletion paradigm. Finally, MyD88 deficiency or microglia depletion largely attenuated glioma expansion in 2 independent in vivo models.
Keywords: brain tumor, invasion, metalloprotease, toll-like receptor
Gliomas are the most frequent primary tumors of the brain, and for highly malignant gliomas (World Health Organization grade III and IV) there is no successful treatment; patients have an average survival time of approximately 1 y after diagnosis. Glioma cells are highly invasive and infiltrate normal brain tissue, and as a result, surgical resection is always incomplete. Degradation of ECM by membrane-bound and secreted metalloproteases facilitates glioma invasion. In particular, the membrane-bound metalloproteases are pivotal for tumor invasion as they very efficiently digest extracellular matrix proteins and also activate secreted metalloproteases (1) like matrix metalloproteinase-2 (MMP-2, also known as gelatinase A), which is one of the major proteases involved in glioma invasion in mouse models (2) and probably also in humans (3). Hence, membrane-inserted metalloproteases like membrane type 1 matrix metalloproteinase (MT1-MMP) can enable gliomas to invade the brain parenchyma as single cells (4).
Microglia are the intrinsic immune cells of the brain; they control the innate and the adaptive immune response in the CNS and are activated by inflammatory or other pathological stimuli (5). Activation of microglial toll-like receptors (TLRs) triggers the innate immune response and can initiate host-defense and tissue repair mechanisms, but also CNS inflammation, neurodegeneration, and trauma (5, 6). As microglial cells are attracted toward glioma in large numbers—glioma tissue consists of as much as 30% microglial cells—and because microglia density in gliomas positively correlates with malignancy, invasiveness, and grading of the tumors (7–9), we investigated if microglia may actively contribute to glioma expansion. Here, we show that soluble factors released from glioma stimulate microglial TLRs, resulting in microglial MT1-MMP expression via the TLR downstream signaling molecules MyD88 and p38 MAPK. In turn, MT1-MMP expression and activity in these immune cells promotes glioma cell invasion and tumor expansion.
Results
Glioma Associated Microglia Over-Express MT1-MMP.
We analyzed the expression pattern of the matrix protease MT1-MMP in mouse and human gliomas and found the enzyme to be expressed predominantly in microglial cells closely associated with the tumors. Whereas tumor-free human brain samples showed virtually no MT1-MMP expression, we detected intense MT1-MMP labeling, especially in higher-grade gliomas. Importantly, in human samples, immunolabeling for the microglial marker Iba1 and for MT1-MMP largely overlapped [supporting information (SI) Fig. S1 A–D and Table S1]. Likewise, after injection of a human glioma cell line (U373 cells) into immunodeficient mice, we detected that microglia represent the predominant cell type contributing intratumoral MT1-MMP expression (see Fig. S1E).
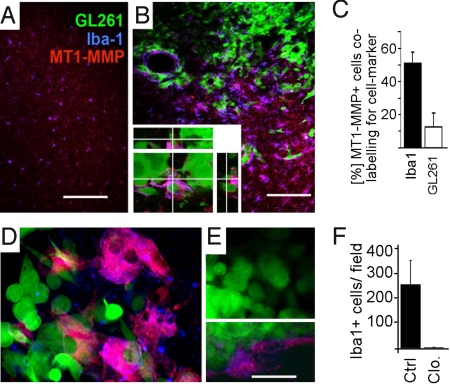
In our in vivo mouse model, the glioma cells were identified by stable expression of EGFP and microglial cells by immunolabeling for Iba1. In sections obtained from mice 2 weeks after intracerebral injection with isogenic glioma cells (GL261 cells), we found an increased density of microglial cells within the tumor and in the immediate vicinity of the glioma compared with the surrounding (i.e., tumor-free) parenchyma or the tissue of the contralateral hemisphere (Fig. 1 A and B; stereological cell counting revealed average microglia densities of 107 cells/mm2 in the contralateral hemisphere and 250 cells/mm2 in the tumor). MT1-MMP immunoreactivity was much more intense in the tumor proper and in the glioma margin, and preponderantly co-localized with labeling for Iba1, but not with GFP-expressing glioma cells (Fig. 1C). Microglial MT1-MMP immunoreactivity was particularly pronounced when the microglia were in close contact with glioma cells. To illustrate this, a single MT1-MMP-expressing microglia cell closely interacting with glioma cells was inspected by confocal microscopy; a stack of optical sections over 10 μm in the Z direction was 3-dimensionally reconstructed and is shown in Fig. 1B. Moreover, the overexpression of MT1-MMP was even increased at the glioma invasive edge compared with the tumor center. A similar observation was made in surgical specimens: we obtained glioma biopsy specimens in which the positional information (resection from the tumor center respectively to tumor border) was preserved and found that MT1-MMP expression was much stronger in the tumor border compared with the center (Fig. S1D). As previously described, microglial cells associated with the glioma had an ameboid morphology, indicating a certain degree of activation (10, 11).
Fig. 1.
