Abstract
Rationale: An objective and simple prognostic model for patients with pulmonary embolism could be helpful in guiding initial intensity of treatment.
Objectives: To develop a clinical prediction rule that accurately classifies patients with pulmonary embolism into categories of increasing risk of mortality and other adverse medical outcomes.
Methods: We randomly allocated 15,531 inpatient discharges with pulmonary embolism from 186 Pennsylvania hospitals to derivation (67%) and internal validation (33%) samples. We derived our prediction rule using logistic regression with 30-day mortality as the primary outcome, and patient demographic and clinical data routinely available at presentation as potential predictor variables. We externally validated the rule in 221 inpatients with pulmonary embolism from Switzerland and France.
Measurements: We compared mortality and nonfatal adverse medical outcomes across the derivation and two validation samples.
Main Results: The prediction rule is based on 11 simple patient characteristics that were independently associated with mortality and stratifies patients with pulmonary embolism into five severity classes, with 30-day mortality rates of 0–1.6% in class I, 1.7–3.5% in class II, 3.2–7.1% in class III, 4.0–11.4% in class IV, and 10.0–24.5% in class V across the derivation and validation samples. Inpatient death and nonfatal complications were ⩽ 1.1% among patients in class I and ⩽ 1.9% among patients in class II.
Conclusions: Our rule accurately classifies patients with pulmonary embolism into classes of increasing risk of mortality and other adverse medical outcomes. Further validation of the rule is important before its implementation as a decision aid to guide the initial management of patients with pulmonary embolism.
Keywords: mortality, prognosis, pulmonary embolism
Acute pulmonary embolism (PE) is a major health problem, with an estimated incidence of 23 to 69 cases per 100,000 persons annually (1, 2). Data from the National Hospital Discharge Survey show that in 2002, 101,000 patients with a primary diagnosis of PE were hospitalized in acute care hospitals in the United States, resulting in 676,700 inpatient days (3). The short-term mortality of this illness varies widely, ranging from less than 2% in many patients with nonmassive PE to more than 95% in patients who experience cardiorespiratory arrest (4–6).
Despite this broad variability in short-term mortality, models for the risk stratification during admission for PE are not well established. An accurate, objective, and simple clinical prediction rule may be helpful in guiding medical decision making. For example, patients estimated to be at low risk could be discharged early or managed entirely as outpatients using low-molecular-weight heparin (7), whereas patients estimated as high risk may benefit from a more intensive surveillance in an intensive care setting. Previous models of risk stratification for PE were limited by their reliance on arterial blood gas values at room air and leg vein ultrasound (8, 9) or by their ability to identify only low-risk patients with PE (10). We therefore sought to develop a practical clinical prediction rule for patients with PE that quantifies the risk of mortality and other adverse medical outcomes across the full spectrum of risk and that relies only on readily available clinical parameters.
METHODS
Patient Identification and Eligibility
We identified patients with PE from January 2000 to November 2002 using the Pennsylvania Health Care Cost Containment Council (PHC4) database (11). We included inpatients 18 years or older who were discharged with a primary International Classification of Diseases, 9th Clinical Modification (ICD-9-CM) diagnosis of PE or a secondary diagnosis for PE and one of the following primary diagnoses that represent complications or treatments of PE: respiratory failure, cardiogenic shock, cardiac arrest, secondary pulmonary hypertension, syncope, thrombolysis, and intubation/mechanical ventilation. A detailed description of the methods is available in the online supplement.
Baseline Predictor Variables and Outcome Measures
The baseline clinical variables used to derive our prediction rule were obtained by linking all eligible patients identified using PHC4 to the MediQual Atlas database (11). To derive our prediction rule, we used clinical variables routinely available at presentation that were previously shown to be associated with mortality in patients with PE or other acute diseases. These variables included demographics (12–15), comorbid conditions (8, 12–14, 16), physical examination findings (8, 14, 15, 17, 18), and laboratory and chest x-ray findings (8, 14, 16, 19–24).
The study outcome used to derive our prediction rule was death from all causes within 30 days of hospitalization based on mortality data from the National Death Index (25). We also assessed whether patients developed severe nonfatal outcomes (cardiogenic shock/cardiorespiratory arrest) during hospitalization.
