Abstract
Rationale: Inhaled nitric oxide (iNO) can reverse neonatal pulmonary hypertension and bronchoconstriction and reduce proliferation of cultured arterial and airway smooth muscle cells.
Objectives: To see if continuous iNO from birth might reduce pulmonary vascular and respiratory tract resistance (PVR, RE) and attenuate growth of arterial and airway smooth muscle in preterm lambs with chronic lung disease.
Methods: Eight premature lambs received mechanical ventilation for 3 weeks, four with and four without iNO (5–15 ppm). Four term lambs, mechanically ventilated without iNO for 3 weeks, served as additional control animals.
Measurements: PVR and RE were measured weekly. After 3 weeks, lung tissue was processed for quantitative image analysis of smooth muscle abundance around small arteries (SMart) and terminal bronchioles (SMtb). Radial alveolar counts were done to assess alveolar number. Endothelial NO synthase (eNOS) protein in arteries and airways was measured by immunoblot analysis.
Main Results: At study's end, PVR was similar in iNO-treated and untreated preterm lambs; PVR was less in iNO-treated preterm lambs compared with term control animals. RE in iNO-treated lambs was less than 40% of RE measured in preterm control animals. SMart was similar in iNO-treated and both groups of control lambs; SMtb in lambs given iNO was significantly less (∼ 50%) than in preterm control animals. Radial alveolar counts of iNO-treated lambs were more than twice that of preterm control animals. eNOS was similar in arteries and airways of iNO-treated preterm lambs compared with control term lambs.
Conclusions: iNO preserves structure and function of airway smooth muscle and enhances alveolar development in preterm lambs with chronic lung disease.
Keywords: airway smooth muscle, bronchopulmonary dysplasia, endothelial nitric oxide synthase, lung growth and development, neonatal chronic lung disease, pulmonary vascular and respiratory tract resistances
Infants who are born at a very early stage of development, notably at less than 28 weeks of gestation, often have respiratory failure because of their immature lungs, primitive respiratory drive, and susceptibility to infection. The frequent need for prolonged assisted ventilation in such infants typically leads to a form of chronic lung injury that was first described by Northway and colleagues (1) as bronchopulmonary dysplasia. The pathologic features of this condition include impaired lung vascular and alveolar development, with excess abundance, tone, and reactivity of pulmonary arterial and airway smooth muscle (2–9). These structural abnormalities of the pulmonary circulation and conducting airways are associated with high resistance to blood flow in the lung vascular bed (10, 11) and high resistance to airflow through the respiratory tract, especially during expiration (12, 13). The etiology of these changes is unknown, but may relate to chronic inflammation that develops in the premature lung exposed to repetitive stretch with oxygen-enriched gas, often complicated by infection in the airways and lung parenchyma.
Most research related to nitric oxide (NO) during fetal and early postnatal lung development has focused on the pulmonary circulation, notably the key role of NO in lowering pulmonary vascular resistance (PVR) after birth, and the benefit of inhaled NO (iNO) in reducing PVR in neonates with persistent pulmonary hypertension. NO plays an important role in regulating smooth muscle tone in the blood vessels of the newborn lung. Blocking NO production lessens the normal postnatal decrease of PVR in newborn sheep (14, 15). Several studies have shown that iNO causes a rapid drop in PVR and improves arterial blood oxygenation in both newborn animals and human infants with pulmonary hypertension (16–23). A recent report indicated that iNO coupled with oxygen breathing caused marked pulmonary vasodilation in children with long-standing pulmonary hypertension from advanced lung vascular disease that began as bronchopulmonary dysplasia (24).
Long-term iNO has been shown to reduce the lung vascular remodeling that occurs in chronically hypoxic rats with pulmonary hypertension (25, 26). At least two studies have shown that NO inhibits proliferation of vascular smooth muscle cells in culture (27, 28). Low-dose iNO has been shown to increase arterial oxygenation, decrease PVR, and lessen lung neutrophil accumulation in preterm lambs with acute respiratory failure (29). In a neonatal piglet model of ischemia-reperfusion lung injury, iNO decreased lung fluid leak and inhibited endothelial dysfunction (30). Because pulmonary hypertension and edema are characteristic features of neonatal chronic lung disease (CLD), early postnatal treatment with iNO might be expected to prevent or at least reduce severity of the lung vascular component of this disease.
Although most studies of iNO have focused on the lung circulation, some investigators have assessed the influence of iNO on airway resistance and bronchiolar smooth muscle. One group showed that iNO decreased respiratory tract resistance (Re) in anesthetized adult guinea pigs with induced bronchoconstriction (31). Another group found that iNO decreased Re in anesthetized newborn piglets (32). NO has been shown to reduce proliferation of airway smooth muscle cells in culture (33). Taken together, these studies indicate that NO can influence smooth muscle abundance and tone in the conducting airways during postnatal development.
We previously created an animal model of CLD, in which fetal sheep were delivered prematurely by cesarean section, given intrapulmonary surfactant, and mechanically ventilated for 3 weeks with sufficient inspired oxygen to maintain normal arterial oxygenation. Physiologic studies showed sustained elevation of PVR and Re, which was associated with postmortem histologic evidence of increased smooth muscle and elastin in the lung circulation and distal airways, a paucity of alveoli and small pulmonary arteries, reduced capillary surface density, and decreased abundance of endothelial NO synthase (eNOS) in blood vessels and airways when compared with lungs of control lambs that were born at term (34–37). We subsequently found that iNO given at 15 ppm for 1 hour consistently reduced PVR by approximately 20% at the end of the first week of mechanical ventilation, but that continued mechanical ventilation was associated with loss of the pulmonary vasodilator response to iNO, possibly related to diminished abundance of eNOS and soluble guanylate cyclase that was noted in the lung circulation at the end of the 3-week studies (38).
These findings, together with accumulating evidence that iNO reduces smooth muscle proliferation and relaxes smooth muscle tone in the lung circulation and conducting airways, led us to consider the possibility that continuous, low-dose iNO, beginning immediately at birth and sustained during long-term mechanical ventilation, might reduce the severity of neonatal lung injury in our ovine model of bronchopulmonary dysplasia. Our hypothesis was that prolonged iNO starting at birth would inhibit vascular and airway smooth muscle growth and thereby reduce PVR and Re. To test this hypothesis, eight preterm lambs were mechanically ventilated for 3 weeks, four of them with iNO at 5 to 15 ppm beginning at birth, and four of them without iNO. PVR after 3 weeks was not significantly different between iNO-treated and control lambs. Re, however, was significantly less in the lambs that received iNO, and postmortem histopathology showed less airway smooth muscle in the lambs treated with iNO compared with control animals. Immunoblot analysis for eNOS in excised small arteries and airways taken from iNO-treated lambs and control lambs born at term showed no difference in abundance of eNOS protein. This result contrasted with previous studies showing reduced eNOS protein in lung blood vessels and airways of chronically ventilated preterm lambs compared with control lambs born at term (36).
We were surprised to discover that alveolarization was significantly greater in iNO-treated lambs than in control lambs, analogous to the beneficial effect on lung septation that was seen with prolonged nasal application of continuous positive airway pressure in very premature baboons (39). These findings indicate that iNO, begun at birth and sustained during 3 weeks of mechanical ventilation, may help to prevent impaired alveolar development, increased lung resistance, overgrowth of airway smooth muscle, and diminished pulmonary expression of eNOS observed in preterm lambs with CLD. Some of the results of this investigation were reported previously in abstract form (40–42).
METHODS
Animals and Experimental Protocol
Animals.