Iba1-positive cells are overexpressing MT1-MMP when associated with experimental gliomas. Mouse brains injected with GL261 glioma cells expressing EGFP (green) were studied for the microglia marker Iba1 (blue) and for the metalloprotease MT1-MMP (red). (A) In the control (i.e., non-injected) hemisphere, the level of MT1-MMP expression is low. (B) The density of microglial cells is much higher in the vicinity of gliomas than in normal brain. MT1-MMP is expressed in microglia associated with glioma. Labeling for MT1-MMP is especially intense in microglia making close contact with glioma cells, whereas GL261 glioma cells express only very low levels of MT1-MMP. A magnified 3D reconstructed micrograph of the tumor area is shown (Inset). (C) Quantification of MT1-MMP expression in microglia and glioma. (D) GFP-expressing glioma cells 5 d after injection into cultivated brain slices shows again that MT1-MMP (blue) is expressed on microglia and endothelia (labeled by isolectin-B4; red). (E) A microglia-depleted brain slice preparation 5 d after injection of GFP-expressing glioma cells was labeled as in D; note that there is no labeling for microglia, whereas labeling for endothelial cells is still present (Inset, E). (F) Quantification of labeling for Iba1 in cultivated brain slices treated with clodronate (Clo.; for microglia depletion) compared with control slices (Ctrl.; i.e., microglia are intact). (Scale bars: 150 μm in A and B, 75 μm and 35 μm in D and E.)
MT1-MMP Expression Is Associated with the Presence of Microglia.
To further establish that MT1-MMP is overexpressed by glioma-associated microglia, but not by the tumor cells, we used a previously established brain slice model in which glioma can grow over a period of several days and in which microglial cells can be selectively depleted. By applying clodronate-filled liposomes to cultured brain slices, microglial cells were completely abrogated whereas the other cell types were not affected (8). After injection of GFP-expressing GL261 cells into these slices, the glioma cells invaded into the parenchyma. In slices containing microglial cells, MT1-MMP labeling was prominently expressed in the tumor area and was largely restricted to microglia (Fig. 1D). In contrast, we could not detect any MT1-MMP overexpression (Fig. 1E) in glioma-injected brain slices after microglia depletion; Fig. 1F shows depletion efficiency. This experimental paradigm did not interfere with cellular mechanisms controlling MT1-MMP expression per se, as the low-level expression of MT1-MMP in endothelia persisted after selective ablation of microglia (Fig. 1F Inset).
Microglial Expression and Activity of MT1-MMP is Up-Regulated after Stimulation with GL261 Conditioned Medium.
To investigate if glioma cells trigger the up-regulation of MT1-MMP expression in microglia (e.g., via a soluble factor), we stimulated cultured microglial cells with glioma-conditioned medium (GCM) and analyzed microglial MT1-MMP expression and activity by reporter-gene assays, RT-PCR, Western blot, and enzyme activity assays. We investigated GCM-triggered transcriptional activation of the MT1-MMP gene in microglia by transfecting cultured murine microglia with constructs containing a full-length or mutated MT1-MMP promoter linked to a luciferase reporter. Two days after transfection, microglia was stimulated for 6 h with GCM and the luciferase activity was assayed. In microglia containing the full-length MT1-MMP promoter, an increase in luciferase activity to more than 210% was observed after GCM stimulation compared with controls (pGL3-Basic and mutant-MT1-MMP reporter vectors; Fig. 2A). No change in reporter gene activity was observed in microglia transfected with control vectors after stimulation with GCM. Additionally, real-time PCR analysis of cultivated rat microglia revealed that stimulation with conditioned medium from rat glioma induces MT1-MMP, but not MMP2, expression (Fig. S1F).
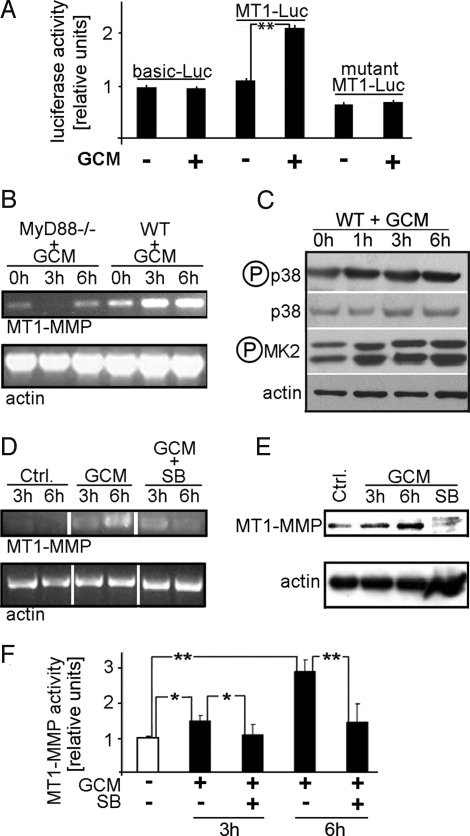
Fig. 2.
A glioma-released factor induces microglial MT1-MMP expression and activity via TLR signaling and the p38 MAPK pathway. (A). MT1-MMP gene expression in microglia stimulated with GCM was measured with a MT1-MMP promoter assay, using constructs containing the full MT1-MMP promoter (MT1-Luc), a promoter-free construct (pGL3-Basic) and a promoter construct containing a mutated MT1-MMP promoter (MT1-Luc-mut). GCM treatment induced the activity of the MT1-MMP promoter to approximately 210%. (B) RT-PCR for MT1-MMP expressed in microglia from WT or MyD88-knockout mice (MyD88-/-), stimulated with GCM for 0, 3, and 6 h (actin serves as loading control); note that MyD88 deficiency prevented the MT1-MMP up-regulation seen in WT. (C) Activation of the p38 MAPK pathway was observed in primary microglia treated for a maximum of 6 h with GCM. The cell lysates were analyzed for activated (i.e., phosphorylated) p38 MAPK (p-p38) and total p38 MAPK (p38). The membranes were then re-probed with anti-phosho-MAPKAPK-2 (p-MK2) antibodies, showing the activation of p38 MAPK downstream kinase (MK2; actin serves as loading control). (D) RT-PCR analysis highlights the p38 dependence of MT1-MMP expression; MT1-MMP mRNA levels (and actin as control) in microglia after stimulation with GCM or with GCM containing SB202190 (GCM+SB). (E) Western blots of microglial protein extracts upon stimulation with GCM or GCM+SB202190; note that GCM induced and co-treatment with SB 202190 prevented expression of MT1-MMP. (F) MT1-MMP activity increase after GCM treatment is mediated by p38 MAPK. Microglial cultures were stimulated with GCM or with GCM containing SB202190 for indicated time periods. The activity is normalized to controls (i.e., the baseline activity was measured in non-stimulated microglia). *Significant at P < 0.05. **Significant at P < 0.01.