Derivation and Internal Validation of the Prediction Rule
The study cohort comprised 15,531 discharged patients with PE treated at 186 hospitals. We randomly selected 10,354 discharged patients (67%) for the derivation and 5,177 (33%) for the internal validation sample. We derived our prediction rule using stepwise logistic regression, with 30-day mortality as the outcome, and the demographic and clinical variables previously described as predictors. In a first step, we constructed our logistic regression model excluding laboratory variables. To quantify the impact of including laboratory tests on model performance, we also estimated a model that included baseline laboratory tests. On the basis of the β-coefficients of the model, we generated a point score that divides patients into five risk classes for 30-day mortality (class I, very low risk; class II, low risk; class III, intermediate risk; class IV, high risk; and class V, very high risk).
External Validation of the Prediction Rule
We validated our rule in an independent patient population using data from 221 inpatients prospectively diagnosed with PE, using spiral computed tomography, at three emergency departments at two university hospitals in Switzerland and one in France (26). Patients who had a contraindication to computed tomography or who were severely ill were not eligible for this study. Follow-up information about mortality, recurrent venous thromboembolism, and major bleeding was obtained by phone interviews of patients, family members, and/or primary care physicians, and hospital chart review.
Statistical Analyses
We compared risk class–specific mortality and rates of nonfatal adverse medical outcomes in the derivation sample to each validation sample using logistic regression with a robust variance estimator or exact χ2 tests. To assess the discriminatory power of our rule to predict 30-day mortality, we also compared the area under the receiver operating characteristic curves of the prediction rule (27).
RESULTS
Patients in the external validation sample had a lower prevalence of most comorbid illnesses and fewer abnormal findings on physical examination compared with those in the derivation and internal validation samples. This reflects the exclusion of more severely ill patients from the external validation sample (Table 1). Thirty-day mortality rates in the derivation, internal, and external validation samples were 9.2, 9.5, and 2.7%, respectively.
TABLE 1.
Comparison of baseline patient characteristics in the derivation and validation samples
| Patient Characteristics* | Derivation Sample, % (n = 10,354) |
Internal Validation Sample, % (n = 5,177) |
External Validation Sample, % (n = 221) |
|---|---|---|---|
| Demographic factors | |||
| Age > 65 yr | 52.8 | 53.1 | 59.3 |
| Male sex | 39.6 | 41.1 | 45.2 |
| Comorbid illnesses | |||
| Cancer | 19.9 | 19.0 | 15.8 |
| Heart failure | 16.1 | 15.3 | 11.8 |
| Chronic lung disease | 18.2 | 19.1 | 8.6 |
| Chronic renal disease | 4.4 | 4.2 | 4.5 |
| Cerebrovascular disease† | 8.9 | 9.9 | 4.5 |
| Clinical findings | |||
| Temperature < 36°C | 16.7 | 16.4 | 3.6 |
| Pulse ⩾ 110/min | 29.2 | 30.0 | 14.0 |
| Systolic blood pressure < 100 mm Hg | 10.6 | 10.2 | 1.8 |
| Respiratory rate ⩾ 30/min | 14.5 | 14.7 | 10.9 |
| Altered mental status‡ | 6.9 | 8.1 | 0 |
| Arterial oxygen saturation < 90%§ | 8.0 | 7.8 | 5.9 |
| Laboratory findings | |||
| Hemoglobin < 12 g/dl | 30.4 | 29.9 | Not available |
| White blood cell count < 4,000 or > 12,000/mm3 | 27.6 | 28.3 | Not available |
| Platelets < 100,000/mm3 | 3.0 | 2.5 | Not available |
| Sodium < 130 or > 150 mmol/L | 2.4 | 2.5 | Not available |
| Blood urea nitrogen ⩾ 11 mmol/L (30 mg/dl) | 13.5 | 13.0 | Not available |
| Creatinine > 177 μmol/L (2.0 mg/dl) | 4.7 | 4.6 | 0.9 |
| Arterial pH < 7.25 | 1.1 | 1.2 | 0.5 |
| PaCO2 < 25 or > 55 mm Hg | 2.9 | 2.9 | 2.3 |
In the derivation and internal validation sample, 1.9% of patients had unknown values for temperature; 1.7% for pulse; 1.4% for systolic blood pressure; 1.9% for respiratory rate; 64.6% for arterial oxygen saturation; 8.1% for hemoglobin; 8.4% for white blood cell count; 9.2% for platelet count; 11.6% for sodium, blood urea nitrogen, and creatinine; 62.0% for arterial pH; and 62.5% for PaCO2. Comorbid conditions were coded as present versus unknown. In the external validation sample, 4.5% of patients had unknown values for temperature, 3.2% for respiratory rate, 45.2% for oxygen saturation, 0.5% for creatinine, 59.7% for pH, and 1.4% for PaCO2. Chronic renal disease and cerebrovascular disease were coded as present versus unknown. For calculating the frequency of baseline patient characteristics, unknown values were assumed to be normal and were included in the denominator.
