Abstract
It generally is assumed that a common neural substrate mediates both the palatability and the reward value of nutritive events. However, recent evidence suggests this assumption may not be true. Whereas opioid circuitry in both the nucleus accumbens and ventral pallidum has been reported to mediate taste-reactivity responses to palatable events, the assignment of reward or inventive value to goal-directed actions has been found to involve the basolateral amygdala. Here we found that, in rats, the neural processes mediating palatability and incentive value are indeed dissociable. Naloxone infused into either the ventral pallidum or nucleus accumbens shell blocked the increase in sucrose palatability induced by an increase in food deprivation without affecting the performance of sucrose-related actions. Conversely, naloxone infused into the basolateral amygdala blocked food deprivation-induced changes in sucrose-related actions without affecting sucrose palatability. This double dissociation of opioid-mediated changes in palatability and incentive value suggests that the role of endogenous opioids in reward processing does not depend on a single neural circuit. Rather, changes in palatability and in the incentive value assigned to rewarding events seem to be mediated by distinct neural processes.
Keywords: incentive value, taste reactivity, instrumental conditioning, opioid receptors, basolateral amygdala
Goal-directed actions are the means by which we exert control over our environment in the service of our desires. The decision to engage in such actions is based largely on the degree to which the goal, or reward, is valued or “desired” (1). Theories regarding the neural bases of reward processing generally assume that the pleasure elicited during contact with rewarding events and the desirability, or incentive value, of those events are mediated by a common process (2–4). However, recent experiments assessing the neural bases of reward have found evidence of multiple candidate regions associated with what seem to be distinct aspects of reward processing. Using consumption and taste-reactivity measures of palatability in rodents, several groups have reported evidence of an opioid receptor-mediated network within the circuitry of the nucleus accumbens shell and ventral pallidum mediating hedonic processing or reward “liking” based on the palatability-enhancing effects of locally infused opioid agonists (5–8). However, although opioid processes often have been proposed to convey the affective properties of natural rewards (9, 10), opioid peptide-containing neurons and receptors are present in multiple basal forebrain regions (11, 12) implicated not in reward hedonia but rather in the learning process that mediates goal-directed actions. In particular, several studies have found the opioid receptor-rich basolateral amygdala (13) to be important for encoding the incentive value of the rewards that support the performance of goal-directed actions in rats (14–17). Although it is possible that opioid-related processes form an integrated reward network (18), the neural processes mediating palatability, or liking, may be distinct from those that establish the desirability of the consequences of goal-directed actions (19, 20).
We sought to evaluate these alternatives by comparing the role of opioid receptor-related processes in the nucleus accumbens shell, the ventral pallidum, and the basolateral amygdala using an animal model that permitted concurrent assessment of changes in liking, or palatability, and the incentive value of a reward. In both humans and rats increased food deprivation increases the palatability of food, as assessed by verbal hedonic evaluation or the incidence of positive facial responses and certain lick patterns, in addition to increasing the performance of food-related actions (21, 22). However, this last effect is not an immediate consequence of the motivational shift; rather, it depends on learning about the food's increased value through consummatory contact in the new state. Thus, in rats, an increase in food deprivation increases the performance of lever-pressing actions that gain access to a palatable food reward only after the rats have been given the opportunity to consume that food in the deprived state (23, 24). This phenomenon, called “incentive learning”, is considered to model the incentive value of rewards or the state of desire evoked to explain the pursuit of goals (23, 24). Here we used this incentive learning paradigm to compare changes in incentive value with changes in the palatability responses that reflect reward liking in rats to assess whether these components of reward processing are mediated by a common neural substrate.
Rats were maintained in a relatively sated state and trained, as illustrated in Table 1, to seek a sucrose reward using a procedure in which they had to press a lever (“seeking lever”) to get access to a second lever (“taking lever”) that delivered the sucrose (15, 25). This task has been found to establish a reward-seeking action specifically sensitive to incentive learning (15, 25). Next, we gave the rats the opportunity for incentive learning by allowing them to consume the sucrose after an increase in food deprivation. To evaluate the role of opioid processes in establishing the current desirability of the sucrose, we gave microinfusions of either the opioid antagonist naloxone or vehicle into the nucleus accumbens shell, ventral pallidum, or basolateral amygdala before consummatory contact (Fig. S1). During this session we evaluated the palatability responses elicited during consumption of the sucrose using a contact lickometer, a measure previously reported to provide an assessment of reward liking similar to that used by Berridge and colleagues (26, 27) (See also SI Results and Fig. S2). We next examined the effects of the naloxone infusions on incentive learning by assessing, off drug, the subsequent performance of goal-directed reward-seeking responses in an unrewarded test (i.e., in the absence of any further experience with the reward).
Table 1.
Design of incentive learning experiments
| Training | Non-Contingent Revaluation Test Day 1 | Test Test Day 2 |
|---|---|---|
| 2 h deprivation LPS → [LPD → Suc] | Naloxone or Vehicle2 h deprivation: Suc |
2 h or 23 h deprivation LPS → [LPD → ø] |
| Naloxone or Vehicle23 h deprivation: Suc |
Rats deprived of food for 2 h were trained to press a seeking lever (LPS) to gain access to a second lever that delivered a sucrose solution (Suc) (LPD→SUC). They then were maintained in the 2 h food-deprived condition or were shifted to 23 h food deprivation and were allowed to consume the sucrose after an infusion of naloxone or vehicle into the nucleus accumbens shell, ventral pallidum, or basolateral amygdala. The effect of the infusions on palatability responses was assessed in this re-evaluation session. The effect of re-exposure and the influence of naloxone on that effect in rats deprived of food for 2 h or 23 h were assessed in a test conducted on the levers in extinction (i.e., in the absence of further experience with the sucrose). (See SI Methods for full details.)
