Abstract
Rationale: Surfactant protein D (SP-D) is a collectin family member with demonstrated immunomodulatory properties in vitro. We hypothesized that SP-D modulates inflammation during noninfectious lung injury in vivo.
Objectives: To investigate the association of alveolar SP-D and injury, we studied the responses of transgenic mice expressing varying levels of SP-D to intratracheal bleomycin (ITB).
Methods: Eight-week old C57/BL6 SP-D–deficient (−/−) mice and syngeneic wild-type (WT) controls or Swiss Black SP-D–overexpressing (SP-D OE) mice and littermate controls received either ITB or saline and were followed for up to 21 d.
Measurements and Results: Kaplan-Meier analysis demonstrated a dose-dependent decrease in survival in ITB SP-D (−/−) mice receiving 2 U/kg bleomycin, with a 14-d mortality of 100% versus 0% mortality for WT receiving 2 U/kg ITB or SP-D (−/−) mice given saline (p < 0.05). At 8 d, ITB SP-D (−/−) mice had greater respiratory distress (frequency/tidal volume) and weight loss than ITB WT mice. Furthermore, bronchoalveolar lavage cellularity, pulmonary parenchymal inflammation, and tissue 3-nitrotyrosine (NO2 Y) were increased to a greater extent in ITB SP-D (−/−) mice. By 21 d, compared with all groups, ITB SP-D (−/−) survivors had increased Trichrome staining and tissue hydroxyproline levels. As proof of principle, SP-D OE mice were highly resistant to bleomycin-induced morbidity and mortality at doses up to 3 U/kg.
Conclusions: These data provide new in vivo evidence for an antiinflammatory role for SP-D in response to noninfectious, subacute lung injury via modulation of oxidative-nitrative stress.
Keywords: bleomycin, collectins, innate immunity, lung, nitric oxide
Surfactant protein D (SP-D) is a 43-kD peptide synthesized by alveolar type II cells and Clara cells of the distal airway (1, 2). Monomeric SP-D contains a collagen-like triple helical domain and a calcium-dependent carbohydrate recognition domain. Biosynthesis of SP-D includes posttranslational glycosylation followed by assembly of monomeric SP-D via disulfide bonding into a dodecamer consisting of four trimers (2). SP-D is a member of a growing family of soluble molecules that play a role in the innate or non–antibody-mediated immune response (3–5). The term collectin (collagen-like lectin) used to describe this family includes, in addition to SP-D, surfactant protein A (SP-A) and non–lung protein members mannose-binding protein, bovine conglutinin, and CL-43.
SP-D has been shown to interact with a variety of pathogens and modulate part of the increasingly complex interplay between host inflammatory responses and invading organism. In vitro, SP-D enhances internalization of Escherichia coli, Streptococcus pneumoniae, S. aureus, and Aspergillus fumigatus conidia by phagocytes (6–9), regulates lymphocyte proliferation (10), modulates chemotaxis and activation of macrophages (11–13), and facilitates presentation of peptide antigen by dendritic cells to lymphocytes thus forging a link between innate and acquired immunity (14). The spatial distribution of SP-D in the distal respiratory tract (15, 16), previous in vitro studies demonstrating that the protein interacts with and modulates the function of macrophages, neutrophils, and lymphocytes, and in vivo data showing alterations in SP-D expression, can be induced by infectious and inflammatory stimuli makes it an ideal candidate for local regulation of lung inflammation (17, 18). Furthermore, variations in frequency of SP-D alleles are associated with differing host responses in humans (susceptibility or protection) to some pulmonary diseases including chronic obstructive pulmonary disease, allergen hyper responsiveness, and viral infection (19–22).
Targeted disruption of the mouse SP-D gene has been reported by two different groups (23, 24). In both cases, regardless of genetic background, the phenotype of the SP-D–deficient mouse (SP-D −/−) is associated with an elevation in the baseline level of pulmonary inflammation. SP-D −/− mice exhibit increases in the number and size of macrophages within the airways, alterations in surfactant phospholipid profiles, increases in metalloproteinase activity, enhancements in lymphocyte activation, and temporal accelerations in airspace enlargement (i.e., emphysema) (11, 23–27). Subsequently, we have shown that the inflammatory response found in SP-D–deficient mice includes an increase in inducible nitric oxide synthase (iNOS) activity and NO production, as well as a shift in the reactive state of NO toward higher oxides (28).
The intratracheal instillation of bleomycin (ITB) in rodents is an established model of subacute lung injury, inflammation, and fibrosis. Its toxicity is related to the production of free radicals that result in endothelial and epithelial cell damage, the appearance of DNA-damage inducible proteins, increased microvascular permeability, and respiratory distress with surfactant dysfunction (18). ITB produces an initial inflammatory response marked by peak levels of tumor necrosis factor-α and transforming growth factor-β 7–10 days after injury mediated by increased activity of nuclear factor-κB (29, 30) that corresponds with maximal inflammatory cell infiltrate and respiratory distress (18, 31). From 14 to 21 d, a transition from inflammation to either extracellular matrix production and fibrosis or to tissue healing and repair ensues (18, 31–33).
Previously, we had shown that administration of ITB to rats resulted in upregulation of SP-D protein content (18). Given the antiinflammatory properties of SP-D and it association with altered NO metabolism, we hypothesized that the inflammatory response to bleomycin could be modulated by alveolar SP-D content. In this report, we demonstrate that ITB administration to SP-D–deficient mice results in increased mortality, in enhanced lung inflammation and tissue injury, and in alterations in nitric oxide metabolism. Furthermore, as proof of principle, mice constitutively overexpressing recombinant SP-D are protected from bleomycin-induced morbidity and mortality. These results establish a critical role for SP-D in the local modulation of the pulmonary inflammatory response to a noninfectious lung injury. Preliminary reports of some of these results have been published (34, 35).
METHODS
Mouse Models
SP-D–deficient mice.
SP-D–deficient mice produced by targeted ablation of the mouse SP-D locus have been previously described (23). Mice bred to homozygosity were backcrossed 10 generations onto the C57BL/6 background. Age-matched wild-type C57BL/6 mice (WT) purchased from either Jackson Laboratories, Inc. (Bar Harbor, ME) or Charles River Laboratories (Wilmington, MA) were used as controls.
SP-D–overexpressing mice.
Heterozygous transgenic mice carrying 1 concatamer of the rat SP-D gene under control of the human SP-C 3.7-kb promoter element have been previously described (36). These mice (termed SP-D OE), backcrossed on to the Swiss Black background, were mated with WT Swiss Black mice (Taconic, Inc., Germantown, NY) to generate 50% SP-D OE mice and 50% transgene negative littermate controls.
Experiments were performed using mixed populations of male and female mice (8–10 wk) housed in a barrier facility. All animal protocols were reviewed and approved by the Animal Care and Use Committee at the University of Pennsylvania.
Model of Intratracheal Bleomycin Injury
Mice (25–35 g) were anesthetized with 50 mg/kg intraperitoneal pentobarbital. With the use of an insulin syringe, 50 μl of either lipopolysaccharide (LPS)-negative saline or clinical grade, sterile, and LPS-free bleomycin sulfate (1.0–3.0 U/kg; Bristol Myers Schwab, Princeton, NJ) were directly injected into the trachea as published (18, 31). The incision was closed using surgical clips. Perioperative mortality in experimental animals was less than 2%.