Defined as transient ischemic attack or stroke.
Defined as disorientation, lethargy, stupor, or coma. Information about mental status was not recorded in the external validation sample. Because patients with cognitive impairment were excluded from the study, mental status was assumed to be normal in all patients in this sample.
With and without administration of supplemental oxygen.
Derivation of the Prediction Rule
The 11 patient factors independently associated with 30-day mortality included two demographic characteristics (age, male sex), three comorbid illnesses (cancer, heart failure, chronic lung disease), and six clinical findings (pulse ⩾ 110/minute, systolic blood pressure < 100 mm Hg, respiratory rate ⩾ 30/minute, temperature < 36°C, altered mental status, and oxygen saturation < 90 mm Hg; Table 2). The scoring system shown in Table 2 was used to quantify the magnitude of the association of each of these 11 factors with mortality. These associations changed only minimally if the 5.5% of discharged patients with recurrent PE during the study period or the 1.8% who were identified using primary ICD-9-CM codes for PE complications or treatments were excluded from the analysis. In the derivation sample, risk class–specific 30-day mortality was 1.1% in risk class I, 3.1% in risk class II, 6.5% in risk class III, 10.4% in risk class IV, and 24.5% in risk class V.
TABLE 2.
Independent predictors of 30-DAY mortality in the derivation sample and points assigned to the risk score
| Predictors | β-Coefficients (95% CI) |
Points Assigned |
|---|---|---|
| Demographic characteristics | ||
| Age, per yr | 0.03 (0.02–0.03) | Age, in yr |
| Male sex | 0.17 (0.02–0.32) | +10 |
| Comorbid illnesses | ||
| Cancer | 0.87 (0.71–1.03) | +30 |
| Heart failure | 0.31 (0.14–0.49) | +10 |
| Chronic lung disease | 0.30 (0.12–0.47) | +10 |
| Clinical findings | ||
| Pulse ⩾ 110/min | 0.60 (0.44–0.76) | +20 |
| Systolic blood pressure < 100 mm Hg | 0.86 (0.67–1.04) | +30 |
| Respiratory rate ⩾ 30/min | 0.41 (0.23–0.58) | +20 |
| Temperature < 36°C | 0.42 (0.25–0.59) | +20 |
| Altered mental status* | 1.50 (1.30–1.69) | +60 |
| Arterial oxygen saturation < 90%† | 0.58 (0.37–0.79) | +20 |
Definition of abbreviation: CI = confidence interval.
A total point score for a given patient is obtained by summing the patient's age in years and the points for each applicable characteristic. Points assignments correspond with the following risk classes: ⩽ 65 class I, very low risk; 66–85 class II, low risk; 86–105 class III, intermediate risk; 106–125 class IV, high risk; > 125 class V, very high risk.
Defined as disorientation, lethargy, stupor, or coma.
With and without the administration of supplemental oxygen.
When laboratory variables also were assessed as potential predictors in the logistic regression model, all of the demographic and clinical variables in the simpler model except heart failure remained independently associated with 30-day mortality. In addition, seven laboratory variables (hemoglobin < 12 g/dl, white blood cell count < 4,000 or > 12,000/mm3, platelets < 100,000/mm3, sodium < 130 or > 150 mmol/L, blood urea nitrogen ⩾ 11 mmol/L [30 mg/dl], arterial pH < 7.25, and PaCO2 < 25 or > 55 mm Hg) were independently associated with mortality. Although this more complex, 17-variable model had a higher discriminatory power than the 11-variable model without laboratory variables (area under the receiver operating characteristic curve, 0.82 vs. 0.78; p < 0.001), the risk class–specific 30-day mortality rates for the more complex model (0.8% in risk class I, 2.5% in class II, 4.3% in class III, 9.9% in class IV, and 27.1% in class V) were similar to those for the simpler model.