Results
Infusing Naloxone into Either the Nucleus Accumbens Shell or Ventral Pallidum Blocks Changes in Palatability Without Affecting Incentive Learning.
As illustrated in Table 1, this experiment was conducted in 3 phases involving initial training, incentive learning, and test. All the rats acquired and maintained lever-pressing performance, and, in the final session of training, performed the seeking lever response at a rate of 9.7 presses (earning 2.3 rewards) per min in the nucleus accumbens shell-cannulated group and 6.3 presses (1.5 rewards) per min in the ventral pallidum-cannulated group.
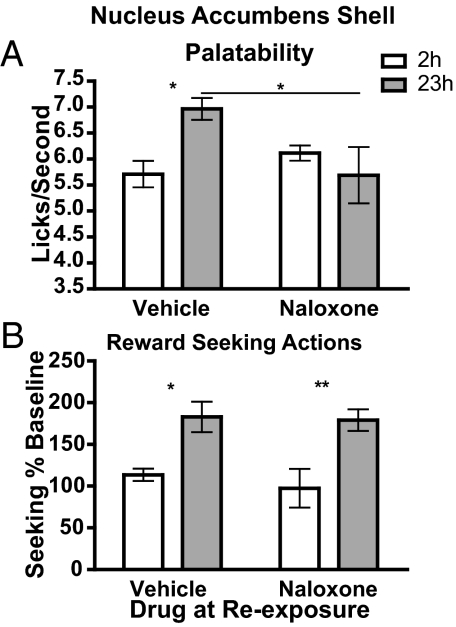
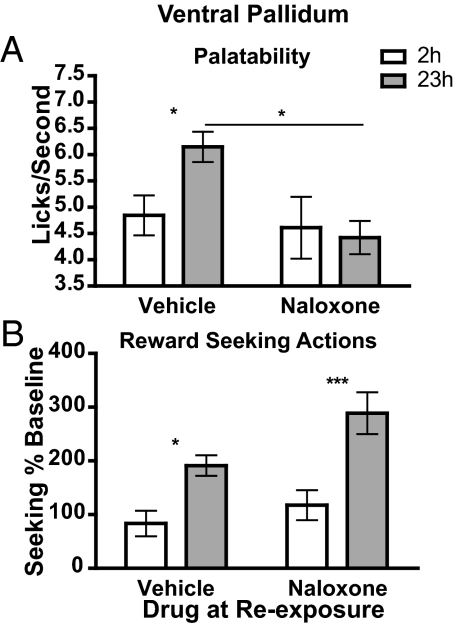
The effect of intra-nucleus accumbens shell and intra-ventral pallidum naloxone infusions on palatability and lever pressing are presented in Figs. 1 and 2, respectively. Figs. 1A and 2A show that infusion of naloxone into the portion of the nucleus accumbens shell or ventral pallidum previously shown to be an opioid receptor-mediated “hedonic hotspot” (5) did not alter sucrose palatability in the 2 h food-deprived condition during the incentive learning phase but did block the significant increase in palatability resulting from increased food deprivation, producing a significant interaction between deprivation and drug treatment. Indeed, rats in the 23 h food-deprived state showed a significant increase in lick frequency, representing an increase in palatability, in the vehicle-treated group but not in the naloxone-treated group. Using a Bayesian analysis (28, 29) of the naloxone group data, we found that the null hypothesis in the accumbens shell and ventral pallidum groups was, respectively, 1.99 and 2.91 times more probable than the alternate hypothesis, confirming that, under intra-accumbens shell or intra-ventral pallidum naloxone, rats did not express a food deprivation-induced increase in sucrose palatability. Other measures of palatability-related licking microstructure were assessed also and showed a similar pattern of effects after infusion into either the nucleus accumbens shell or the ventral pallidum (see Table S1).
Fig. 1.
Naloxone infused into the nucleus accumbens shell blocks food deprivation-induced increases in palatability without affecting incentive learning. (A) Palatability. Rats, deprived of food for either 2 h (control) or 23 h, received an infusion of vehicle or naloxone into the nucleus accumbens shell immediately before re-exposure to sucrose; during the exposure, licking frequency data were measured. Assessment by 2-way ANOVA of lick frequency found no main effect of drug (F1,24 = 2.11, P = 0.16) or deprivation (F1,24 = 1.91, P = 0.18) but did find a significant interaction between drug and deprivation (F1,24 = 7.88, P = 0.009). (The y axis is truncated at 3.5 licks/s based on our observation of this frequency as the floor licking rate). (B) Incentive learning. Incentive learning was assessed off drug in a test of lever-press performance conducted unrewarded. Reward-seeking response rates on test were normalized to response rates during baseline training. Assessment by 2-way ANOVA found a main effect of deprivation (F1,22 = 21.65, P = 0.0001), no effect of drug (F1,22 = 0.37 P = 0.54), and no interaction between drug and deprivation (F1,22 = 0.14 P = 0.71). Error bars represent standard error of the mean. n = 16. *, P < 0.05; **, P < 0.01.
Fig. 2.