Measurement of Pulmonary Function
Respiratory rates and tidal volumes were determined in conscious, unrestrained, spontaneously breathing mice in a whole-body plethysmograph (Buxco Electronics Incorporated, Troy, NY).
Preparation and Analysis of Bronchoalveolar Lavage
Total bronchoalveolar lavage (BAL) cell counts were determined using a Z1 Counter particle counter (Beckman-Coulter, Inc., Miami, FL) as published (37). Differential cell counts were performed manually on cytospins stained with Dif-Quik (37). The size (diameter) of each macrophage identified during differential counting was determined using an eyepiece objective containing a grid of known size and magnification (Model WH10X2-H; Olympus America, Inc., Melville, NY). Data were stratified into two groups, greater and less than 25 μm in diameter, and recorded as a percent of total macrophage number.
Cell-free BAL supernatants were separated into two fractions by centrifugation as described previously (38) producing a biophysically active large-aggregate form in the pellet and a supernatant that contained soluble proteins and biophysically inactive surfactant forms (small-aggregate fraction. Total protein content of fractions was determined by the Bradford method with bovine IgG as a standard (39). Phospholipid contents were determined by the method of Bartlett (40).
Polyacrylamide Gel Electrophoresis and Immunoblotting
SP-D expression was determined by Western blotting using NuPAGE 10% Bis-Tris gels (Novex, San Diego, CA) as previously described (38, 41). Bands visualized using enhanced chemiluminescence (ECL Kit; Amersham, Inc., Arlington Heights, IL) and a monospecific, polyclonal anti–SP-D (42) were quantitated by densitometry using Kodak 440 image analysis software as described (38).
Solid-Phase ELISA for Nitrotyrosine and SP-A
Nitrotyrosine content of lung homogenates was determined by solid-phase ELISA (immuno dot-blot) using a monoclonal anti-nitrotyrosine antibody (generous gift of Dr. Harry Ischiropolous, Children's Hospital of Philadelphia, PA) as described (28, 37, 43, 44). Relative nitrotyrosine contents were calculated by comparison to a standard of nitrated bovine serum albumin.
SP-A content in total BAL fluid was determined in an identical fashion using the monospecific, polyclonal anti-rat SP-A antibody (PA3) (45, 46) as described (37).
Nitrogen Oxide Measurements
The analysis of NO metabolites was performed using the Ionics/Sievers Nitric Oxide Analyzer 280 (NOA 280) as described (28). Total NO was determined by treatment with an excess of vanadium chloride reducing nitrates and nitrites to release NO. Nitrite was measured independently using a KI and acetic acid mixture (47) and subtracted from total NO to obtain a calculated nitrate content.
Determination of Lung Inflammation and Fibrosis
Lung histology.
Paraffin sections prepared from the lungs of WT and SP-D deficient mice as described previously were stained each with Trichrome and with hematoxylin and eosin.
Hydroxyproline Content
The right lungs of mice harvested at Day 21 were analyzed for hydroxyproline content according to the method of Brown and colleagues (48). After hydrolysis and oxidation with chloramine-T (Acros, Morris Plains, NJ), color developed using Ehrlich's reagent was quantitated by absorbance at 570 nm. The amount of hydroxyproline was determined against a standard curve hydroxyproline (Sigma, Inc., St. Louis, MO).
Data Analysis
All parametric data from experimental and control groups were expressed as mean ± standard error of mean (X ± SE). Groups were compared using unpaired two-tailed Student's t test analyzed with a standard statistical software package (iStat3 for MacIntosh v 3.03; Graph Pad Software, Inc., San Diego, CA). In all cases, a p value of < 0.05 was considered significant.
RESULTS
Morbidity and Mortality Are Increased in SP-D–deficient Mice Receiving Bleomycin
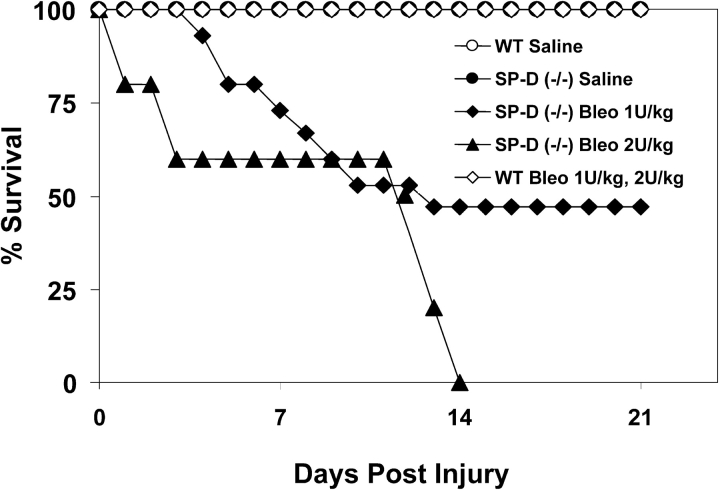
Administration of intratracheal bleomycin at doses up to 2 U/kg produced a dose-dependent increase in mortality exclusively in SP-D (−/−) mice. As Figure 1 demonstrates, Kaplan-Meier analysis showed a 14-day mortality of 100% for SP-D–deficient mice receiving 2 U/kg ITB and 53% mortality at 1 U/kg compared with a 0% mortality for age- and sex-matched SP-D (−/−) mice receiving saline. C57BL/6 controls receiving ITB at doses up to 2 U/kg also had a 100% survival regardless of breeding source (Charles River or Jackson Laboratories).
Figure 1.
Surfactant protein D (SP-D)-deficient mice have increased susceptibility to intratracheal bleomycin. SP-D–deficient mice and C57BL/6 control mice (Jackson Research Laboratories) 8 to 9 wk of age received either saline (n = 5 for each strain), 1 U/kg intratracheal bleomycin (n = 15 for each strain), or 2 U/kg intratracheal bleomycin (n = 15 for each strain) as described in METHODS. Survival for each group was recorded throughout a 21-d observation period and plotted as Kaplan-Meier survival analysis. Comparison of mortality at Day 14 revealed enhanced susceptibility at both 1 U/kg and 2 U/kg bleomycin in the SP-D (−/−) groups (p < 0.05 versus corresponding control groups). Data are representative of three separate experiments.
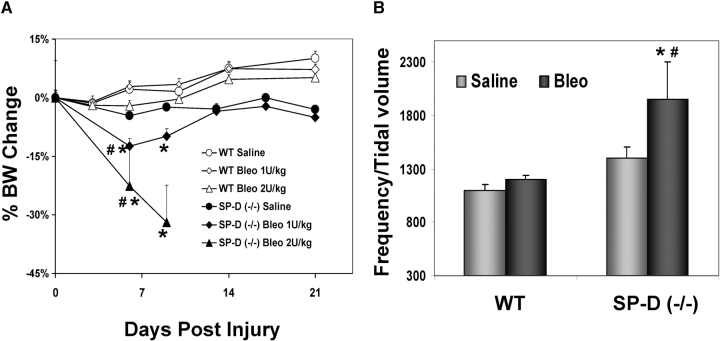
The increased mortality observed in SP-D (−/−) mice receiving ITB was accompanied by systemic and pulmonary morbidity. At doses of ITB up to 2 U/kg, SP-D–deficient mice exhibited statistically significant weight loss at Days 6 and 9 after administration (Figure 2A). When assessed at 8 d after bleomycin injury, surviving SP-D–deficient mice were also found to exhibit greater degrees of respiratory distress as measured using respiratory frequency/tidal volume ratios (Figure 2B). Statistically significant differences were also noted in respiratory frequency alone between ITB-treated SP-D (−/−) and ITB-treated WT mice (SP-D (−/−) = 417 ± 4 bpm, n = 5 versus 364 ± 12, n = 5; p = 0.038). By 21 d, frequency/tidal volume ratio was similar among survivors for both WT and SP-D (−/−) groups (data not shown).