The GCM-Induced MT1-MMP Expression and Activity in Microglia Is Mediated by TLR Signaling and p38 MAPK Activity.
MT1-MMP mRNA expression is up-regulated in murine WT microglia after 3 and 6 h of stimulation with GCM as demonstrated by RT-PCR analysis (Fig. 2B). Deletion of the TLR adapter protein, MyD88 (MyD88-/-), prevented the GCM-induced overexpression of MT1-MMP in microglia. The mRNA levels for MT1-MMP in MyD88-/- microglia are even reduced after 3 h of stimulation with GCM and return to basal levels after 6 h (Fig. 2B). These results firmly suggest that GCM stimulates microglial MT1-MMP overexpression via TLR signaling.
Recent reports indicate that the p38 MAPK pathway is activated downstream of TLR and MyD88 signaling (12) and that MT1-MMP expression is triggered by p38 MAPK activity (13, 14). To test if the p38 MAPK was activated by GCM, we analyzed microglial lysates by Western blotting with antibodies that selectively recognize the phosphorylated (i.e., activated) form of p38 MAPK. The membranes were re-probed to evaluate total MAPK expression. As shown in Fig. 2C, GCM increased p38 MAPK phosphorylation over a time course of 6 h. Additionally, we examined the ability of GCM to activate MAPK-activated protein kinase-2 (MK2; a downstream substrate of p38 MAPK) in microglia. MK2 phosphorylation (activation) increased along with p38 MAPK phosphorylation and peaked at 6 h after GCM treatment (Fig. 2C and Fig. S2). This demonstrates that microglial p38 MAPK is indeed activated by stimulation with GCM. The GCM-induced p38 MAPK activation then stimulates overexpression of MT1-MMP mRNA and protein, as demonstrated in Fig. 2 D and E, in which we show that the specific p38 MAPK blocker SB202190 (5 μM) abolished the GCM-triggered increase in microglial MT1-MMP. Furthermore, a urokinase activity assay also indicated that GCM-promoted microglial MT1-MMP activity was p38 MAPK-dependent, as SB202190 abrogated the GCM-induced MT1-MMP activity, both at 3 and 6 hours of stimulation (Fig. 2F).
Blocking the MT1-MMP Expression by shRNA in Microglia Results in Diminished Activation of Pro-MMP-2 Released from Glioma.
Our results indicated that a glioma-derived factor activates the microglial TLR signaling cascade via MyD88 and thereby stimulates MT1-MMP expression and function. We therefore tested whether microglial MT1-MMP may in turn convert glioma-derived pro-MMP-2 into active MMP-2. Hence, we attenuated microglial MT1-MMP expression by a sequence-specific shRNA and thus down-modulated MT1-MMP activity (Fig. 3A). However, in this experiment, we noted that the basal MT1-MMP activity (without GCM stimulation) was slightly increased (by 8%) after transfection with an shRNA for MT1-MMP. This may be explained by the immunomodulatory function of double-stranded RNAs (like siRNAs or shRNAs). Double-stranded RNAs are recognized by TLRs as foreign genetic information and can trigger immune responses (15). As TLR signaling also induces MT1-MMP expression, shRNAs may not be able to fully suppress MT1-MMP activity. This is supported by our observation that shRNAs unspecific for MT1-MMP raise the basal MT1-MMP activity even further (not shown).
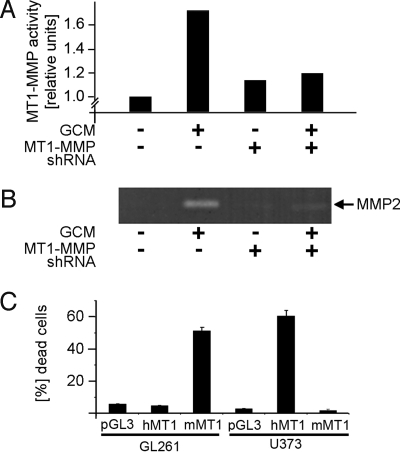
Fig. 3.
Microglial MT1-MMP activation of glioma-derived MMP-2 and the impact of glioma MT1-MMP expression on cell survival. (A) MT1-MMP shRNA blocks the GCM-stimulated MT1-MMP activity in microglia. The activity is measured with an enzymatic assay and is normalized to controls (i.e., the baseline activity was measured in non-stimulated microglia). (B) The gelatin zymography demonstrates that activation of MMP-2 is diminished in microglia transfected with an shRNA for MT1-MMP. (C) Forced expression of MT1-MMP in glioma cells induces cell death. The GL261 (mouse) and U373 (human) glioma cells were transfected with empty vector-pGL3, human MT1-MMP vector (hMT1), or mouse MT1-MMP vector (mMT1). Forty-eight hours after transfection, the cells were stained with propidium iodide (which labels dead cells) and Hoechst dye (which labels all cells). The rate of cell death was expressed as percentage of total cells.