Validation of the Prediction Rule
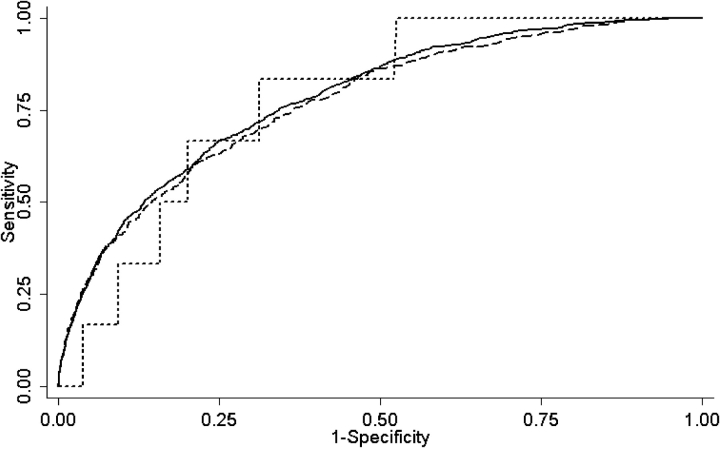
The prediction rule classified similar proportions of patients in each of the five risk classes across the derivation and internal validation samples (Table 3). In the external validation sample, a higher proportion of patients was classified in risk classes I–III and a lower proportion was classified in risk classes IV and V, reflecting the exclusion of severely ill patients from this sample. Thirty-day mortality rates in each risk class were not significantly different between the derivation and the validation samples; in the internal validation sample, these rates ranged from 1.6% in the very-low-risk (class I) patients to 23.9% in the very-high-risk (class V) patients (Table 4). The 30-day mortality was lower overall in the external validation sample and ranged from 0% among patients in risk class I to 10.0% among patients in class V. The rule's discriminatory power for 30-day mortality was nearly identical in the derivation and internal and external validation samples, with an area under the receiver operating characteristic curve of 0.78, 0.77, and 0.79, respectively (Figure 1).
TABLE 3.
Risk class distributions in the derivation and validation samples
| Risk Class | Derivation Sample, % (95% CI) (n = 10,354) |
Internal Validation Sample, % (95% CI) (n = 5,177) | External Validation Sample, % (95% CI) (n = 221) |
|---|---|---|---|
| Class I, very low risk | 19.4 (18.7–20.2) | 19.6 (18.5–20.7) | 24.4 (18.9–30.7) |
| Class II, low risk | 21.5 (20.7–22.3) | 21.2 (20.1–22.4) | 27.1 (21.4–33.5) |
| Class III, intermediate risk | 21.7 (20.9–22.5) | 22.2 (21.0–23.3) | 28.1 (22.2–34.5) |
| Class IV, high risk | 16.4 (15.7–17.1) | 15.8 (14.8–16.8) | 11.3 (7.5–16.2) |
| Class V, very high risk | 21.0 (20.3–21.8) | 21.3 (20.2–22.4) | 9.0 (5.6–13.6) |
For definition of abbreviation, see Table 2.
TABLE 4.