Figure 2.
Bleomycin-challenged SP-D–deficient mice exhibit increased morbidity. (A) Weight changes. After administration of intratracheal instillation of bleomycin (ITB) to either wild-type (WT) or SP-D (−/−) mice, body weights were recorded up to Day 21 as indicated. Data are expressed as a percent of change from the baseline body weight before surgical installation of bleomycin or saline (X ± SE; n = 5 for saline treated groups; n = 15 for bleomycin-treated groups). At Day 6, SP-D (−/−) mice receiving 1 or 2 U /kg ITB had a significantly greater weight loss then either SP-D (−/−) mice receiving saline or WT mice receiving bleomycin [*p < 0.05 versus SP-D (−/−) saline group; #p < 0.05 versus WT bleomycin 2 U/kg group]. (B) Respiratory distress. SP-D (−/−) and C57BL/6 mice receiving saline or 1 U/kg bleomycin were subjected to pulmonary function testing using a BUXCO whole body plethysmograph. Conscious, unrestrained, nonsedated mice underwent determination of respiratory frequency and tidal volume. Data are expressed as a respiratory distress index consisting of respiratory frequency divided by tidal volume. (X ± SE, n = 4–5 per group; *p < 0.05 versus corresponding control groups, #p < 0.05 versus corresponding WT group.)
SP-D–deficient Mice Demonstrate Increased Lung Inflammation after Bleomycin
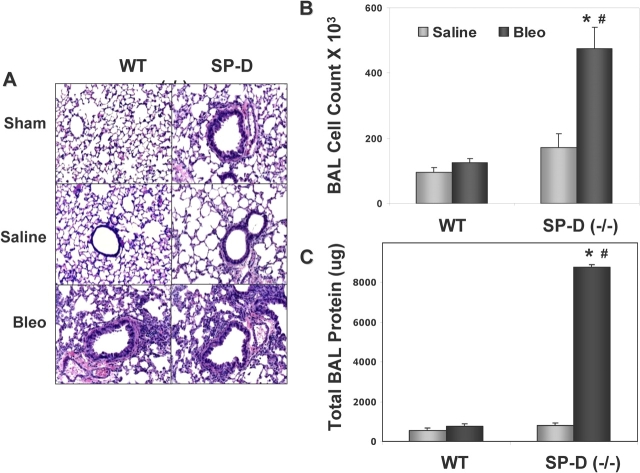
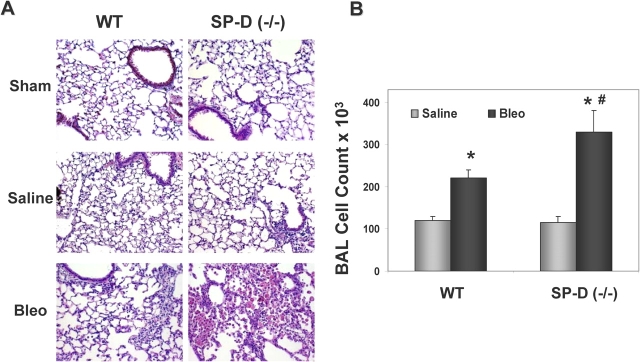
Physiologic morbidity in ITB-treated SP-D (−/−) mice was accompanied by increased parenchymal lung inflammation and BAL cellularity. In Figure 3A, lung histology obtained at Day 8 (during maximum respiratory distress) showed increased inflammatory cell infiltrates in lung tissue in SP-D (−/−) mice receiving 1 U/kg ITB. The histopathologic changes were reflected by commensurate increases in total BAL cells in this group (Figure 3B). The threefold increase in total cell count in SP-D (−/−) mice receiving ITB consisted predominantly of macrophages with lesser amounts of lymphocytes. There were no statistically significantly differences in differential cell percentages between SP-D (−/−) and WT mice receiving ITB (data not shown).
Figure 3.
SP-D (−/−) mice demonstrate increased inflammation and injury after bleomycin. SP-D (−/−) mice and C57BL/6 WT mice were each inoculated with 1 U/kg bleomycin sulfate. At 8 d, all mice were killed and both BAL and inflation fixation for histopathology were performed as described in METHODS. (A) Representative hematoxylin and eosin–stained lung sections from WT and SP-D (−/−) mice receiving sham surgery (upper panels), intratracheal saline (middle panels), or bleomycin (lower panels) shown at equal magnification (×400). Compared with WT, lungs from ITB-treated SP-D (−/−) mice show increased cell infiltrates. BAL cell counts (B) and BAL total protein (C) from the same time point (n = 4–5 per group) were determined as described in METHODS. Data for each group are all expressed as X ± SE. *p < 0.05 versus saline-treated SP-D (−/−) group; #p < 0.05 versus bleomycin-treated WT group.
The increase in cellular infiltration was accompanied by an increase in lung leak. On Day 8, BAL total protein was markedly elevated in ITB-treated SP-D (−/−) mice compared with either saline treated SP-D (−/−) mice or with WT mice that received ITB (Figure 3C).
Reactive Nitrogen Species and SP-A Are Increased in ITB-Treated SP-D–deficient Mice
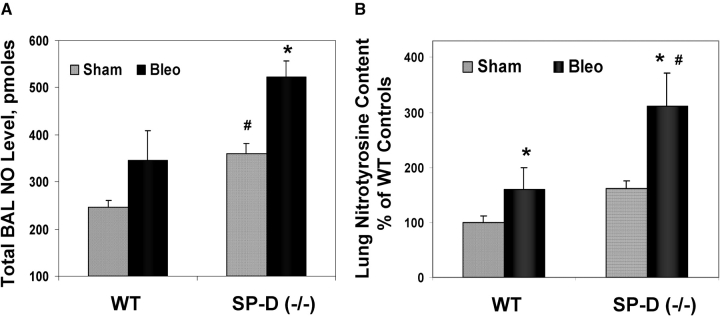
Previous studies have shown that SP-D–deficient mice demonstrate increases in production of both oxygen radicals and reactive nitrogen species (11, 28). When assayed at the peak of lung inflammation (Day 8), although WT and SP-D–deficient mice treated with bleomycin each show modest increases in total BAL NO production (Figure 4A), the lung homogenates from bleomycin-injured SP-D (−/−) mice had a proportionately greater (twofold) increase in total lung nitrotyrosine compared with WT mice (Figure 4B). NO2Y staining of BAL cell pellets was similarly qualitatively increased (data not shown).
Figure 4.
NO and 3-nitrotyrosine are increased in bleomycin-treated SP-D (−/−) mice. BAL and lung tissue from SP-D (−/−) and C57BL/6 WT mice was harvested 8 d after administration of 1 U/kg bleomycin sulfate. (A) Total bronchoalveolar lavage (BAL) NO was determined as previously published (28). Data were expressed as total BAL NO expressed in pmoles (X ± SE, n = 3–5 per group; *p < 0.05 versus WT sham control. #p < 0.05 versus bleomycin-treated WT group). (B) Relative 3-nitrotyrosine levels in lung homogenates were determined by solid phase ELISA as previously described (28, 44). Data were expressed as a percent of sham operated WT controls (*p < 0.05 versus corresponding sham group; #p < 0.05 versus bleomycin-treated WT group; X ± SE, n = 3–5 per group).