Next, we measured GCM-triggered MMP2 activity with and without MT1-MMP knock-down. For this experiment we applied GCM to microglia for 6 h, subsequently replaced the GCM with medium without serum for 16 h, and then determined the MMP-2 levels in this newly harvested medium by gelatin zymography. Application of GCM to control microglia (i.e., microglia expressing a control construct; Fig. 3B) led to an increase of MMP-2 activity, whereas microglia expressing an shRNA for MT1-MMP released no detectable MMP-2 activity. This experiment suggests that glioma-induced expression of MT1-MMP in microglia may indeed serve to activate glioma-derived MMP-2.
Overexpression of MT1-MMP in GL261 Cells Induces Cell Death.
To investigate if the expression of higher levels of MT1-MMP in the glioma cells proper would be detrimental for the tumor cells (16), we transfected mouse and human MT1-MMP full-length expressing vectors in GL261 (mouse) and U373 (human) cells, and incubated the cells 48 h after transfection with propidium iodide to label the fraction of dead cells and Hoechst dye to label the entire cell population. In cells expressing homologous MT1-MMP, the rate of cell death was massively increased. Indeed, 53% of GL261 cells and 61% of U373 cells were positive for propidium iodide after overexpression of MT1-MMP (Fig. 3C). In contrast, in glioma cells expressing empty vectors or full-length heterologous MT1-MMP vectors (human MT1-MMP GL261 cells and mouse MT1-MMP in U373 gliomas), the percentage of dead glioma cells versus live glioma cells was less than 5%.
Deletion of Microglial MT1-MMP Impairs Glioma Invasion.
To investigate whether microglial MT1-MMP expression has an influence on glioma expansion, we used the organotypic brain slice culture model described earlier (8, 17). We compared glioma invasion in brain slice cultures from MT1-MMP+/+ MT1-MMP+/−MT1-MMP−/− mice. EGFP-transfected glioma cells were injected and glioma size was quantified as established previously (8, 10) by measuring the projected fluorescent area covered by the EGFP-transfected glioma cells within the slice 5 d after injection. These surface measurements give a good correlate for the invasiveness of a tumor (ref. 10; see also Fig. S3); additionally we used live-cell imaging to document that the invasive behavior of individual glioma cells is decreased in microglia-depleted brain slices, compared with microglia-containing controls (see Fig. S4). The average tumor size was significantly smaller in brain slice cultures obtained from MT1-MMP−/− mice, at 46% of MT1-MMP+/+ mice. Tumor size in brain slice cultures from MT1-MMP+/− was 78% of WT (Fig. 4A).
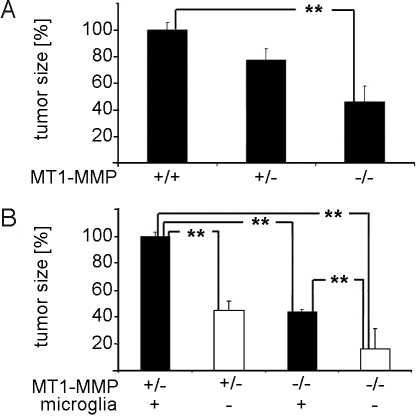
Fig. 4.
Microglial MT1-MMP mediates increased tumor size. (A) Invasion of glioma cells within organotypic brain slice cultures was determined from MT1-MMP WT (MT1-MMP+/+), MT1-MMP heterozygous (MT1-MMP+/−), and knockout (MT1-MMP−/−) mice 5 d after tumor injection. It is evident that the tumor invasion promoting MT1-MMP activity stems from the parenchyma. Defining the tumor size in organotypic brain slice cultures from MT1-MMP+/+ as 100%, the average tumor size from MT1-MMP−/− mice was 46% and that from MT1-MMP+/+ was 57.5%. (B) The tumor size from MT1-MMP+/− mice was defined as 100% and microglia depletion resulted in a tumor size of 45%. Tumor size of control and microglia-depleted organotypic brain slice cultures containing from MT1-MMP−/− mice was 43% and 16%, respectively. Significance was accessed by Wilcoxon signed-rank test; **P < 0.01.
To verify that the effect of MT1-MMP deletion on glioma growth is mediated by microglial cells, we depleted microglia from the organotypic slice as described earlier (8). We could confirm the previous observation that depletion of microglial cells leads to a reduction of glioma expansion (8) (Fig. S5 and Movie S1 and Movie S2). We compared normal and microglia-depleted slices from MT1-MMP+/− and MT1-MMP−/− mice. Defining the tumor size of microglia-containing MT1-MMP+/− brain slices as 100%, the microglia depletion in MT1-MMP+/− brain slices reduced glioma size to 45%, MT1-MMP−/− in microglia-containing slices to 43%, and microglia-depleted MT1-MMP−/− to a mere 16%. These data indicate that MT1-MMP is the major—but not the exclusive—glioma-promoting factor in microglia (Fig. 4B). Furthermore, this suggests that microglia is not the exclusive cell type expressing MT1-MMP, which is consistent with our immunohistochemical data showing that MT1-MMP expression is also detected on a low level in a subset of glioma cells and in some endothelia (see Fig. 1 C and E).
Glioma-Induced TLR Signaling in Microglia Promotes Parenchymal MT1-MMP Expression and Tumor Expansion in Vivo.
Our in vitro studies suggested that a soluble factor from glioma stimulates microglial TLRs, triggers MyD88 and p38MAPK signaling, and thereby induces microglial MT1-MMP expression. Increased parenchymal MT1-MMP in turn promoted accelerated glioma expansion, presumably also by activation of glioma-derived MMP2. To test whether this pathway may also be initiated in gliomas in vivo, we performed experiments with 2 different animal models. First, we evaluated the role of microglia-glioma interaction for tumor expansion in animals lacking most TLR signaling (MyD88-/-), and second, we used an approach to perform microglia depletion in vivo [by using the CD11b-HSVTK mouse (18)].