Risk class–specific medical outcomes in the derivation and validation samples
| Medical Outcomes | Derivation Sample, % (95% CI) (n = 10,354) |
Internal Validation Sample, % (95% CI) (n = 5,177) |
External Validation Sample, % (95% CI) (n = 221) |
p Value§ | p Value‖ |
|---|---|---|---|---|---|
| 30-day mortality | |||||
| Class I | 1.1 (0.7–1.7) | 1.6 (0.9–2.6) | 0 (0–6.6) | 0.32 | 0.66 |
| Class II | 3.1 (2.5–4.0) | 3.5 (2.5–4.7) | 1.7 (0–8.9) | 0.63 | 0.72 |
| Class III | 6.5 (5.5–7.6) | 7.1 (5.7–8.7) | 3.2 (0.4–11.2) | 0.51 | 0.43 |
| Class IV | 10.4 (9.0–11.9) | 11.4 (9.3–13.8) | 4.0 (0.1–20.4) | 0.44 | 0.36 |
| Class V | 24.5 (22.7–26.4) | 23.9 (21.4–26.5) | 10.0 (1.2–31.7) | 0.69 | 0.19 |
| Inpatient mortality* | |||||
| Class I | 0.8 (0.5–1.3) | 1.1 (0.5–1.9) | 0 (0–6.6) | 0.42 | 1.00 |
| Class II | 1.8 (1.3–2.4) | 1.9 (1.2–2.9) | Not available‡ | 0.75 | — |
| Class III | 4.2 (3.4–5.1) | 4.7 (3.6–6.1) | Not available‡ | 0.49 | — |
| Class IV | 5.9 (4.8–7.1) | 7.0 (5.3–9.0) | Not available‡ | 0.31 | — |
| Class V | 15.8 (14.3–17.4) | 17.2 (15.1–19.6) | Not available‡ | 0.29 | — |
| Nonfatal cardiogenic shock or cardiorespiratory arrest† |
|||||
| Class I | 0.6 (0.3–1.0) | 1.0 (0.5–1.8) | Not available‡ | 0.24 | — |
| Class II | 1.3 (0.9–1.9) | 1.2 (0.6–2.0) | Not available‡ | 0.77 | — |
| Class III | 2.1 (1.6–2.8) | 2.0 (1.3–3.0) | Not available‡ | 0.86 | — |
| Class IV | 1.9 (1.3–2.7) | 2.1 (1.2–3.3) | Not available‡ | 0.82 | — |
| Class V | 4.6 (3.8–5.6) | 5.3 (4.0–6.8) | Not available‡ | 0.40 | — |
For definition of abbreviation, see Table 2.
Patients who remained in the hospital for > 30 days were censored at 30 days.
During the initial hospital stay only.
Inpatient complications such as death, cardiogenic shock, and cardiorespiratory arrest were not explicitly recorded in the external validation sample.
For comparisons of outcomes in the derivation and internal validation samples.
For comparisons of outcomes in the derivation and external validation samples.
Figure 1.
Receiver operating characteristic curves for 30-day mortality in the derivation and validation samples. The area under the receiver operating characteristic curves were 0.78 (95% confidence interval [CI], 0.77–0.80) in the derivation sample, 0.77 (95% CI, 0.75–0.79) in the internal validation sample, and 0.79 (95% CI, 0.65–0.93) in the external validation sample (derivation vs. internal validation sample, p = 0.35; derivation vs. external validation sample, p = 0.93). Solid line = derivation; dashed line = internal validation; dotted line = external validation.
Inpatient mortality rates in risk class I (where comparable data were available) were similar across the three study samples (Table 4). The rates of nonfatal cardiogenic shock or cardiorespiratory arrest in each risk class in the derivation and internal validation samples also were similar, and ranged from 0.6 to 1.0% in class I to 4.6 to 5.3% in class V.
In the external validation sample, no patients in class I or II had nonfatal recurrent venous thromboembolism or major bleeding at 30 days after presentation. Overall, six patients died during follow-up in the external validation sample (three died of PE, one died of possible PE, and one from major bleeding). The rates of nonfatal recurrent venous thromboembolism were 1.6% in class III, 0% in class IV, and 0% in class V. The rates of nonfatal major bleeding were 1.6% in class III, 8.0% in class IV, and 0% in class V.
DISCUSSION
In this study to develop a clinical prediction rule for prognosis of PE, we identified 11 clinical findings from the history and physical examination that classify patients into five risk classes of increasing risk of death and other adverse medical outcomes. When validated in a retrospectively identified internal validation sample and a prospectively identified external validation sample, the performance of the rule was highly reliable. In all three study samples, mortality increased in a stepwise fashion with increasing risk class, and no significant differences in risk class specific mortality were observed across risk classes I–V.