The SP-D and SP-A genes both map to the same chromosomal locus in the mouse (49). Baseline levels of SP-A– in SP-D–deficient mice are reported to be approximately 50 to 60% that of strain-matched controls (23, 28, 37). To ensure that the inflammatory changes observed in SP-D–deficient mice were not accompanied by a commensurate loss of SP-A after ITB, immuno dot-blot analysis of total BAL was performed. Consistent with previous reports, when corrected for total protein content, 8 d after inoculation, saline-treated SP-D (−/−) mice had 62 ± 7.7% of the BAL SP-A protein content versus saline inoculated WT mice (p < 0.05). ITB treatment of SP-D (−/−) mice produced a nearly fivefold increase in alveolar SP-A protein [486 ± 142% versus saline-inoculated SP-D (−/−) mice, p < 0.05].
Late Inflammatory Responses and Fibrosis Are Increased in SP-D–deficient Mice
Among mice surviving 21 d, ITB-treated SP-D (−/−) mice had increases in each of lung tissue inflammation, BAL cellularity, and fibrosis. Compared with syngeneic controls, parenchymal infiltration by mononuclear cells was more prominent in SP-D (−/−) mice receiving 1 U/kg ITB (Figure 5A) with parallel increases in BAL cellularity (Figure 5B).
Figure 5.
Late inflammation in bleomycin-treated SP-D (−/−) mice. SP-D (−/−) and WT mice receiving 1 U/kg bleomycin sulfate were killed 21 d after treatment. BAL and lung tissue were prepared as in Figure 3. (A) Representative hematoxylin and eosin–stained lung sections from WT and SP-D (−/−) mice receiving sham surgery (upper panels), intratracheal saline (middle panels), or bleomycin (lower panels) shown at equal magnification (×400) reveal increased inflammatory cells infiltrates in bleomycin-treated SP-D (−/−) mice; (B) BAL cell counts were done using a UZ1 hemocytometer as described in METHODS. Data for each group are expressed as X ± SE (n = 5 per saline group, n = 7–13 for bleomycin-treated groups). Total cell counts in ITB-treated SP-D (−/−) mice were elevated versus both WT controls and SP-D (−/−) mouse controls (*p < 0.05 versus corresponding saline-treated group; #p < 0.05 versus bleomycin-treated WT group).
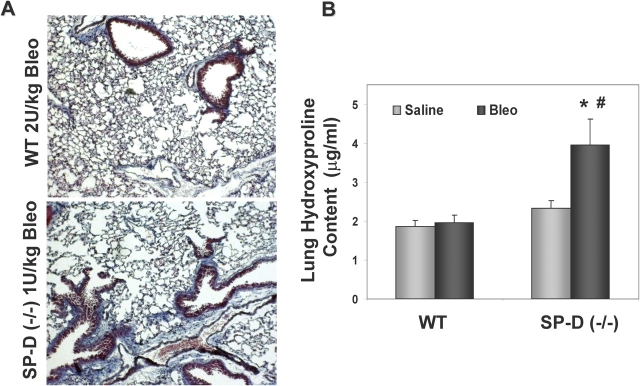
A fibrotic response observed in mice receiving bleomycin was enhanced in the absence of SP-D. Trichrome staining for collagen expression was increased in lungs from SP-D–deficient mice receiving 1 U/kg ITB compared with minimal changes seen in C57BL/6 controls receiving twofold more ITB (2 U/kg) (Figure 6A). The qualitative increases in lung fibrosis were reflected by quantitative increases in lung hydroxyproline content (Figure 6B). Confirming the histologic findings, a twofold increase in lung hydroxyproline content was also observed in bleomycin-treated SP-D (−/−) mice compared with WT controls. These results indicate that throughout the course of the lung injury produced by ITB administration, SP-D (−/−) mice demonstrated not only increases in lung inflammation and oxidative-nitrative stress, but in end-organ damage, manifesting as an enhanced fibrotic response.
Figure 6.
SP-D (−/−) mice develop more fibrosis after bleomycin. (A) Representative trichrome staining of lung obtained 21 d after bleomycin-induced lung injury demonstrates increased fibrosis in surviving SP-D (−/−) mice receiving 1 U/kg ITB compared with minimal fibrosis seen in WT mice receiving 2 U/kg; (B) hydroxyproline content of lung homogenates from groups described in A was measured as described in METHODS and expressed as μg hydroxyproline/ml homogenate (X ± SE; n = 5 per saline group, n = 7–13 for bleomycin-treated groups). ITB increased hydroxyproline content twofold in lungs from SP-D (−/−) mice compared with saline-treated SP-D (−/−) mice and to ITB treated wild-type mice (*p < 0.05 versus saline-treated SP-D (−/−) group, #p < 0.05 versus bleomycin-treated WT group).
SP-D–overexpressing Mice Are Protected from Bleomycin Injury
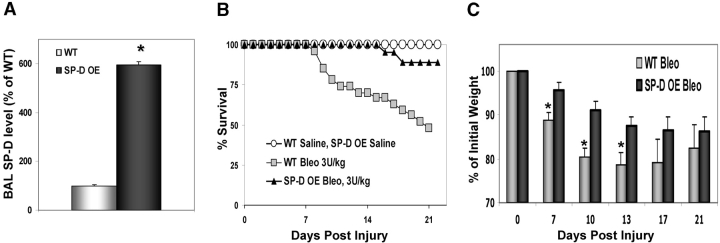
To verify that the differential response to bleomycin observed in the SP-D–deficient mouse model was attributable to a lack of endogenous SP-D, we performed similar challenges with ITB in a transgenic mouse model that constitutively overexpresses recombinant rat SP-D (rSP-D) under the control of the human SP-C promoter (SP-D OE) (36). By Western blot analysis, SP-D OE mice containing single concatamers of the SP-D transgene had sixfold elevations in baseline levels of total alveolar SP-D content compared with littermate controls (Figure 7A). When challenged with ITB (3 U/kg), littermate controls (Swiss black background) had 47% mortality at 21 d. In contrast, SP-D OE mice treated identically demonstrated a 90% survival (Figure 7B). At 21 d, by Western blotting, BAL fluid from surviving SP-D OE mice receiving bleomycin had a nearly sixfold higher levels of SP-D protein than saline-treated SP-D OE (684 ± 111% of SP-D OE saline, n = 4 in each group) and nearly 16-fold higher SP-D levels than bleomycin-treated littermate controls (1,692 ± 274% of littermate WT controls).
Figure 7.
SP-D–overexpressing mice demonstrate increased survival to ITB. Transgenic mice expressing recombinant rat SP-D under control of the SP-C promoter and littermate controls were inoculated with 3 U/kg of bleomycin sulfate or saline as described in METHODS. (A) Western blot analysis for baseline SP-D production in noninoculated SP-D–overexpressing mice (n = 4) and WT littermate controls (n = 5) using 5 μg of total protein. SP-D band density was determined and SP-D content per total BAL recovered was calculated. Data, normalized to % WT level, are expressed as X ± SE. (*p < 0.05 versus WT group). (B) Kaplan-Meier survival curves for SP-D OE mice (n = 19) and littermate controls (Swiss Black background, n = 27) receiving ITB. SP-D OE mice given ITB demonstrate 90% survival versus 47% for identically treated littermate controls with the increase of mortality noted at 8 to 9 d after ITB saline. Saline-treated WT (n = 5) and SP-D OE (n = 7) groups had 100% survival. (C) Weights of SP-D OE and littermate controls treated with ITB were determined up to 21 d after instillation. Data were normalized as a percent of initial weight and expressed as X ± SEM (n = 9–13 for OE and n = 6–18 for WT at each time point). At all time points following injury, both SP-D OE and WT mice developed statistically significant weight loss in comparison to starting weight. *p < 0.05 versus bleomycin-treated WT (littermate) mice at corresponding time point.