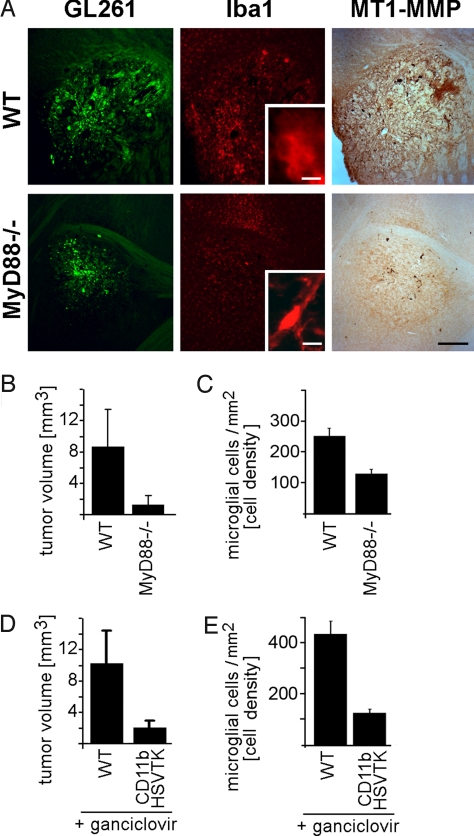
When we inspected tissue from MyD88-/- mice 14 d after glioma inoculation, we noted a considerable reduction in immunohistochemical labeling for MT1-MMP in the tumor area (and especially in the border between gliomas and the parenchyma; Fig. 5A) and a prominent reduction in tumor volume compared with WT controls (glioma size in MyD88-/- was reduced by 85% compared with WT controls; Fig. 5B). The reduction in tumor volume in MyD88-/- mice is likely a result of reduced glioma invasion. The distances that individual glioma cells migrated away from the tumor center is much greater in WT mice than in Myd88-/-, whereas the proliferative index of glioma cells in WT and knockouts is similar (see Fig. S5). The reduction in tumor size directly correlated with a massive reduction in microglia density in the tumor area (microglia density in MyD88-/- mice was reduced by 49% compared with WT), as obtained after quantification of microglia cell numbers in the whole tumor area. Furthermore, we noted that microglia in WT mice had an ameboid shape, which is generally observed in tumor-associated microglia and microglia in pathologic tissue, whereas glioma in Myd88-/- animals were associated with ramified microglia as found in healthy tissue (Fig. 5A Inset, highlighting Iba1 staining). This suggests that TLR signaling partakes in glioma-induced microglia activation.
Fig. 5.
Glioma-induced TLR signaling in microglia promotes parenchymal MT1-MMP expression and tumor expansion in vivo. Mice with reduced TLR-signaling (MyD88-/-; n = 8) and WT controls (n = 8) were intracerebrally inoculated with GFP-expressing glioma cells (GL261, green) and, 14 d later, immunohistochemically analyzed for Iba1 (red) MT1-MMP (brown DAB precipitate) staining (A); note that tumor size, intra- and peri-tumoral microglia density, and labeling intensity for MT1-MMP are all reduced in MyD88-/- animals; tumor volume (B) and microglia density (C) in and around glioma from MyD88-/- and WT were also quantified by stereology. Glioma cells were injected into the brain of transgenic CD11b-HSVTK (n = 8) and WT (n = 8) mice with subsequent intratumoral microglia depletion by intracerebral ganciclovir infusion in transgenic mice. Tumor volume (D) and intra- as well as peri-tumoral microglia density (E) were quantified by a morphometric stereologic analysis; note the significant microglia depletion and reduced tumor size. (Scale bar: 500 μm in A, 10 μm in A Insets.)
We also measured glioma volume in the CD11b-HSVTK mouse model (Fig. 5 D and E). Here, we modified the previously described paradigm (18) for interference with microglial function in vivo (19). Seven days after intracerebral glioma inoculation in WT or CD11b-HSVTK mice, we infused ganciclovir (a specific substrate for the viral thymidine kinase HSVTK) via mini-pumps [similarly as described by others (19)] into the tumor area for a further 7 d of in vivo experimentation. Only the monocytes of transgenic mice convert ganciclovir into highly toxic triphosphates. Hence, infusion of ganciclovir into the brain specifically and highly efficiently ablates microglia (by 71%; see Fig. 5E), which was evaluated by counting microglia cell numbers in the tumor border (as the tumor center was missing after retraction of the mini-pump cannula). Microglia depletion resulted in an 80% reduction in glioma volume (Fig. 5D).
In both models, Myd88-/- and CD11b-HSVTK, the strong reduction in microglia density in gliomas should only marginally contribute to an overall reduction in tumor size. Microglia comprise as much as 30% of the tumor mass, and a 71% depletion of intra-tumoral microglia (like in the CD116-HSVTK model) should reduce the entire glioma mass by 21%, which is only a minor effect compared with the 80% reduction in total tumor volume observed in this model.
Discussion
MT1-MMP Is Predominantly Expressed by Tumor-Associated Microglia.
Up-regulation of MT1-MMP expression in glioma has also been reported previously for human tissue samples. It was shown that MT1-MMP is up-regulated at the tumor edge compared with the center (3). However, in that previous study, the MT1-MMP expressing cell was not identified. Our data indicate that MT1-MMP expression is up-regulated in human, mouse, and rat microglia when they are in close contact with glioma cells. In the normal brain, MT1-MMP expression in microglia is low and detectable only in the white matter (20).