We used eight lambs that were delivered prematurely by cesarean section at 124 ± 3 days' gestation (term, 147 days). Four lambs had mechanical ventilation and received iNO continuously for 3 weeks beginning immediately after birth, and four lambs had mechanical ventilation for 3 weeks without iNO (preterm control lambs). Table 1 lists descriptive data for the two groups of lambs. Gestational age and weights at birth and death were similar in both groups. Detailed descriptions of operative procedures and early postnatal management have been published previously (35) and can be viewed in the online supplement.
TABLE 1.
Demographic data (mean ± sd) for the two groups of four preterm lambs
| Gestation (d) | Birth Weight (kg) | Death Weight (kg) | Male:Female | |
|---|---|---|---|---|
| Control lambs, no iNO | 124 ± 4 | 2.85 ± 0.45 | 3.36 ± 0.95 | 1:3 |
| Treated with iNO | 124 ± 3 | 2.70 ± 0.86 | 3.46 ± 0.73 | 1:3 |
Definition of abbreviation: iNO = inhaled nitric oxide.
Experimental protocol.
All eight of the studies with iNO-treated and control preterm lambs were done within a 15-month period at the University of Utah. Before delivery, four lambs were preassigned to receive iNO continuously beginning immediately after birth, and four control lambs were preassigned not to receive iNO during 3 weeks of mechanical ventilation. For lambs assigned to receive iNO, the gas was allowed to flow into the inspiratory limb of the ventilator circuit from a cylinder of NO (Scott Specialty Gases, Plumsteadville, PA) that contained 2,200 ppm, with nitrogen as the balance gas. The NO concentration in the inspired gas was measured frequently by a chemiluminescent analyzer (Model 2108; Dasibi Environmental Corp., Glendale, CA), which was calibrated before and intermittently during each 3-week study using a gas cylinder containing a known concentration of NO. The flow of NO was adjusted to keep measured NO concentrations between 5 and 15 ppm. NO flow was reduced if blood methemoglobin concentration, which was measured frequently (Model 300; Radiometer, Copenhagen, Denmark), exceeded 2%.
Physiologic studies included weekly measurements of steady-state pulmonary arterial and left atrial pressures and cardiac output (measured in triplicate by thermodilution using Model SP 1425, Cardiac Output Computer; Gould Inc., Oxnard, CA) to determine PVR. We measured vascular pressures with calibrated pressure transducers (BT3DC; Statham Instruments, Oxnard, CA) connected to an eight-channel amplifier-recorder (Model 7D; Grass Instruments, Quincy, MA). Respiratory variables were assessed weekly from simultaneous measurements of proximal airway and pleural pressures and gas flow, which was measured using a calibrated pneumotachograph connected to a PEDS Pulmonary Evaluation and Diagnosis System (Medical Associated Services, Hatfield, PA) (43).
Studies performed with control term lambs.
To better assess the impact of iNO on lung structure and function of mechanically ventilated preterm sheep, we did four additional studies on newborn lambs that were born vaginally at term gestation and mechanically ventilated for 3 weeks without iNO. This enabled us to compare physiologic and histologic variables of premature versus mature newborn lambs with ventilator-induced lung injury, and to determine the extent to which iNO attenuates the abnormalities that occur in the incompletely developed lung exposed to prolonged cyclic stretch. The design of the studies performed with term lambs was similar to the design of the studies done with preterm lambs, except that the term lambs were born vaginally after spontaneous onset of labor at an average gestational age of 144 ± 1 days, and cardiac output was measured with a flow meter (Model T106; Transonic Systems, Inc., Ithaca, NY) connected by a cable to a 12-mm ultrasonic flow probe (Transonic Systems) that was surgically implanted around the main pulmonary artery.
All surgical procedures and experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Utah School of Medicine.
Postmortem Studies
At the end of each 3-week study, the lambs were anesthetized with intravenous pentobarbital sodium, 35 mg/kg, followed by a thoracotomy to excise the lungs for subsequent histopathologic, biochemical, and molecular assays. We used quantitative methods, as previously described (34, 35), to study postmortem lung histopathology.
Quantitative histology was used to estimate the following: (1) smooth muscle thickness in the walls of small pulmonary arteries and airways, (2) numbers of small pulmonary blood vessels, (3) radial alveolar counts, (4) surface density of capillaries in the walls of distal airspaces, and (5) elastin content of distal lung parenchyma. The results reflect two-dimensional area measurements. Three-dimensional estimates were not made because the entire lung was not available for volume displacement measurements or structural analysis. The alveolar number across terminal respiratory units was estimated by the radial alveolar count method described by Emery and Mithal (44). We used immunohistochemistry and morphometry to estimate the surface density of capillaries in the walls of distal airspaces (35). Details of the quantitative histologic methods can be viewed in the online supplement.
Immunoblot analysis of eNOS protein.
The caudal lobe of the right lung was used to dissect third- and fourth-generation intrapulmonary arteries and airways, which were stored at −80°C for subsequent immunoblot analysis of eNOS protein as previously described (36). We used lungs from three spontaneously born term lambs that were killed 1 day after birth to measure eNOS protein abundance in lung blood vessels and airways of control term lambs for comparison with measurements made on lung arteries and airways of iNO-treated preterm lambs. We previously reported results of eNOS protein measurements made on excised intrapulmonary arteries and airways of chronically ventilated preterm lambs that did not receive iNO (the control group for this study) and of control term lambs that breathed spontaneously for either 1 day or 3 weeks after birth (36). The dissections were performed at 4°C. The excised lung lobe was rinsed with cold sterile saline, followed by dissection of third- and fourth-generation intrapulmonary arteries and adjacent airways, which were placed immediately in liquid nitrogen for later processing, as described in the online supplement.
Portions of both lungs were used for measurement of extravascular lung water by a previously described gravimetric method (45).
Statistical Analysis
Data in the text, tables, and figures are expressed as mean ± SD. When comparing datasets that displayed a normal Gaussian distribution, we used Student's unpaired t test to assess for significant differences in physiologic and histologic data between the two groups of preterm lambs that were mechanically ventilated for 3 weeks with or without iNO (46). For datasets in which there was a skewed non-Gaussian distribution, we applied the nonparametric Mann-Whitney test to assess for significant differences. We used one-way analysis of variance and Student-Newman-Keuls multiple comparison tests to identify differences in physiologic and histologic variables between the two groups of preterm lambs and the group of term lambs that were mechanically ventilated for 3 weeks, and for assessment of differences in the immunoblot measurements of eNOS protein between experimental and control groups. Statistical analysis was done using a commercially available computer program (StatView SE+ Graphics; Abacus Concepts, Inc., Berkeley, CA) and standard statistical tables (46). Differences were considered statistically significant if the p value was less than 0.05.