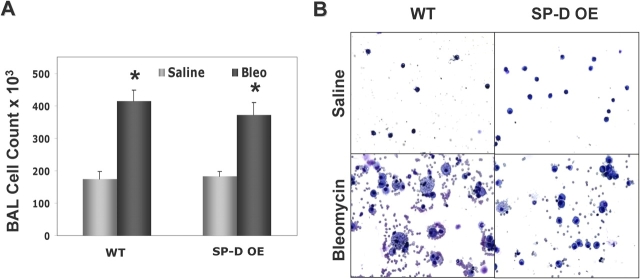
The decreased mortality observed in SP-D OE mice receiving bleomycin was accompanied by less systemic morbidity. After ITB, SP-D OE mice exhibited less weight loss at 7, 10, and 13 d after administration (Figure 7C). The decreased morbidity and mortality observed in ITB SP-D OE mice occurred despite similar degrees of BAL cellularity. In Figure 8A, at 8 d, total BAL cell counts increased two- to threefold in both ITB-treated groups. There were no statistically significantly differences in cell percentages between SP-D OE and WT mice receiving ITB in which cell differentials were again composed of predominantly macrophages and lesser amounts of lymphocytes and neutrophils (data not shown). However, between various transgenic models of collectin-expressing mice, there was a striking difference in the morphology of the recovered macrophages after ITB. As Figure 8B demonstrates, DiffQuik staining of BAL cell pellets revealed that a significant portion of the macrophages obtained from ITB-treated littermates (WT) had increased size with a prominent foamy cytoplasm. In contrast, macrophages from SP-D OE mice receiving ITB appeared to be more normal in size and appearance. Quantitative analysis was done by identification and counting of macrophages in BAL cytopsins followed by stratification according to size. In BAL from ITB-treated WT mice, the percentage of macrophages greater than 25 μm in diameter (3 times' normal size) was significantly increased (32 ± 3% macrophages > 25 μm for bleomycin-treated WT versus 18 ± 5% for bleomycin-treated OE, n = 30–50 macrophages/cytospin sample × 6–8 cytospins in each group, p < 0.01). Consistent with previously published data, SP-D (−/−) macrophages, which have baseline increases in size and granularity, developed further enlargement and more granularity than C57Bl/6 controls (not shown).
Figure 8.
BAL cell counts and macrophage morphology after ITB. SP-D OE mice and littermate controls were inoculated intratracheally with 3 U/kg of bleomycin or saline as described in METHODS. Eight days after treatment, animals were killed and BAL performed. (A) Total BAL cell counts for each group were determined as described in METHODS. Data are expressed as X ± SEM, n = 4–10 mice per group; *p < 0.05 versus corresponding saline-treated group. (B) Representative Diff-Quik–stained cytospins from SP-D–over-expressing mice and transgene negative littermates are shown. (B, upper panels) Saline-treated SP-D OE and WT controls as labeled each demonstrate >99% macrophages, which appear normal in size and shape. (B, lower panels) In littermate controls (WT), injury by ITB produced increases in the number and size of foamy alveolar macrophages. Despite administration of ITB, SP-D OE mice maintained relatively normal macrophage morphology.
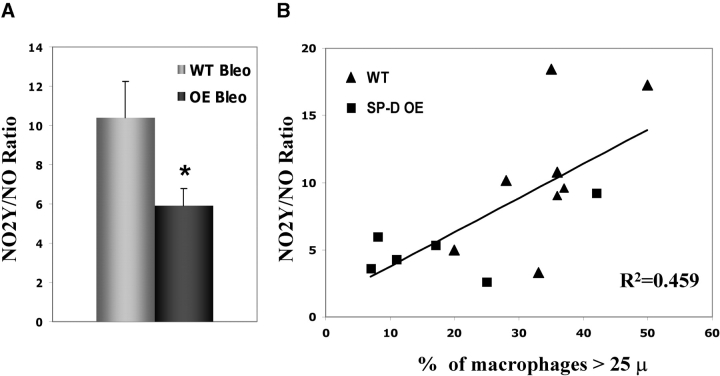
In addition to differences in macrophage appearance, basal overexpression of SP-D limited the changes in NO metabolism by the local lung environment observed previously in SP-D (−/−) mice receiving ITB. When assessed at Day 8 after injury, ITB increased total BAL NO levels in both Swiss-Black littermate controls (saline WT = 138 ± 10 pmoles NO versus ITB WT = 246 ± 39 pmoles NO, n = 4–10, p = 0.024) and in SP-D OE mice (saline SP-D OE = 247 ± 36 pmoles NO versus ITB SP-D OE = 338 ± 39 pmoles NO, n = 6–8 per group, p = 0.034). However, in contrast to both Swiss-Black littermate controls and SP-D (−/−) mice, despite the increase in the baseline total NO level, lungs from bleomycin-injured SP-D OE mice generated proportionately less 3-nitrotyrosine (NO2Y) in response to ITB. As shown in Figure 9A, when expressed as the ratio of NO2Y/NO, overexpression of SP-D significantly limited the production of higher oxides of NO. Furthermore, the NO2Y/NO ratio had a significant positive correlation with the increase in macrophage size observed after ITB (Figure 9B). Collectively, these data indicate that in SP-D OE mice, the response to the ITB results in continued mononuclear cell recruitment to the lung, but that increasing amounts of SP-D also limit the amount of reactive nitrogen species generated in response to this stress.
Figure 9.
3-nitrotyrosine/NO ratio and macrophage size are increased after intratracheal bleomycin. BAL and lung tissue from SP-D OE and littermate control mice were harvested 8 d after administration of 3 U/kg bleomycin. (A) BAL NO levels and tissue 3-nitrotyrosine (NO2Y) content were measured as described in METHODS. Data, expressed as the ratio of NO2Y to NO, demonstrate an increase in NO2Y/NO in ITB-treated WT mice compared with ITB-treated SP-D OE mice. *p < 0.05. (B) Manual counting and determination of macrophage size were performed on cytospins (n = 30–50 macrophages/cytospin sample × 6–8 cytospins) in bleomycin-treated SP-D OE (square) and littermate (triangle) mice. NO2Y/NO (y axis) plotted as a function of the percentage of macrophages greater that 25 μ in diameter (x axis) positively correlated with macrophages size (r = 0.459, n = 6–8).