Glioma Cells Induce Microglial MT1-MMP Expression via TLR Signaling and the p38 MAPK Pathway.
GCM rapidly up-regulated microglial MT1-MMP expression and activity. Induction of MT1-MMP depended on TLR signaling (via the TLR adaptor MyD88) and activation of the p38 MAPK pathway, which is in agreement with studies showing that p38 MAPK activation is induced downstream of TLR and MyD88 signaling (12) and that MT1-MMP expression is regulated through p38 MAPK (13, 14).
TLRs are pattern recognition receptors that sense pathogens and induce the innate immune response (6). Moreover, it is becoming increasingly accepted that TLRs have functions beyond recognition of infections and may generally serve to monitor “well-being” in tissues (21). Microglia represent the predominant TLR expressing cell type in the CNS (5), and microglia are continuously scanning the brain for pathological insults (22). Furthermore, we show here that microglial cells receive signals from gliomas via TLRs. Gliomas can constitutively release heat-shock proteins, the high-mobility group box 1 protein, and hyluronan (23–25) and all these factors have been shown to stimulate TLRs (26). It is possible that these factors were present in our GCM, which was used to stimulate microglial MT1-MMP expression and activity in vitro. Moreover, in vivo TLRs can also be stimulated by factors liberated after cell necrosis, which is a frequent event in high-grade gliomas. Additionally, TLR signaling can be desinhibited through degradation of the extracellular matrix (27). In glioma this may lead to a vicious cycle in which TLRs induce metalloproteinase activity that in turn facilitates further TLR signaling. These in vivo effects may further explain the strong reduction in tumor size in vivo in our animal models (MyD88 knockout vs. WT or CD11b-HSVTK vs. WT).
In a glioma model similar to our CD11b-HSVTK model (also using GL261 cells), it previously was suggested that microglia are anti-tumorigenic and that microglia depletion can promote tumor growth (28). However, these findings are based on systemic ganciclovir administration upon bone marrow transplantation including irradiation. For this systemic approach the CD11b-HSVTK mice were reconstituted with WT bone marrow resulting in a reduction of microglia by approximately 30%, This experimental approach produced a rather small “inverse” effect, namely a slight (33%) increase in the overall glioma volume. These results indicate that microglia may have a pro- and an anti-tumorigenic function: such function may crucially depend on the microglial activity status and/or the amount of microglia present within or next to gliomas, thus possibly promoting a pro-pathological net effect of tumor-associated microglia (Fig. 5 D and E).
Glioma Depend on MT1-MMP Expressing Microglia to Promote Tumor Expansion.
We investigated the physiological role of MT1-MMP over-expression in glioma-associated microglia. Interestingly, microglial cells may boost glioma invasion not only by the matrix digesting activity of MT1-MMP alone, but this enzyme may serve to activate the excess pro-MMP-2 from glioma. Indeed, our zymography experiments showed that exposure of microglia to GCM led to a conversion of glioma derived pro-MMP-2 into active MMP-2, which depended entirely on microglial MT1-MMP expression.
Glioma cells relied on microglial MT1-MMP expression to sufficiently activate their pro-MMP content, as glioma cells underwent cell death when they themselves over-expressed MT1-MMP. This is in accordance with a recent study showing that MT1-MMP may have an additional role in regulating glioma cell homeostasis and that increased MT1-MMP expression can induce necrosis in glioma (16). Such a specific signal transduction role is also suggested by our experiments, as the death promoting effect of MT1-MMP in glioma was species-specific and could not be mimicked by heterologous MT1-MMP expression.
As the MT1-MMP-knockout (MT1-MMP−/−) mice develop dwarfism, are weaker than their WT litter-mates, have a reduced life span, and start to die after only 30 postnatal days (29), it was not possible to test the glioma-related effects in MT1-MMP-knockout mice in vivo. However, these adverse affects were not apparent at the age used for brain slice preparation (16 postnatal days).
Overall, our data highlight that glioma manipulate tumor associated microglial cells—via TLR signaling—to express MT1-MMP and thereby to support glioma expansion. Our study suggests that the pro-tumorigenic role of microglial cells is substantial and may put microglial cells into focus as a target for new brain tumor therapies. Therapeutic TLR blockade, which may be achieved with TLR subtype-specific antagonists, could serve as a future tool to attenuate microglia-promoted tumor invasion.
Materials and Methods
Cell Culture.
GL261 cells (National Cancer Institute) and microglia were grown as previously described (8, 30).
Organotypic Brain Slice Model.
Organotypic brain slice culture preparation was performed as described previously (8). Brain tissue was derived from 16-d-old male C57BL/6 mice, and coronal brain sections (250 μm) were injected with 10,000 GFP-transfected GL261 tumor cells.
Cell Transfection.
Primary microglial cultures were transfected with jetPEI-macrophage transfecting reagent according to manufacturer instructions (Polyplus); 1.2 μg MT1-MMP luciferase and 0.3 μg Renilla luciferase plasmids for reporter assay experiments or 3 μg of MT1-MMP shRNA for MT1-MMP activity assay or gelatin zymography, for 48 h.
Reporter Assay.
A full-length fragment (7.2 kbp) of the human MT1-MMP promoter region (31) was cloned in front of luciferase gene and the resulting construct was transfected into microglia. The activity of MT1-MMP-Luc was compared with the mutated MT1-MMP-Luc promoter construct (in the Sp-1 site) (32) using a dual-luciferase assay (Promega).
Western Blotting.
Whole-cell protein extracts were prepared from microglial cells as described (33). Antibodies recognizing phosphorylated kinases and total kinases were from Cell Signaling. Rabbit anti-MT1-MMP antibody was from Sigma.