Naloxone infused into the ventral pallidum blocks food deprivation-induced increases in palatability without affecting incentive learning. (A) Palatability: see the legend for Fig. 1A. Assessment by 2-way ANOVA of lick frequency data found a main effect of drug (F1,36 = 5.21, P = 0.03) but no effect of deprivation (F1,36 = 1.68, P = 0.20) or an interaction between drug and deprivation (F1,36 = 3.01, P = 0.09). (B) Incentive learning: see the legend for Fig.1B. Assessment by 2-way ANOVA of lever press data found a main effect of deprivation (F1,27 = 25.31, P < 0.001) and drug (F1,27 = 5.64, P = 0.02) but no interaction between drug and deprivation (F1,27 = 1.30, P = 0.26). n = 18. *, P < 0.05; ***, P < 0.001.
Figs. 1B and 2B illustrate the effect of intra-nucleus accumbens shell and intra-ventral pallidum naloxone infusion on incentive learning during exposure to sucrose after 23 h of food deprivation. An increase in seeking actions induced by exposure to the sucrose in the food-deprived condition would suggest that the incentive value of the sucrose was increased as a consequence of that experience (25, 30) and that this information was used to direct subsequent reward-seeking actions. A clear incentive learning effect was observed in rats given vehicle infusion into the nucleus accumbens shell and ventral pallidum and in those given naloxone infusion. In rats given accumbens shell infusions, there was a main effect of the re-exposure in the deprivation state on reward seeking, but no effect of drug and no interaction between these factors. Similarly, in the ventral pallidal-infused rats there was an effect of the re-exposure in the deprived state and also an effect of drug (resulting from the slightly enhanced incentive learning effect in the group re-exposed under naloxone) on reward-seeking actions, but, importantly, there was no interaction between these factors. A similar incentive learning effect also was observed in both groups in a subsequent off-drug rewarded test in which actions on the chain were rewarded with sucrose (see Table S2).
Importantly, therefore, despite its effectiveness in blocking the deprivation-induced increase in sucrose palatability, neither intra-accumbens shell nor intra-ventral pallidum naloxone was found to have any effect on incentive learning or on incentive learning-induced changes in reward seeking.
Infusing Naloxone into the Basolateral Amygdala Blocks Incentive Learning Without Affecting Palatability.
The lack of effect of intra-ventral pallidum or intra-nucleus accumbens shell naloxone on incentive learning strongly counters the claim that opioid processes in these regions mediate the process through which the desirability of rewarding events is established. The suggestion that the opioid receptor-rich (13) basolateral amygdala plays an important role in incentive learning (14–17) led us next to evaluate the influence of naloxone infusion into that structure on palatability responses and incentive learning using the procedure described in the previous sections (see Table 1). During the first (training) phase, all rats acquired and maintained performance of 9.00 lever presses (2.05 rewards) per min on the seeking lever.
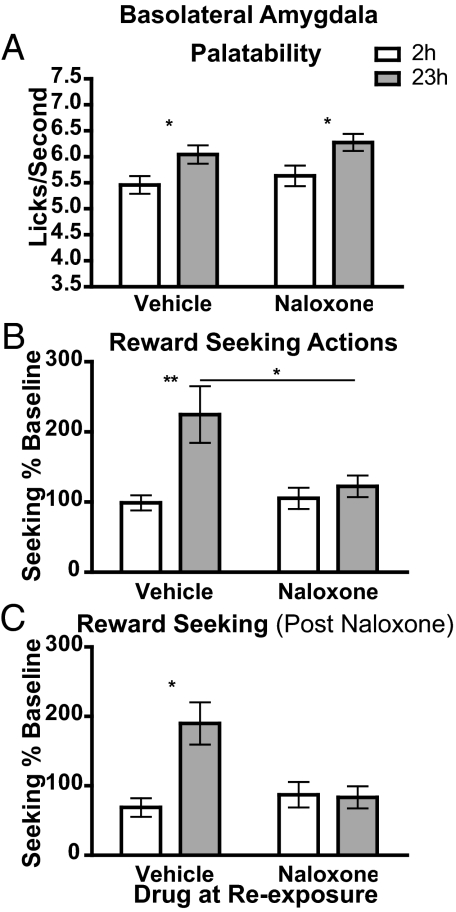
The results from the palatability and incentive learning assessments are presented in Fig. 3. As shown in Fig. 3A, and in contrast to its effects in the nucleus accumbens shell and ventral pallidum, intra-basolateral amygdala naloxone seemed to have no effect on the food deprivation-induced increase in palatability responses. The increase in food deprivation was found to increase licking frequency significantly, with neither an effect of drug treatment nor an interaction between deprivation and drug treatment; both the naloxone-infused and vehicle-infused rats showed a clear increase in palatability response at 23-h vs. 2-h food deprivation. Intra-basolateral amygdala naloxone did not affect other measures of licking microstructure thought to reflect palatability (see Table S1).
Fig. 3.