Our rule accurately identifies patients who are at low risk of fatal and nonfatal medical outcomes: class I and class II patients had a 30-day mortality of 1.6% or less and 3.5% or less, respectively. Nonfatal cardiogenic shock or cardiorespiratory arrest occurred in 1.0% or less of patients in class I and 1.3% or less in class II, and no patient in these two risk classes had nonfatal major bleeding or recurrent venous thromboembolism. Recent evidence suggests that many patients with nonmassive PE can be safely treated entirely as outpatients using low-molecular-weight heparins or discharged early (28–32). On the basis of this evidence, the British Thoracic Society recommends outpatient treatment for clinically stable patients with PE (7). Thus, our rule provides clinicians with an explicit tool for identifying very-low-risk (class I) and low-risk (class II) patients with PE who may be potential candidates for outpatient treatment or early hospital discharge. If applied on a health system or national level, outpatient treatment or early discharge of only a small proportion of patients with PE is likely to result in substantial cost savings (33). However, it is important to note that our rule is intended to supplement, not replace, clinical judgment. The initial site of treatment decision for patients with PE must also consider psychosocial contraindications to outpatient care (e.g., frailty, lack of treatment adherence due to psychiatric or substance abuse problems) or the availability of outpatient systems of health care. Likewise, physicians would be unlikely to discharge a previously healthy 40-year-old woman who has severe hypoxemia and no additional pertinent prognostic factors, even if she was classified as very low risk (class I) by the rule.
Our rule also accurately identifies patients who are at higher risk of short-term death and other adverse medical outcomes. Patients in class V had 30-day mortality rates of up to 24.5% and rates of nonfatal cardiogenic shock or cardiorespiratory arrest of up to 5.3%. Whether these high-risk patients with PE could potentially benefit from more intensive forms of surveillance and care (e.g., in an intensive care unit setting) remains to be shown.
Our prediction rule has several distinctive strengths compared with a prior model of prognosis after PE (8, 9). First, it consists of clearly defined, routinely available predictors and does not require any laboratory tests or radiographic procedures not routinely performed in the management of PE. Second, the accuracy and generalizability of the rule are supported by its derivation (patients in the United States only) and validation in 15,752 patients from 189 hospitals in the United States, Switzerland, and France. Third, our study samples represent a broad disease spectrum, ranging from nonmassive PE to PE with cardiorespiratory arrest.
Our study also has potential limitations. First, patients in our derivation and internal validation samples were identified using ICD-9-CM codes for PE rather than standardized radiographic criteria, and therefore patient eligibility may be subject to study selection biases due to hospital coding procedures. However, two prior studies demonstrated that up to 96% of patients with specific ICD-9-CM codes for PE had objectively documented disease on the basis of chart review criteria (34–36). Furthermore, we cannot rule out the possibility that patients who were identified using a secondary ICD-9-CM code for PE with a primary diagnosis of possible PE complications (e.g., cardiogenic shock) actually developed PE as a consequence of one of these other conditions. Yet, the performance of our rule did not change when these patients were excluded from our analyses. Second, the study used to externally validate our rule was not originally designed for this task, and information about mental status was not explicitly recorded (26). Although it is very unlikely that more than a few patients had an altered mental status in the external validation sample, we cannot exclude the possibility that disease severity may have been underestimated in these patients. The external validation sample also excluded more severely ill patients (e.g., those who were hemodynamically unstable). As a result, there were fewer higher risk patients in this sample and the 95% confidence interval for risk class–specific mortality was relatively wide among patients in risk classes IV and V. Finally, we could not assess the occurrence of recurrent venous thromboembolism and major bleeding in the derivation and internal validation samples and cardiogenic shock or cardiorespiratory arrest in the external validation sample because these complications were not reliably documented in our databases.
In conclusion, we derived and validated a practical bedside tool for risk stratification that accurately classifies patients with PE at increasing risk of death and other adverse outcomes. Outpatient management or early hospital discharge of patients with PE identified as very low risk (class I) and low risk (class II) has the potential to result in large cost savings without added risk to patients. However, before this rule can be implemented into clinical practice, its clinical usefulness should be tested in a prospective study.