MT1-MMP Activity Assay.
This ELISA based assay measures amounts of active MT1-MMP. It was performed according to manufacturers instructions in a 96 well plate, using a mutated pro-urokinase for detection (GE-Healthcare).
Gelatin Zymography.
Activity of gelatinases (MMP-2 and MMP-9) was analyzed with the gelatin zymography procedure (34) containing 1% gelatin. After electrophoresis, the gel was washed, equilibrated, and stained in 0.5% Coomassie blue solution. Enzymatic activity resulted in gelatin digestion, visible as clear bands on the dark blue background. The different gelatinase types were identified by their corresponding molecular weights.
Semi-Quantitative and Quantitative RT-PCR.
RNA was isolated with an RNeasy kit (Qiagen) and first-strand cDNA was synthesized with SuperScript II reverse transcriptase (Invitrogen) using 1 μg RNA and oligo-dT primer. PCR was performed with a PCR kit (Invitrogen).
Quantitative real-time PCR was carried out using SYBR Green. Total RNAs isolated from 4 independent experiments were used for these analyses. cDNAs were synthesized by extension of oligo(dT)15 primers with. An aliquot of each RT reaction (cDNA equivalent to 50 ng RNA) was amplified in duplicate in. All primers are presented in SI Text.
shRNA.
shRNA targeting mouse MT1-MMP was purchased from Sigma. The sequence CGGGCAGTGATGAAGTCTTCACATCTCGAGATGTGAAGACTTCACACTGCTTTTG was inserted into the pLKO1 lentiviral vector.
In Vivo Models. WT C57BL/6 (Charles River), MyD88-/- and CD11b-HSVTK mice were bred and maintained as described (9, 18, 35, 36). Mice were immobilized and mounted onto a stereotactic head holder as described (9). Briefly, 1 μL (2 × 104 cells/μL) of GL261 EGFP cell suspension was injected 1 mm anterior and 1.5 mm lateral to the bregma and mice were kept for 14 d after surgery. Seven days after tumor implantation CD11b-HSVTK and WT mice received intracerebral mini-pumps, delivering 0.5 μL/h of 1 mg/mL ganciclovir (G2536; Sigma) diluted in artificial cerebrospinal fluid for a period of 7 d.
Human Biopsies.
A tissue array containing paraffin-embedded specimens from human astrocytomas of all grades and tumor-free brain tissue was obtained from US-Biomax.
Immunolabeling.
All staining was performed as described previously (9). Additional primary antibodies were rabbit anti-Iba1 (Wako) and rabbit anti-MT1-MMP (Sigma); MT1-MMP staining on paraffin-embedded samples was enhanced by tyramide amplification (Perkin-Elmer) according to manufacturer instructions.
Cell Counting and Unbiased Stereology.
Glioma volume was quantified according to the Cavalieri principle by determining tumor area in every twelfth 40-μm brain slice and then multiplying by 12 × 40 μm (see also ref. 9).
To determine microglia density, we used a previously established (17) unbiased approach using the optical fractionator procedure (StereoInvestigator; MicroBrightField). The coefficient of error of the probe was consistently 0.08 or lower.
Supplementary Material
Acknowledgments.
We gratefully acknowledge Dr. Maciek Lipko's contribution of real-time PCRs for MT1-MMP in rodent microglia. We thank Dr. Stefan Momma (Frankfurt, Germany) for donating human glioma material, Dr. Jouko Lohi (Helsinki, Finland) for donating MT1-MMP luciferase constructs, and Irene Haupt for providing excellent technical help. This study was supported by a joint German-Polish grant from BMFT (German Ministry of Research and Technology), Deutsche Forschungsgemeinschaft, and National Institutes of Health/National Institute of Neurological Disorders and Stroke Grant R01 NS046006 (to F.L.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804273106/DCSupplemental.
References
- 1.Osenkowski P, Toth M, Fridman R. Processing, shedding, and endocytosis of membrane type 1-matrix metalloproteinase (MT1-MMP) J Cell Physiol. 2004;200:2–10. doi: 10.1002/jcp.20064. [DOI] [PubMed] [Google Scholar]
- 2.Mayes DA, et al. PAX6 suppresses the invasiveness of glioblastoma cells and the expression of the matrix metalloproteinase-2 gene. Cancer Res. 2006;66:9809–9817. doi: 10.1158/0008-5472.CAN-05-3877. [DOI] [PubMed] [Google Scholar]
- 3.Guo P, et al. Up-regulation of angiopoietin-2, matrix metalloprotease-2, membrane type 1 metalloprotease, and laminin 5 gamma 2 correlates with the invasiveness of human glioma. Am J Pathol. 2005;166:877–890. doi: 10.1016/s0002-9440(10)62308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3:489–501. doi: 10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- 5.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 6.Crack PJ, Bray PJ. Toll-like receptors in the brain and their potential roles in neuropathology. Immunol Cell Biol. 2007;85:476–480. doi: 10.1038/sj.icb.7100103. [DOI] [PubMed] [Google Scholar]
- 7.Watters JJ, Schartner JM, Badie B. Microglia function in brain tumors. J Neurosci Res. 2005;81:447–455. doi: 10.1002/jnr.20485. [DOI] [PubMed] [Google Scholar]
- 8.Markovic DS, Glass R, Synowitz M, Rooijen N, Kettenmann H. Microglia stimulate the invasiveness of glioma cells by increasing the activity of metalloprotease-2. J Neuropathol Exp Neurol. 2005;64:754–762. doi: 10.1097/01.jnen.0000178445.33972.a9. [DOI] [PubMed] [Google Scholar]