Intra-basolateral amygdala naloxone blocks incentive learning but does not affect changes in palatability or the retrieval of incentive value. (A) Palatability: see the legend for Fig. 1A. Assessment by 2-way ANOVA of lick frequency data following intra-basolateral amygdala infusion found a main effect of deprivation (F1,127 = 11.88, P = 0.0008), no effect of drug (F1,127 = 1.29, P = 0.25), and no interaction between drug and deprivation (F1,127 = 0.02, P = 0.88). (B) Incentive learning: see the legend for Fig. 1B. Assessment by 2-way ANOVA found a main effect of deprivation (F1,85 = 8.30, P = 0.005) and drug (F1,85 = 3.72, P = 0.05) and an interaction between drug and deprivation (F1,85 = 4.79, P = 0.03). (C) Naloxone on test: seeking response rates, normalized to training baseline, are shown for the rats receiving naloxone immediately before the non-rewarded test. Assessment by 3-way ANOVA found a main effect of deprivation (F1,127 = 11.90, P = 0.001) and of drug during re-exposure (F1,127 = 5.95, P = 0.016) but no effect of drug on test (F1,127 = 2.62, P = 0.11). There was an interaction between drug and deprivation during re-exposure (F1,127 = 9.58, P = 0.002). No other interactions were significant (all Fs < 1). n = 26 in both replications. *, P < 0.05; **, P < 0.01.
The results of the incentive learning test are presented in Fig. 3B. Unlike the effects of naloxone in the accumbens shell and ventral pallidum, intra-basolateral amygdala naloxone seemed to abolish incentive learning completely. Thus, a clear incentive learning effect was observed in reward-seeking actions in rats re-exposed to the sucrose when food deprived after an infusion of vehicle, but no elevation in performance was observed in those re-exposed when food deprived after an infusion of naloxone (Fig. 3B). Intra-basolateral amygdala naloxone had no effect in the 2-h food-deprived condition, indicating that naloxone did not reduce the sucrose incentive value but, rather, acted to block the increase in value. Bayesian analysis (28, 29) supported the notion that intra-basolateral amygdala naloxone blocked the incentive learning effect; the null hypothesis that there was no difference in seeking responses in the 2-h and 23-h conditions when re-exposed under naloxone was found to be 3.99 times more probable than the alternate hypothesis. Finally, as previously, rats subsequently were tested on the chain under rewarded conditions and, when food deprived for 23 h, were found to be capable of updating the incentive value of the sucrose. They increased their performance of seeking actions, irrespective of re-exposure drug treatment, indicating that naloxone infusion did not produce any permanent damage (see Table S2).
Generally, therefore, although blocking opioid receptors in the basolateral amygdala did not affect reward palatability (Fig. 3A), it clearly blocked incentive learning (Fig. 3B), consistent with the argument that activation of opioid receptors within the basolateral amygdala is necessary for encoding changes in the incentive value of the outcome of goal-directed actions.
Naloxone in the Basolateral Amygdala Affects Encoding but Not the Retrieval of Incentive Value.
Although it has been claimed that amygdala-related emotional or somatic states are required for both the encoding and the retrieval of incentive value information (the “somatic marker hypothesis”) (3), several experiments testing this claim have found data suggesting that incentive values are encoded more abstractly and that their retrieval is not dependent on the processes through which they were originally encoded (23, 31). Therefore, in an extension of the experiment reported in the previous sections, we assessed the role of basolateral amygdala opioid receptors in the retrieval of incentive value information. This experiment was conducted as described earlier, with rats deprived of food for either 2 h or 23 h being re-exposed to sucrose after an intra-basolateral amygdala infusion of either vehicle or naloxone. In this case, however, rats also received an infusion of either vehicle or naloxone into the basolateral amygdala before the non-rewarded incentive learning test.
The data from the rats receiving naloxone on test are presented in Fig. 3C. These data show that infusion of naloxone before the non-rewarded test did not influence the current incentive value; that is, the rate of responding reflected the incentive value encoded during re-exposure, and this value was not affected further by naloxone infusion before the test. Thus, although, as we found in the experiments described earlier, there was an interaction between deprivation and drug during re-exposure, the drug condition on test had no effect on seeking actions in the non-rewarded test. There was a significant increase in the rate of seeking responses in the 23-h food-deprived condition (relative to 2-h deprivation) in the rats that received intra-basolateral amygdala vehicle during re-exposure and naloxone on test. These data suggest that the effect of naloxone on re-exposure (Fig. 3B) was not drug-state dependent; the group that received infusions of naloxone both before re-exposure and before test did not show incentive learning. More importantly, these data suggest that activation of the endogenous opioid system in the basolateral amygdala is necessary for the encoding but not for the retrieval of incentive value information necessary to select a course of action.
Dynamic Effects During On-Baseline Changes in Incentive Value on Goal-Directed Action.
The previous results suggest that activation of endogenous opioid receptors in the basolateral amygdala is necessary to establish the desirability of the sucrose and thus to regulate the performance of goal-directed actions that earn it. However, because rats were allowed to learn about the change in the incentive value of the sucrose during a re-exposure session on the day before the test, the time-course of the effects of opioid receptor blockade remain unclear. Furthermore, neither the specificity of the basolateral infusion of naloxone within the amygdala nor the effects of opioid receptor blockade in the nucleus accumbens shell and ventral pallidum on test were explored. The following experiments were designed to address these issues (see Table 2). Rats were trained on the reward-seeking chain as before, except that they were food deprived, receiving 10–12 g of food per day. At test, rats were given an infusion of vehicle or naloxone into the nucleus accumbens shell, ventral pallidum, amygdala central nucleus, or the basolateral amygdala immediately before being allowed to respond on the reward-seeking chain, first during an unrewarded period to assess any unconditioned effects and then, immediately afterward during the same session, in a period when the sucrose reward was earned by lever-pressing performance. All animals were tested twice; once under vehicle and once under naloxone in counterbalanced order with re-training between tests. At issue was whether naloxone infusions into these areas would influence the performance of reward-seeking actions for sucrose and, if so, with what time-course.
Table 2.