RESULTS
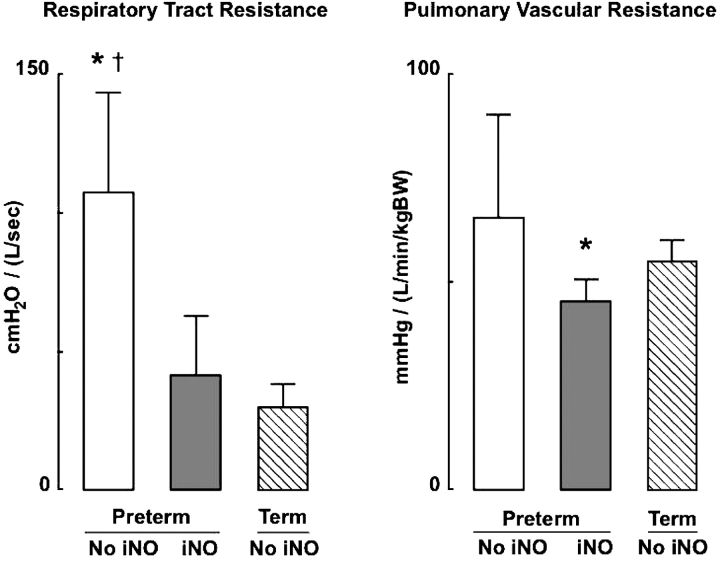
Table 2 shows results of key respiratory variables that were measured at the end of Week 3 in the two groups of preterm lambs. There was considerable variability in the severity of pulmonary dysfunction in the lambs that did not receive iNO. Consequently, there were no significant differences between the two groups with respect to FiO2, arterial blood gas tensions, peak inflation pressure, or dynamic lung compliance (Cl). Re, however, was significantly less in preterm lambs that received iNO than it was in untreated preterm lambs. Re was not significantly different in iNO-treated preterm lambs compared with control term lambs that did not receive iNO (Figure 1). Although Cl was not significantly different between the two groups of preterm lambs, it is noteworthy that Cl was significantly less in preterm lambs that did not receive iNO (0.51 ± 0.21 [ml/cm H2O]/kg bodyweight) compared with term lambs (0.98 ± 0.15 [ml/cm H2O]/kg bodyweight), whereas Cl was similar in iNO-treated preterm lambs (0.88 ± 0.35 [ml/cm H2O]/kg bodyweight) and term lambs that did not receive iNO.
TABLE 2.
Respiratory data (mean ± sd) for the two groups of preterm lambs at the end of the 3-WEEK study
| Airway Pressure (cm H2O)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| iNO | FIO2 | PaO2 (mm Hg) | PaCO2 (mm Hg) | pH | Peak | Mean | VT (ml/kg) | Dynamic Compliance ([ml/cm H2O]/kg BW) |
Expiratory Resistance (cm H2O/[L/s]) |
| − | 0.48 ± 0.35 | 70 ± 11 | 43 ± 30 | 7.35 ± 0.13 | 41 ± 24 | 13 ± 5 | 13 ± 5 | 0.51 ± 0.21 | 106.8 ± 37.3 |
| + | 0.29 ± 0.09 | 70 ± 9 | 31 ± 3 | 7.41 ± 0.04 | 24 ± 8 | 9 ± 2 | 14 ± 4 | 0.88 ± 0.35 | 40.3*± 22.4 |
Definition of abbreviations: BW = bodyweight; iNO = inhaled nitric oxide.
Significant difference compared to preterm lambs not treated with iNO, p < 0.05.
Figure 1.
Respiratory tract and pulmonary vascular resistances (mean and SD) in four preterm lambs that received iNO at 5–15 ppm (dark bars) compared with four preterm lambs that did not receive inhaled nitric oxide (iNO; white bars) and four term lambs that did not receive iNO (hatched bars) during 3 weeks of mechanical ventilation. *Significant difference compared with the group of four term lambs, p < 0.05; †significant difference compared with the group of four preterm lambs given iNO, p < 0.05. BW = bodyweight.
Table 3 shows results of important cardiovascular variables that were measured at the end of Week 3 in the two groups of preterm lambs. There was marked variability of mean pulmonary arterial blood pressure 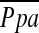 among the four preterm lambs that did not receive iNO. Consequently, there was no statistically significant difference (p > 0.15) in either
among the four preterm lambs that did not receive iNO. Consequently, there was no statistically significant difference (p > 0.15) in either 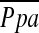 or PVR between treated and untreated preterm lambs. It is noteworthy that PVR was less in preterm lambs that received iNO than it was in control term lambs that did not receive iNO (Figure 1).
or PVR between treated and untreated preterm lambs. It is noteworthy that PVR was less in preterm lambs that received iNO than it was in control term lambs that did not receive iNO (Figure 1).
TABLE 3.
Cardiovascular data (mean ± sd) for the two groups of preterm lambs at the end of the 3-WEEK study
| Pulmonary Artery Pressure (mm Hg) |
Left Atrial Pressure (mm Hg) |
Pulmonary Blood Flow ([L/min]/kg BW) |
Pulmonary Vascular Resistance (mm Hg/[L/min]/kg BW) |
|
|---|---|---|---|---|
| Controls, no iNO | 26 ± 13 | 5 ± 2 | 0.35 ± 0.17 | 65.2 ± 25.1 |
| Treated with iNO | 16 ± 3 | 6 ± 2 | 0.21 ± 0.03 | 44.8 ± 5.4 |
For definition of abbreviation, see Table 2.
There were no significant differences.
Although there was a tendency for extravascular lung water to be greater in the lambs that received iNO, this difference between the two groups of lambs was not statistically significant (5.35 ± 0.91 g/g dry lung in lambs that did not receive iNO, compared with 6.62 ± 1.31 g/g dry lung in lambs that received iNO, p > 0.15). Extravascular lung water was significantly greater in both sets of preterm lambs than it was in term lambs that were ventilated for 3 weeks without iNO (their extravascular lung water was 4.56 ± 0.38 g/g dry lung).
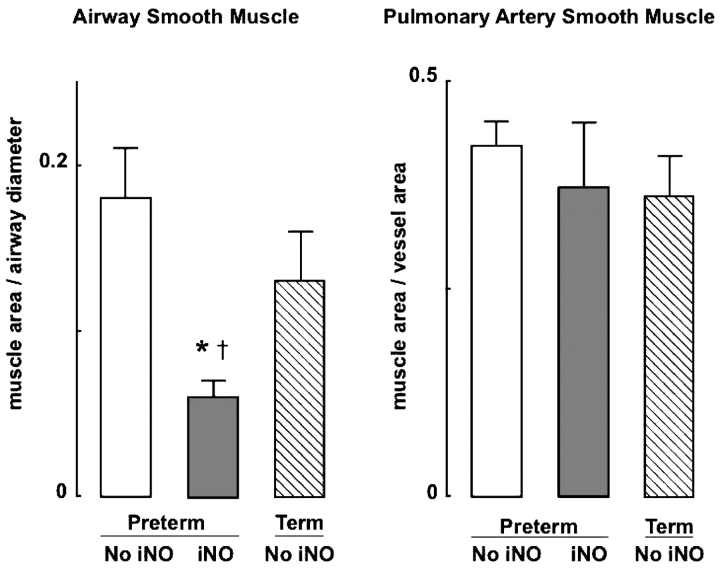
Quantitative assessment of lung histopathology showed significantly less airway smooth muscle in preterm lambs that received iNO than in the control group of preterm lambs (Figures 2 and 3). Airway smooth muscle in iNO-treated preterm lambs also was less than it was in control term lambs that did not receive iNO. There was no significant difference in pulmonary artery smooth muscle abundance between the two groups of preterm lambs, nor was there a difference in vascular smooth muscle between either group of preterm lambs when compared with the group of control term lambs (Figure 2).
Figure 2.
Airway and pulmonary artery smooth muscle area (mean and SD) in four preterm lambs that received iNO at 5–15 ppm (dark bars) compared with four preterm lambs that did not receive iNO (white bars) and four term lambs that did not receive iNO (hatched bars) during 3 weeks of mechanical ventilation. *Significant difference compared with the group of four preterm lambs not given iNO, p < 0.05; †significant difference compared with the group of four term lambs, p < 0.05.