Supplementary Material
Supported by a grant from the National Heart, Lung, and Blood Institute (1 R21 HL075521-01A1). D.A. was supported by the Swiss Foundation in Medicine and Biology and the Swiss Medical Association, and the Clinical Epidemiology Center, University of Lausanne. M.J.F. was supported in part by a career development award (K24 AI001769) from the National Institute of Allergy and Infectious Diseases.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Anderson FA Jr, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, Forcier A, Dalen JE. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism: the Worcester DVT Study. Arch Intern Med 1991;151:933–938. [PubMed] [Google Scholar]
- 2.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ III. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 1998;158:585–593. [DOI] [PubMed] [Google Scholar]
- 3.Kozak LJ, Owings MF, Hall MJ. National hospital discharge survey: 2002 annual summary with detailed diagnosis and procedure data. National Center for Health Statistics. Vital Health Stat 13 2005;158:1–199. [PubMed] [Google Scholar]
- 4.Kurkciyan I, Meron G, Sterz F, Janata K, Domanovits H, Holzer M, Berzlanovich A, Bankl HC, Laggner AN. Pulmonary embolism as a cause of cardiac arrest: presentation and outcome. Arch Intern Med 2000;160:1529–1535. [DOI] [PubMed] [Google Scholar]
- 5.Simonneau G, Sors H, Charbonnier B, Page Y, Laaban JP, Azarian R, Laurent M, Hirsch JL, Ferrari E, Bosson JL, et al. A comparison of low-molecular-weight heparin with unfractionated heparin for acute pulmonary embolism. The THESEE Study Group (Tinzaparine ou Heparine Standard: Evaluations dans l'Embolie Pulmonaire). N Engl J Med 1997;337:663–669. [DOI] [PubMed] [Google Scholar]
- 6.Buller HR, Davidson BL, Decousus H, Gallus A, Gent M, Piovella F, Prins MH, Raskob G, van den Berg-Segers AE, Cariou R, et al. Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med 2003;349:1695–1702. [DOI] [PubMed] [Google Scholar]
- 7.British Thoracic Society. British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax 2003;58:470–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wicki J, Perrier A, Perneger TV, Bounameaux H, Junod AF. Predicting adverse outcome in patients with acute pulmonary embolism: a risk score. Thromb Haemost 2000;84:548–552. [PubMed] [Google Scholar]
- 9.Nendaz MR, Bandelier P, Aujesky D, Cornuz J, Roy PM, Bounameaux H, Perrier A. Validation of a risk score identifying patients with acute pulmonary embolism, who are at low risk of clinical adverse outcome. Thromb Haemost 2004;91:1232–1236. [DOI] [PubMed] [Google Scholar]
- 10.Aujesky D, Obrosky DS, Stone RA, Auble TE, Perrier A, Cornuz J, Roy PM, Fine MJ. A prediction rule to identify low-risk patients with pulmonary embolism. Arch Intern Med (In press) [DOI] [PubMed]
- 11.Auble TE, Hsieh M, Gardner W, Cooper GF, Stone RA, McCausland JB, Yealy DM. A prediction rule to identify low-risk patients with heart failure. Acad Emerg Med 2005;12:514–521. [DOI] [PubMed] [Google Scholar]
- 12.Carson JL, Kelley MA, Duff A, Weg JG, Fulkerson WJ, Palevsky HI, Schwartz JS, Thompson BT, Popovich J Jr, Hobbins TE, et al. The clinical course of pulmonary embolism. N Engl J Med 1992;326:1240–1245. [DOI] [PubMed] [Google Scholar]
- 13.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ III. Predictors of survival after deep vein thrombosis and pulmonary embolism: a population-based, cohort study. Arch Intern Med 1999;159:445–453. [DOI] [PubMed] [Google Scholar]
- 14.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999;353:1386–1389. [DOI] [PubMed] [Google Scholar]
- 15.Grifoni S, Olivotto I, Cecchini P, Pieralli F, Camaiti A, Santoro G, Conti A, Agnelli G, Berni G. Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation 2000;101:2817–2822. [DOI] [PubMed] [Google Scholar]
- 16.Giannitsis E, Muller-Bardorff M, Kurowski V, Weidtmann B, Wiegand U, Kampmann M, Katus HA. Independent prognostic value of cardiac troponin T in patients with confirmed pulmonary embolism. Circulation 2000;102:211–217. [DOI] [PubMed] [Google Scholar]