On-baseline assessment of incentive learning
| Training(23-h deprivation) | Test(23-h deprivation) Naloxone or Vehicle | |
|---|---|---|
| LPS → [LPD → Suc] | Non-Rewarded | Rewarded |
| LPS → [LPD→ ø] | LPS → [LPD → Suc] | |
Rats deprived of food for 23 h were trained to press a seeking lever (LPS) to gain access to a second lever (LPD). A single press on the second lever delivered a sucrose solution (LPD→SUC). While in this food deprived state rats subsequently were administered naloxone or vehicle into the nucleus accumbens shell, ventral pallidum, basolateral amygdala, or amygdala central nucleus and were tested immediately on the seeking-delivery chain, first non-rewarded and then, immediately thereafter, with the sucrose reward delivered. The effects of the infusions were assessed across time during these non-rewarded and rewarded periods.
In a preliminary study we found evidence that peripheral administration of naloxone 15 min before test reduced both sucrose palatability and, gradually, after ≈12 min, reward-seeking responses (see SI Results and Fig. S3). Given the effects of naloxone infusions into the nucleus accumbens shell or the ventral pallidum, shown in Figs. 1A and 2A, it seems likely that this effect on palatability was mediated in part by the blockade of opioid receptors in these regions. Interestingly, in the current study, we found that on-baseline infusion of naloxone into the nucleus accumbens shell or the ventral pallidum had no effect on reward-seeking responses during either the non-rewarded or rewarded periods of the test (see Figs S4 A and B and Results for statistical analysis).
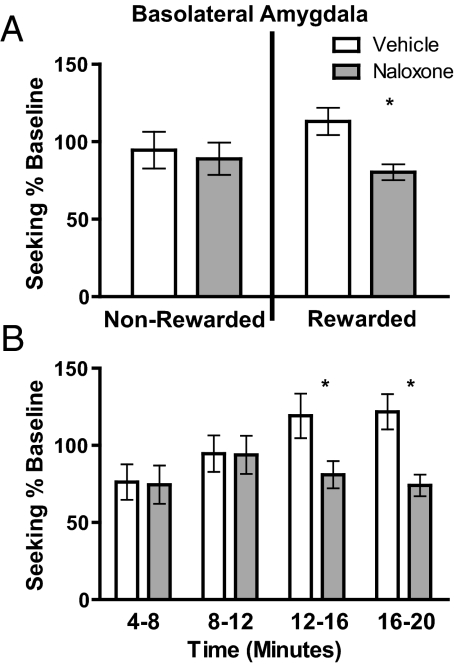
A very different pattern of results was observed after infusion of naloxone into the basolateral amygdala. In the non-rewarded component of the test, intra-basolateral amygdala naloxone did not affect performance (Fig. 4A, Left). During the rewarded period, however, it clearly reduced seeking actions (Fig. 4A, Right) and did so roughly after 12 min of rewarded performance (Fig. 4B). Bayesian analysis found that the null hypothesis predicting no difference between the vehicle and naloxone conditions was 3.66 times more probable than the alternate hypothesis in the non-rewarded condition, but the alternate hypothesis, proposing a difference between drug conditions, was 8.84 times more probable than the null hypothesis in the rewarded test. This finding suggests that intra-basolateral amygdala naloxone had no effect on reward-seeking actions or on the representation of incentive value when the reward itself was absent, but that intra-basolateral amygdala naloxone significantly reduced reward-seeking actions when the reward was available, and therefore its incentive value constantly updated for use in directing subsequent actions. Finally, it should be noted that this effect of intra-basolateral amygdala naloxone on goal-directed action was unlikely to have been caused by diffusion of the drug outside this area and was limited to this region of the amygdala. Similar infusions of naloxone into the amygdala central nucleus had no effect on seeking responses in either the non-rewarded or rewarded tests (see Fig. S4 and Results for statistical analysis).
Fig. 4.
Temporal control of incentive learning. Intra-basolateral amygdala naloxone reduces lever pressing across time in an on-baseline test of reward seeking. We conducted 2 sequential tests of lever pressing under a single food-deprivation condition; the first was non-rewarded, and the second, immediately thereafter, was under rewarded conditions. Naloxone or vehicle was infused into the basolateral amygdala immediately before the testing sequence. (A) Seeking responses normalized to baseline responding, for both the non-rewarded (Left) and rewarded (Right) tests. Assessment by 2-way repeated measures ANOVA found no effect of test (F1,17 = 1.34, P = 0.26) but an effect of drug (F1,17 = 4.35, P = 0.05) and a marginal interaction between test and drug (F1,17 = 3.61, P = 0.07). (B) The temporal dynamics of the effect of intra-basolateral amygdala naloxone on reward-seeking responses presented in 4-min bins over the course of the rewarded test, expanding the data presented in the shaded portion of A. Assessment by 2-way repeated measures ANOVA found no overall effect of time (F3,51 = 2.15 P = 0.11) but a significant effect of drug (F1,17 = 6.53 P = 0.02) and an interaction between time and drug (F3,51 = 3.73, P = 0.02). n = 18. *, P < 0.05
This specific effect of naloxone on lever pressing in the rewarded test is consistent with the claim that opioid receptors in the basolateral amygdala are important for updating the incentive value used to control the performance of goal-directed action. Importantly, the reduction in incentive value induced by opioid receptor blockade was dynamic and was significant only after ≈12 min of rewarded responding (i.e., after the rats had earned an average of 24 sucrose rewards). These data suggest that opioid receptor blockade in the basolateral amygdala, although not affecting the expression of reward palatability, disrupts the evaluative processes through which animals encode the incentive value of the rewards associated with their actions.