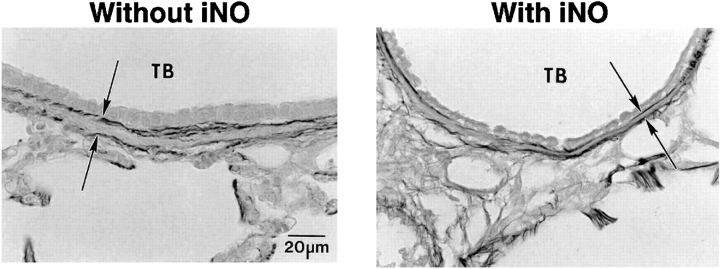
Figure 3.
Representative photomicrographs showing smooth muscle wall thickness between the internal and external elastic laminae (arrows; Hart's elastin stain) of terminal bronchioles (TB) in lungs of preterm lambs that were mechanically ventilated for 3 weeks either without iNO (control, left) or with iNO (5–15 ppm, right).
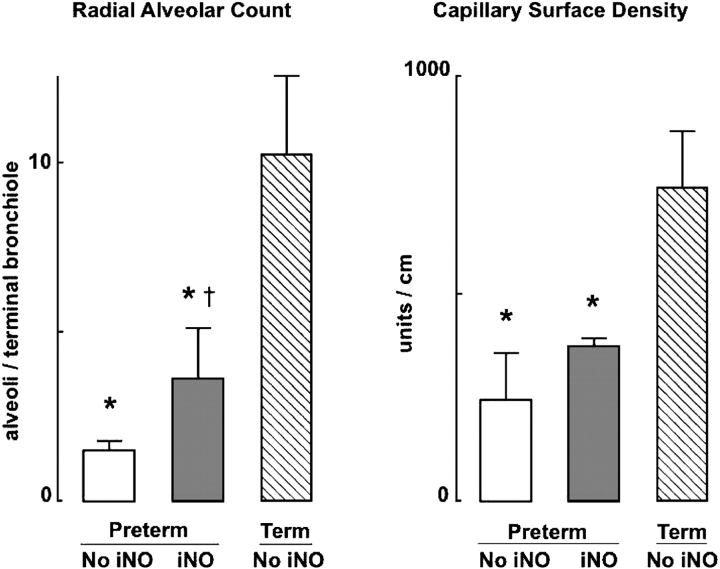
Lambs that received iNO had significantly more alveoli, as assessed by radial alveolar counts, than control preterm lambs (Figures 4 and 5). Capillary surface density tended to be greater in preterm lambs that received iNO (p = 0.06), consistent with the apparent difference in radial alveolar counts between the two groups of preterm lambs. Both alveolar number and capillary surface density were less in the two groups of preterm lambs than in the group of term lambs (Figure 5). These structural differences between preterm and term lambs are not surprising, because alveolar and lung vascular development progresses rapidly in sheep during late gestation.
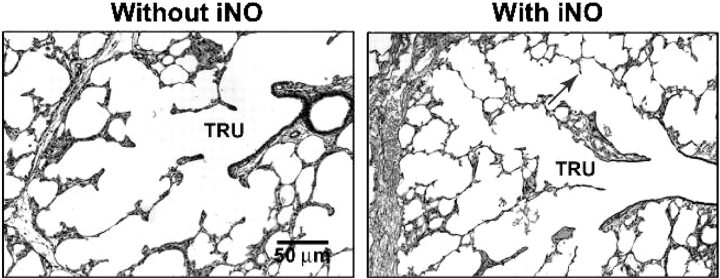
Figure 4.
Lung tissue images illustrating differences in radial alveolar counts for preterm lambs that were mechanically ventilated for 3 weeks without iNO (left) or with iNO (right). Radial alveolar counts averaged 1.5 ± 0.3 alveoli/terminal bronchiole in lambs that did not receive iNO and 3.6 ± 1.5 alveoli/terminal bronchiole in lambs that received iNO. The arrow points to a secondary crest (alveolar septa). TRU = terminal respiratory unit, which is the landmark for measuring radial alveolar counts.
Figure 5.
Radial alveolar count and capillary surface density (mean and SD) in lungs of four preterm lambs that received iNO at 5–15 ppm (dark bars) compared with four preterm lambs that did not receive iNO (white bars) and four term lambs that did not receive iNO (hatched bars) during 3 weeks of mechanical ventilation. *Significant difference compared with the group of four term lambs, p < 0.05; †significant difference compared with the group of four preterm lambs not given iNO, p < 0.05.
Elastin content in lung parenchyma, which was assessed by quantitative image analysis of 6 to 10 histologic tissue sections per animal, was not different in the two groups of preterm lambs (15.8 ± 6.4% with iNO vs. 19.1 ± 2.6% without iNO, expressed as a percentage of parenchymal area). Compared with the four term lambs that were mechanically ventilated without iNO for 3 weeks, however, both groups of preterm lambs had greater elastin accumulation in their distal lung tissue than term lambs (3.7 ± 1.2%).
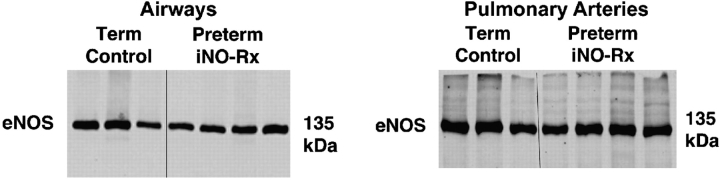
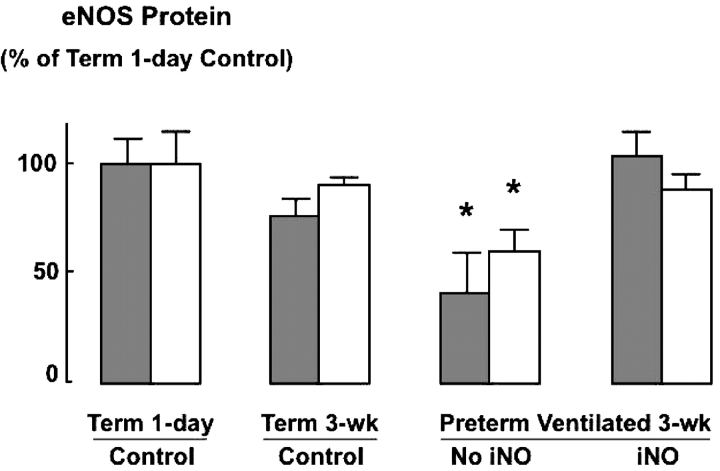
Immunoblot analysis of eNOS protein abundance in excised airways and small pulmonary arteries of preterm lambs that received iNO, compared with unventilated control lambs born at term, showed no significant differences (Figure 6). This finding contrasts with previous results that demonstrated reduced eNOS protein abundance, as assessed both by immunohistochemistry and by immunoblot analysis, in small pulmonary arteries and airways of chronically ventilated preterm lambs (similar to the control lambs reported here) compared with unventilated control lambs born at term (36). We compared eNOS protein measured in small pulmonary arteries and airways of chronically ventilated preterm lambs, with or without iNO treatment from birth, versus eNOS protein measured in small pulmonary arteries and airways of control lambs that were 1 day old (same postconceptional age as the preterm lambs) and 3 weeks old (same postnatal age as the preterm lambs) after birth at term gestation: eNOS protein was significantly less in blood vessels and airways obtained from preterm lambs that did not receive iNO, but there was no difference between preterm lambs that received iNO versus either group of term lambs (Figure 7).
Figure 6.