DISCUSSION
A convincing body of data is emerging to support the role of SP-D in the modulation of antiinflammatory responses required to limit lung injury during infectious and inflammatory challenge. SP-D–deficient mice, previously generated by two different groups with the use of comparable transgenic technologies, each demonstrate alterations in surfactant component homeostasis accompanied by baseline inflammation within the lung manifested as peribronchiolar mononuclear infiltrates, increased BAL cellularity, macrophage activation, oxidative stress, matrix metalloproteinase production, and, ultimately, in premature emphysema. We have used mice derived from one of these lines and backcrossed to the C57BL/6 strain to study the response to bleomycin-induced lung injury. In a previous report, we found that SP-D–deficient mice had significant increases in the levels of large aggregate SP-B and phospholipid, a mild reduction in surfactant protein A content, increases in total BAL cells, and alterations in nitric oxide production and metabolism (28). The results presented within this article extend these observations with new studies demonstrating that in the absence of SP-D protein, the murine lung demonstrates an exaggerated inflammatory and fibrotic response to subacute lung injury produce by challenge with intratracheal bleomycin. ITB in SP-D (−/−) mice produced an increased total cellular influx in the lung parenchyma and alveolar space that was accompanied by abnormalities in nitric oxide metabolism with generation of 3-nitrotyrosine. This resulted in enhanced lung injury and increased mortality in the SP-D–deficient model. Furthermore, the constitutive overexpression of SP-D that results in 5- to 10-fold increases in alveolar SP-D content in transgenic mice results in protection from ITB lung injury. These results represent the first report linking alveolar levels of SP-D in the lung with alterations in the response to a noninfectious inflammatory challenge.
The role of SP-D in the modulation of host defense and inflammatory responses has been studied predominantly in vitro. In addition to the well-known interactions with variety of microorganisms including viruses (influenza A and herpes simplex), bacteria (Pseudomonas aeruginosa), and fungi (Cryptococcus neoformans, A. fumigatus, and Pneumocystis), SP-D is also known to interact with and modulate the activity of a variety of effector cells in the lung (9). SP-D can stimulate chemotaxis of macrophages, but appears to have no effect on monocyte migration. SP-D has also been shown to alter the state of macrophage activation in response to an inflammatory stimulus such as LPS and can affect the degree of macrophage apoptosis. After in vivo replacement of SP-D, either from the use of exogenous SP-D or in transgenic mice using inducible promoter systems, both surfactant lipid abnormalities as well as inflammatory mediators can be ameliorated (12, 36, 50). Taken together, the data suggest a key role for SP-D in the local modulation of lung inflammation.
Previously, we had shown that administration of ITB to rats was also accompanied by an elevation in BAL SP-D protein levels (18). Furthermore in many other animal models of infection and inflammation including Pneumocystis carinii pneumonia, antigen-mediated airways hyperresponsiveness (A. fumigatus or ovalbumin), and hyperoxia, the inflammatory injury is accompanied by selective increases in SP-D (17, 51, 52). SP-D administered to mice sensitized with ovalbumin downregulates airway hyperresponsiveness and inflammation (53). Similar findings have been observed in humans. Asthmatic patients subjected to segmental antigen challenge respond in part with increases in BAL SP-D (54). In patients with cystic fibrosis, indices of inflammation (e.g., cytokine production) have been shown to correlate inversely with the level of SP-D found in the BAL (55). Collectively, these findings support the notion that SP-D represents an acute phase response important to counteract proinflammatory events in the lung.
The SP-D–deficient mouse has been used to study the inflammatory response to pathogens. Previously, pulmonary infection of SP-D (−/−) mice by influenza A resulted in enhanced markers of inflammation (56, 57). More recently, we have demonstrated that the SP-D–deficient mouse model had an increasingly susceptibility to pulmonary infection by P. carinii and that the pneumonia is accompanied by an increase in lung inflammation characterized by rises in BAL protein, wet-to-dry ratios, inflammatory infiltrates, and reactive nitrogen species production. Because of the known direct effects of many of these pathogens on the alveolar epithelium, we sought to extend the understanding of the role of SP-D in the counterregulation and control of inflammation using a noninfectious model of lung injury. ITB is a well-recognized model of subacute lung injury characterized by the production of both reactive oxygen species as well as pro-inflammatory cytokines. Administration of ITB to SP-D (−/−) mice resulted in enhanced mortality and markers of inflammation. Thus it is likely that these observations obtained in the SP-D–deficient mouse are independent of the inciting organism.
The protection from ITB observed in SP-D–overexpressing mice was associated with an increased level of SP-D in BAL at baseline and a further marked elevation at 21 d after injury. In contrast to littermate controls (WT), SP-D OE mice were protected from injury despite equivalent increases in mononuclear cell migration into the alveolar space (Figure 8A). However, in contrast to ITB-treated WT mice, macrophages from SP-D OE mice receiving ITB were shown to have marked differences in size and appearance. Cytologic examination of BAL cells from SP-D OE mice given bleomycin revealed a greater proportion of normal size macrophages with an absence of cytoplasmic granularity (Figure 8B). We had shown previously that in SP-D–deficient mice, the presence of large foamy macrophages was associated with increases in reactive oxygen species/reactive nitrogen species (28, 37). Interestingly, in this study, after ITB, for both groups larger macrophage size (> 25 μm) was positively correlated with increased tissue nitrotyrosine, a marker of RNS production (Figure 9). Furthermore, lungs from bleomycin-injured SP-D OE mice contained proportionately less NO2Y than did ITB littermate controls. These data suggest a critical role for SP-D in the modulation of lung inflammation via effects on NO metabolism and reactive oxygen-nitrogen species. Additional studies utilizing novel inhibitors of iNOS and NO production in vivo to further address this issue are in progress.
In summary, this report highlights the emerging importance of the lung collectin SP-D in modulation of the inflammatory response after subacute lung injury. The injury in response to ITB is associated with an enhanced oxidative-nitrative stress in the lung, which produces lung parenchymal injury that can be modulated by the level of alveolar SP-D. Based on the data presented in this study as well as studies the literature, inflammation in vivo appears to inversely correlate with alveolar levels of SP-D. This suggests that interventions such as the administration of SP-D either prophylactically or as therapy have the potential to limit the inflammatory response through effects of alveolar macrophages. Given the nature of the molecular mechanisms underlying bleomycin-induced lung injury, SP-D may have broader therapeutic implication in other forms for acute lung injury such as those produced by ischemia-reperfusion injury or hyperoxia.
Acknowledgments
The authors are indebted to Dr. Frank McCormack for provision of breeding pairs of SP-D–overexpressing transgenic mice. The authors also acknowledge the technical assistance of Adam Inch, Seth Scanlon, Pamela Scott, and Joseph Foley.
Supported by NIH P50-AT-00428 (M.F.B.), RO1 HL 64520 (M.F.B.), HL 62472 (R.C.S.), HL073896 (R.C.S.), HL-24075 (SH), and HL 58047 (S.H.), Fellowship Research Grant, Forest Pharmaceuticals, Inc. (J.C.).