Discussion
The results of these experiments demonstrate that the endogenous opioid receptor-related processes mediating palatability and those necessary for the assignment of incentive value to instrumental outcomes are doubly dissociable. Endogenous opioid receptor activation in the nucleus accumbens shell and ventral pallidum was found to mediate the expression of increases in sucrose palatability (Figs. 1A and 2A). This finding is consistent with the work of Smith and Berridge (6), who reported that opioid receptor-related processes influence reward liking based on ratings of changes in facial reactions, such as rhythmic tongue protrusions. However, the intra-accumbens shell and pallidal infusions of naloxone in the current study failed to influence either the short-term use (Fig. S4 A and B), or the longer-term assignment of incentive value to an instrumental outcome (Figs. 1B and 2B). Taken together, it therefore appears that, whereas the infusion of naloxone into the accumbens shell or ventral pallidum immediately before consummatory contact with the sucrose blocked the increased palatability induced by increased food deprivation, it had no effect on the increase in incentive value of the sucrose induced by such deprivation; incentive learning still was reflected as an increase in subsequent reward-seeking activity. Thus, although the palatability responses of these animals indicated no apparent increase in experienced sucrose liking, the rats nevertheless increased their reward-seeking responses. Similarly, intra-accumbens shell and intra-pallidal naloxone administration given at the same dose, volume, and site shown to produce reliable palatability effects had no effect on reward-seeking responses when administered immediately before test.
In contrast, endogenous opioid processes in the basolateral amygdala, but not in the amygdala central nucleus, were found to be important for both the short-term evaluation (Fig. 4A) and longer-term encoding (Fig. 3B) of the incentive value of sucrose for use in directing reward-seeking actions but were not necessary for reward palatability (Fig. 3A). Importantly, therefore, we found that different neural structures mediate opioid control of palatability and incentive value, suggesting that these aspects of reward processing, although both involve endogenous opioid activity, are independent. Furthermore, opioid activity in the basolateral amygdala was not necessary for the retrieval of incentive value information once encoded (Fig. 3C). As a consequence, these data do not support the view advanced in Damasio's somatic marker hypothesis (3) that comparable processes are engaged during the encoding and retrieval of incentive values. Rather, it seems that retrieval of incentive information does not depend on re-experiencing the original emotional effects of the reward they represent (23, 31); that is, an opioid receptor-related process in the basolateral amygdala was found to be necessary for the encoding of incentive value information, but, once encoded, this process no longer was necessary to retrieve and use this information. This suggestion implies that a separate system independent of basolateral amygdala opioid processes, which may or may not involve endogenous opioid peptides in other regions, mediates the storage and retrieval of incentive value information.
The current data are consistent with the previously described role of the basolateral amygdala in goal-specific incentive processes in rats (16, 32) and monkeys (33), as well as with data suggesting the basolateral amygdala mediates the representation of sensory aspects of motivationally significant events (34, 35). Indeed, work from our laboratory suggests that protein synthesis within the basolateral amygdala is important for consolidation and reconsolidation of reward-related memories during incentive learning (14). The present data establish the importance of endogenous opioids in inter-neuronal communication within this structure mediating incentive learning. Furthermore, these results suggest that opioid activity in the basolateral amygdala functions as the proximal source of incentive learning and of the assignment of value to the outcomes of goal-directed actions and that these values are not reducible to or dependent on the palatability controlled by accumbens shell and ventral pallidum. Hence, although opioid-related palatability responses are considered to reflect reward liking, they are not necessarily indicative of the incentive value controlling goal-directed actions through which animals seek access to rewarding events. Logically, this suggestion raises the possibility that a third system, with which the accumbens shell, ventral pallidum, and basolateral amygdala are associated, distributes the affective signals elicited by specific commodities across distinct functional systems to control reward seeking as well as, presumably, reward prediction. At present we do not have any direct evidence for a system of this kind, but indirect evidence suggests it may reside within the motivationally rich circuits linking hypothalamic and brainstem viscerogenic structures such as the parabrachial nucleus (36, 37).
This study provides evidence that, although incentive value and palatability both depend on opioid receptor activation, they are functionally and neuroanatomically dissociable, with activation of opioid receptors in the accumbens shell and ventral pallidum being important for palatability and those in the basolateral amygdala important for encoding the incentive value used to drive goal-directed actions. Moreover, we found that opioid receptors within the basolateral amygdala are not necessary for the retrieval of incentive value information. These dissociations may have implications for understanding decision-making, particularly as it relates to current distinctions in the literature between “wanting” and “liking,” the former characterizing a state in which rats' tendency to pursue a commodity is dissociated from palatability-related taste reactivity elicited during consummation of that commodity (38). Indeed, this dissociation recently has been extended to human models of food choice and consumption in eating disorders and obesity (39, 40). These theories focus on a dissociation of liking and the Pavlovian influence that reward-associated cues exert on reward seeking. Here we dissociate liking from the desire for, or the incentive value of the reward itself, that influences the performance of goal-directed actions and decision-making. The neuroanatomical dissociation of reward liking and desire in the current study may shed light on the involvement of endogenous opioid systems in disorders, such as addiction, that are marked by inappropriate reward-related decision-making. Indeed, a dual role of endogenous opioids in the affective experience associated with drug taking and the incentive learning process involved in reward seeking may underlie the intensely addictive property of opiate drugs and other substances that induce release of these peptides. Targeting these anatomically dissociable opioid-mediated processes may provide treatments for these disorders that improve the integration of emotional and cognitive processes to result in more appropriate desire and decision-making.