Immunoblot results for endothelial NO synthase (eNOS) in dissected third- and fourth-generation intrapulmonary airways (left) and arteries (right) excised from lungs of 1-day-old term lambs and preterm lambs given iNO, 5–15 ppm, during 3 weeks of mechanical ventilation. Term lambs were at the same postconceptional age as the preterm lambs, but did not receive mechanical ventilation or iNO. Densitometry showed no significant differences for either airways or arteries.
Figure 7.
Summary data (mean and SD) for eNOS protein measured by immunoblot analysis of excised third- and fourth-generation intrapulmonary arteries (dark bars) and airways (light bars) from four groups of lambs: unventilated 1-day-old and 3-week-old lambs born at term (controls), and 3-week-old preterm lambs that received mechanical ventilation for 3 weeks either without iNO (third group from left) or with iNO (right). Results are plotted relative to 1-day-old term lambs (left) that were at the same postconceptional age as the ventilated preterm lambs. Note that eNOS protein abundance was similar in arteries and airways obtained from term control lambs and from preterm lambs that received iNO, whereas eNOS protein was significantly less in airways and arteries of preterm lambs that were ventilated for 3 weeks without iNO. Reference 36 displays all immunoblot results that are depicted in this figure except for those of iNO-treated preterm lambs, which are shown in Figure 6. *Significant difference compared with 1-day-old and 3-week-old term lambs and preterm lambs that were mechanically ventilated with iNO for 3 weeks, p < 0.05.
DISCUSSION
When we began this study, we expected that iNO would have its greatest effect on the lung vasculature in this ovine model of neonatal CLD, because we previously had discovered that PVR consistently decreased by approximately 20 to 25% when chronically ventilated preterm lambs received iNO for 1 hour early in the course of evolving CLD. The pulmonary vasodilator response to iNO was either diminished or lost after 3 weeks of mechanical ventilation, a finding that we attributed to deficient eNOS and soluble guanylate cyclase protein abundance measured in the lungs of preterm lambs with established CLD (36, 38). We speculated that early and continuous administration of iNO to chronically ventilated preterm lambs might inhibit early lung inflammation, as previous studies had suggested (29), thereby preventing the adverse effects of long-term mechanical ventilation on the premature pulmonary circulation during postnatal development. The observation that iNO had a greater effect on the structure and function of the respiratory tract than it had on the pulmonary circulation of our lambs with established CLD was unexpected, perhaps reflecting direct exposure of the respiratory tract to iNO, as opposed to the potential barrier between airspaces and the pulmonary circulation resulting from lung inflammation, edema, and atelectasis in CLD.
We also were surprised to discover that continuous long-term administration of iNO did not reduce the expression of eNOS in the lungs of the lambs, which is the explanation sometimes given to account for the apparent rise of PVR and reduction of PaO2 that often occur when iNO therapy is discontinued in infants with persistent pulmonary hypertension. Our findings that eNOS protein abundance remained at control levels in both arteries and airways of preterm lambs that received iNO during mechanical ventilation, however, are consistent with the previous report that NO donors increased NOS expression in cultured pulmonary artery endothelial cells derived from fetal sheep (47). Preservation of vascular eNOS, however, yielded no significant benefit with respect to either PVR or arterial smooth muscle abundance.
The importance that NO plays in modulating the structure and function of smooth muscle in the respiratory tract has received much less attention than has the role of NO on smooth muscle abundance, tone, and reactivity in lung blood vessels. A number of recent studies have indicated that NO may be a key regulatory element within the developing respiratory tract, just as it is in the vasculature. It is noteworthy that all three isoforms of NO synthase (eNOS, neuronal NOS, and inducible NOS) are expressed in the respiratory epithelium of both sheep and primates during fetal development (48, 49). We found that eNOS protein expression is reduced in both the pulmonary endothelium and respiratory epithelium of preterm lambs after 3 weeks of mechanical ventilation (36), an observation that recently was confirmed in a nonhuman primate model of CLD: both eNOS protein and total NOS activity were reduced in the lungs of preterm baboons with CLD (50). Lack of NOS activity could contribute to the increased abundance and tone of smooth muscle found in both airways and pulmonary arteries of preterm lambs with CLD compared with control lambs at the same postconceptional age (34, 35), because NO has been shown to inhibit proliferation of vascular and airway smooth muscle cells in culture (27, 28, 33, 51) and to relax airway smooth muscle in newborn animals (32, 52).
We were surprised to discover that the number of alveoli was significantly greater in lungs of preterm lambs that received continuous iNO from birth when compared with preterm lambs that did not receive iNO. Recent studies, however, have shown that NO may have a critical role in lung growth, affecting new formation of both alveoli and pulmonary blood vessels. In a study of compensatory lung growth after left pneumonectomy, eNOS-deficient mice showed no increase of alveolar cell proliferation, alveolar surface density, or volume of the respiratory region in the right lung (53). Compensatory lung growth after pneumonectomy also was impaired in wild-type mice that were treated with an NOS inhibitor, NG-nitro-l-arginine methyl ester (l-NAME). In another study comparing wild-type and eNOS-deficient mice, a 10-day exposure to mild hypoxia (FiO2, 0.16) during the postnatal period of rapid lung growth was associated with fewer alveoli and less blood vessel volume density in lungs of eNOS-deficient neonatal mice compared with age-matched control pups (54). This inhibition of hypoxia-induced lung septation and vasculogenesis was accompanied by reduced lung protein abundance of vascular endothelial growth factor receptor 2 (VEGF-R2), which led to speculation that NO may preserve normal growth of distal lung during hypoxia through preservation of VEGF-R2 signaling. A subsequent study by this group showed that pulmonary hypertension, induced in newborn rats by treatment with the VEGF-R2 inhibitor SU-5416, was reduced, and that lung growth, assessed by radial alveolar counts, was enhanced in newborn rats that were treated for 3 weeks after birth with 10 ppm iNO (55). Another recent study showed that eNOS-deficient mice display major defects in lung morphogenesis, which cause early postnatal death from respiratory failure (56). Lungs of transgenic newborn mice lacking eNOS, compared with wild-type pups, had thickened saccular septae and a paucity of distal lung arterioles, in addition to reduced surfactant phosphatidylcholine in their lung lavage fluid. These striking abnormalities of lung structure and function in eNOS-deficient mice underscore the critical role of NO in lung development and offer insights into why iNO beginning at birth, with preservation of lung eNOS in treated newborn sheep, might have facilitated formation of alveoli and lung capillaries in the face of prolonged mechanical ventilation with oxygen-enriched gas.
The mechanism by which continuous exposure to iNO enhanced lung development during prolonged mechanical ventilation of preterm lambs remains unclear. Studies done with the fawn-hooded rat, an inbred strain in which lung expression of eNOS is reduced, showed not only abnormal lung vascular development and severe pulmonary hypertension when exposed to mild hypoxia (57), but these rats also displayed deficient postnatal alveolar development (58). Related studies conducted with cultured explants of fetal lung from Sprague-Dawley rats showed that NO donors induced a dose-dependent stimulation of lung branching, which was attenuated by NOS inhibition with either l-NAME or NG-monomethyl-l-arginine (59). This observation was consistent with a previous report that NO contributes to the accelerated respiratory tract development that occurs with hypoxia in Drosophila (60). Because NO has been shown to inhibit alveolar epithelial cell apoptosis induced by either hyperoxia, stretch, or ischemia-reperfusion injury (61–63), it is possible that continuous exposure to iNO during prolonged mechanical ventilation of preterm lambs helped to preserve alveolar growth by preventing lung epithelial cell, and perhaps endothelial cell, death.