- 9.Glass R, et al. Glioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survival. J Neurosci. 2005;25:2637–2646. doi: 10.1523/JNEUROSCI.5118-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sliwa M, et al. The invasion promoting effect of microglia on glioblastoma cells is inhibited by cyclosporin A. Brain. 2007;130(Pt 2):476–489. doi: 10.1093/brain/awl263. [DOI] [PubMed] [Google Scholar]
- 11.Strik HM, Stoll M, Meyermann R. Immune cell infiltration of intrinsic and metastatic intracranial tumours. Anticancer Res. 2004;24:37–42. [PubMed] [Google Scholar]
- 12.Watters TM, Kenny EF, O'Neill LA. Structure, function and regulation of the Toll/IL-1 receptor adaptor proteins. Immunol Cell Biol. 2007;85:411–419. doi: 10.1038/sj.icb.7100095. [DOI] [PubMed] [Google Scholar]
- 13.Boyd PJ, Doyle J, Gee E, Pallan S, Haas TL. MAPK signaling regulates endothelial cell assembly into networks and expression of MT1-MMP and MMP-2. Am J Physiol Cell Physiol. 2005;288:C659–C668. doi: 10.1152/ajpcell.00211.2004. [DOI] [PubMed] [Google Scholar]
- 14.Munshi HG, et al. Differential regulation of membrane type 1-matrix metalloproteinase activity by ERK 1/2- and p38 MAPK-modulated tissue inhibitor of metalloproteinases 2 expression controls transforming growth factor-beta1-induced pericellular collagenolysis. J Biol Chem. 2004;279:39042–39050. doi: 10.1074/jbc.M404958200. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal S, Kandimalla ER. Role of Toll-like receptors in antisense and siRNA [corrected] Nat Biotechnol. 2004;22:1533–1537. doi: 10.1038/nbt1042. [DOI] [PubMed] [Google Scholar]
- 16.Belkaid A, Fortier S, Cao J, Annabi B. Necrosis induction in glioblastoma cells reveals a new “bioswitch” function for the MT1-MMP/G6PT signaling axis in proMMP-2 activation versus cell death decision. Neoplasia. 2007;9:332–340. doi: 10.1593/neo.07142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Synowitz M, et al. A1 adenosine receptors in microglia control glioblastoma-host interaction. Cancer Res. 2006;66:8550–8557. doi: 10.1158/0008-5472.CAN-06-0365. [DOI] [PubMed] [Google Scholar]
- 18.Heppner FL, et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 19.Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Yamada T, et al. White matter microglia produce membrane-type matrix metalloprotease, an activator of gelatinase A, in human brain tissues. Acta Neuropathol (Berl) 1995;90:421–424. doi: 10.1007/BF00294800. [DOI] [PubMed] [Google Scholar]
- 21.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by toll-like receptor 4. J Immunol. 2002;168:5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 22.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 23.Guzhova I, et al. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914:66–73. doi: 10.1016/s0006-8993(01)02774-3. [DOI] [PubMed] [Google Scholar]
- 24.Bassi R, et al. HMGB1 as an autocrine stimulus in human T98G glioblastoma cells: role in cell growth and migration. J Neurooncol. 2008;87:23–33. doi: 10.1007/s11060-007-9488-y. [DOI] [PubMed] [Google Scholar]
- 25.Gately CL, et al. In vitro studies on the cell-mediated immune response to human brain tumors. II. Leukocyte-induced coats of glycosaminoglycan increase the resistance of glioma cells to cellular immune attack. J Immunol. 1984;133:3387–3395. [PubMed] [Google Scholar]
- 26.Aravalli RN, Hu S, Lokensgard JR. Inhibition of toll-like receptor signaling in primary murine microglia. J Neuroimmune Pharmacol. 2008;3:5–11. doi: 10.1007/s11481-007-9097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson GB, Brunn GJ, Platt JL. Cutting edge: an endogenous pathway to systemic inflammatory response syndrome (SIRS)-like reactions through toll-like receptor 4. J Immunol. 2004;172:20–24. doi: 10.4049/jimmunol.172.1.20. [DOI] [PubMed] [Google Scholar]
- 28.Galarneau H, Villeneuve J, Gowing G, Julien JP, Vallieres L. Increased glioma growth in mice depleted of macrophages. Cancer Res. 2007;67:8874–8881. doi: 10.1158/0008-5472.CAN-07-0177. [DOI] [PubMed] [Google Scholar]
- 29.Holmbeck K, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 30.Prinz M, Hanisch UK. Murine microglial cells produce and respond to interleukin-18. J Neurochem. 1999;72:2215–2218. doi: 10.1046/j.1471-4159.1999.0722215.x. [DOI] [PubMed] [Google Scholar]
- 31.Lohi J, Lehti K, Valtanen H, Parks WC, Keski-Oja J. Structural analysis and promoter characterization of the human membrane-type matrix metalloproteinase-1 (MT1-MMP) gene. Gene. 2000;242:75–86. doi: 10.1016/s0378-1119(99)00549-1. [DOI] [PubMed] [Google Scholar]
- 32.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 33.Ciechomska I, Pyrzynska B, Kazmierczak P, Kaminska B. Inhibition of Akt kinase signalling and activation of Forkhead are indispensable for upregulation of FasL expression in apoptosis of glioma cells. Oncogene. 2003;22:7617–7627. doi: 10.1038/sj.onc.1207137. [DOI] [PubMed] [Google Scholar]
- 34.Heussen C, Dowdle EB. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- 35.Adachi O, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 36.Lehnardt S, et al. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J Immunol. 2006;177:583–592. doi: 10.4049/jimmunol.177.1.583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.