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Akiyama J, Hoffman A, Brown C, Allen L, Edmondson J, Poulain F, Hawgood S. Tissue distribution of surfactant proteins A and D in the mouse. J Histochem Cytochem 2002;50:993–996. [DOI] [PubMed] [Google Scholar]
- 2.Crouch EC. Structure, biologic properties, and expression of surfactant protein D (SP-D). Biochim Biophys Acta 1998;1408:278–289. [DOI] [PubMed] [Google Scholar]
- 3.Crouch E, Hartshorn K, Ofek I. Collectins and pulmonary innate immunity. Immunol Rev 2000;173:52–65. [DOI] [PubMed] [Google Scholar]
- 4.Crouch EC. Collectins and pulmonary host defense. Am J Respir Cell Mol Biol 1998;19:177–201. [DOI] [PubMed] [Google Scholar]
- 5.Wright JR. Immunomodulatory functions of surfactant. Physiol Rev 1997;77:931–962. [DOI] [PubMed] [Google Scholar]
- 6.Madan T, Eggleton P, Kishore U, Strong P, Aggrawal SS, Sarma PU, Reid KBM. Binding of pulmonary surfactant proteins A and D to Aspergillus fumigatus conidia enhances phagocytosis and killing by human neutrophils and alveolar macrophages. Infect Immun 1997;65:3171–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeVine AM, Whitsett JA, Gwozdz JA, Richardson TR, Fisher JH, Burhans MS, Korfhagen TR. Distinct effects of surfactant protein A or D deficiency during bacterial infection on the lung. J Immunol 2000;165:3934–3940. [DOI] [PubMed] [Google Scholar]
- 8.Bufler P, Schmidt B, Schikor D, Bauernfeind A, Crouch EC, Griese M. Surfactant Protein A and D differentially regulate the immune response to nonmucoid Pseudomonas aeruginosa and its lipopolysaccharide. Am J Respir Cell Mol Biol 2003;28:249–256. [DOI] [PubMed] [Google Scholar]
- 9.Crouch EC, Wright JR. Surfactant proteins A and D and pulmonary host defense. Annu Rev Physiol 2001;63:521–554. [DOI] [PubMed] [Google Scholar]
- 10.Borron PJ, Crouch EC, Lewis JF, Wright JR, Possmayer F, Fraher LJ. Recombinant rat surfactant-associated protein D inhibits human T lymphocyte proliferation and IL-2 production. J Immunol 1998;161:4599–4603. [PubMed] [Google Scholar]
- 11.Yoshida M, Korfhagen TR, Whitsett JA. Surfactant protein D regulates NF-kappa B and matrix metalloproteinase production in alveolar macrophages via oxidant-sensitive pathways. J Immunol 2001;166:7514–7519. [DOI] [PubMed] [Google Scholar]
- 12.Clark H, Palaniyar N, Strong P, Edmondson J, Hawgood S, Reid KBM. Surfactant protein D reduces alveolar macrophage apoptosis in vivo. J Immunol 2002;169:2892–2899. [DOI] [PubMed] [Google Scholar]
- 13.Reid KB. Functional roles of the lung surfactant proteins SP-A and SP-D in innate immunity. Immunobiology 1998;199:200–207. [DOI] [PubMed] [Google Scholar]
- 14.Brinker KG, Martin E, Borron P, Mostaghel E, Doyle C, Harding CV, Wright JR. Surfactant protein D enhances bacterial antigen presentation by bone marrow-derived dendritic cells. Am J Physiol Lung Cell Mol Physiol 2001;281:L1453–L1463. [DOI] [PubMed] [Google Scholar]
- 15.Crouch E, Parghi D, Kuan SF, Persson A. Surfactant Protein-D—subcellular localization in nonciliated bronchiolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 1992;263:L60–L66. [DOI] [PubMed] [Google Scholar]
- 16.Mason RJ, Greene K, Voelker DR. Surfactant protein A and surfactant protein D in health and disease. Am J Physiol Lung Cell Mol Physiol 1998;275:L1–13. [DOI] [PubMed] [Google Scholar]
- 17.Haczku A, Atochina EN, Tomer Y, Chen H, Scanlon ST, Russo SJ, Xu J, Panettieri RA Jr, Beers MF. Aspergillus fumigatus-induced allergic airway inflammation alters surfactant homeostasis and lung function in BALB/c mice. Am J Respir Cell Mol Biol 2001;25:45–50. [DOI] [PubMed] [Google Scholar]
- 18.Savani RC, Godinez RI, Godinez MH, Wentz E, Zaman A, Cui Z, Pooler PM, Guttentag SH, Beers MF, Gonzales LW, et al. Respiratory distress after intratracheal bleomycin: selective deficiency of surfactant proteins B and C. Am J Physiol Lung Cell Mol Physiol 2001;281:L685–L696. [DOI] [PubMed] [Google Scholar]
- 19.Floros J, Hoover RR. Genetics of the hydrophilic surfactant proteins A and D. Biochim Biophys Acta 1998;1408:312–322. [DOI] [PubMed] [Google Scholar]
- 20.Lin Z, Pearson C, Chinchilli V, Pietschmann SM, Luo J, Pison U, Floros J. Polymorphisms of human SP-A, SP-B, and SP-D genes: association of SP-B Thr131Ile with ARDS. Clin Genet 2000;58:181–191. [DOI] [PubMed] [Google Scholar]
- 21.Guo X, Lin HM, Lin Z, Montano M, Sansores R, Wang G, DiAngelo S, Pardo A, Selman M, Floros J. Surfactant protein gene A, B, and D marker alleles in chronic obstructive pulmonary disease of a Mexican population. Eur Respir J 2001;18:482–490. [DOI] [PubMed] [Google Scholar]
- 22.Husby S, Herskind AM, Jensenius JC, Holmskov U. Heritability estimates for the constitutional levels of the collectins mannan-binding lectin and lung surfactant protein D: a study of unselected like-sexed mono- and dizygotic twins at the age of 6–9 years. Immunology 2002;106:389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Botas C, Poulain F, Akiyama J, Brown C, Allen L, Goerke J, Clements J, Carlson E, Gillespie AM, Epstein C, et al. Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D. Proc Natl Acad Sci U S A 1998;95:11869–11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korfhagen TR, Sheftelyevich V, Burhans MS, Bruno MD, Ross GF, Wert SE, Stahlmann MT, Jobe AH, Ikegami M, Whitsett JA, et al. Surfactant protein-D regulates surfactant phospholipid homeostasis in vivo. J Biol Chem 1998;273:28438–28443. [DOI] [PubMed] [Google Scholar]
- 25.Wert SE, Yoshida M, LeVine AM, Ikegami M, Jones T, Ross GF, Fisher JH, Korfhagen TR, Whitsett JA. Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene-inactivated mice. Proc Natl Acad Sci USA 2000;97:5972–5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikegami M, Whitsett JA, Jobe A, Ross G, Fisher J, Korfhagen T. Surfactant metabolism in SP-D gene-targeted mice. Am J Physiol Lung Cell Mol Physiol 2000;279:L468–L476. [DOI] [PubMed] [Google Scholar]
- 27.Fisher JH, Larson J, Cool C, Dow SW. Lymphocyte activation in the lungs of SP-D null mice. Am J Respir Cell Mol Biol 2002;27:24–33. [DOI] [PubMed] [Google Scholar]
- 28.Atochina EN, Beers MF, Hawgood S, Poulain F, Davis C, Fusaro TT, Gow AJ. Surfactant protein-D, a mediator of innate lung immunity, alters the products of nitric oxide metabolism. Am J Respir Cell Mol Biol 2003;30:271–279. [DOI] [PubMed] [Google Scholar]
- 29.Huang WX, Wang GR, Phelps DS, Al Mondhiry H, Floros J. Combined SP-A-bleomycin effect on cytokines by THP-1 cells: impact of surfactant lipids on this effect. Am J Physiol Lung Cell Mol Physiol 2002;283:L94–L102. [DOI] [PubMed] [Google Scholar]