As noted above, recent reports have called attention to the possible role of VEGF and its receptors in regulating alveolar, as well as lung vascular, development (64, 65). Several studies have implicated NO as an important downstream mediator of VEGF, contributing to VEGF-induced angiogenesis in a number of in vivo and in vitro experimental models (66–69). There is also evidence that NO can induce synthesis of VEGF in vascular smooth muscle cells (70). Because pulmonary expression of VEGF and its receptors are reduced in lungs of infants dying of bronchopulmonary dysplasia and in animal models of this disease (71, 72), it is possible that delivery of iNO to the developing lung during long-term mechanical ventilation enabled VEGF signaling through preservation of the tyrosine kinase receptor Flk-1 (VEGF-R2), just as it preserved eNOS expression in lung blood vessels and airways.
Because of the arduous and costly nature of our lengthy life-support studies, the number of animals in each group was small (four/group), thus increasing the chance of missing important differences between groups. Despite this potential pitfall, significant differences in key respiratory variables between iNO-treated and untreated lambs were apparent after 3 weeks of mechanical ventilation.
Because of the instability of very premature lambs maintained in an intensive care setting over a prolonged period, there was considerable variability of respiratory and cardiovascular measurements at the end of Week 3 in the group of preterm lambs that did not receive iNO. This could have contributed to the absence of significant differences in physiologic variables that previously have been attributed to NO, such as the early postnatal improvement in Cl and reduced pulmonary artery pressure reported in premature primates treated with iNO (73).
By comparing the two groups of preterm lambs to a group of term lambs that also were mechanically ventilated for 3 weeks, we discovered that Cl was significantly less in preterm lambs that did not receive iNO than it was in term lambs, whereas Cl was similar in preterm lambs that received iNO compared with term lambs without iNO. In addition, iNO-treated preterm lambs had significantly lower PVR values after 3 weeks of mechanical ventilation than term lambs, whereas untreated preterm lambs did not have lower values. These observations indicate that iNO may afford a major benefit in terms of inhibiting pulmonary hypertension, as well as reducing resistance and improving compliance of the respiratory system during evolution of CLD after premature birth and lengthy mechanical ventilation.
A recent report described better lung function and lung growth, compared with control animals, in chronically ventilated preterm baboons that received continuous iNO at a lower concentration (5 ppm) and for a shorter duration of mechanical ventilation (2 weeks) than our lambs (73). In this study, iNO begun 1 hour after birth was associated with increased Cl and reduced Re during the first week, and postmortem lung histology after 2 weeks showed longer secondary crests but no significant differences in the number of secondary crests or in lung volume measurements between iNO-treated and untreated baboons. In contrast to our results with preterm lambs, morphometric analysis of pulmonary arteries and bronchioles of preterm primates showed no significant difference in the amount of vascular or airway smooth muscle in iNO-treated compared with untreated baboons. In addition, excess accumulation of lung elastin, which is characteristic of CLD in human infants and newborn lambs (6, 37), including iNO-treated lambs, was absent in the baboons that received iNO. The reason for these histologic differences between the two species is unclear. It is possible that the greater concentration of iNO and the longer duration of mechanical ventilation in preterm lambs compared with baboons yielded a greater reduction in airway smooth muscle and allowed for improved alveolarization, without impacting lung elastin deposition in the lambs. Irrespective of these differences, however, the apparent benefit of iNO on structure and function of the developing lung in two different species and under two different sets of experimental conditions underscores the need to explore the mechanisms by which NO might contribute to lung development. These reports also provide a rationale for testing the therapeutic efficacy and safety of this intervention in disorders associated with impaired lung growth.
Supplementary Material
Acknowledgments
This work would not have been possible without the help of numerous people who are not listed as authors. The present authors are especially grateful for the technical assistance provided by several research associates, including Philip Clair, MarJanna Dahl, Katherine Lagerquist, and Jiancheng Sun, and for the steadfast efforts of many University of Utah medical students, part-time technicians, and respiratory therapists who assisted in the daily management of the lambs. We also thank Dr. Edmund Egan (Ony, Inc.) for generously providing bovine surfactant (Infasurf) and Dr. Ronald Day for advice regarding the circuitry used to deliver iNO.
Supported in part by March of Dimes Birth Defects Foundation grant 6FY97-0138 (R.D.B.); National Heart, Lung, and Blood Institute grants HL-62512 (R.D.B.), HL-56401, and HL-62875 (K.H.A.); SCOR Project V (R.D.B); and the Vera Moulton Wall Center for Cardiopulmonary Research at Stanford University.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respiratory therapy of hyaline membrane disease: bronchopulmonary dysplasia. N Engl J Med 1967;276:357–368. [DOI] [PubMed] [Google Scholar]
- 2.Chambers HM, van Velzen D. Ventilator-related pathology in the extremely immature lung. Pathology 1989;21:79–83. [DOI] [PubMed] [Google Scholar]
- 3.Coalson JJ. Pathology of chronic lung disease of early infancy. In: Bland RD, Coalson JJ, editors. Chronic lung disease in early infancy. New York: Marcel Dekker; 2000. pp. 85–124.
- 4.Hislop AA, Haworth SG. Pulmonary vascular damage and the development of cor pulmonale following hyaline membrane disease. Pediatr Pulmonol 1990;9:152–161. [DOI] [PubMed] [Google Scholar]
- 5.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 1998;29:710–717. [DOI] [PubMed] [Google Scholar]
- 6.Margraf LR, Tomashefski JF Jr, Bruce MC, Dahms BB. Morphometric analysis of the lung in bronchopulmonary dysplasia. Am Rev Respir Dis 1991;143:391–400. [DOI] [PubMed] [Google Scholar]
- 7.Sward-Comunelli SL, Mabry SM, Truog WE, Thibeault DW. Airway muscle in preterm infants: changes during development. J Pediatr 1997;130:570–576. [DOI] [PubMed] [Google Scholar]
- 8.Tomashefski JF Jr, Oppermann HC, Vawter GF, Reid LM. Bronchopulmonary dysplasia: a morphometric study with emphasis on the pulmonary vasculature. Pediatr Pathol 1984;2:469–487. [DOI] [PubMed] [Google Scholar]
- 9.Van Lierde S, Cornelis A, Devlieger H, Moerman P, Lauweryns J, Eggermont E. Different patterns of pulmonary sequelae after hyaline membrane disease: heterogeneity of bronchopulmonary dysplasia? A clinicopathologic study. Biol Neonate 1991;60:152–162. [DOI] [PubMed] [Google Scholar]
- 10.Abman SH, Wolfe RR, Accurso FJ, Koops BL, Bowman CM, Wiggins JW Jr. Pulmonary vascular response to oxygen in infants with severe bronchopulmonary dysplasia. Pediatrics 1985;75:80–84. [PubMed] [Google Scholar]
- 11.Berman W, Yabek SM, Dillon T, Burstein R, Corlew S. Evaluation of infants with BPD using cardiac catheterization. Pediatrics 1982;70:708–712. [PubMed] [Google Scholar]