Methods
Full details of the materials and methods used can be found in the SI Methods.
Briefly, male Long Evans rats (Harlan), food deprived for either 2 or 23 h, were trained to earn 20% sucrose solution on a heterogeneous seeking-delivery chain (Tables 1 and 2). They then were implanted bilaterally with 22-gauge stainless steel cannulae (Plastics One) into the ventral pallidum [anterior-posterior (AP): −0.8; medial-lateral (ML): ± 2.9; ventral (V): 7.8], nucleus accumbens shell (AP: +1.7; ML: ±.75; V: 6.6), basolateral amygdala (AP: −3.0; ML: ± 5.1; V: 8.0), or the central nucleus of the amygdala (AP: −2.3; ML: ± 4.0; V: 7.6). For histological assessment see Fig. S1. Following recovery rats were tested as follows.
Incentive Learning Test.
For the incentive learning tests (Figs. 1–3), all rats were trained after 2 h of food deprivation. After training, half the rats were deprived of food for 23 h, and half were maintained on 2-h food deprivation before receiving either intra-basolateral amygdala (n = 26 initial and n = 26 for replication), intra-nucleus accumbens shell (n = 16), or intra-ventral pallidum (n = 18) naloxone or vehicle immediately before being given 30 non-contingent sucrose presentations over 40 min. Lickometer measures were collected during this phase. The next day, the rate of responding on the chain was assessed in a 4-min test in the absence of reward in either the 2-h or 23-h deprivation state off drug. This non-rewarded test was conducted just as in training, with rats responding on the seeking lever on a random ratio of 4 (RR-4) to receive the second delivery lever, which, when pressed, was retracted without delivering a reward. On the following day, the rats were given a 16-min rewarded test. Rats then were given 2 days without training in which they were food deprived for 2 h per day and then were retrained for 2 days on the 2-h food-deprived schedule. They then were allowed again to consume the sucrose in the opposite deprivation and drug condition to the first exposure session and were tested for their responding in the chain under non-rewarded and rewarded conditions, as described previously.
An additional set of rats (n = 26) was trained and tested as described previously but was given an infusion of either vehicle or naloxone into the basolateral amygdala before the non-rewarded test. Thus there were 4 groups: vehicle at re-exposure and vehicle on test, vehicle at re-exposure and naloxone on test, naloxone at re-exposure and vehicle on test, and naloxone at re-exposure and naloxone on test.
On-Baseline Incentive Learning Test.
Rats, trained and food deprived for 23 h, were infused bilaterally with naloxone or vehicle into the basolateral amygdala (n = 18), amygdala central nucleus (n = 15), nucleus accumbens shell (n = 15), or ventral pallidum (n = 16), and then immediately were allowed to respond on the chain. The first test was a 4-min non-rewarded test. The test then was switched immediately to a progressive-ratio rewarded test in which the first press on the seeking lever was rewarded and the subsequent series of presses rewarded on a random ratio RR-2 then RR-4 schedule. Rats subsequently were retrained for 2 sessions and then were tested again under the opposite drug condition.
Supplementary Material
ACKNOWLEDGMENTS.
The authors thank Ingrid Cely for technical assistance. This research was supported by Grant MH56446 from the National Institute of Mental Health (to B.B.), Grants DA09359 and DA05010 from the National Institute on Drug Abuse (to N.T.M.), Training Grant T32MH017140 (to S.B.O.), and a Ruth L. Kirschstein National Research Service Award and Hatos scholarship (to K.M.W.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905874106/DCSupplemental.
References
- 1.Balleine BW, Dickinson A. Goal-directed instrumental action: Contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37(4–5):407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- 2.Cabanac M. Pleasure: The common currency. J Theor Biol. 1992;155(2):173–200. doi: 10.1016/s0022-5193(05)80594-6. [DOI] [PubMed] [Google Scholar]
- 3.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 4.Panksepp J. Affective consciousness: Core emotional feelings in animals and humans. Consciousness and Cognition. 2005;14(1):30–80. doi: 10.1016/j.concog.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: Where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25(50):11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith KS, Berridge KC. Opioid limbic circuit for reward: Interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27(7):1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley AE. Ventral striatal control of appetitive motivation: Role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27(8):765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology. 2007;191(3):497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- 9.Kelley AE, et al. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76(3):365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 10.Doyle TG, Berridge KC, Gosnell BA. Morphine enhances hedonic taste palatability in rats. Pharmacol Biochem Behav. 1993;46(3):745–749. doi: 10.1016/0091-3057(93)90572-b. [DOI] [PubMed] [Google Scholar]
- 11.Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol. 1996;367(3):375–402. doi: 10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Poulin JF, Chevalier B, Laforest S, Drolet G. Enkephalinergic afferents of the centromedial amygdala in the rat. J Comp Neurol. 2006;496(6):859–876. doi: 10.1002/cne.20956. [DOI] [PubMed] [Google Scholar]