- 17.Peres Bota D, Lopes Ferreira F, Melot C, Vincent JL. Body temperature alterations in the critically ill. Intensive Care Med 2004;30:811–816. [DOI] [PubMed] [Google Scholar]
- 18.Kanich W, Brady WJ, Huff JS, Perron AD, Holstege C, Lindbeck G, Carter CT. Altered mental status: evaluation and etiology in the ED. Am J Emerg Med 2002;20:613–617. [DOI] [PubMed] [Google Scholar]
- 19.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002;87:978–982. [DOI] [PubMed] [Google Scholar]
- 20.Hodkinson HM. Value of admission profile tests for prognosis in elderly patients. J Am Geriatr Soc 1981;29:206–210. [DOI] [PubMed] [Google Scholar]
- 21.Lee CT, Guo HR, Chen JB. Hyponatremia in the emergency department. Am J Emerg Med 2000;18:264–268. [DOI] [PubMed] [Google Scholar]
- 22.Terzian C, Frye EB, Piotrowski ZH. Admission hyponatremia in the elderly: factors influencing prognosis. J Gen Intern Med 1994;9:89–91. [DOI] [PubMed] [Google Scholar]
- 23.Steen PM, Brewster AC, Bradbury RC, Estabrook E, Young JA. Predicted probabilities of hospital death as a measure of admission severity of illness. Inquiry 1993;30:128–141. [PubMed] [Google Scholar]
- 24.Konstantinides S, Geibel A, Olschewski M, Kasper W, Hruska N, Jackle S, Binder L. Importance of cardiac troponins I and T in risk stratification of patients with acute pulmonary embolism. Circulation 2002;106:1263–1268. [DOI] [PubMed] [Google Scholar]
- 25.MacMahon B. The national death index. Am J Public Health 1983;73:1247–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perrier A, Roy PM, Aujesky D, Chagnon I, Howarth N, Gourdier AL, Leftheriotis G, Barghouth G, Cornuz J, Hayoz D, et al. Diagnosing pulmonary embolism in outpatients with clinical assessment, D-dimer measurement, venous ultrasound, and helical computed tomography: a multicenter management study. Am J Med 2004;116:291–299. [DOI] [PubMed] [Google Scholar]
- 27.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- 28.Wells PS, Kovacs MJ, Bormanis J, Forgie MA, Goudie D, Morrow B, Kovacs J. Expanding eligibility for outpatient treatment of deep venous thrombosis and pulmonary embolism with low-molecular-weight heparin: a comparison of patient self-injection with homecare injection. Arch Intern Med 1998;158:1809–1812. [DOI] [PubMed] [Google Scholar]
- 29.Kovacs MJ, Anderson D, Morrow B, Gray L, Touchie D, Wells PS. Outpatient treatment of pulmonary embolism with dalteparin. Thromb Haemost 2000;83:209–211. [PubMed] [Google Scholar]
- 30.Lim AY, Parr DG, Stableforth DE, Fellows M, Fontaine R, Fegan CD. Early discharge and home supervision of patients with pulmonary embolism treated with low-molecular weight heparin. Eur J Intern Med 2003;14:89–93. [DOI] [PubMed] [Google Scholar]
- 31.Beer JH, Burger M, Gretener S, Bernard-Bagattini S, Bounameaux H. Outpatient treatment of pulmonary embolism is feasible and safe in a substantial proportion of patients. J Thromb Haemost 2003;1:186–187. [DOI] [PubMed] [Google Scholar]
- 32.Wells PS, Anderson DR, Rodger MA, Forgie MA, Florack P, Touchie D, Morrow B, Gray L, O'Rourke K, Wells G, et al. A randomized trial comparing 2 low-molecular-weight heparins for the outpatient treatment of deep vein thrombosis and pulmonary embolism. Arch Intern Med 2005;165:733–738. [DOI] [PubMed] [Google Scholar]
- 33.Aujesky D, Smith KJ, Cornuz J, Roberts MS. Cost-effectiveness of low-molecular-weight heparin for treatment of pulmonary embolism. Chest (In press) [DOI] [PubMed]
- 34.White RH, Gettner S, Newman JM, Trauner KB, Romano PS. Predictors of rehospitalization for symptomatic venous thromboembolism after total hip arthroplasty. N Engl J Med 2000;343:1758–1764. [DOI] [PubMed] [Google Scholar]
- 35.White RH, Romano PS, Zhou H, Rodrigo J, Bargar W. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med 1998;158:1525–1531. [DOI] [PubMed] [Google Scholar]
- 36.Murin S, Romano PS, White RH. Comparison of outcomes after hospitalization for deep venous thrombosis or pulmonary embolism. Thromb Haemost 2002;88:407–414. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.