- 12.Gerhardt T, Hehre D, Feller R, Reifenberg L, Bancalari E. Serial determination of pulmonary function in infants with chronic lung disease. J Pediatr 1987;110:448–456. [DOI] [PubMed] [Google Scholar]
- 13.Motoyama EK, Fort MD, Klesh KW, Mutich RL, Guthrie RD. Early onset of airway reactivity in premature infants with bronchopulmonary dysplasia. Am Rev Respir Dis 1987;136:50–57. [DOI] [PubMed] [Google Scholar]
- 14.Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol Heart Circ Physiol 1990;259:H1921–H1927. [DOI] [PubMed] [Google Scholar]
- 15.Fineman JR, Wong J, Morin FC, Wild LM, Soifer SJ. Chronic nitric oxide inhibition in utero produces persistent pulmonary hypertension in newborn lambs. J Clin Invest 1994;93:2675–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, Roy BJ, Keszler M, Kinsella JP. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med 2000;342:469–474. [DOI] [PubMed] [Google Scholar]
- 17.Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM. Inhaled nitric oxide: a selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation 1991;83:2038–2047. [DOI] [PubMed] [Google Scholar]
- 18.Kinsella JP, Neish SR, Shaffer E, Abman SH. Low-dose inhalational nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 1992;340:819–820. [DOI] [PubMed] [Google Scholar]
- 19.Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med 1997;336:597–604. [DOI] [PubMed] [Google Scholar]
- 20.Roberts JD Jr, Fineman JR, Morin FC III, Shaul PW, Rimar S, Schreiber MD, Polin RA, Zwass MS, Zayek MM, Gross I, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. N Engl J Med 1997;336:605–610. [DOI] [PubMed] [Google Scholar]
- 21.Roberts JD Jr, Polaner DM, Lang P, Zapol WM. Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 1992;340:818–819. [DOI] [PubMed] [Google Scholar]
- 22.Roberts JD Jr, Chen T-Y, Kawai N, Wain J, Dupuy P, Shimouchi A, Bloch K, Polaner D, Zapol WM. Inhaled nitric oxide reverses pulmonary vasoconstriction in the hypoxic and acidotic newborn lamb. Circ Res 1993;72:246–254. [DOI] [PubMed] [Google Scholar]
- 23.Zayek M, Cleveland D, Morin FC III. Treatment of persistent pulmonary hypertension in the newborn lamb by inhaled nitic oxide. J Pediatr 1993;122:743–750. [DOI] [PubMed] [Google Scholar]
- 24.Mourani PM, Ivy DD, Gao D, Abman SH. Pulmonary vascular effects of inhaled nitric oxide and oxygen tension in bronchopulmonary dysplasia. Am J Respir Crit Care Med 2004;170:1006–1013. [DOI] [PubMed] [Google Scholar]
- 25.Kouyoumdjian C, Adnot S, Levame M, Eddahibi S, Bousbaa H, Rafestin B. Continuous inhalation of nitric oxide protects against development of pulmonary hypertension in chronically hypoxic rats. J Clin Invest 1994;94:578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts JD Jr, Roberts CT, Jones RC, Zapol WM, Bloch KD. Continuous nitric oxide inhalation reduces pulmonary arterial structural changes, right ventricular hypertrophy and growth retardation in the hypoxic newborn rat. Circ Res 1995;76:215–222. [DOI] [PubMed] [Google Scholar]
- 27.Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest 1989;83:1774–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomae KR, Nakayama DK, Billiar TR, Simmons RL, Pitt BR, Davies P. The effect of nitric oxide on fetal pulmonary artery smooth muscle growth. J Surg Res 1995;59:337–343. [DOI] [PubMed] [Google Scholar]
- 29.Kinsella JP, Parker TA, Galan H, Sheridan BC, Halbower AC, Abman SH. Effects of inhaled nitric oxide on pulmonary edema and lung neutrophil accumulation in severe experimental hyaline membrane disease. Pediatr Res 1997;41:457–463. [DOI] [PubMed] [Google Scholar]
- 30.Barbotin-Larrieu F, Mazmanian M, Baudet BHD, Chapelier A, Libert JM, Cartevelle P, Herve P. Prevention of ischemia-reperfusion lung injury by inhaled nitric oxide in neonatal piglets. J Appl Physiol 1996;80:782–788. [DOI] [PubMed] [Google Scholar]
- 31.Dupuy PM, Shorz SA, Drazen JM, Frostell C, Hill WA, Zapol WM. Bronchodilator action of inhaled nitric oxide in guinea pigs. J Clin Invest 1992;90:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potter CF, Dreshaj IA, Haxhiu MA, Stork EK, Chatburn RL, Martin RJ. Effect of exogenous and endogenous nitric oxide on the airway and tissue components of lung resistance in the newborn piglet. Pediatr Res 1997;41:886–891. [DOI] [PubMed] [Google Scholar]
- 33.Hamad AM, Johnson SR, Knox AJ. Antiproliferative effects of NO and ANP in cultured human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 1999;277:L910–L918. [DOI] [PubMed] [Google Scholar]
- 34.Albertine KH, Kim BI, Kullama LK, Starcher BC, Cho SC, Carlton DP, Bland RD. Chronic lung injury in preterm lambs: disordered respiratory tract development. Am J Respir Crit Care Med 1999;159:945–958. [DOI] [PubMed] [Google Scholar]
- 35.Bland RD, Albertine KH, Carlton DP, Kullama LK, Davis PL, Cho SC, Kim B, Dahl M, Tabatabaei N. Chronic lung injury in preterm lambs: abnormalities of the pulmonary circulation and lung fluid balance. Pediatr Res 2000;48:64–74. [DOI] [PubMed] [Google Scholar]
- 36.MacRitchie AN, Albertine KH, Sun J, Lei PS, Jensen SC, Freestone AA, Clair PM, Dahl MJ, Godfrey EA, Carlton DP, et al. Reduced endothelial nitric oxide synthase in lungs of chronically ventilated preterm lambs. Am J Physiol Lung Cell Mol Physiol 2001;281:L1011–L1020. [DOI] [PubMed] [Google Scholar]
- 37.Pierce RA, Albertine KH, Starcher BC, Bohnsack JF, Carlton DP, Bland RD. Chronic lung injury in preterm lambs: disordered pulmonary elastin deposition. Am J Physiol Lung Cell Mol Physiol 1997;272:L452–L460. [DOI] [PubMed] [Google Scholar]
- 38.Bland RD, Ling CY, Albertine KH, Carlton DP, MacRitchie AJ, Day RW, Dahl M. Pulmonary vascular dysfunction in preterm lambs with chronic lung disease. Am J Physiol Lung Cell Mol Physiol 2003;285:L76–L85. [DOI] [PubMed] [Google Scholar]
- 39.Thomson MA, Yoder BA, Winter VT, Martin H, Catland D, Siler-Khodr TM, Coalson JJ. Treatment of immature baboons for 28 days with early nasal continuous positive airway pressure. Am J Respir Crit Care Med 2004;169:1054–1062. [DOI] [PubMed] [Google Scholar]
- 40.Bland RD, Albertine KH, Carlton DP, Day RW, Stolworthy LD, Jones GP, Lagerquist K. Continuous inhalation of nitric oxide from birth decreases airway resistance and bronchiolar smooth muscle in chronically ventilated preterm lambs [abstract]. Pediatr Res 1998;42:275A. [Google Scholar]
- 41.Bland RD, Carlton DP, Day RW, MacRitchie AN, Albertine KH, Lagerquist K. Continuous inhalation of nitric oxide from birth preserves the pulmonary vasoconstrictor response to hypoxia in chronically ventilated preterm lambs [abstract]. Pediatr Res 1998;42:276A. [Google Scholar]
- 42.MacRitchie AN, Albertine KH, Qu K, Carlton DP, Bland RD. Regulation of endothelial nitric oxide synthase expression in small pulmonary arteries and airways of chronically ventilated preterm lambs [abstract]. Pediatr Res 1998;43:290A. [Google Scholar]
- 43.Bhutani VK, Sivieri EM, Abbasi S, Shaffer TH. Evaluation of neonatal pulmonary mechanics and energetics: a two factor least mean square analysis. Pediatr Pulmonol 1988;4:150–158. [DOI] [PubMed] [Google Scholar]
- 44.Emery JL, Mithal A. The number of alveoli in the terminal respiratory unit of man during late intrauterine life and childhood. Arch Dis Child 1960;35:544–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pearce ML, Yamashita J, Beazell J. Measurement of pulmonary edema. Circ Res 1965;16:482–488. [DOI] [PubMed] [Google Scholar]
- 46.Zar JH. Biostatistical analysis, 2nd ed. Englewood Cliffs, NJ: Prentice-Hall; 1984.