- 13.Paden CM, Krall S, Lynch WC. Heterogeneous distribution and upregulation of mu, delta and kappa opioid receptors in the amygdala. Brain Res. 1987;418(2):349–355. doi: 10.1016/0006-8993(87)90102-8. [DOI] [PubMed] [Google Scholar]
- 14.Wang SH, Ostlund SB, Nader K, Balleine BW. Consolidation and reconsolidation of incentive learning in the amygdala. J Neurosci. 2005;25(4):830–835. doi: 10.1523/JNEUROSCI.4716-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbit LH, Balleine BW. Instrumental and Pavlovian incentive processes have dissociable effects on components of a heterogeneous instrumental chain. J Exp Psychol Anim Behav Process. 2003;29(2):99–106. doi: 10.1037/0097-7403.29.2.99. [DOI] [PubMed] [Google Scholar]
- 16.Balleine BW, Killcross AS, Dickinson A. The effect of lesions of the basolateral amygdala on instrumental conditioning. J Neurosci. 2003;23(2):666–675. doi: 10.1523/JNEUROSCI.23-02-00666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostlund SB, Balleine BW. Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. J Neurosci. 2008;28(17):4398–4405. doi: 10.1523/JNEUROSCI.5472-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson LR, Aylward RL, Hussain Z, Totterdell S. Input from the amygdala to the rat nucleus accumbens: Its relationship with tyrosine hydroxylase immunoreactivity and identified neurons. Neuroscience. 1994;61(4):851–865. doi: 10.1016/0306-4522(94)90408-1. [DOI] [PubMed] [Google Scholar]
- 19.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26(3):321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 20.Dayan P, Balleine BW. Reward, motivation, and reinforcement learning. Neuron. 2002;36(2):285–298. doi: 10.1016/s0896-6273(02)00963-7. [DOI] [PubMed] [Google Scholar]
- 21.Rolls ET, Rolls BJ, Rowe EA. Sensory-specific and motivation-specific satiety for the sight and taste of food and water in man. Physiol Behav. 1983;30:85–92. doi: 10.1016/0031-9384(83)90003-3. [DOI] [PubMed] [Google Scholar]
- 22.Berridge KC. Modulation of taste affect by hunger, caloric satiety, and sensory specific satiety in the rat. Appetite. 1991;16(2):103–120. doi: 10.1016/0195-6663(91)90036-r. [DOI] [PubMed] [Google Scholar]
- 23.Balleine BW. Neural bases of food seeking: Affect, arousal and reward in corticostriatolimbic circuits. Physiol Behav. 2005;86:717–730. doi: 10.1016/j.physbeh.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 24.Dickinson A, Balleine BW. Motivational control of goal-directed action. Anim Learn Behav. 1994;22:1–18. [Google Scholar]
- 25.Balleine BW, Garner C, Gonzalez F, Dickinson A. Motivation control of heterogeneous instrumental chains. J Exp Psychol Anim Behar Process. 1995;21:203–217. [Google Scholar]
- 26.Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci. 1992;106(1):217–228. [PubMed] [Google Scholar]
- 27.Davis JD, Smith GP. Analysis of lick rate measures the positive and negative feedback effects of carbohydrates on eating. Appetite. 1988;11(3):229–238. doi: 10.1016/s0195-6663(88)80005-9. [DOI] [PubMed] [Google Scholar]
- 28.Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychonomic Bull Rev. 2009;16(2):225–237. doi: 10.3758/PBR.16.2.225. [DOI] [PubMed] [Google Scholar]
- 29.Gallistel CR. The importance of proving the null. Psychol Rev. 2009;116(2):439–453. doi: 10.1037/a0015251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balleine B. Instrumental performance following a shift in primary motivation depends on incentive learning. J Exp Psychol Anim Behav Process. 1992;18(3):236–250. [PubMed] [Google Scholar]
- 31.Balleine BW, Dickinson A. Consciousness: The interface between affect and cognition. In: Cornwell J, editor. Consciousness and Human Identity. Oxford: Oxford Univ Press; 1998. pp. 57–85. [Google Scholar]
- 32.Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. J Neurosci. 2005;25(4):962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wellman LL, Gale K, Malkova L. GABAA-mediated inhibition of basolateral amygdala blocks reward devaluation in macaques. J Neurosci. 2005;25(18):4577–4586. doi: 10.1523/JNEUROSCI.2257-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blundell P, Hall G, Killcross S. Lesions of the basolateral amygdala disrupt selective aspects of reinforcer representation in rats. J Neurosci. 2001;21(22):9018–9026. doi: 10.1523/JNEUROSCI.21-22-09018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dwyer DM, Killcross S. Lesions of the basolateral amygdala disrupt conditioning based on the retrieved representations of motivationally significant events. J Neurosci. 2006;26(32):8305–8309. doi: 10.1523/JNEUROSCI.1647-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson RH, Swanson LW. Structural characterization of a hypothalamic visceromotor pattern generator network. Brain Res Brain Res Rev. 2003;41(2–3):153–202. doi: 10.1016/s0165-0173(02)00232-1. [DOI] [PubMed] [Google Scholar]
- 37.Norgren R, Hajnal A, Mungarndee SS. Gustatory reward and the nucleus accumbens. Physiol Behav. 2006;89(4):531–535. doi: 10.1016/j.physbeh.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berridge KC. Food reward: Brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20(1):1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 39.Finlayson G, King N, Blundell JE. Is it possible to dissociate ‘liking’ and ‘wanting’ for foods in humans? A novel experimental procedure. Physiol Behav. 2007;90(1):36–42. doi: 10.1016/j.physbeh.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 40.Finlayson G, King N, Blundell J. The role of implicit wanting in relation to explicit liking and wanting for food: Implications for appetite control. Appetite. 2008;50(1):120–127. doi: 10.1016/j.appet.2007.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.