- 30.Koptides M, Umstead TM, Floros J, Phelps DS. Surfactant protein A activates NF-kappa B in the THP-1 monocytic cell line. Am J Physiol Lung Cell Mol Physiol 1997;17:L382–L388. [DOI] [PubMed] [Google Scholar]
- 31.Savani RC, Zhou Z, Arguiri E, Wang S, Vu D, Howe CC, DeLisser HM. Bleomycin-induced pulmonary injury in mice deficient in SPARC. Am J Physiol Lung Cell Mol Physiol 2000;279:L743–L750. [DOI] [PubMed] [Google Scholar]
- 32.Blommuilwijk MC, Vriesendorp R, Veninga TS, Hofstra W, Sleyfer DT, Wieringa RA, Konings AWT. Pulmonary toxicity after treatment with bleomycin alone or in combination with hyperoxia—studies in the rat. Br J Anaesth 1988;60:91–97. [DOI] [PubMed] [Google Scholar]
- 33.Lindenschmidt RC, Tryka AF, Godfrey GA, Frome EL, Witschi H. Intratracheal versus intravenous administration of bleomycin in mice—acute effects. Toxicol Appl Pharmacol 1986;85:69–77. [DOI] [PubMed] [Google Scholar]
- 34.Casey JA, Tomer Y, Savani R, Gow A, Hawgood S, Beers MF. SP-D deficiency predisposes to more severe lung inflammation and oxidative stress in response to bleomycin. Pediatr Res 2004;55:453A. [Google Scholar]
- 35.Kaplan JH, Casey JA, Atochina EN, McCormack FX, Fisher JH, Tomer Y, Kadire H, Beers MF. Bleomycin induced lung injury is modulated by alveolar SP-D content [abstract]. Proc Am Thorac Soc 2005;2:A87. [Google Scholar]
- 36.Fisher JH, Sheftelyevich V, Ho YS, Fligiel S, McCormack FX, Korfhagen TR, Whitsett JA, Ikegami M. Pulmonary-specific expression of SP-D corrects pulmonary lipid accumulation in SP-D gene-targeted mice. Am J Physiol Lung Cell Mol Physiol 2000;278:L365–L373. [DOI] [PubMed] [Google Scholar]
- 37.Atochina EN, Gow AJ, Beck JM, Haczku A, Inch A, Kadire H, Tomer Y, Davis C, Preston AM, Poulain F, et al. Surfactant protein D deficient mice exhibit delayed clearance of Pneumocystis lung infection with increased inflammation and altered nitric oxide metabolism. J Infect Dis 2004;189:1528–1539. [DOI] [PubMed] [Google Scholar]
- 38.Atochina EN, Beers MF, Scanlon ST, Preston AM, Beck JM. P. carinii induces selective alterations in component expression and biophysical activity of lung surfactant. Am J Physiol Lung Cell Mol Physiol 2000;278:L599–L609. [DOI] [PubMed] [Google Scholar]
- 39.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ann Biochem 1976;72:248–254. [DOI] [PubMed] [Google Scholar]
- 40.Bartlett GR. Phosphorous assay in column chromatography. J Biol Chem 1959;234:466–468. [PubMed] [Google Scholar]
- 41.Haczku A, Cao Y, Atochina E, Scanlon S, Tomer Y, Russo S, Campbell C, Beers M. Interleukin (IL)-4 regulates surfactant protein (SP)-D over production during development of allergic inflammation in mice. Clin Immunol 2002;103:S61–S62. [Google Scholar]
- 42.Cao Y, Tao JQ, Bates S, Beers M, Haczku A. IL-4 induces production of the lung collectin surfactant protein-D. J Allergy Clin Immunol 2004;113:439–444. [DOI] [PubMed] [Google Scholar]
- 43.Gow AJ, McClelland M, Garner SE, Malcolm S, Ischiropoulos H. The determination of nitrotyrosine residues in proteins. Methods Mol Biol 1998;100:291–299. [DOI] [PubMed] [Google Scholar]
- 44.Ischiropoulos H, Beers MF, Ohnishi ST, Fisher D, Garner S, Thom SR. Nitric oxide production and perivascular tyrosine nitration in brain following carbon monoxide poisoning in the rat. J Clin Invest 1996;97:2260–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beers MF, Bates SR, Fisher AB. Differential extraction for the rapid purification of bovine surfactant protein B. Am J Physiol Lung Cell Mol Physiol 1992;262:L773–L778. [DOI] [PubMed] [Google Scholar]
- 46.Wali A, Beers MF, Dodia C, Feinstein SI, Fisher AB. ATP and cAMP stimulate the synthesis of surfactant protein SP-A in rat lung. Am J Physiol Lung Cell Mol Physiol 1993;264:L431–L437. [DOI] [PubMed] [Google Scholar]
- 47.Fang KZ, Ragsdale NV, Carey RM, Macdonald T, Gaston B. Reductive assays for S-nitrosothiols: implications for measurements in biological systems. Biochem Biophys Res Commun 1998;252:535–540. [DOI] [PubMed] [Google Scholar]
- 48.Brown S, Worsfold M, Sharp C. Microplate assay for the measurement of hydroxyproline in acid-hydrolyzed tissue samples. Biotechniques 2001;30:38–41. [DOI] [PubMed] [Google Scholar]
- 49.Akiyama J, Volik SV, Plajzer-Frick I, Prince A, Sago H, Weier HUG, Vanderbilt JN, Hawgood S, Poulain FR. Characterization of the mouse collectin gene locus. Am J Respir Cell Mol Biol 1999;21:193–199. [DOI] [PubMed] [Google Scholar]
- 50.Clark H, Reid K. The potential of recombinant surfactant protein D therapy to reduce inflammation in neonatal chronic lung disease, cystic fibrosis, and emphysema. Arch Dis Child 2003;88:981–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atochina EN, Beck JM, Scanlon ST, Preston AM, Beers MF. Pneumocystis carinii pneumonia alters expression and distribution of lung collectins SP-A and SP-D. J Lab Clin Med 2001;137:429–439. [DOI] [PubMed] [Google Scholar]
- 52.Aderibigbe AO, Thomas RF, Mercer RR, Auten RL. Brief exposure to 95% oxygen alters surfactant protein D and mRNA in adult rat alveolar and bronchiolar epithelium. Am J Respir Cell Mol Biol 1999;20:219–227. [DOI] [PubMed] [Google Scholar]
- 53.Strong P, Reid KBM, Clark H. Intranasal delivery of a truncated recombinant human SP-D is effective at down-regulating allergic hypersensitivity in mice sensitized to allergens of Aspergillus fumigatus. Clin Exp Immunol 2002;130:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng G, Ueda T, Numao T, Kuroki Y, Nakajima H, Fukushima Y, Motojima S, Fukuda T. Increased levels of surfactant protein A and D in bronchoalveolar lavage fluids in patients with bronchial asthma. Eur Respir J 2000;16:831–835. [DOI] [PubMed] [Google Scholar]
- 55.Noah TL, Murphy PC, Alink JJ, Leigh MW, Hull WM, Stahlman MT, Whitsett JA. Bronchoalveolar lavage fluid surfactant protein-A and surfactant protein-D are inversely related to inflammation in early cystic fibrosis. Am J Respir Crit Care Med 2003;168:685–691. [DOI] [PubMed] [Google Scholar]
- 56.Hawgood S, Brown C, Edmondson J, Stumbaugh A, Allen L, Goerke J, Clark H, Poulain F. Pulmonary collectins modulate strain-specific influenza A virus infection and host responses. J Virol 2004;78:8565–8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.LeVine AM, Whitsett JA, Hartshorn KL, Crouch EC, Korfhagen TR. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J Immunol 2001;167:5868–5873. [DOI] [PubMed] [Google Scholar]