- 47.Yuhanna IS, MacRitchie AN, Lantin-Hermoso RL, Wells LB, Shaul PW. Nitric oxide (NO) upregulates NO synthase expression in fetal intrapulmonary artery endothelial cells. Am J Respir Cell Mol Biol 1999;21:629–636. [DOI] [PubMed] [Google Scholar]
- 48.Shaul PW, Afshar S, Gibson LL, Sherman TS, Kerecman JD, Grubb PH, Yoder BA, McCurnin DC. Developmental changes in nitric oxide synthase isoform expression and nitric oxide production in fetal baboon lung. Am J Physiol Lung Cell Mol Physiol 2002;283:L1192–L1199. [DOI] [PubMed] [Google Scholar]
- 49.Sherman TS, Chen Z, Yuhanna IS, Lau KS, Margraf LR, Shaul PW. Nitric oxide synthase isoform expression in the developing lung epithelium. Am J Physiol Lung Cell Mol Physiol 1999;276:L383–L390. [DOI] [PubMed] [Google Scholar]
- 50.Afshar S, Gibson LL, Yuhanna IS, Sherman TS, Kerecman JD, Grubb PH, Yoder BA, McCurnin DC, Shaul PW. Pulmonary NO synthase expression is attenuated in a fetal baboon model of chronic lung disease. Am J Physiol Lung Cell Mol Physiol 2003;284:L749–L758. [DOI] [PubMed] [Google Scholar]
- 51.Yang W, Ando J, Korenaga R, Toyo-oka T, Kamiya A. Exogenous nitric oxide inhibits proliferation of cultured vascular endothelial cells. Biochem Biophys Res Commun 1994;203:1160–1167. [DOI] [PubMed] [Google Scholar]
- 52.Martin RJ, Mhanna MJ, Haxhiu MA. The role of endogenous and exogenous nitric oxide on airway function. Semin Perinatol 2002;26:432–438. [DOI] [PubMed] [Google Scholar]
- 53.Leuwerke SM, Kaza AK, Tribble CG, Kron IL, Laubach VE. Inhibition of compensatory lung growth in endothelial nitric oxide synthase-deficient mice. Am J Physiol Lung Cell Mol Physiol 2002;282:L1272–L1278. [DOI] [PubMed] [Google Scholar]
- 54.Balasubramaniam V, Tang JR, Maxey A, Plopper CG, Abman SH. Mild hypoxia impairs alveolarization in the endothelial nitric oxide synthase-deficient mouse. Am J Physiol Lung Cell Mol Physiol 2003;284:L964–L971. [DOI] [PubMed] [Google Scholar]
- 55.Tang JR, Markham NE, Lin YJ, McMurtry IF, Maxey A, Kinsella JP, Abman SH. Inhaled nitric oxide attenuates pulmonary hypertension and improves lung growth in infant rats after neonatal treatment with a VEGF receptor inhibitor. Am J Physiol Lung Cell Mol Physiol 2004;287:L344–L351. [DOI] [PubMed] [Google Scholar]
- 56.Han RN, Babaei S, Robb M, Lee T, Ridsdale R, Ackerley C, Post M, Stewart DJ. Defective lung vascular development and fatal respiratory distress in endothelial NO synthase-deficient mice: a model of alveolar capillary dysplasia? Circ Res 2004;94:1115–1123. [DOI] [PubMed] [Google Scholar]
- 57.Le Cras TD, Kim DH, Markham NE, Abman AS. Early abnormalities of pulmonary vascular development in the fawn-hooded rat raised at Denver's altitude. Am J Physiol Lung Cell Mol Physiol 2000;279:L283–L291. [DOI] [PubMed] [Google Scholar]
- 58.Le Cras TD, Kim DH, Gebb S, Markham NE, Shannon JM, Tuder RM, Abman SH. Abnormal lung growth and the development of pulmonary hypertension in the fawn-hooded rat. Am J Physiol Lung Cell Mol Physiol 1999;277:L709–L718. [DOI] [PubMed] [Google Scholar]
- 59.Young SL, Evans K, Eu JP. Nitric oxide modulates branching morphogenesis in fetal rat lung explants. Am J Physiol Lung Cell Mol Physiol 2002;282:L379–L385. [DOI] [PubMed] [Google Scholar]
- 60.Wingrove JA, O'Farrell PH. Nitric oxide contributes to behavioral, cellular, and developmental responses to low oxygen in Drosophila. Cell 1999;98:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edwards YS, Sutherland LM, Murray AW. NO protects alveolar type II cells from stretch-induced apoptosis: a novel role for macrophages in the lung. Am J Physiol Lung Cell Mol Physiol 2000;279:L1236–L1242. [DOI] [PubMed] [Google Scholar]
- 62.Howlett CE, Hutchison JS, Veinot JP, Chiu A, Merchant P, Fliss H. Inhaled nitric oxide protects against hyperoxia-induced apoptosis in rat lungs. Am J Physiol Lung Cell Mol Physiol 1999;277:L596–L605. [DOI] [PubMed] [Google Scholar]
- 63.Yamashita H, Akamine S, Sumida Y, Inoue M, Sawada T, Nagayasu T, Oka T. Inhaled nitric oxide attenuates apoptosis in ischemia-reperfusion injury of the rabbit lung. Ann Thorac Surg 2004;78:292–297. [DOI] [PubMed] [Google Scholar]
- 64.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 2000;279:L600–L607. [DOI] [PubMed] [Google Scholar]
- 65.Le Cras TD, Markham NE, Tuder RM, Voelkel NF, Abman SH. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol Lung Cell Mol Physiol 2002;283:L555–L562. [DOI] [PubMed] [Google Scholar]
- 66.Morbidelli L, Chang CH, Douglas JG, Granger HJ, Ledda F, Ziche M. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endotheliuim. Am J Physiol Heart Circ Physiol 1996;270:H411–H415. [DOI] [PubMed] [Google Scholar]
- 67.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest 1997;100:3131–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest 1998;101:731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest 1997;99:2625–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dulak J, Jozkowicz A, Dembinska-Kiec A, Guevara I, Zdzienicka A, Zmudzinska-Grochot D, Florek I, Wojtowicz A, Szuba A, Cooke JP. Nitric oxide induces the synthesis of vascular endothelial growth factor by rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2000;20:659–666. [DOI] [PubMed] [Google Scholar]
- 71.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;164:1971–1980. [DOI] [PubMed] [Google Scholar]
- 72.Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H. Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol 2002;282:L811–L823. [DOI] [PubMed] [Google Scholar]
- 73.McCurnin D, Pierce R, Chang L, Gibson L, Osborne-Lawrence S, Yoder B, Kerecman J, Albertine K, Winter V, Coalson J, et al. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol 2005;288:L450–L459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.